Physiological Responses of a Grapefruit Orchard to Irrigation with Desalinated Seawater
Abstract
1. Introduction
2. Results and Discussion
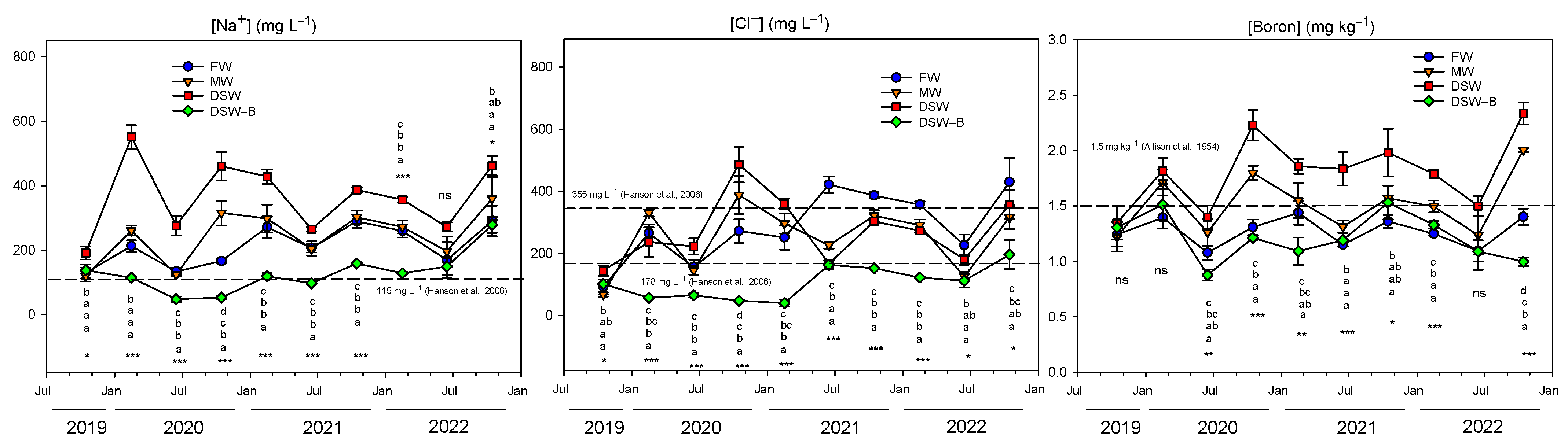
2.1. Irrigation Water Quality

2.2. Evolution of Toxic Elements in the Soil
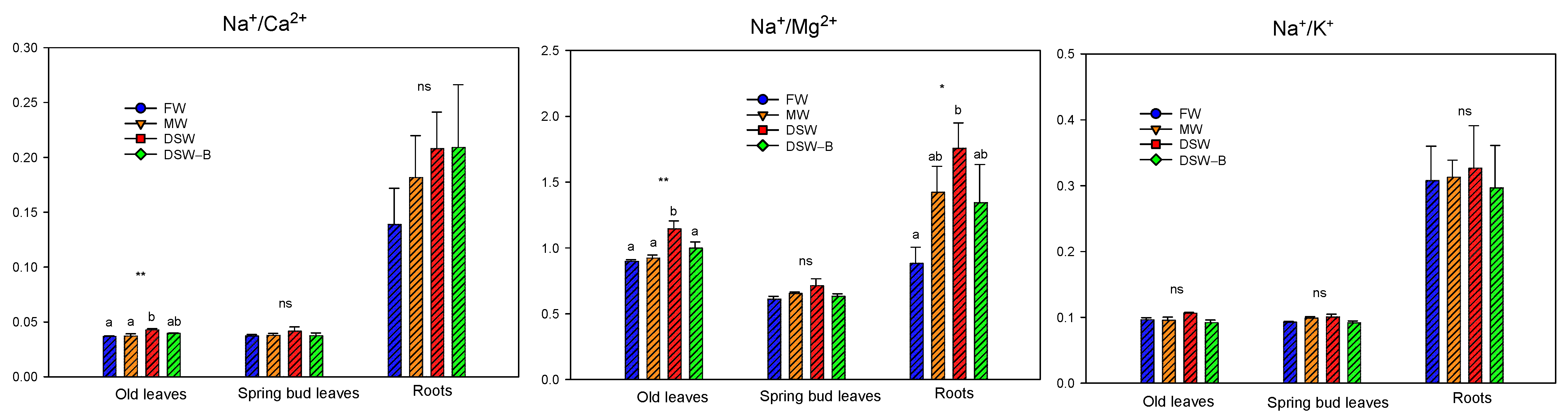
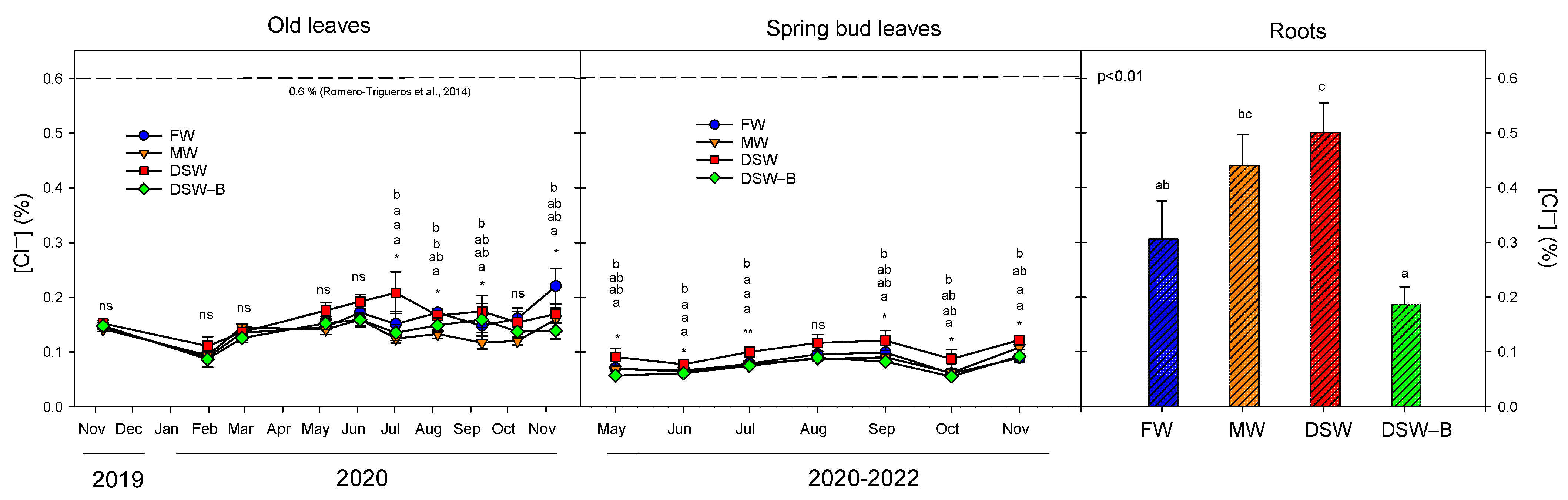
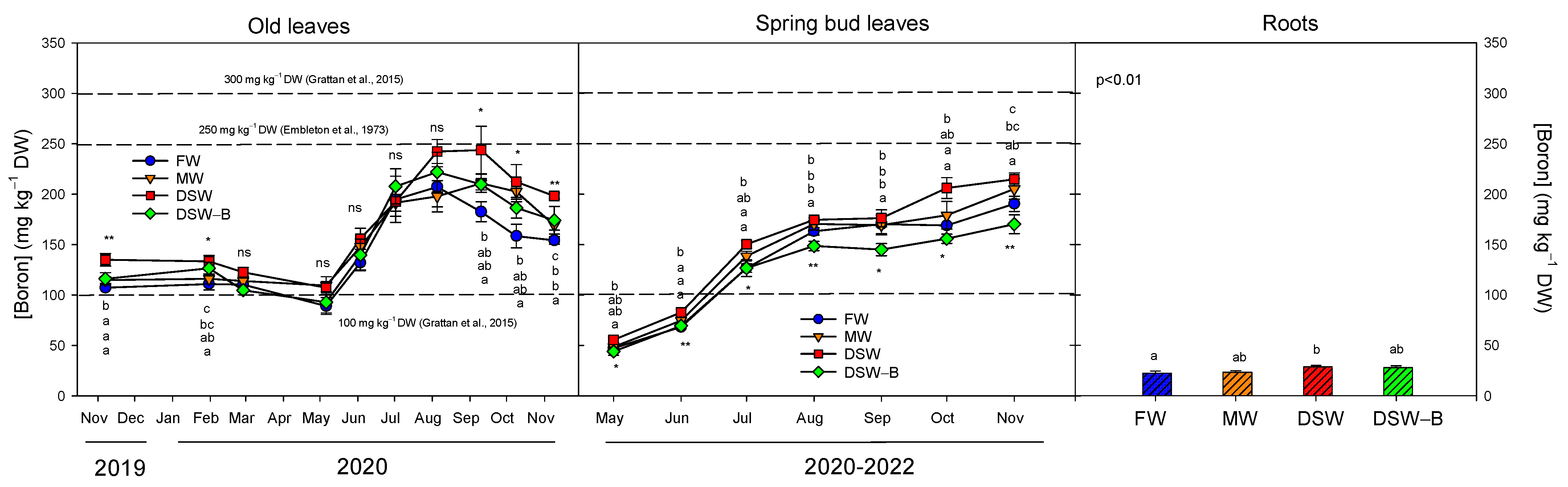
2.3. The Accumulation and Partitioning of Phytotoxic Elements in the Trees
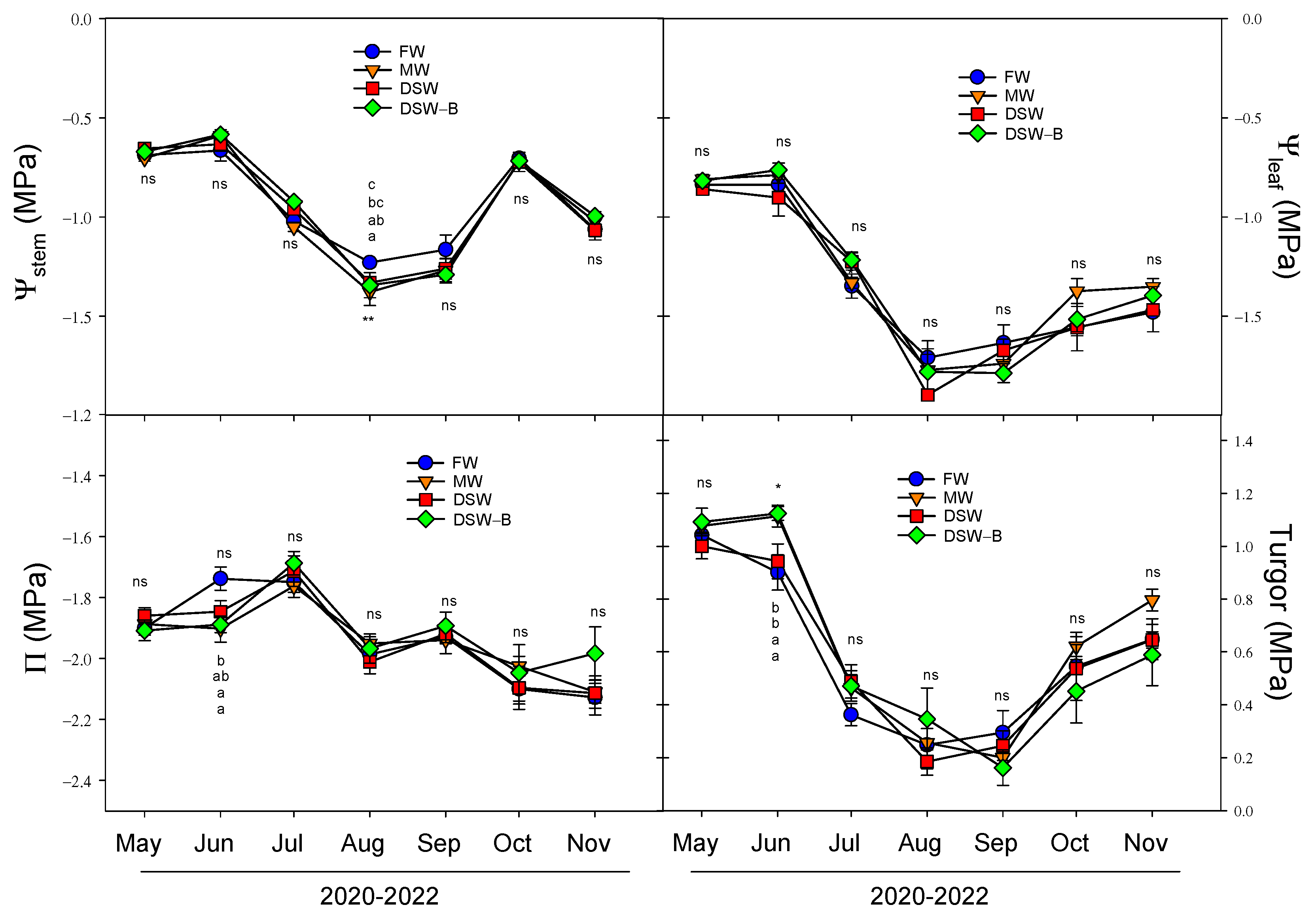
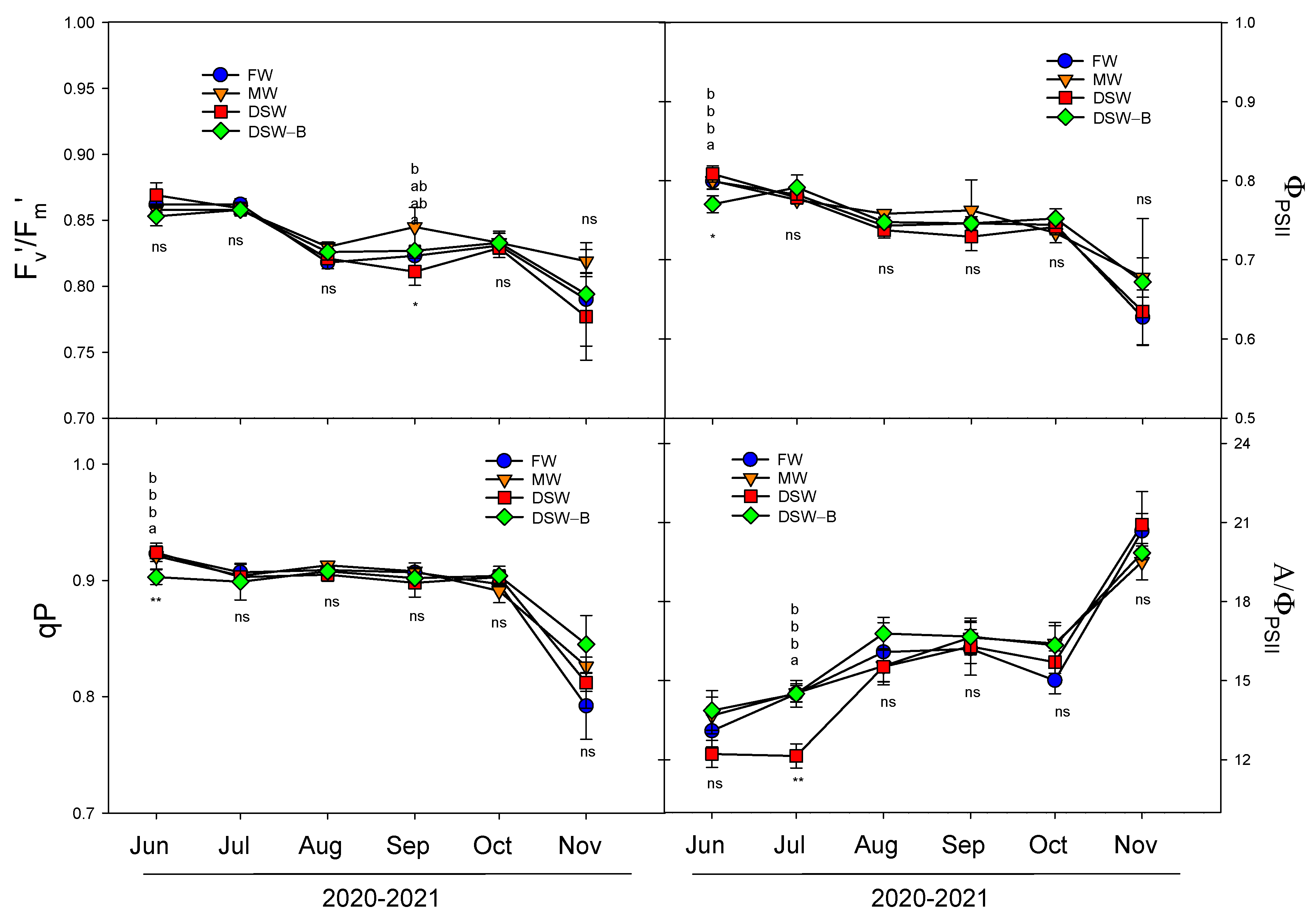
2.4. Plant Water Status and Physiological Responses
2.5. Plant Growth and Fruit Yield
3. Materials and Methods
3.1. Experimental Plot, Vegetal Material, and Crop Management
3.2. Treatments and Experimental Design
3.3. Water Quality and Soil Analysis
3.4. Plant Mineral Analysis
3.5. Plant Water Relations
3.6. Gas Exchange Parameters and Chlorophyll Fluorescence
3.7. Osmolytes and Chlorophyll Determinations
3.8. Vegetative Growth and Yield Fruit
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Alvarez, V.; Imbernón-Mulero, A.; Gallego-Elvira, B.; Soto-García, M.; Maestre-Valero, J.F. Multidisciplinary assessment of the agricultural supply of desalinated seawater in south-eastern Spain. Desalination 2023, 548, 116252. [Google Scholar] [CrossRef]
- Martinez-Mate, M.A.; Martin-Gorriz, B.; Martínez-Alvarez, V.; Soto-García, M.; Maestre-Valero, J.F. Hydroponic system and desalinated seawater as an alternative farm-productive proposal in water scarcity areas: Energy and greenhouse gas emissions analysis of lettuce production in southeast Spain. J. Clean. Prod. 2018, 172, 1298–1310. [Google Scholar] [CrossRef]
- Maestre-Valero, J.F.; Martínez-Álvarez, V.; Jódar-Conesa, F.J.; Acosta, J.A.; Martin-Gorriz, B.; Robles, J.M.; Pérez-Pérez, J.G.; Navarro, J.M. Short-term response of young mandarin trees to desalinated seawater irrigation. Water 2020, 12, 159. [Google Scholar] [CrossRef]
- Pellicer-Martinez, F.; Martínez-Paz, J.M. Climate change effects on the hydrology of the headwaters of the Tagus River: Implications for the management of the Tagus-Segura transfer. Hydrol. Earth Syst. Sci. 2018, 22, 6473–6491. [Google Scholar] [CrossRef]
- European Environmental Agency (EEA). Use of Freshwater Resources in Europe. 2022. Available online: www.eea.europa.eu/airs/2018/resource-efficiency-and-low-carbon-economy/freshwater-use (accessed on 19 January 2023).
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Informe Anual de Indicadores, Agricultura Pesca y Alimentación. 2021. Available online: https://www.mapa.gob.es/es/ministerio/servicios/analisis-y-prospectiva/iai2021_version_final_web_tcm30-626537.pdf (accessed on 2 February 2023).
- Martínez-Álvarez, V.; Martin-Gorriz, B.; Soto-García, M. Seawater desalination for crop irrigation—A review of current experiences and revealed key issues. Desalination 2016, 381, 58–70. [Google Scholar] [CrossRef]
- Redondo-Orts, J.A.; López-Ortiz, M.I. The economic impact of drought on the irrigated crops in the Segura River Basin. Water 2020, 12, 2955. [Google Scholar] [CrossRef]
- Martínez-Álvarez, V.; González-Ortega, M.J.; Martin-Gorriz, B.; Soto-García, M.; Maestre-Valero, J.F. The use of desalinated seawater for crop irrigation in the Segura River Basin (south-eastern Spain). Desalination 2017, 422, 153–164. [Google Scholar] [CrossRef]
- Hilal, N.; Kim, G.J.; Somerfield, C. Boron removal from saline water: A comprehensive review. Desalination 2011, 273, 23–35. [Google Scholar] [CrossRef]
- Navarro, L. The Spanish citrus industry. Acta Hortic. 2015, 1065, 41–48. [Google Scholar] [CrossRef]
- Ben Amor, R.; de Miguel Gomez, M.D. Competitiveness of Spanish orange sector in the Mediterranean area. Acta Hortic. 2020, 1292, 23–30. [Google Scholar] [CrossRef]
- Voutchkov, N.; Semiat, R. Seawater desalination. In Advanced Membrane Technology and Applications, 1st ed.; Li, N.N., Fane, A.G., Ho, W.W., Matsuura, T., Eds.; John Wiley &Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 47–86. [Google Scholar]
- Nable, R.O.; Banuelos, G.S.; Paull, J.G. Boron toxicity. Plant Soil 1997, 193, 181–198. [Google Scholar] [CrossRef]
- Storey, R.; Walker, R.R. Citrus and salinity. Sci. Hort. 1999, 78, 39–81. [Google Scholar] [CrossRef]
- Maas, E.V. Salinity and citriculture. Tree Physiol. 1993, 12, 195–216. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Jifon, J.; Carvajal, M.; Syvertsen, J.P. Gas exchange, chlorophyll and nutrient contents in relation to Na+ and Cl− accumulation in ‘Sunburst’ mandarin grafted on different rootstock. Plant Sci. 2002, 162, 705–712. [Google Scholar] [CrossRef]
- Garcia-Sanchez, F.; Syvertsen, J.P. Salinity tolerance of Cleopatra mandarin and Carrizo citrange citrus rootstock seedling is affected by CO2 enrichment during growth. J. Am. Soc. Hortic. Sci. 2006, 131, 24–31. [Google Scholar] [CrossRef]
- Mesquita, G.L.; Zambrosi, F.C.B.; Tanaka, F.A.O.; Boaretto, R.M.; Quaggio, J.A.; Ribeiro, R.V.; Mattos, D., Jr. Anatomical and physiological responses of citrus trees to varying boron availability are dependent on rootstock. Front. Plant Sci. 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Antolinos, V.; Robles, J.M.; Botía, P. Citrus irrigation with desalinated seawater under a climate change scenario. Front. Plant Sci. 2022, 13, 909083. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Antolinos, V.; Botía, P.; Robles, J.M. Deficit irrigation applied to lemon trees grafted on two rootstocks and irrigated with desalinated seawater. Plants 2023, 12, 2300. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Moreno, J.L.; García, C.; Nicolás, E.; Bastida, F. Agro-physiological and soil microbial responses to desalinated seawater irrigation in two crops. Ecotoxicol. Environ. Saf. 2023, 250, 114507. [Google Scholar] [CrossRef] [PubMed]
- Imbernón-Mulero, A.; Gallego-Elvira, B.; Martínez-Alvarez, V.; Martin-Gorriz, B.; Molina-del-Toro, R.; Jodar-Conesa, F.J.; Maestre-Valero, J.F. Boron removal from desalinated seawater for irrigation with an on-farm reverse osmosis system in southeastern Spain. Agronomy 2022, 12, 611. [Google Scholar] [CrossRef]
- Murugaiyan, K.; Sivakumar, K. Seasonal variation in elemental composition of Stoechospermum marginatum (Ag.) Kutz and Sargassum wightii (Greville Mscr.) J.G. Agardh in relation to chemical composition of seawater. Colloids Surf. B 2008, 64, 140–144. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Grattan, S.R.; Diaz, F.J.; Pedrero, F.; Vivaldi, G.A. Assessing the suitability of saline wastewaters for irrigation of Citrus spp.: Emphasis on boron and specific-ion interactions. Agric. Water Manag. 2015, 157, 48–58. [Google Scholar] [CrossRef]
- Levy, Y.; Syvertsen, J. Irrigation water quality and salinity effects in citrus trees. Hortic. Rev. 2004, 30, 37–82. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper 29 Rev. 1; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; 174p. [Google Scholar]
- Hanson, B.; Grattan, S.; Fulton, A. Agricultural Salinity and Drainage; Water Management Series Publication No. 3375; University of California: Davis, CA, USA, 2006; p. 157. [Google Scholar]
- Dydo, P.; Turek, M.; Milewski, A. Removal of boric acid, monoborate and boron complexes with polyols by reverse osmosis membrane. Desalination 2013, 334, 39–45. [Google Scholar] [CrossRef]
- Martínez-Álvarez, V.; Maestre-Valero, J.F.; González-Ortega, M.J.; Gallego-Elvira, B.; Martin-Gorriz, B. Characterization of the agricultural supply of desalinated seawater in southeastern Spain. Water 2019, 11, 1233. [Google Scholar] [CrossRef]
- Díaz, F.J.; Tejedor, M.; Jiménez, C.; Grattan, S.R.; Dorta, M.; Hernández, J.M. The imprint of desalinated seawater on recycled wastewater: Consequences for irrigation in Lanzarote Island, Spain. Agric. Water Manag. 2013, 116, 62–72. [Google Scholar] [CrossRef]
- Allison, L.E.; Brown, J.W.; Hayward, H.E.; Richards, L.A.; Bernstein, L.; Fireman, M.; Pearson, G.A.; Wilcox, L.V.; Bower, C.A.; Hatcher, J.T.; et al. Diagnosis and Improvement of Saline and Alkali Soils; Agriculture Handbook 60; United States Department of Agriculture: Washington, DC, USA, 1954; p. 160.
- de Abreu, C.A.; van Raij, B.; de Abreu, M.F.; González, A.P. Routine soil testing to monitor heavy metals and boron. Sci. Agric. 2005, 62, 564–571. [Google Scholar] [CrossRef]
- Eaton, F.M. Deficiency, toxicity and accumulation of boron in plants. J. Agric. Res. 1944, 69, 237–277. [Google Scholar]
- Grattan, S.R.; Grieve, C.M. Mineral element acquisition and growth response of plants grown in saline environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Behboudian, M.H.; Törökfalvy, E.; Walker, R.R. Effects of salinity on ionic content, water relations, and gas exchange parameters in some citrus scion-rootstock combinations. Sci. Hort. 1986, 28, 105–116. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Nortes, P.A.; Pedrero, F.; Mounzer, O.; Alarcón, J.J.; Bayona, J.M.; Nicolás, E. Assessment of the viability of using saline reclaimed water in grapefruit in medium to long term. Span. J. Agric. Res. 2014, 12, 1137–1148. [Google Scholar] [CrossRef]
- Maas, E. Crop salt tolerance. In Salinity Assessment and Management; Tanji, K.K., Ed.; American Society of Civil Engineers: New York, NY, USA, 1990. [Google Scholar]
- Embleton, T.W.; Jones, W.W.; Labanauskas, C.K.; Reuther, W. Leaf analysis as a diagnostic tool and guide to fertilization. In The Citrus Industry; Reuther, V., Ed.; University of California Press: Berkeley, CA, USA, 1973; Volume III, pp. 183–210. [Google Scholar]
- Simón-Grao, S.; Nieves, M.; Martínez-Nicolás, J.J.; Cámara-Zapata, J.M.; Alfosea-Simón, M.; García-Sánchez, F. Response of three citrus genotypes used as rootstocks grown under boron excess conditions. Ecotoxicol. Environ. Saf. 2018, 159, 10–19. [Google Scholar] [CrossRef]
- Simón-Grao, S.; Nieves, M.; Martínez-Nicolás, J.J.; Alfosea-Simón, M.; Cámara-Zapata, J.M.; Fernández-Zapata, J.C.; García-Sánchez, F. Arbuscular mycorrhizal symbiosis improves tolerance of Carrizo citrange to excess boron supply by reducing leaf B concentration and toxicity in the leaves and roots. Ecotoxicol. Environ. Saf. 2019, 173, 322–330. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Dimassi, N.; Bosabalidis, A.M.; Therios, I.N.; Patakas, A.; Giannakoula, A. Boron toxicity in Clementine mandarin plants on two rootstocks. Plant Sci. 2004, 166, 539–547. [Google Scholar] [CrossRef]
- Gimeno, V.; Simon, I.; Nieves, M.; Martinez, V.; Camara-Zapata, J.M.; Garcia, A.L.; García-Sánchez, F. The physiological and nutritional responses to an excess of boron by Verna lemon trees that were grafted on four contrasting rootstocks. Trees 2012, 26, 1513–1526. [Google Scholar] [CrossRef]
- Ortuño, M.F.; García-Orellana, Y.; Conejero, W.; Ruiz, M.C.; Alarcón, J.J.; Torrecillas, A. Stem and leaf water potentials, gas exchange, sap flow, and trunk diameter fluctuations for detecting water stress in lemon trees. Trees 2006, 20, 1–8. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Seasonal and diurnal changes in photosynthetic limitation of young sweet orange trees. Environ. Exp. Bot. 2009, 66, 203–211. [Google Scholar] [CrossRef]
- Veste, M.; Ben-Gal, A.; Shani, U. Impact of thermal tress and high VPD on gas exchange and chlorophyll fluorescence of Citrus grandis under desert conditions. Acta Hortic. 2000, 531, 143–149. [Google Scholar] [CrossRef]
- Macho-Rivero, M.A.; Herrera-Rodríguez, M.B.; Brejcha, R.; Schäffner, A.R.; Tanaka, N.; Fujiwara, T.; González-Fontes, A.; Camacho-Cristóbal, J.J. Boron toxicity reduces water transport from root to shoot in Arabidopsis plants. Evidence for a reduced transpiration rate and expression of major PIP aquaporin genes. Plant Cell Physiol. 2018, 59, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Cai, Z.-J.; Wen, S.-X.; Guo, P.; Ye, X.; Lin, G.-Z.; Chen, L.-S. Effects of boron toxicity on root and leaf anatomy in two Citrus species differing in boron tolerance. Trees 2014, 28, 1653–1666. [Google Scholar] [CrossRef]
- Cakmak, I.; Römheld, V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil. 1997, 193, 71–83. [Google Scholar] [CrossRef]
- Rhoades, J.D. Soluble salts. In Methods of Soil Analysis Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Kenney, D.R., Eds.; Agronomy Monograph; Soil Science Society of America: Madison, WI, USA, 1982; Volume 9, pp. 167–178. [Google Scholar]
- McCutchan, H.; Shackel, K.A. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef]
- Turner, N.C. Measurements of plant water status by pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Sinclair, T.T.; Allen, L.H. Carbon dioxide and water vapour exchange of leaves on field-grown citrus trees. J. Exp. Bot. 1982, 33, 1166–1175. Available online: https://www.jstor.org/stable/23690488 (accessed on 15 January 2023).
- Bates, L.; Waldren, R.; Teare, I.D. 1973. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.; Grattan, S. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficient of chlorophyll a and b in N,N-Dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef]
- Turrell, F.M. Growth and photosynthesis area of citrus. Bot. Gaz. 1961, 122, 284–298. [Google Scholar] [CrossRef]




| Water Source | EC | Ca2+ | Mg2+ | K+ | NO3− | PO43− | SO42− |
|---|---|---|---|---|---|---|---|
| FW | 1.23 d | 71.4 d | 42.7 d | 5.88 b | 4.92 b | 0.88 bc | 209.2 c |
| MW | 1.00 c | 45.8 c | 20.4 c | 6.22 b | 2.57 c | 1.15 c | 90.6 b |
| DSW | 0.89 b | 24.5 b | 5.3 b | 6.89 b | 1.31 d | 0.36 ab | 7.4 a |
| DSW–B | 0.17 a | 2.7 a | 1.0 a | 0.41 a | 0.78 a | 0.16 a | 1.3 a |
| ANOVA | *** | *** | *** | *** | *** | ** | *** |
| [Na+]water | [Na+]soil | [Na+]leaf | [Cl−]water | [Cl−]soil | [Cl−]leaf | [B]water | [B]soil | [B]leaf | |
|---|---|---|---|---|---|---|---|---|---|
| [Na+]water | - | 0.525 *** | 0.180 | 0.982 *** | 0.547 *** | 0.187 | 0.661 *** | 0.365 * | 0.129 |
| [Na+]soil | 0.525 *** | - | 0.107 | 0.517 ** | 0.725 *** | 0.021 | 0.720 *** | 0.812 ** | 0.521 ** |
| [Na+]leaf | 0.180 | 0.107 | - | 0.234 | 0.0756 | 0.203 | 0.236 | 0.245 | 0.529 *** |
| [Cl−]water | 0.982 *** | 0.517 ** | 0.234 | - | 0.526 *** | 0.204 | 0.691 *** | 0.380 * | 0.113 |
| [Cl−]soil | 0.547 *** | 0.725 *** | 0.076 | 0.526 *** | - | −0.244 | 0.425 ** | 0.588 *** | 0.439 ** |
| [Cl−]leaf | 0.187 | 0.021 | 0.203 | 0.204 | −0.244 | - | 0.293 | 0.024 | 0.135 |
| [B]water | 0.661 *** | 0.720 *** | 0.236 | 0.691 *** | 0.420 ** | 0.293 | - | 0.725 *** | 0.302 |
| [B]soil | 0.365 * | 0.812 ** | 0.245 | 0.380 * | 0.588 *** | 0.024 | 0.725 *** | - | 0.570 *** |
| [B]leaf | 0.129 | 0.521 ** | 0.529 *** | 0.113 | 0.439 ** | 0.135 | 0.302 | 0.570 *** | - |
| Water Source | Yield (kg tree−1) | Fruit Number | Fruit Weight (g) |
|---|---|---|---|
| FW | 85.1 ± 4.2 | 229.5 ± 16.1 | 380.5 ± 13.7 |
| MW | 81.0 ± 6.2 | 222.0 ± 15.4 | 367.6 ± 5.9 |
| DSW | 91.5 ± 4.7 | 246.5 ± 16.7 | 382.8 ± 22.8 |
| DSW–B | 90.4 ± 6.6 | 248.4 ± 18.4 | 368.8 ± 22.8 |
| ANOVA | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, J.M.; Imbernón-Mulero, A.; Robles, J.M.; Hernández-Ballester, F.M.; Antolinos, V.; Gallego-Elvira, B.; Maestre-Valero, J.F. Physiological Responses of a Grapefruit Orchard to Irrigation with Desalinated Seawater. Plants 2024, 13, 781. https://doi.org/10.3390/plants13060781
Navarro JM, Imbernón-Mulero A, Robles JM, Hernández-Ballester FM, Antolinos V, Gallego-Elvira B, Maestre-Valero JF. Physiological Responses of a Grapefruit Orchard to Irrigation with Desalinated Seawater. Plants. 2024; 13(6):781. https://doi.org/10.3390/plants13060781
Chicago/Turabian StyleNavarro, Josefa M., Alberto Imbernón-Mulero, Juan M. Robles, Francisco M. Hernández-Ballester, Vera Antolinos, Belén Gallego-Elvira, and José F. Maestre-Valero. 2024. "Physiological Responses of a Grapefruit Orchard to Irrigation with Desalinated Seawater" Plants 13, no. 6: 781. https://doi.org/10.3390/plants13060781
APA StyleNavarro, J. M., Imbernón-Mulero, A., Robles, J. M., Hernández-Ballester, F. M., Antolinos, V., Gallego-Elvira, B., & Maestre-Valero, J. F. (2024). Physiological Responses of a Grapefruit Orchard to Irrigation with Desalinated Seawater. Plants, 13(6), 781. https://doi.org/10.3390/plants13060781









