Eucalypt Extracts Prepared by a No-Waste Method and Their 3D-Printed Dosage Forms Show Antimicrobial and Anti-Inflammatory Activity
Abstract
1. Introduction
2. Results
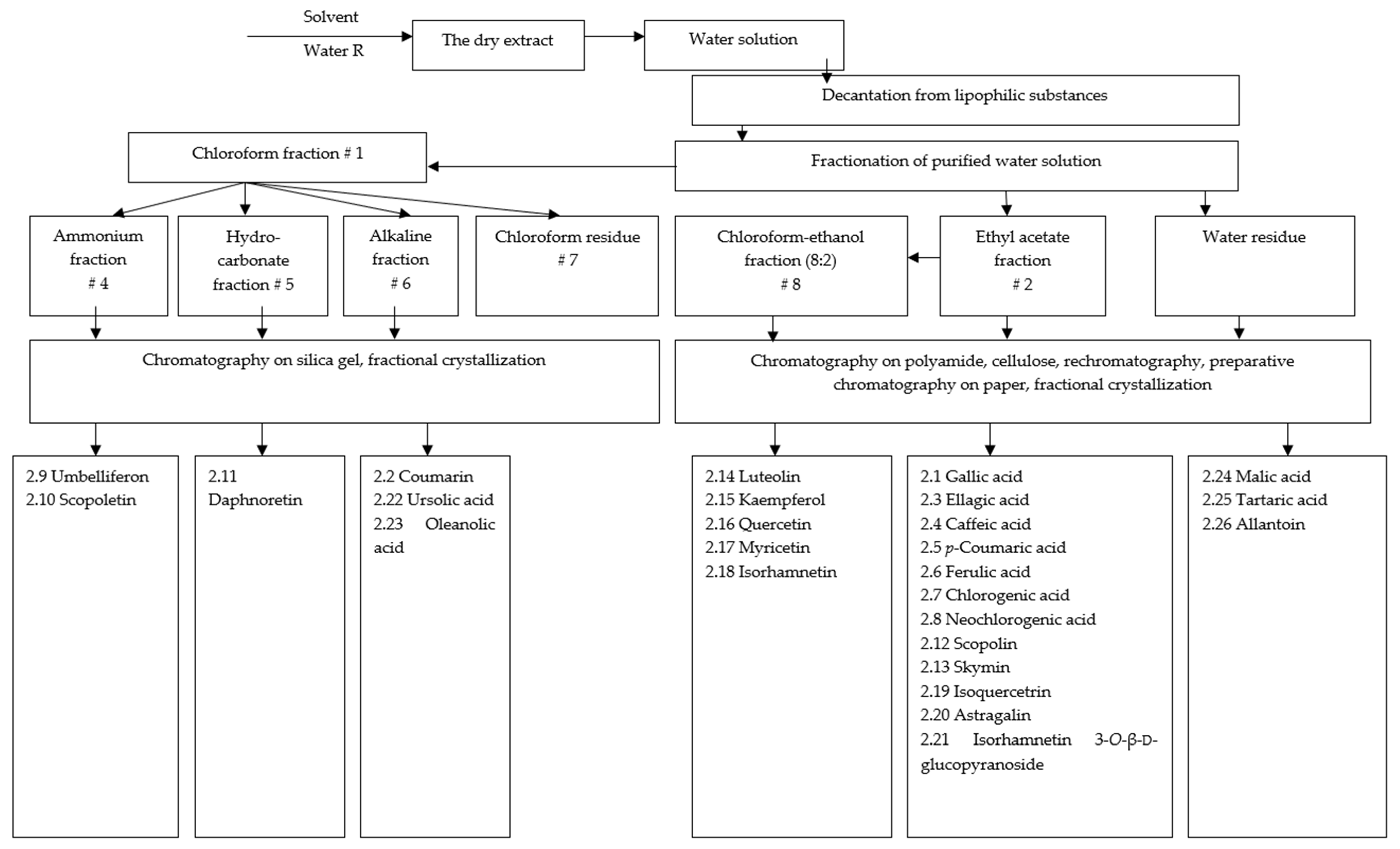
2.1. Phytochemical Study of the Eucalypt Extracts
2.2. Pharmacological Studies of the Eucalypt Extracts
2.2.1. Study of Antimicrobial Activity
2.2.2. Anti-Inflammatory Activity of Dry Eucalypt Extract
3. Discussion
4. Materials and Methods
4.1. Raw Materials
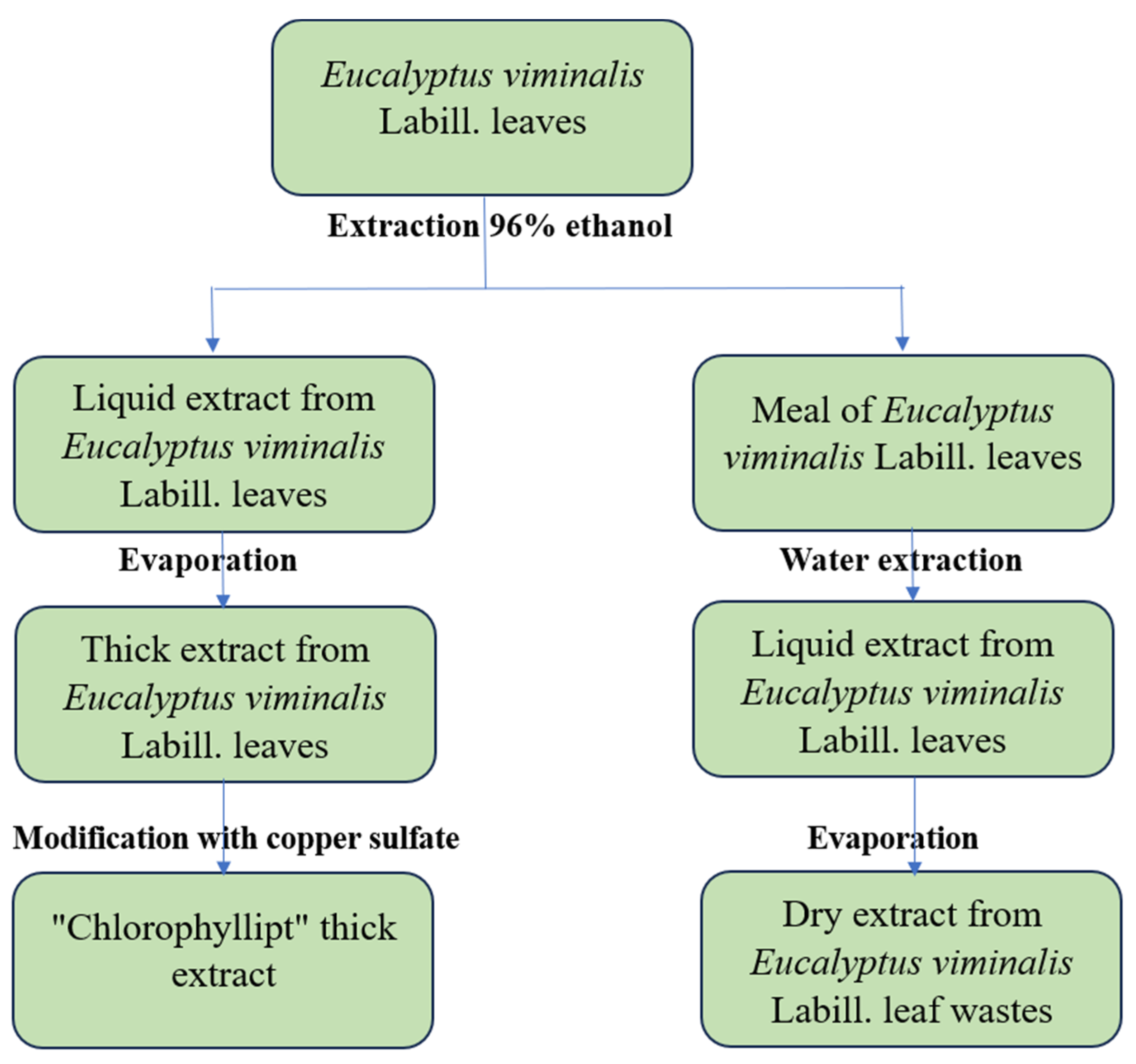
4.2. Preparation of Eucalypt Extracts
4.3. Preparation of Eucalypt-Extracts-Loaded Gels for 3D Printing
4.4. 3D Printing of the Eucalypt Extracts
4.5. Phytochemical Analysis
4.6. Study of Antimicrobial Activity
4.7. Study of Anti-Inflammatory Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, N.; Madrid-López, C.; Villalba-Méndez, G.; Talens-Peiró, L. New Techniques for Assessing Critical Raw Material Aspects in Energy and Other Technologies. Environ. Sci. Technol. 2022, 56, 17236–17245. [Google Scholar] [CrossRef] [PubMed]
- Sepp, J.; Koshovyi, O.; Jakstas, V.; Žvikas, V.; Botsula, I.; Kireyev, I.; Tsemenko, K.; Kukhtenko, O.; Kogermann, K.; Heinämäki, J.; et al. Phytochemical, Technological, and Pharmacological Study on the Galenic Dry Extracts Prepared from German Chamomile (Matricaria Chamomilla L.) Flowers. Plants 2024, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.; Vovk, G.; Akhmedov, E.; Komissarenko, A.N. The Study of the Chemical Composition and Pharmacological Activity of Salvia Officinalis Leaves Extracts Getting by Complex Processing. Azerbaijan Pharm. Pharmacother. J. 2015, 15, 30–34. [Google Scholar]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and Pharmacological Screening of a Monarda Fistulosa L. Dry Extract Based on a Hydrodistilled Residue By-Product. Front. Pharmacol. 2021, 12, 563436. [Google Scholar] [CrossRef]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Extraction of High-Value Chemicals from Plants for Technical and Medical Applications. Int. J. Mol. Sci. 2022, 23, 10334. [Google Scholar] [CrossRef]
- Offiah, V.; Kontogiorgos, V.; Falade, K.O. Extrusion Processing of Raw Food Materials and By-Products: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2979–2998. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Kang, S.-S.; Shin, H.-S. Pharmaceutical Importance of Some Promising Plant Species with Special Reference to the Isolation and Extraction of Bioactive Compounds: A Review. Curr. Pharm. Biotechnol. 2022, 23, 15–29. [Google Scholar] [CrossRef]
- Kovalenko, V.N. Compendium 2020. In Medicines; MORION: Kyiv, Ukraine, 2020; p. 2700. [Google Scholar]
- Branco, S.; Videira, N.; Branco, M.; Paiva, M.R. A Review of Invasive Alien Species Impacts on Eucalypt Stands and Citrus Orchards Ecosystem Services: Towards an Integrated Management Approach. J. Environ. Manag. 2015, 149, 17–26. [Google Scholar] [CrossRef]
- Mansfield, S. New Communities on Eucalypts Grown Outside Australia. Front. Plant Sci. 2016, 7, 1812. [Google Scholar] [CrossRef]
- Poke, F.S.; Vaillancourt, R.E.; Potts, B.M.; Reid, J.B. Genomic Research in Eucalyptus. Genetica 2005, 125, 79–101. [Google Scholar] [CrossRef]
- Eschler, B.M.; Foley, W.J. A New Sideroxylonal from Eucalyptus melliodora. Aust. J. Chem. 1999, 52, 157. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Morita, H.; Takeya, K.; Itokawa, H. Eucalyptone from Eucalyptus globulus. Phytochemistry 1995, 40, 183–184. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Haruna, M.; Ito, K.; Crow, W.D.; Paton, D.M. Euglobal-In-1, a New Euglobal from Eucalyptus Incrassata. Chem. Pharm. Bull. 1994, 42, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Haruna, M.; Ito, K.; Yoshida, S. Four Euglobals from Eucalyptus Blakelyi. Chem. Pharm. Bull. 1994, 42, 2177–2179. [Google Scholar] [CrossRef]
- Murata, M.; Yamakoshi, Y.; Homma, S.; Aida, K.; Hori, K.; Ohashi, Y. Macrocarpal A, a Novel Antibacterial Compound from Eucalyptus macrocarpa. Agric. Biol. Chem. 1990, 54, 3221–3226. [Google Scholar] [CrossRef]
- Guan, X.; Guo, Q.; Huang, X.; Wang, Y.; Ye, W. A new flavonoid glycoside from leaves of Eucalyptus robusta. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2015, 40, 4868–4872. [Google Scholar]
- Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S.; Neto, C.P.; Domingues, P. New Glucosides from Eucalyptus Globulus Wood, Bark and Kraft Pulps. Holzforschung 2004, 58, 501–503. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Haruna, M.; Ito, K.; Shingu, T. Structures of Euglobals-G1, -G2, -G3, -G4, and -G5 from Eucalyptus grandis. ChemInform 1995, 26, 199535229. [Google Scholar] [CrossRef]
- Begum, S.; Sultana, I.; Siddiqui, B.S.; Shaheen, F.; Gilani, A.H. Structure and Spasmolytic Activity of Eucalyptanoic Acid from Eucalyptus camaldulensis Var. Obtusa and Synthesis of Its Active Derivative from Oleanolic Acid. J. Nat. Prod. 2002, 65, 1939–1941. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Hosking, H.; Ashwath, N.; Walsh, K.B.; Neilsen, P.M.; Broszczak, D.A.; Naiker, M. Antioxidative and Therapeutic Potential of Selected Australian Plants: A Review. J. Ethnopharmacol. 2021, 268, 113580. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, Medicinal and Toxicological Significance of Eucalyptus Leaf Essential Oil: A Review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef] [PubMed]
- Nadtoka, V.L.; Bozhko, N.G.; Grizhko, A.O. The Method of Obtaining Chlorophyllipt. No. 2753048/SU. Patent 5242 Ukraine, IPC A61K35/78. Application 25.04.79, 28 December 1994. Bull. No. 7-1, 7p. (In Ukrainian). [Google Scholar]
- Ravanbakhsh, H.; Bao, G.; Luo, Z.; Mongeau, L.G.; Zhang, Y.S. Composite Inks for Extrusion Printing of Biological and Biomedical Constructs. ACS Biomater. Sci. Eng. 2021, 7, 4009–4026. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Alomgir Hossen, M.; Dai, J.; Qin, W.; Liu, Y. Facile Fabrication of Sandwich-like Anthocyanin/Chitosan/Lemongrass Essential Oil Films via 3D Printing for Intelligent Evaluation of Pork Freshness. Food Chem. 2022, 370, 131082. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Zang, Z.; Sun, X.; Tan, H.; Si, X.; Tian, J.; Teng, W.; Wang, J.; Liang, Q.; et al. 3D Food Printing: Applications of Plant-Based Materials in Extrusion-Based Food Printing. Crit. Rev. Food Sci. Nutr. 2022, 62, 7184–7198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with Precise Layer-Wise Dose Adjustments for Paediatric Use via Pressure-Assisted Microsyringe Printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A.W. Pressure-Assisted Microsyringe 3D Printing of Oral Films Based on Pullulan and Hydroxypropyl Methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.; Heinämäki, J.; Laidmäe, I.; Topelius, N.S.; Grytsyk, A.; Raal, A. Semi-Solid Extrusion 3D-Printing of Eucalypt Extract-Loaded Polyethylene Oxide Gels Intended for Pharmaceutical Applications. Ann. 3D Print. Med. 2023, 12, 100123. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Raal, A.; Laidmäe, I.; Topelius, N.S.; Komisarenko, M.; Komissarenko, A. Pharmaceutical 3D-Printing of Nanoemulsified Eucalypt Extracts and Their Antimicrobial Activity. Eur. J. Pharm. Sci. 2023, 187, 106487. [Google Scholar] [CrossRef]
- Miguel, M.; Gago, C.; Antunes, M.; Lagoas, S.; Faleiro, M.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Figueiredo, A. Antibacterial, Antioxidant, and Antiproliferative Activities of Corymbia Citriodora and the Essential Oils of Eight Eucalyptus Species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef]
- Tumanov, V.N.; Chiruk, S.L.; GrGU im., I. Qualitative and Quantitative Methods for Studying Photosynthesis Pigments; GrGU im. I. Kupala: Grodno, Belarus, 2007. [Google Scholar]
- Balhaddad, A.A.; AlSheikh, R.N. Effect of Eucalyptus Oil on Streptococcus Mutans and Enterococcus Faecalis Growth. BDJ Open 2023, 9, 26. [Google Scholar] [CrossRef]
- State Pharmacopoeia of Ukraine, 2nd ed.; Ukrainian Scientific Pharmacopoeial Center of Drugs Quality: Kharkiv, Ukraine, 2015.
- Marzullo, L.; Ochkur, O.; Orlandini, S.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Del Bubba, M. Quality by Design in Optimizing the Extraction of (Poly)Phenolic Compounds from Vaccinium Myrtillus Berries. J. Chromatogr. A 2022, 1677, 463329. [Google Scholar] [CrossRef] [PubMed]
- Viidik, L.; Seera, D.; Antikainen, O.; Kogermann, K.; Heinämäki, J.; Laidmäe, I. 3D-Printability of Aqueous Poly(Ethylene Oxide) Gels. Eur. Polym. J. 2019, 120, 109206. [Google Scholar] [CrossRef]
- Riegel, J.; Mayer, W.; Havre, Y.V. FreeCAD. 2001. Available online: https://www.freecadweb.org/ (accessed on 22 December 2023).
- Koshovyi, O.; Raal, A.; Kovaleva, A.; Myha, M.; Ilina, T.; Borodina, N.; Komissarenko, A. The Phytochemical and Chemotaxonomic Study of Salvia Spp. Growing in Ukraine. J. Appl. Biol. Biotechnol. 2020, 8, 29–36. [Google Scholar] [CrossRef]
- Koshevoi, O.N. Amino-Acid and Monosaccharide Compositions of Salvia Officinalis Leaves. Chem. Nat. Compd. 2011, 47, 492–493. [Google Scholar] [CrossRef]
- Kovaleva, A.M.; Georgievskyi, G.V.; Kovalev, V.M.; Komissarenko, A.M.; Timchenko, M.M. Development of the Method of Standardization of the New Medicinal Product Piflamin. Pharmacom 2002, 2, 92–97. (In Ukrainian) [Google Scholar]
- Xue, T.; Ruan, K.; Tang, Z.; Duan, J.; Xu, H. Isolation, Structural Properties, and Bioactivities of Polysaccharides from Althaea Officinalis Linn.: A Review. Int. J. Biol. Macromol. 2023, 242, 125098. [Google Scholar] [CrossRef]
- Koshovyi, O.M.; Zagayko, A.L.; Kolychev, I.O.; Akhmedov, E.Y.; Komissarenko, A.N. Phytochemical Study of the Dry Extract from Bilberry Leaves. Azerbaijan Pharm. Pharmacother. J. 2016, 16, 18–23. [Google Scholar]
- Shinkovenko, I.L.; Kashpur, N.V.; Ilyina, T.V.; Kovalyova, A.M.; Goryacha, O.V.; Koshovyi, O.M.; Toryanyk, E.L.; Kryvoruchko, O.V. The Immunomodulatory Activity of the Extracts and Complexes of Biologically Active Compounds of Galium verum L. Herb. Ceska A Slov. Farm. 2018, 67, 25–29. [Google Scholar]
- Vlasova, I.; Gontova, T.; Grytsyk, L.; Zhumashova, G.; Sayakova, G.; Boshkayeva, A.; Shanaida, M.; Koshovyi, O. Determination of Standardization Parameters of Oxycoccus Macrocarpus (Ait.) Pursh and Oxycoccus Palustris Pers. Leaves. Sci. Pharm. Sci. 2022, 3, 48–57. [Google Scholar] [CrossRef]
- Khokhlova, K.; Zdoryk, O.; Vyshnevska, L. Chromatographic Characterization on Flavonoids and Triterpenes of Leaves and Flowers of 15 Crataegus L. Species. Nat. Prod. Res. 2020, 34, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Belikov, V.V. Method for Quantitative Determination of Tannin. A.S. 741149 USSR, MK2G01 N 31/16. No. 2570841. Application 01/18/76, 21 February 1980. Bulletin. No. 22. [Google Scholar]
- GOST 4565-79; Sumac Leaf. 02/26/1979, No. 754. State Standard GOST: Moscow, Russia,, 1979.
- Khvorost, O.P.; Belikov, V.V.; Serbin, A.G.; Komissarenko, N.F. Comparative Quantitative Assessment of the Content of Tannins in Alnus glutinosa (L) Gaerth. Rastit. Resour. 1986, 22, 258–262. [Google Scholar]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and Pharmacological Research in Agrimonia eupatoria L. Herb Extract with Anti-Inflammatory and Hepatoprotective Properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef]
- Stefanov, O.V. Preclinical Studies of Drugs; Avicenna: Kyiv, Ukraine, 2001. [Google Scholar]
- Lapach, S.N.; Chubenko, A.V.; Babich, P.N. Statistical Methods in Biomedical Research Using Excel; MORION: Kyiv, Ukraine, 2000. [Google Scholar]
- Methodological Recommendations for Experimental (Preclinical) Study of Drugs for Local Treatment of Purulent Wounds; Ministry of Health: Moscow, Russia, 1989.
- European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes; Council of Europe: Strasbourg, France, 1986; Volume 222, pp. 31–37.
- Council Directive 2010/63/EU of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; Council of Europe: Strasbourg, France, 2010; pp. 33–79.
- The Order of the Ministry of Health of Ukraine No. 944 dated 14 December 2009 “On Approval of the Procedure for Preclinical Study of Medicinal Products and Examination of Materials of Preclinical Study of Medicinal Products”. Available online: https://zakon.rada.gov.ua/laws/show/z0972-01#Text (accessed on 1 February 2010).
- The Law of Ukraine “On the Protection of Animals from Cruel Treatment” dated 12/15/2009. Available online: https://zakon.rada.gov.ua/laws/show/3447-15#Text (accessed on 8 September 2021).
- Regulating the Application of Principles of Good Laboratory Practice and the Verification of Their Applications for Tests on Chemical Substances; European Union: Brussels, Belgium, 1986; Volume 1, pp. 145–146.
- Ihnatova, T.; Kaplaushenko, A.; Frolova, Y.; Pryhlo, E. Synthesis and Antioxidant Properties of Some New 5-Phenethyl-3-Thio-1,2,4-Triazoles. Pharmacia 2021, 68, 129–133. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022.
| Substance | Assay (%) of the Volatile Fraction of the Extract |
|---|---|
| α-Phellandrene | 3.13 |
| 1,8-Cineol | 11.15 |
| trans-Pinocarveol | 1.48 |
| Pinocarvone | 0.41 |
| Terpinen-4-ol | 0.68 |
| α-Terpineol | 1.5 |
| α-Terpinyl acetate | 0.57 |
| Geranyl acetate | 0.47 |
| α-Guryunen | 1.81 |
| Kalaren | 1.15 |
| Aromadendren | 26.01 |
| Allo-aromadendrene | 3.95 |
| Leden | 2.55 |
| Dehydroaromadendrene | 0.47 |
| Epiglobulol | 3.47 |
| Globulol | 14.66 |
| Viridiflorol | 2.57 |
| Epi-γ-eudesmol | 1.16 |
| Epi-β-eudesmol | 1.17 |
| Kubenol | 3.29 |
| β-Eudesmol | 1,05 |
| α-Eudesmol | 1.15 |
| Posifoliol | 2.06 |
| Palmitic acid | 0.89 |
| Ethyl palmitate | 1.01 |
| Phytol | 0.51 |
| Ethyl oleate | 0.32 |
| Ethyl linolenate | 0.68 |
| 7 unidentified compounds | 4.24 |
| # | Substance | Tmelt, °C | , Degree | UV Spectrum, λmax, nm | RF in Solvent Systems | |
|---|---|---|---|---|---|---|
| System | RF | |||||
| Derivatives of benzoic acid | ||||||
| 1. | 2.1 Gallic acid (3,4,5-trihydroxybenzoic acid) | 226–228 | - | 272 | 1 | 0.65 |
| 2. | 2.3 Ellagic acid (hexahydroxydiphenic acid dilactone) | 360 distr. | - | 366 | 1 2 | 0.12 0.06 |
| Derivatives of cinnamic acid | ||||||
| 3. | 2.4 Caffeic acid (3,4-dihydroxycinnamic acid) | 194–195 | - | 325 300 235 | 1 3 | 0.8 0.5 |
| 4. | 2.5 p-Coumaric acid (4- hydroxycinnamic acid) | 212–214 | - | 310 228 217 | 1 3 | 0.9 0.6 |
| 5. | 2.6 Ferulic acid (4-hydroxy-5-methoxy-cinnamic acid) | 168–170 | - | 320 290 234 | 1 3 | 0.88 0.55 |
| 6. | 2.7 Chlorogenic acid (5-O-caffeyl-d-quinic acid) | 203–205 | −32 (methanol) | 325 300 245 | 1 3 | 0.62 0.7 |
| 7. | 2.8 Neochlorogenic acid (3-O-caffeyl-d-quinic acid) | Amorphous | +2.6 (ethanol) | 325 300 243 | 1 3 | 0.64 0.75 |
| Coumarin derivatives | ||||||
| 8. | 2.2 Coumarin | 67–69 | - | - | 4 | 0.2 |
| 9. | 2.9 Umbelliferon (7-hydroxycoumarin) | 228–230 | - | 231 258 327 | 5 | 0.36 |
| 10. | 2.10 Scopoletin (6-methoxy-7-hydroxycoumarin) | 202–204 | - | 230 255 296 346 | 5 | 0.58 |
| 11. | 2.11 Daphnoretin (2-methoxy-6-oxy-3,7′-dicoumarin ether) | 254–256 | - | - | 5 | 0.85 |
| 12. | 2.12 Scopolin (6-methoxy-7-(O-(-d-glucopyranosyl)-coumarin) | 218–220 | –8.5 DMF | 231 330 | 6 | 0.24 |
| 13. | 2.13 Skymin (7-(O-(-d-glucopyranosyl)-coumarin) | 218–220 | −80 (methanol) | 220 252 325 | 5 | 0.38 |
| Flavones | ||||||
| 14. | 2.14 Luteolin (5,7,3′,4′-tetrahydroxyflavone) | 232–241 | - | 255 318 350 410 | 1 3 | 0.82 0.11 |
| Flavonols | ||||||
| 15. | 2.15 Kaempferol (3,5,7,4′-tetrahydroxyflavone) | 273–274 | - | 366 266 | 1 7 | 0.83 0.55 |
| 16. | 2.16 Quercetin (3,5,7,3′,4′-pentahydroxyflavone) | 310–312 | - | 375 268 256 | 1 7 | 0.69 0.32 |
| 17. | 2.17 Myricetin (3,5,7,3′,4′,5′-hexahydroxyflavone) | 350–354 distr. | - | 374 272 254 | 7 | 0.18 |
| 18. | 2.18 Isorhamnetin (3,5,7,4′-tetrahydroxy-3′-methoxyflavone) | 167–170 | - | 370 265 254 | 1 7 | 0.85 0.73 |
| Flavonol glycosides | ||||||
| 19. | 2.19 Isoquercetrin (quercetin-3-O-β-d-glucopyranoside) | 227–229 | −12.5 (methanol) | 355 267 256 | 1 3 | 0.52 0.36 |
| 20. | 2.20 Astragalin (kaempferol-3-O-β-d-glucopyranoside) | 196–198 | −6.8 (ethanol) | 375 270 | 1 3 | 0.69 0.37 |
| 21. | 2.21 Isorhamnetin 3-O-β-d-glucopyranoside | 317–319 | −30 (DMF) | 357 302 255 | 1 3 | 0.46 0.59 |
| Triterpenoids | ||||||
| 22. | 2.22 Ursolic acid | 280–283 | +62.5 (chloroform) | - | 1 | 0.89 |
| 23. | 2.23 Oleanolic acid | 300–303 | +79.0 (chloroform) | - | 1 8 | 0.9 0.44 |
| Organic acids | ||||||
| 24. | 2.24 Malic acid | 100–101 | ||||
| 25. | 2.25 Tartaric acid | 170–171 | +11.9 (ethanol) | - | 9 | 0.48 |
| Monosugars | ||||||
| 26. | D-glucose | 1 | 0.23 | |||
| 27. | D-galactose | 1 | 0.17 | |||
| 28. | D-xylose | 1 | 0.31 | |||
| 29. | L-rhamnose | 1 | 0.43 | |||
| Amino acids | ||||||
| 30. | Cysteine * | |||||
| 31. | Taurine * | |||||
| 32. | Phosphoethanolamine * | |||||
| 33. | Aspartic acid * | |||||
| 34. | Threonine * | |||||
| 35. | Serin * | |||||
| 36. | Asparagine * | |||||
| 37. | Glutamic acid * | |||||
| 38. | Proline * | |||||
| 39. | Glycine * | |||||
| 40. | Alanine * | |||||
| 41. | Citrulline * | |||||
| 42. | α-amino-n-butyrin * | |||||
| 43. | Valine * | |||||
| 44. | Cystine * | |||||
| 45. | Cystathionine * | |||||
| 46. | Methionine * | |||||
| 47. | Isoleucine * | |||||
| 48. | Tyrosine * | |||||
| 49. | Phenylalanine * | |||||
| 50. | β-Alanine * | |||||
| 51. | Ethanolamine * | |||||
| 52. | Ornithine * | |||||
| 53. | Lysine * | |||||
| 54. | 1-methylhistidine * | |||||
| 55. | 3-methylhistidine * | |||||
| 56. | Arginine * | |||||
| Urea derivatives | ||||||
| 57. | 2.26 Allantoin | 234–235 | - | - | 1 | 0.35 |
| The BAS Group That Was Determined and the Method Used | Assay in the Extract, % |
|---|---|
| Amino acids: | |
| Amino-acid analyzer | 0.21 |
| Spectrophotometric method for leucine | 0.19 ± 0.01 |
| Polysaccharides: | |
| Gravimetric method | 17.42 ± 0.68 |
| Spectrophotometric method for glucose | 12.91 ± 0.53 |
| Hydroxycinnamic acid | |
| Spectrophotometric method for chlorogenic acid | 3.38 ± 0.22 |
| Flavonoids: | |
| Spectrophotometric method for rutin | 4.69 ± 0.11 |
| Spectrophotometric method for quercetin | 3.7 ± 0.09 |
| Polyphenolic compounds: | |
| Complexometric method | 3.92 ± 0.07 |
| Spectrophotometric method for gallic acid | 2.0 ± 0.59 |
| Microorganisms | MIC of Eucalypt Extract, mg/mL |
|---|---|
| S. aureus ATCC 25923 | 25–35 |
| S. aureus ATCC 6538 | 25–35 |
| E. coli ATCC 25922 | 35–45 |
| P. vulgaris NCTC 4636 | 45–50 |
| P. aeruginosa ATCC 27853 | 50–55 |
| P. aeruginosa ATCC9027 | 50–60 |
| B. subtilis ATCC 6633 | 25–35 |
| C. albicans ATCC 885/653 | 45–65 |
| S. typhimurium 144 | 45–50 |
| S. paratyphi A 290 | 45–50 |
| S. flexneri 170 | 45–50 |
| C. diphtheriae gravis 14 tox+ | 45–50 |
| C. diphtheriae mitis 6 tox+ | 35–45 |
| S. aureus (tonsillitis) | 50–70 |
| S. aureus (bronchitis) | 50–70 |
| S. pyogenes (bronchitis) | 45–55 |
| E. coli (purulent wound) | 60–80 |
| P. aeruginosa (purulent wound) | 60–80 |
| P. aeruginosa (burn) | 100–150 |
| C. albicans (vaginitis) | 45–60 |
| K. pneumoniae (pneumonia) | 100–120 |
| Microorganisms | Growth Inhibition Zones Diameter, mm | ||
|---|---|---|---|
| The Dry-Extract Solution | 1% Alcohol Solution of the Soft Extract | ||
| 1% | 10% | ||
| S. aureus ATCC 25923 | 14 | 16 | 23 |
| S. aureus ATCC 6538 | 14 | 14 | 24 |
| E. coli ATCC 25922 | 14 | 15 | 13 |
| P. vulgaris NCTC 4636 | 13 | 14 | growth |
| P. aeruginosa ATCC 27853 | 17 | 18 | growth |
| P. aeruginosa ATCC 9027 | 13 | 14 | growth |
| B. subtilis ATCC 6633 | 20 | 22 | growth |
| C. albicans ATCC 885/653 | 17 | 19 | growth |
| S. enterica Typhimurium 144 | 14 | 14 | ns |
| S. enterica Paratyphi A 290 | 13 | 15 | ns |
| S. flexneri 170 | 14 | 15 | ns |
| C. diphtheriae gravis 14 tox+ | 16 | 19 | ns |
| C. diphtheriae mitis 6 tox+ | 17 | 20 | ns |
| S. aureus (tonsillitis) | 12 | 14 | ns |
| S. aureus (bronchitis) | 15 | 19 | ns |
| S. pyogenes (bronchitis) | 16 | 21 | ns |
| E. coli (purulent wound) | 14 | 14 | ns |
| P. aeruginosa (purulent wound) | 15 | 13 | ns |
| P. aeruginosa (burn) | Growth | growth | ns |
| C. albicans (vaginitis) | 16 | 17 | ns |
| K. pneumoniae (pneumonia) | 14 | 16 | ns |
| Microbe | Inhibition Zone Around the Discs (mm) with the Eucalypt Extracts | ||||||
|---|---|---|---|---|---|---|---|
| The Soft Extract, mg/mL | The Dry Extract, mg/mL | The Combined Extract, mg/mL | |||||
| 10 | 5 | 10 | 20 | 100 | 10 + 10 | ||
| S. aureus | ATCC 29213 | 4 | 0 | 0 | 0 | 1 | 2 |
| S. aureus | HUMB 19417 | 4 | 0 | 0 | 0 | 2 | 4 |
| S. aureus | HUMB 5630 | 4 | 0 | 0 | 0 | 4 | 3 |
| E. coli | ATCC 25922 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. coli | HUMB 7024 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. coli | HUMB 5666 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. albicans | ATCC 10231 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. albicans | HUMB 05355 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. albicans | HUMB 19373 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. pyogenes | DSM 25943 | 3 | 0 | 0 | 0 | 1 | 2 |
| S. pyogenes | HUMB 18939 | 3 | 0 | 0 | 0 | 0.5 | 2 |
| S. pyogenes | HUMB 18966 | 3 | 0 | 0 | 0 | 1 | 2 |
| S. mutans | HUMB 13076 | 1 | 0 | 0 | 0 | 0 | 0 |
| S. mutans | HUMB 13034 | 1 | 0 | 0 | 0 | 0 | 0 |
| S. mutans | HUMB 13033 | 1 | 0 | 0 | 0 | 0 | 0 |
| S. sobrinus | HUMB 13087 | 1 | 0 | 0 | 1 | 0 | 0 |
| S. sobrinus | HUMB 13104 | 1 | 0 | 0 | 1 | 0 | 0 |
| S. sobrinus | HUMB 13038 | 1 | 0 | 1 | 0 | 0 | 0 |
| Agent | Dose, mg/kg | Average Value of Swelling (after 3 h) | Antiexudative Effect, % |
|---|---|---|---|
| Eucalypt extract | 20 | 0.378 ± 0.031 | 61.5 |
| Voltaren | 2.3 | 0.358 ± 0.061 | 63.5 |
| Control | 0 | 0.982 ± 0.111 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshovyi, O.; Komisarenko, M.; Osolodchenko, T.; Komissarenko, A.; Mändar, R.; Kõljalg, S.; Heinämäki, J.; Raal, A. Eucalypt Extracts Prepared by a No-Waste Method and Their 3D-Printed Dosage Forms Show Antimicrobial and Anti-Inflammatory Activity. Plants 2024, 13, 754. https://doi.org/10.3390/plants13060754
Koshovyi O, Komisarenko M, Osolodchenko T, Komissarenko A, Mändar R, Kõljalg S, Heinämäki J, Raal A. Eucalypt Extracts Prepared by a No-Waste Method and Their 3D-Printed Dosage Forms Show Antimicrobial and Anti-Inflammatory Activity. Plants. 2024; 13(6):754. https://doi.org/10.3390/plants13060754
Chicago/Turabian StyleKoshovyi, Oleh, Mykola Komisarenko, Tatyana Osolodchenko, Andrey Komissarenko, Reet Mändar, Siiri Kõljalg, Jyrki Heinämäki, and Ain Raal. 2024. "Eucalypt Extracts Prepared by a No-Waste Method and Their 3D-Printed Dosage Forms Show Antimicrobial and Anti-Inflammatory Activity" Plants 13, no. 6: 754. https://doi.org/10.3390/plants13060754
APA StyleKoshovyi, O., Komisarenko, M., Osolodchenko, T., Komissarenko, A., Mändar, R., Kõljalg, S., Heinämäki, J., & Raal, A. (2024). Eucalypt Extracts Prepared by a No-Waste Method and Their 3D-Printed Dosage Forms Show Antimicrobial and Anti-Inflammatory Activity. Plants, 13(6), 754. https://doi.org/10.3390/plants13060754








