Exploring Information Exchange between Thesium chinense and Its Host Prunella vulgaris through Joint Transcriptomic and Metabolomic Analysis
Abstract
1. Introduction
2. Results
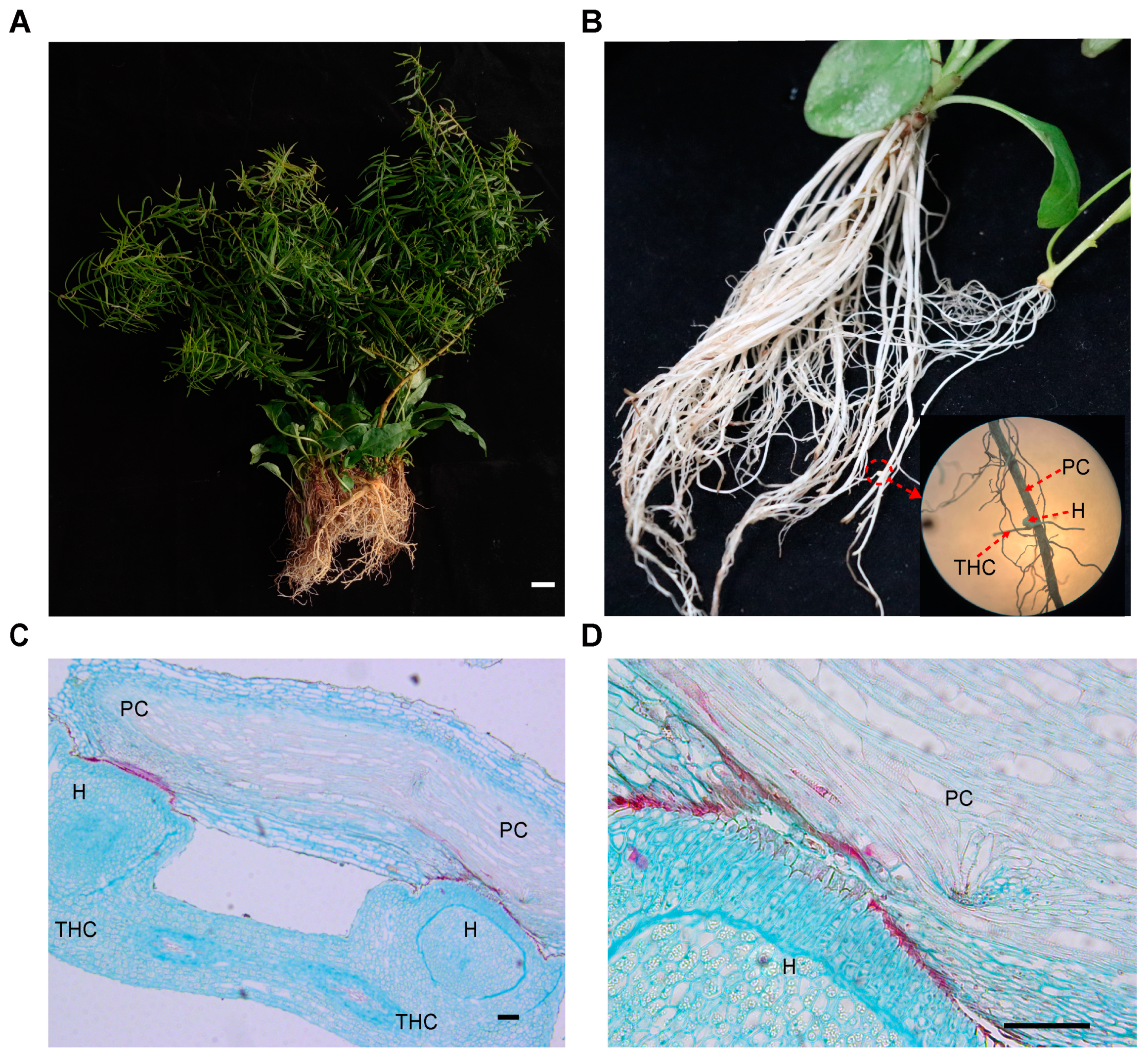
2.1. Root Morphology of T. chinense and Its Host P. vulgaris Post Parasition
2.2. Metabolomic Changes in T. chinense and Its Host P. vulgaris Post Symbiosis
2.3. The Exchanges of Metabolites between T. chinense and Its Host P. vulgaris during Parasitism
2.4. Transcriptomic Changes in T. chinense and Its Host P. vulgaris Post Symbiosis
2.5. The Mobile Genes between T. chinense and Its Host P. vulgaris
2.6. The Conjoint Analysis of Genes and Metabolites Related to Haustoria Formation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Collection
4.2. Metabolomic Analysis
4.3. Screening of Differentially Accumulated Metabolites
4.4. RNA Extraction, Library Construction and Sequencing
4.5. RNA-Seq Analysis
4.6. Integrated Metabolomic and Transcriptomic Analysis
4.7. Constructing the Network of Metabolites and Genes Related to Haustoria Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westwood, J.; Yoder, J.; Timko, M.; DePamphilis, C. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef]
- Goyet, V.; Wada, S.; Cui, S.; Wakatake, T.; Shirasu, K.; Montiel, G.; Simier, P.; Yoshida, S. Haustorium Inducing Factors for Parasitic Orobanchaceae. Front. Plant Sci. 2019, 10, 1056. [Google Scholar] [CrossRef]
- González-Fuente, M. Parasitic plants are one step ahead: Cuscuta responds transcriptionally to different hosts. Plant Physiol. 2023, 194, kiad524. [Google Scholar] [CrossRef]
- Leso, M.; Kokla, A.; Feng, M.; Melnyk, C. Pectin modifications promote haustoria development in the parasitic plant Phtheirospermum japonicum. Plant Physiol. 2023, 194, 343. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The Haustorium, a Specialized Invasive Organ in Parasitic Plants. Annu. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L.; García, M.A. Lacomucinaea, a new monotypic genus in Thesiaceae (Santalales). Phytotaxa 2015, 224, 173–184. [Google Scholar] [CrossRef]
- Parveen, Z.; Deng, Y.; Saeed, M.; Dai, R.; Ahamad, W.; Yu, Y. Antiinflammatory and analgesic activities of Thesium chinense Turcz extracts and its major flavonoids, kaempferol and kaempferol-3-o-glucoside. Yakugaku Zasshi 2007, 127, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, J.; Deng, P.; Ren, F.; Li, N. Chemical Constituents of Thesium chinense Turcz and Their In Vitro Antioxidant, Anti-Inflammatory and Cytotoxic Activities. Molecules 2023, 28, 2685. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, C.; Ma, W.; Ma, J.; Liu, Z.; Ren, F.; Li, N. Antibacterial Activity of Thesium chinense Turcz Extract Against Bacteria Associated with Upper Respiratory Tract Infections. Infect. Drug Resist. 2023, 16, 5091–5105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, L.; Qi, K.; Jiang, L. Sensitivity test of effective extracts from Thesium chinense to seven kinds of bacteria. Guizhou Med. 2006, 6, 564–566. [Google Scholar]
- Liu, C.; Li, X.; Cheng, R.; Han, Z.; Yang, L.; Song, Z.; Wang, Z. Anti-oral common pathogenic bacterial active acetylenic acids from Thesium chinense Turcz. J. Nat. Med. 2018, 72, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, S.; Ming, L. Analgesic effect of Bairui buccal tablet on mice. J. Huaihai Med. 2001, 1, 17–18. [Google Scholar]
- Shao, L.; Sun, Y.; Liang, J.; Li, M.; Li, X. Decolorization affects the structural characteristics and antioxidant activity of polysaccharides from Thesium chinense Turcz: Comparison of activated carbon and hydrogen peroxide decolorization. Int. J. Biol. Macromol. 2020, 155, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Tang, D.; Bian, J.; Hu, S.; Hu, J.; Fan, Z. Therapeutic effects of Thesium chinense on adriamycin-induced nephropathy rats. J. Pharm. Pract. 2012, 30, 443–446. [Google Scholar]
- Chen, P.; Chen, X.; Wu, C.; Meng, Y.; Cao, J. Research progress on the development and utilization of Thesium chinense Turcz. Chin. Wild Plant Resour. 2020, 39, 48–52. [Google Scholar]
- Li, D.; Li, J.; Yuan, Y.; Zhou, J.; Xiao, Q.; Yang, T.; Li, Y.; Jiang, L.; Gao, H. Risk factors and prognosis of acute lactation mastitis developing into a breast abscess: A retrospective longitudinal study in China. PLoS ONE 2020, 17, e0273967. [Google Scholar] [CrossRef]
- Li, G.; Fang, K.; Yang, K.; Cheng, X.; Wang, X.; Shen, T.; Lou, H. Thesium chinense Turcz.: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2021, 273, 113950. [Google Scholar] [CrossRef]
- Suetsugu, K.; Kawakita, A.; Kato, M. Host range and selectivity of the hemiparasitic plant Thesium chinense (Santalaceae). Ann. Bot. 2008, 102, 49–55. [Google Scholar] [CrossRef]
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L. A Review of its Ethnopharmacology, Phytochemistry, Quality Control and Pharmacological Effects. Front. Pharmacol. 2022, 13, 903171. [Google Scholar] [CrossRef]
- David-Schwartz, R.; Runo, S.; Townsley, B.; Machuka, J.; Sinha, N. Long-distance transport of mRNA via parenchyma cells and phloem across the host-parasite junction in Cuscuta. New Phytol. 2008, 179, 1133–1141. [Google Scholar] [CrossRef]
- Roney, J.; Khatibi, P.; Westwood, J. Cross-species translocation of mRNA from host plants into the parasitic plant dodder. Plant Physiol. 2007, 143, 1037–1043. [Google Scholar] [CrossRef]
- LeBlanc, M.; Kim, G.; Patel, B.; Stromberg, V.; Westwood, J. Quantification of tomato and Arabidopsis mobile RNAs trafficking into the parasitic plant Cuscuta pentagona. New Phytol. 2013, 200, 1225–1233. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, J.; Lei, Y.; Xu, Y.; Wu, J. Between-Plant Signaling. Annu. Rev. Plant Biol. 2023, 74, 367–386. [Google Scholar] [CrossRef]
- Saucet, S.; Shirasu, K. Molecular Parasitic Plant-Host Interactions. PLoS Pathog. 2016, 12, e1005978. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Axtell, M. Small RNA warfare: Exploring origins and function of trans-species microRNAs from the parasitic plant Cuscuta. Curr. Opin. Plant Biol. 2019, 50, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, H.; Xu, R.; Liu, S.; Wei, J.; Guo, K.; Qiao, H.; Xu, C. Combined Metabolome and Transcriptome Analysis Highlights the Host’s Influence on Cistanche deserticola Metabolite Accumulation. Int. J. Mol. Sci. 2023, 24, 7968. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; LeBlanc, M.; Wafula, E.; dePamphilis, C.; Westwood, J. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 2014, 345, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shen, G.; Xu, Y.; Liu, H.; Zhang, J.; Li, S.; Li, J.; Zhang, C.; Qi, J.; Wang, L.; et al. Extensive Inter-plant Protein Transfer between Cuscuta Parasites and Their Host Plants. Mol. Plant 2019, 13, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Choi, D.; Shirasu, K.; Ichihashi, Y.; Hahn, Y. A novel RNA virus, Thesium chinense closterovirus 1, identified by high-throughput RNA-sequencing of the parasitic plant Thesium chinense. Acta Virol. 2022, 66, 206–215. [Google Scholar] [CrossRef]
- Ma, J.; Wei, J.; Chen, G.; Yan, X.; Sun, H.; Li, N. Extracts of Thesium chinense inhibit SARS-CoV-2 and inflammatio in vitro. Pharm. Biol. 2023, 61, 1446–1453. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Kusano, M.; Kobayashi, M.; Suetsugu, K.; Yoshida, S.; Wakatake, T.; Kumaishi, K.; Shibata, A.; Saito, K.; Shirasu, K. Transcriptomic and Metabolomic Reprogramming from Roots to Haustoria in the Parasitic Plant, Thesium chinense. Plant Cell Physiol. 2018, 59, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Li, C.; Thiombiano, B.; Rahimi, M.; Dong, L. Adaptation of the parasitic plant lifecycle: Germination is controlled by essential host signaling molecules. Plant Physiol. 2021, 185, 1292–1308. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, T.; Zhou, X.; Tang, X.; Gao, R.; Xu, L.; Wang, L.; Zhou, Z.; Lin, J.; Hu, Y. Network pharmacology based virtual screening of active constituents of Prunella vulgaris L. and the molecular mechanism against breast cancer. Sci. Rep. 2020, 10, 15730. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Inaba, S.; Suzaki, T.; Yoshida, S. Developing for nutrient uptake: Induced organogenesis in parasitic plants and root nodule symbiosis. Curr. Opin. Plant Biol. 2023, 76, 102473. [Google Scholar] [CrossRef] [PubMed]
- Wakatake, T.; Ogawa, S.; Yoshida, S.; Shirasu, K. An auxin transport network underlies xylem bridge formation between the hemi-parasitic plant Phtheirospermum japonicum and host Arabidopsis. Development 2020, 147, dev187781. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Wakatake, T.; Yoshida, S.; Takebayashi, Y.; Kasahara, H.; Wafula, E.; dePamphilis, C.; Namba, S.; Shirasu, K. Local Auxin Biosynthesis Mediated by a YUCCA Flavin Monooxygenase Regulates Haustorium Development in the Parasitic Plant Phtheirospermum japonicum. Plant Cell 2016, 28, 1795–1814. [Google Scholar] [CrossRef]
- Ashapkin, V.; Kutueva, L.; Aleksandrushkina, N.; Vanyushin, B.; Teofanova, D.; Zagorchev, L. Genomic and Epigenomic Mechanisms of the Interaction between Parasitic and Host Plants. Int. J. Mol. Sci. 2023, 24, 2647. [Google Scholar] [CrossRef]
- Aly, R.; Cholakh, H.; Joel, D.M.; Leibman, D.; Steinitz, B.; Zelcer, A.; Naglis, A.; Yarden, O.; Gal-On, A. Gene silencing of mannose 6-phosphate reductase in the parasitic weed Orobanche aegyptiaca through the production of homologous dsRNA sequences in the host plant. Plant Biotechnol. J. 2009, 7, 487–498. [Google Scholar] [CrossRef]
- Patel, T.; Williamson, J. Mannitol in Plants, Fungi, and Plant-Fungal Interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef]
- Rivera-Cuevas, Y.; Carruthers, V. The multifaceted interactions between pathogens and host ESCRT machinery. PLoS Pathog. 2023, 19, e1011344. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhao, Q.; Duan, H.; Bi, S.; Hao, X.; Xu, R.; Bai, R.; Yu, R.; Lu, W.; Bao, T.; et al. Large-scale mRNA transfer between Haloxylon ammodendron (Chenopodiaceae) and herbaceous root holoparasite Cistanche deserticola (Orobanchaceae). iScience 2022, 26, 105880. [Google Scholar] [CrossRef]
- Guo, C.; Qin, L.; Ma, Y.; Qin, J. Integrated metabolomic and transcriptomic analyses of the parasitic plant Cuscuta japonica Choisy on host and non-host plants. BMC Plant Biol. 2022, 22, 393. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Fraga, C.; Clowers, B.; Moore, R.; Zink, E. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Dai, B.; Luo, Y.; Ding, Y. Integrated analysis of multiple metabolome and transcriptome revealed the accumulation of flavonoids and associated molecular regulation mechanisms in Rubus chingii Hu at different developmental stages. Plant Physiol. Bioch. 2023, 204, 108085. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 27, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, R.; Zhang, Y.; Liao, Y.; Zhao, J.; Jia, Y.; Tan, M.; Xiang, Z. The phenotypic variation mechanisms of Atractylodes lancea post-cultivation revealed by conjoint analysis of rhizomic transcriptome and metabolome. Plant Physiol. Bioch. 2023, 203, 108025. [Google Scholar] [CrossRef] [PubMed]
- Olson, W.; Martorelli Di Genova, B.; Gallego-Lopez, G.; Dawson, A.; Stevenson, D.; Amador-Noguez, D.; Knoll, L. Dual metabolomic profiling uncovers Toxoplasma manipulation of the host metabolome and the discovery of a novel parasite metabolic capability. PLoS Pathog. 2020, 16, e1008432. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.; Haas, B.; Yassour, M.; Levin, J.; Thompson, D.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Mao, Y.; Yang, J.; Li, F.; Fu, H. Uncovering nutritional metabolites and candidate genes involved in flavonoid metabolism in Houttuynia cordata through combined metabolomic and transcriptomic analyses. Plant Physiol. Bioch. 2023, 203, 108059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liu, X.; Liu, L.; Hou, Y.; Han, Z.; Zhang, L. Integrated metabolomics and transcriptomic analysis reveals metabolic changes of flavor compounds of Camellia assamica host plant after parasitized by Viscum articulatum. Plant Physiol. Bioch. 2023, 205, 108157. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, D.; Fang, L.; Zhou, Q.; Liu, W.; Liu, Z. Bidirectional lncRNA transfer between Cuscuta parasites and their host plant. Int. J. Mol. Sci. 2022, 23, 561. [Google Scholar] [CrossRef]
- Hong, Z.; Peng, D.; Tembrock, L.; Liao, X.; Xu, D.; Liu, X.; Wu, Z. Chromosome-level genome assemblies from two sandalwood species provide insights into the evolution of the Santalales. Commun. Biol. 2023, 6, 587. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]




| Compounds | CAS | Category | Type | |
|---|---|---|---|---|
| THvsTHC | PvsPC | |||
| 2,3,19-Trihydroxyurs-12-en-28-oic acid | - | Terpenoids | up | up |
| Pinfaensic acid | - | Terpenoids | up | up |
| N,N′-Dimethylarginine; SDMA | 30344-00-4 | Amino acids and derivatives | up | up |
| N-Monomethyl-L-arginine | 17035-90-4 | Amino acids and derivatives | up | up |
| L-Isoleucyl-L-Aspartate | - | Amino acids and derivatives | up | up |
| L-Aspartyl-L-Phenylalanine | 13433-09-5 | Amino acids and derivatives | up | up |
| Candelabrone 12-methyl ether | - | Terpenoids | up | up |
| 19-Hydroxyursolic acid | - | Terpenoids | up | up |
| Homoarginine | 156-86-5 | Amino acids and derivatives | up | up |
| NG,NG-Dimethyl-L-arginine | 30315-93-6 | Amino acids and derivatives | up | up |
| 2-Deoxyribose-1-phosphate | 17210-42-3 | Nucleotides and derivatives | up | up |
| 6′-O-Feruloyl-D-sucrose | 118230-77-6 | Phenolic acids | up | up |
| Jasmonic acid | 77026-92-7 | Organic acids | up | up |
| 2-Acetoxymethyl-anthraquinone | - | Quinones | up | up |
| Propyl 4-hydroxybenzoate | 94-13-3 | Phenolic acids | up | up |
| 5-hydroxy-1-phenyl-7-3-heptanone | - | Others | up | up |
| 2,2-Dimethylsuccinic acid | 597-43-3 | Organic acids | up | up |
| L-Tartaric acid | 87-69-4 | Organic acids | up | down |
| Tachioside | 109194-60-7 | Phenolic acids | down | down |
| Isotachioside | 31427-08-4 | Phenolic acids | down | down |
| 1-O-Salicyloyl-β-D-glucose | 60517-74-0 | Phenolic acids | down | down |
| Salicylic acid-2-O-glucoside | 10366-91-3 | Phenolic acids | down | down |
| p-Hydroxypheny-β-D-allopyranoside | - | Phenolic acids | down | down |
| Arbutin | 497-76-7 | Phenolic acids | down | down |
| Sinapoyl malate | 92344-58-6 | Phenolic acids | down | down |
| 2-O-Caffeoylglucaric Acid | - | Phenolic acids | down | down |
| Oleic acid | 112-80-1 | Lipids | down | down |
| N-Methyl-Trans-4-Hydroxy-L-Proline | 4252-82-8 | Amino acids and derivatives | down | down |
| 2,6-Dimethoxy-4-hydroxyphenol-1-O-ß-D-glucopyranoside | - | Others | down | down |
| Methoxyindoleacetic acid | 3471-31-6 | Alkaloids | down | up |
| Tryptamine | 61-54-1 | Alkaloids | down | up |
| L-Tryptophan | 73-22-3 | Amino acids and derivatives | down | up |
| 3-Indoleacetonitrile | 771-51-7 | Alkaloids | down | up |
| 1-Methoxy-indole-3-acetamide | - | Alkaloids | down | up |
| Indole | 120-72-9 | Alkaloids | down | up |
| 3-Indolepropionic acid | 830-96-6 | Alkaloids | down | up |
| 3-Indoleacrylic acid | 1204-06-4 | Alkaloids | down | up |
| γ-glutamylmethionine | 17663-87-5 | Amino acids and derivatives | down | up |
| 2-Aminoethanesulfonic acid | 107-35-7 | Organic acids | down | up |
| p-Coumaric acid methyl ester | 19367-38-5 | Phenolic acids | down | up |
| Roseoside | 54835-70-0 | Others | down | up |
| Isoquinoline | 119-65-3 | Alkaloids | down | up |
| 4-caffeoylshikimic acid | - | Phenolic acids | down | up |
| L-Histidine | 71-00-1 | Amino acids and derivatives | down | up |
| Phlorizin | 60-81-1 | Flavonoids | down | up |
| 3,4-Methylenedioxy cinnamyl alcohol | 58095-76-4 | Lignans and Coumarins | down | up |
| Kaurenoic Acid | 6730-83-2 | Terpenoids | down | up |
| LysoPC 15:0 | 108273-89-8 | Lipids | down | up |
| Melibiose | 585-99-9 | Others | down | up |
| 3-amino-2-naphthoic acid | - | Alkaloids | down | up |
| L-Lysine-Butanoic Acid | 80407-71-2 | Amino acids and derivatives | down | up |
| cyclo-(Gly-Phe) | 10125-07-2 | Amino acids and derivatives | down | up |
| Trans-Citridic acid | 4023-65-8 | Organic acids | down | up |
| 1-O-Sinapoyl-β-D-glucose | - | Phenolic acids | down | up |
| Linarin | 480-36-4 | Flavonoids | down | up |
| Syringaresinol-4′-O-glucoside | 7374-79-0 | Lignans and Coumarins | down | up |
| Compounds | CAS | Category | TH | THC | PC | p |
|---|---|---|---|---|---|---|
| Ethylsalicylate | 118-61-6 | Phenolic acids | - | 35,397 | 500,949 | 542,212 |
| Eriodictyol-7-O-glucoside | 38965-51-4 | Flavonoids | - | 29,411 | 3,851,867 | 4,694,065 |
| Aromadendrin-7-O-glucoside | 28189-90-4 | Flavonoids | - | 113,418 | 1,191,540 | 604,233 |
| Pruvuloside B | - | Terpenoids | - | 1,789 | 54,923 | 40,759 |
| 2-Ethylpyrazine | 13925-00-3 | Alkaloids | 49,686 | 43,058 | 78,849 | - |
| Unigene ID | Gene | Annotation | FPKM | |||
|---|---|---|---|---|---|---|
| TH | THC | PC | p | |||
| T. chinense → P. vulgaris | ||||||
| Cluster-12122.6 | R10A | 60S ribosomal protein L10a | 924.6 | 1404.5 | 3.6 | 0.0 |
| Cluster-13284.0 | RAN1 | GTP-binding nuclear protein Ran1 | 452.4 | 907.1 | 8.0 | 0.0 |
| Cluster-14148.4 | TIC32 | Short-chain dehydrogenase TIC 32, chloroplastic | 982.1 | 2251.9 | 4.4 | 0.0 |
| Cluster-16015.3 | METK5 | S-adenosylmethionine synthase 5 | 433.0 | 3149.3 | 5.5 | 0.0 |
| Cluster-16686.0 | WRK40 | Probable WRKY transcription factor 40 | 1142.9 | 1043.8 | 10.9 | 0.0 |
| Cluster-16839.1 | SUNN | Leucine-rich repeat receptor-like kinase | 49.3 | 46.8 | 4.9 | 0.0 |
| Cluster-1723.0 | UNC13 | Protein unc-13 homolog | 32.9 | 46.3 | 6.2 | 0.0 |
| Cluster-18022.0 | ORM1 | ATORM1, OROSOMUCOID-LIKE 1 ORM1 | 125.2 | 148.4 | 6.4 | 0.0 |
| Cluster-22492.4 | PPA29 | Probable inactive purple acid phosphatase 29 | 917.8 | 1041.0 | 4.6 | 0.0 |
| Cluster-23922.0 | LTI6B | Hydrophobic protein LTI6B | 365.2 | 891.6 | 4.6 | 0.0 |
| Cluster-23995.0 | EMB8 | Embryogenesis-associated protein EMB8 | 88.3 | 49.9 | 11.0 | 0.0 |
| Cluster-24355.1 | KNAP3 | Homeobox protein knotted-1-like 3 | 257.7 | 238.1 | 5.9 | 0.1 |
| Cluster-24679.1 | BAGP1 | BAG-associated GRAM protein 1 | 37.1 | 74.0 | 3.7 | 0.0 |
| Cluster-24856.0 | C7A12 | Cytochrome P450 CYP736A12 | 174.8 | 199.3 | 3.2 | 0.0 |
| Cluster-25215.2 | EP1L3 | EP1-like glycoprotein 3 | 395.6 | 467.9 | 3.1 | 0.0 |
| Cluster-25707.0 | PMT1 | Probable methyltransferase PMT1 | 28.0 | 67.0 | 5.1 | 0.0 |
| Cluster-26296.0 | RTNLB | Reticulon-like protein B2 | 226.7 | 269.3 | 9.2 | 0.1 |
| Cluster-26397.0 | RL24 | 60S ribosomal protein L24 | 56.8 | 102.6 | 3.8 | 0.0 |
| Cluster-2641.0 | VP371 | Vacuolar protein-sorting-associated protein 37 | 104.5 | 151.3 | 11.6 | 0.0 |
| Cluster-27486.3 | KAD7 | Probable adenylate kinase 7, mitochondrial | 538.7 | 480.3 | 3.6 | 0.1 |
| Cluster-28157.0 | DG | DNA glycosylase superfamily protein | 7.6 | 92.6 | 9.6 | 0.0 |
| Cluster-28431.7 | ALFC6 | Fructose-bisphosphate aldolase 6, cytosolic | 706.9 | 1620.3 | 3.6 | 0.0 |
| Cluster-28487.3 | ADS3 | Palmitoyl-monogalactosyldiacylglycerol delta-7 desaturase | 1432.6 | 1611.5 | 3.3 | 0.0 |
| Cluster-30032.0 | GRP | Glycine-rich protein A3 | 2030.6 | 2754.2 | 4.5 | 0.0 |
| Cluster-31395.0 | TRAPPC3 | Transport protein particle (TRAPP) | 95.3 | 98.7 | 10.1 | 0.0 |
| Cluster-31481.0 | CAMT | Caffeoyl-CoA O-methyltransferase | 468.8 | 1485.6 | 5.5 | 0.0 |
| Cluster-34709.1 | IF5A | Eukaryotic translation initiation factor 5A | 1030.8 | 908.8 | 3.1 | 0.0 |
| Cluster-36607.0 | PRU1 | Major allergen Pru ar 1 | 2404.5 | 7930.2 | 8.7 | 0.3 |
| Cluster-37697.0 | TCTP | Translationally-controlled tumor protein | 8294.9 | 9520.8 | 5.7 | 0.1 |
| Cluster-4150.5 | NIN1 | Neutral/alkaline invertase 1, mitochondrial | 195.7 | 202.0 | 3.8 | 0.0 |
| Cluster-4583.0 | PER52 | Peroxidase 52 | 39.1 | 207.4 | 4.7 | 0.0 |
| Cluster-6216.6 | GBLP | Guanine nucleotide-binding protein subunit beta | 147.5 | 161.5 | 5.6 | 0.0 |
| Cluster-7222.7 | GSTUP | Glutathione S-transferase U25 | 333.6 | 304.3 | 4.4 | 0.0 |
| Cluster-7227.1 | RS202 | 40S ribosomal protein | 442.1 | 546.1 | 6.7 | 0.0 |
| Cluster-7844.0 | ZCF37 | ZCF37 AT1G10220; IMPGSAL1N27970 | 56.3 | 70.4 | 3.2 | 0.0 |
| Cluster-11481.5 | G6PD | Glucose-6-phosphate 1-dehydrogenase | 156.0 | 470.6 | 3.9 | 0.0 |
| Cluster-13110.1 | SD18 | Receptor-like serine/threonine-protein kinase | 73.9 | 94.1 | 13.7 | 0.0 |
| Cluster-23641.1 | XTH23 | Xyloglucan endotransglucosylase/hydrolase protein 23 | 460.3 | 981.2 | 3.8 | 0.0 |
| Cluster-25384.10 | IPYR4 | Soluble inorganic pyrophosphatase 4 | 458.4 | 438.7 | 3.4 | 0.0 |
| Cluster-26866.5 | ALA9 | Phospholipid-transporting ATPase 9 | 72.1 | 32.5 | 6.7 | 0.0 |
| Cluster-28356.0 | CH62 | Chaperonin CPN60-2, mitochondrial | 205.4 | 149.5 | 3.7 | 0.0 |
| Cluster-28583.0 | PMTE | Methyltransferase PMT14 | 65.8 | 104.8 | 3.3 | 0.0 |
| Cluster-29004.0 | RS11 | 40S ribosomal protein S11 | 246.9 | 542.3 | 9.9 | 0.0 |
| Cluster-29660.0 | COPD | Coatomer subunit delta | 110.9 | 159.9 | 3.0 | 0.0 |
| P. vulgaris → T. chinense | ||||||
| Cluster-73329.0 | EFTU | Elongation factor Tu, plastid | 0.1 | 14.6 | 4.3 | 4.9 |
| Cluster-83509.0 | RL401 | Ubiquitin-60S ribosomal protein | 0.3 | 4.1 | 5.7 | 17.0 |
| Cluster-86098.6 | BiP | Luminal-binding protein | 0.1 | 3.5 | 9.0 | 14.4 |
| Cluster-43539.64 | -- | -- | 0.3 | 3.5 | 5.9 | 23.1 |
| Cluster-92949.1 | PAT | Glutamate/aspartate-prephenate aminotransferase | 0.0 | 10.7 | 96.7 | 19.8 |
| Cluster-26621.39 | YCF68 | Uncharacterized protein | 0.7 | 65.8 | 18.8 | 3.4 |
| Unigene ID | Gene | Annotation | FPKM | |||
|---|---|---|---|---|---|---|
| TH | THC | PC | p | |||
| Cluster-28098.16 | 1433D_SOYBN | 14-3-3 protein | 277.4 | 291.9 | 0.2 | 0.0 |
| Cluster-30463.1 | ACT11_ARATH | Actin | 0.3 | 5.2 | 1.7 | 0.0 |
| Cluster-30642.1 | ACT1_ORYSI | Actin | 0.3 | 1.2 | 7.2 | 0.1 |
| Cluster-26673.1 | AMT11_SOLLC | Ammonium Transporter Family | 234.2 | 428.8 | 2.1 | 0.0 |
| Cluster-37206.1 | CAF1K_ARATH | CAF1 family ribonuclease | 294.3 | 412.9 | 0.2 | 0.0 |
| Cluster-28144.0 | CAES_ARATH | Carbohydrate esterase, sialic acid-specific acetylesterase | 34.6 | 179.0 | 1.2 | 0.0 |
| Cluster-40689.0 | CHIT_PERAE | Chitinase class I | 82.1 | 604.1 | 2.2 | 0.0 |
| Cluster-2905.1 | ALPL_ARATH | DDE superfamily endonuclease | 243.2 | 350.0 | 2.6 | 0.0 |
| Cluster-33686.3 | -- | Dehydrin | 3321 | 6625 | 4.0 | 0.1 |
| Cluster-25215.6 | EP1L4_ARATH | D-mannose binding lectin | 499.1 | 735.5 | 1.1 | 0.0 |
| Cluster-28520.1 | DUF4228 | Domain of unknown function | 309.4 | 405.8 | 0.3 | 0.0 |
| Cluster-31749.0 | DUF4723 | Domain of unknown function | 50.4 | 604.3 | 2.0 | 0.1 |
| Cluster-28199.1 | ESSS | ESSS subunit of NADH:ubiquinone oxidoreductase | 475.7 | 553.4 | 0.6 | 0.0 |
| Cluster-86868.1 | ERM | Ezrin/radixin/moesin family | 0.0 | 0.1 | 0.1 | 0.0 |
| Cluster-28808.1 | BBE21_ARATH | FAD binding domain | 80.6 | 336.6 | 1.5 | 0.0 |
| Cluster-31390.0 | CASL1_CANSA | FAD binding domain | 88.5 | 126.5 | 1.7 | 0.0 |
| Cluster-24876.1 | DUF716 | Family of unknown function | 15.3 | 18.8 | 0.7 | 0.0 |
| Cluster-26009.1 | FB119_ARATH | F-box-like | 34.9 | 118.7 | 0.2 | 0.0 |
| Cluster-20986.5 | GSTF_HYOMU | Glutathione S-transferase, C-terminal domain | 249.2 | 502.4 | 1.3 | 0.0 |
| Cluster-20103.7 | GADPH | Glyceraldehyde 3-phosphate dehydrogenase | 395 | 576.7 | 0.8 | 0.0 |
| Cluster-23641.1 | XTH23_ARATH | Glycosyl hydrolases family 16 | 460.3 | 981.2 | 3.8 | 0.0 |
| Cluster-26468.1 | ERLL1_ARATH | Hydrophobic seed protein | 136.7 | 490.7 | 0.1 | 0.0 |
| Cluster-29998.1 | LEA14_GOSHI | Late embryogenesis abundant protein | 425.5 | 842.2 | 0.2 | 0.0 |
| Cluster-28203.4 | GILP_ARATH | LITAF-like zinc ribbon domain | 67.1 | 80.9 | 0.1 | 0.0 |
| Cluster-27980.8 | FPPS1_LUPAL | Polyprenyl synthetase | 43.3 | 217.8 | 0.2 | 0.0 |
| Cluster-29223.2 | MSK3_MEDSA | Protein kinase domain | 204.5 | 265.7 | 1.2 | 0.0 |
| Cluster-26011.1 | CRK7_ARATH | Protein tyrosine kinase | 37.4 | 46.4 | 0.5 | 0.0 |
| Cluster-15047.1 | SPE1_PEA | Pyridoxal-dependent decarboxylase | 489.5 | 618.4 | 1.0 | 0.0 |
| Cluster-25493.2 | RL72_ARATH | Ribosomal L30 N-terminal domain | 323.6 | 331.5 | 0.3 | 0.0 |
| Cluster-28869.1 | RL72_ARATH | Ribosomal L30 N-terminal domain | 139.9 | 210.5 | 0.1 | 0.0 |
| Cluster-27350.3 | RL3_ORYSJ | Ribosomal protein L3 | 1144 | 1163 | 0.7 | 0.0 |
| Cluster-25988.1 | RL262_ARATH | Ribosomal proteins L26 | 382.7 | 481.3 | 0.3 | 0.0 |
| Cluster-29252.2 | RICI_RICCO | Ricin-type beta-trefoil lectin domain | 765.1 | 2370 | 1.4 | 0.0 |
| Cluster-29448.5 | CSE_ARATH | Serine aminopeptidase, S33 | 6.9 | 43.7 | 0.0 | 0.0 |
| Cluster-72533.2 | STC | Stanniocalcin family | 0.1 | 0.5 | 1.0 | 0.9 |
| Cluster-37697.0 | TCTP_ELAGV | Translationally controlled tumour protein | 8295 | 9521 | 5.7 | 0.1 |
| Cluster-25717.1 | SSRA_ARATH | Translocon-associated protein (TRAP) alpha | 89.6 | 106.1 | 0.9 | 0.0 |
| Cluster-27790.5 | LHT1_ARATH | Transmembrane amino acid transporter protein | 32.4 | 138.4 | 0.9 | 0.0 |
| Cluster-30943.0 | TBA_EUGGR | Tubulin C-terminal domain | 0.1 | 0.5 | 2.2 | 0.0 |
| Cluster-43188.0 | TBB_CHLIN | Tubulin/FtsZ family, GTPase domain | 0.4 | 1.7 | 4.4 | 0.1 |
| Cluster-26035.2 | U73D1_ARATH | UDP-glucoronosyl and UDP-glucosyl transferase | 53.1 | 120.9 | 0.2 | 0.0 |
| Cluster-44341.8 | ZFP | Zinc finger C-x8-C-x5-C-x3-H type | 0.0 | 0.1 | 0.3 | 0.0 |
| Cluster-27579.1 | EXLB1_ARATH | Expansin | 294.2 | 1724 | 1.2 | 0.0 |
| Cluster-92147.2 | ARI4_ARATH | E3 ubiquitin-protein ligase | 0.1 | 0.1 | 0.7 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, A.; Wang, R.; Liu, J.; Meng, W.; Zhang, Y.; Chen, G.; Hu, G.; Tan, M.; Xiang, Z. Exploring Information Exchange between Thesium chinense and Its Host Prunella vulgaris through Joint Transcriptomic and Metabolomic Analysis. Plants 2024, 13, 804. https://doi.org/10.3390/plants13060804
Ding A, Wang R, Liu J, Meng W, Zhang Y, Chen G, Hu G, Tan M, Xiang Z. Exploring Information Exchange between Thesium chinense and Its Host Prunella vulgaris through Joint Transcriptomic and Metabolomic Analysis. Plants. 2024; 13(6):804. https://doi.org/10.3390/plants13060804
Chicago/Turabian StyleDing, Anping, Ruifeng Wang, Juan Liu, Wenna Meng, Yu Zhang, Guihong Chen, Gang Hu, Mingpu Tan, and Zengxu Xiang. 2024. "Exploring Information Exchange between Thesium chinense and Its Host Prunella vulgaris through Joint Transcriptomic and Metabolomic Analysis" Plants 13, no. 6: 804. https://doi.org/10.3390/plants13060804
APA StyleDing, A., Wang, R., Liu, J., Meng, W., Zhang, Y., Chen, G., Hu, G., Tan, M., & Xiang, Z. (2024). Exploring Information Exchange between Thesium chinense and Its Host Prunella vulgaris through Joint Transcriptomic and Metabolomic Analysis. Plants, 13(6), 804. https://doi.org/10.3390/plants13060804







