Transcriptome Analysis of Meloidogyne javanica and the Role of a C-Type Lectin in Parasitism
Abstract
1. Introduction
2. Results
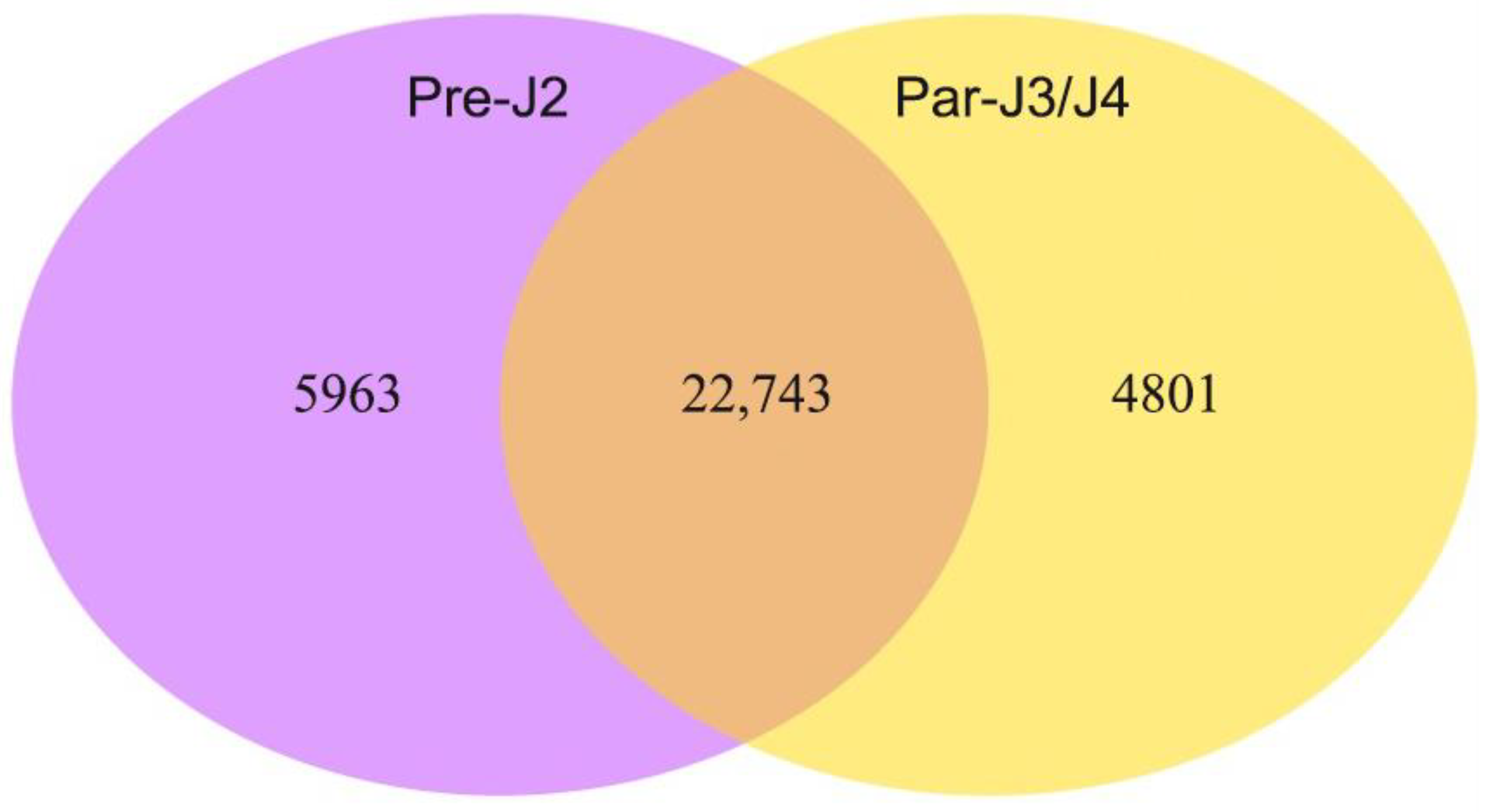
2.1. Overview of M. javanica Transcriptome Sequencing, Assembly and Annotations
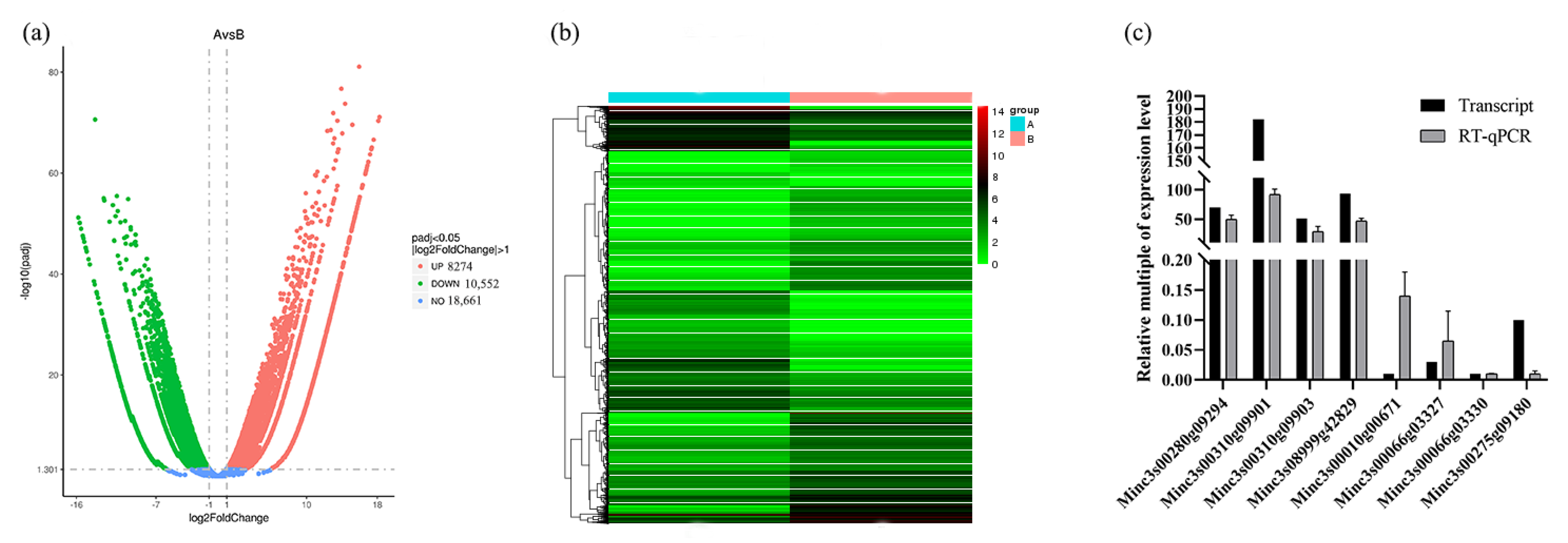
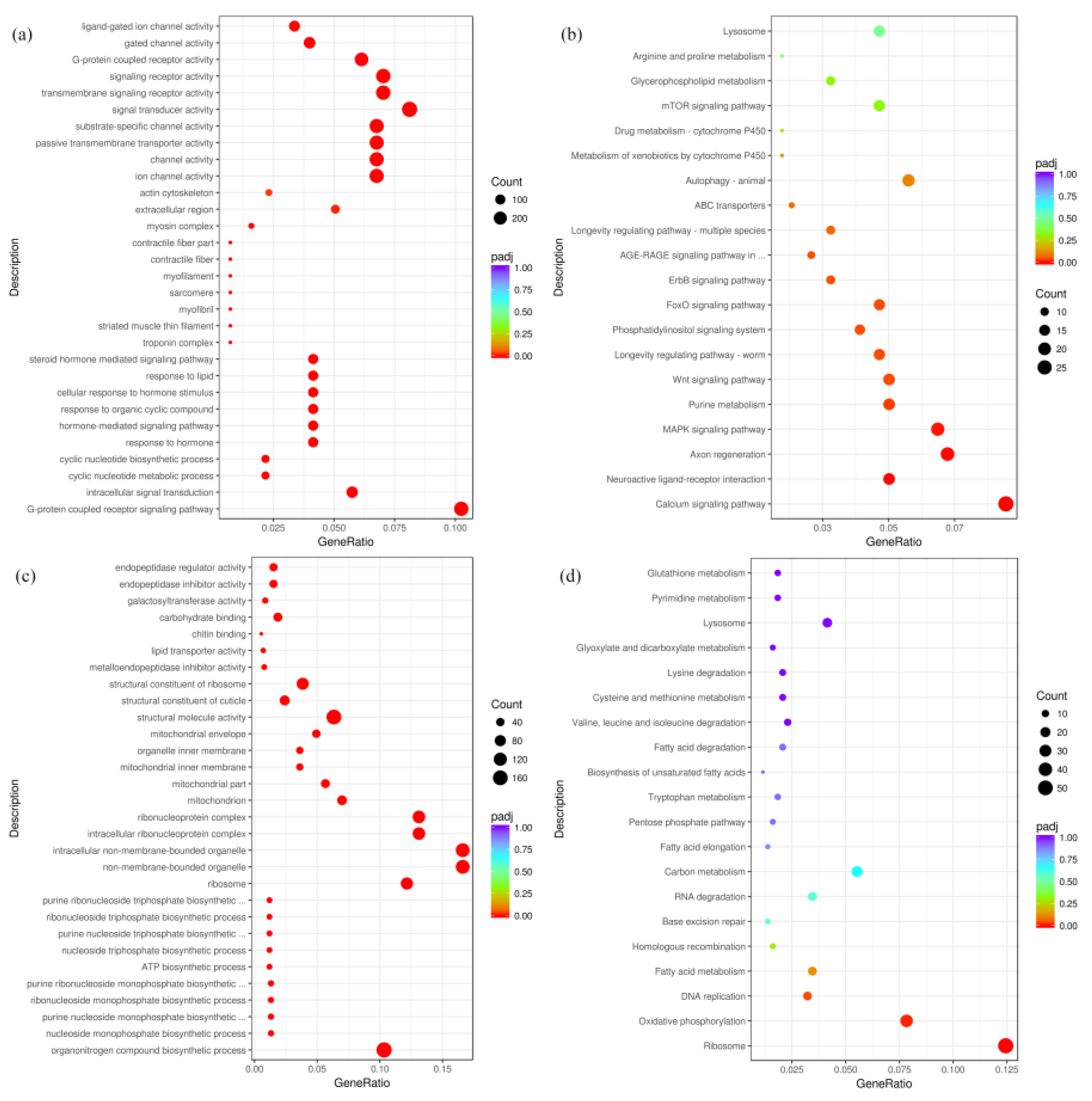
2.2. Identification and Verificationof DEGs in Transcriptome
2.3. Screening of Lectins and Sequence Alignment of the Mj-CTL Genes
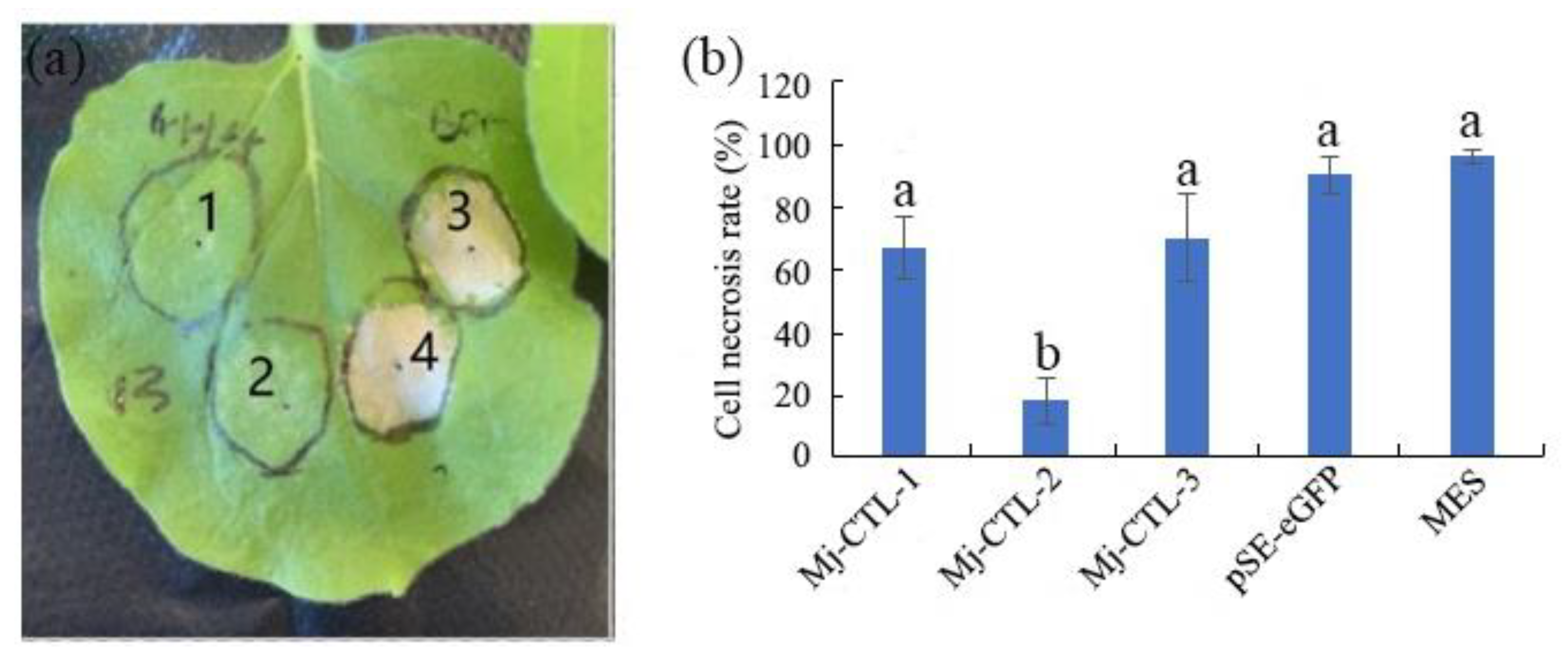
2.4. Suppression of Gpa2/RBP-1-Induced Cell Death by Mj-CTL-2
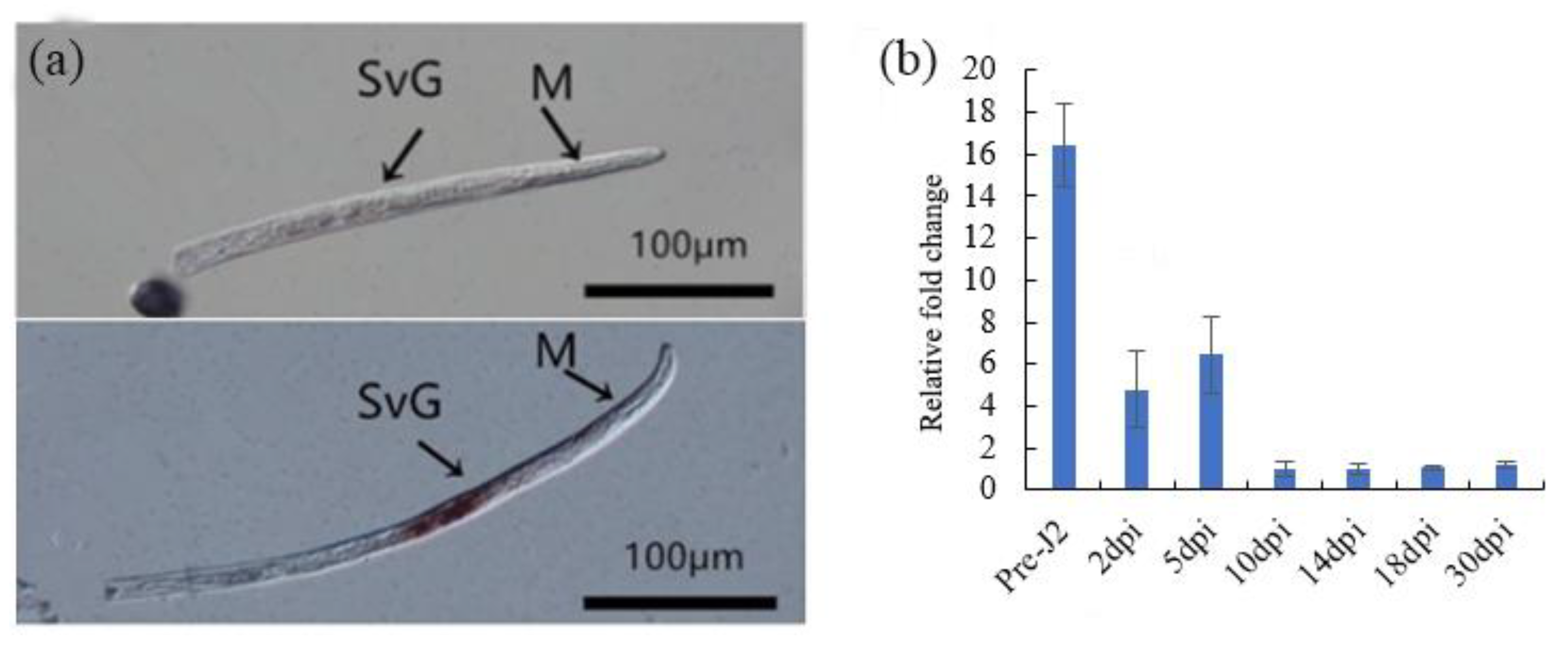
2.5. Spatial and Developmental Expression Analysis of Mj-CTL-2
2.6. Inhibitory Effect of Mj-CTL-2 on the ROS Burst
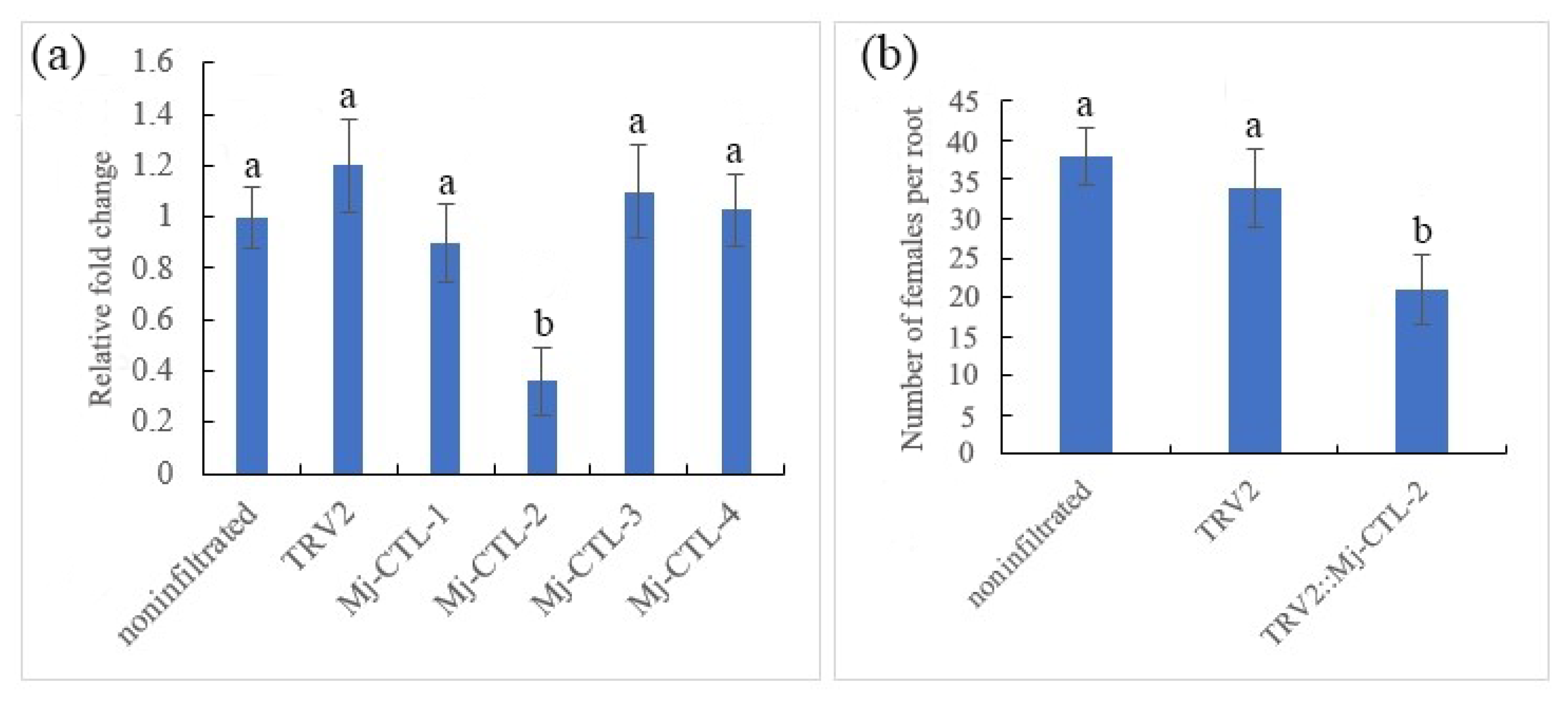
2.7. Attenuating Effect of in Planta RNA Interference (RNAi) of Mj-CTL-2 on Nematode Parasitism
2.8. Interactions between Mj-CTL-2 and Solanum Lycopersicum Catalase: SlCAT2
3. Discussion
4. Materials and Methods
4.1. Nematode Culture
4.2. RNA Extraction and Library Preparation
4.3. Reads Mapping to the Reference Genome and Functional Classification
4.4. Quantification of Gene Expression Level and Differential Expression Analysis
4.5. Validation of DEGs by qPCR
4.6. In Situ Hybridization
4.7. Developmental Expression Analysis
4.8. Inhibitory Effect of CTLs of M. javanica on Gpa2/RBP-1-Induced Tobacco Cell Death
4.9. Detection of ROS Burst
4.10. In Planta RNAi
4.11. Y2H Co-Transformation and BiFC Assays
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blok, V.C.; Jones, J.T.; Phillips, M.S.; Trudgill, D.L. Parasitism genes and host range disparities in biotrophic nematodes: The conundrum of polyphagy versus specialisation. BioEssays 2008, 30, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Fitoussi, N.; Borrego, E.; Kolomiets, M.V.; Qing, X.; Bucki, P.; Sela, N.; Belausov, E.; Braun Miyara, S. Oxylipins are implicated as communication signals in tomato–root-knot nematode (Meloidogyne javanica) interaction. Sci. Rep. 2021, 11, 326. [Google Scholar] [CrossRef]
- Hu, M.X.; Zhuo, K.; Liao, J.L. Multiplex PCR for the simultaneous identification and detection of Meloidogyne incognita, M. enterolobii, and M. javanica using DNA extracted directly from individual galls. Phytopathology 2011, 101, 1270. [Google Scholar] [CrossRef]
- Abad, P.; Gouzy, G.J.; Aury, J. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Opperman, C.H.; Bird, D.M.; Williamson, V.M.; Rokhsar, D.S.; Burke, M.; Cohn, J.; Cromer, J.; Diener, S.; Gajan, J.; Graham, S.; et al. Sequence and Genetic Map of Meloidogyne hapla: A Compact Nematode Genome for Plant Parasitism. Proc. Natl. Acad. Sci. USA 2008, 105, 14802–14807. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.; Niu, J.; Zhao, J.; Jian, H. De Novo Analysis of the Transcriptome of Meloidogyne enterolobii to Uncover Potential Target Genes for Biological Control. Int. J. Mol. Sci. 2016, 17, 1442. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Bourne, Y.; Van Damme, E.J.; Rougé, P. Overview of the Structure-Function Relationships of Mannose-Specific Lectins from Plants, Algae and Fungi. Int. J. Mol. Sci. 2019, 20, 254. [Google Scholar] [CrossRef]
- Baigent, S.J.; McCauley, J.W. Influenza type A in humans, mammals and birds: Determinants of virus virulence, host-range and interspecies transmission. BioEssays 2003, 25, 657–671. [Google Scholar] [CrossRef]
- Wang, Y.; Narain, R.; Liu, Y. Study of Bacterial Adhesion on Different Glycopolymer Surfaces by Quartz Crystal Microbalance with Dissipation. Langmuir 2014, 30, 7377–7387. [Google Scholar] [CrossRef]
- Bauters, L.; Naalden, D.; Gheysen, G. The Distribution of Lectins across the Phylum Nematoda: A Genome-Wide Search. Int. J. Mol. Sci. 2017, 18, 91. [Google Scholar] [CrossRef]
- Zelensky, A.N.; Gready, J.E. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef]
- Zhuo, K.; Naalden, D.; Nowak, S.; Huy, N.X.; Bauters, L.; Gheysen, G. A Meloidogyne graminicola C-type lectin, Mg01965, is secreted into the host apoplast to suppress plant defence and promote parasitism. Mol. Plant Pathol. 2019, 20, 346–355. [Google Scholar] [CrossRef]
- De Boer, J.M.; Mcdermott, J.P.; Wang, X.; Maier, T.; Qui, F.; Hussey, R.S.; Davis, E.L.; Baum, T.J. The use of DNA microarrays for the developmental expression analysis of cDNAs from the oesophageal gland cell region of Heterodera glycines. Mol. Plant Pathol. 2002, 3, 261–270. [Google Scholar] [CrossRef]
- Urwin, P.E.; Lilley, C.J.; Atkinson, H.J. Ingestion of Double-Stranded RNA by Preparasitic Juvenile Cyst Nematodes Leads to RNA Interference. Mol. Plant-Microbe Interact. 2002, 15, 747–752. [Google Scholar] [CrossRef]
- Ganji, S.; Jenkins, J.N.; Wubben, M.J. Molecular characterization of the reniform nematode C-type lectin gene family reveals a likely role in mitigating environmental stresses during plant parasitism. Gene 2014, 537, 269–278. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Q.; Quentin, M.; Ling, J.; Abad, P.; Zhang, X.; Li, Y.; Yang, Y.; Favery, B.; Mao, Z.; et al. A Meloidogyne incognita C-type lectin effector targets plant catalases to promote parasitism. New Phytol. 2021, 232, 2124–2137. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Hwang, B.K. Induction of some defense-related genes and oxidative burst is required for the establishment of systemic acquired resistance in Capsicum annuum. Planta 2005, 221, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, D.; Wang, Z.; Dong, A.; Liu, L.; Wang, B.; Chen, Q.; Liu, X. Transcriptomic analysis of the rice white tip nematode, Aphelenchoides besseyi (Nematoda: Aphelenchoididae). PLoS ONE 2014, 9, e91591. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, C.-L.; Yang, S.-H.; Li, J.-Y.; Wang, H.-L.; Zhang, Z.-X.; Chen, C.; Xie, H. Life-stage specific transcriptomes of a migratory endoparasitic plant nematode, Radopholus similis elucidate a different parasitic and life strategy of plant parasitic nematodes. Sci. Rep. 2019, 9, 6277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, D.; Shuang, L.; Xiao, D.; Xuan, Y.; Duan, Y.; Chen, L.; Wang, Y.; Liu, X.; Fan, H.; et al. Transcriptome Analysis of Rice Roots in Response to Root-Knot Nematode Infection. Int. J. Mol. Sci. 2020, 21, 848. [Google Scholar] [CrossRef]
- Alvares, S.M.; Mayberry, G.A.; Joyner, E.Y.; Lakowski, B.; Ahmed, S. H3K4 demethylase activities repress proliferative and postmitotic aging. Aging Cell 2014, 13, 245–253. [Google Scholar] [CrossRef]
- Alvarez, J.; Alvarez-Illera, P.; García-Casas, P.; Fonteriz, R.I.; Montero, M. The Role of Ca2+ Signaling in Aging and Neurodegeneration: Insights from Caenorhabditis elegans Models. Cells 2020, 9, 204. [Google Scholar] [CrossRef]
- McFarlane, I.G. Hepatic clearance of serum glycoproteins. Clin. Sci. 1983, 64, 127–135. [Google Scholar] [CrossRef]
- Loukas, A.; Maizels, R.M. Helminth C-type lectins and host-parasite interactions. Parasitol. Today Regul. Ed. 2000, 16, 333–339. [Google Scholar] [CrossRef]
- Harcus, Y.; Nicoll, G.; Murray, J.; Filbey, K.; Gomez-Escobar, N.; Maizels, R.M. C-type lectins from the nematode parasites Heligmosomoides polygyrus and Nippostrongylus brasiliensis. Parasitol. Int. 2009, 58, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Roze, E.; Hanse, B.; Mitreva, M.; Vanholme, B.; Bakker, J.; Smant, G. Mining the secretome of the root-knot nematode Meloidogyne chitwoodi for candidate parasitism genes. Mol. Plant Pathol. 2007, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cambi, A.; Koopman, M.; Figdor, C.G. How C-type lectins detect pathogens. Cell Microbiol. 2005, 7, 481–488. [Google Scholar] [CrossRef]
- Davis, E.L.; Hussey, R.S.; Mitchum, M.G.; Baum, T.J. Parasitism proteins in nematode-plant interactions. Curr. Opin. Plant Biol. 2008, 11, 360–366. [Google Scholar] [CrossRef]
- Huang, G.; Dong, R.; Allen, R.; Davis, E.L.; Baum, T.J.; Hussey, R.S. Two chorismate mutase genes from the root-knot nematode Meloidogyne incognita. Mol. Plant Pathol. 2005, 6, 23–30. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- De Boer, J.M.; Yan, Y.; Smant, G.; Davis, E.L.; Baum, T.J. In-situ hybridisation to messenger RNA in Heterodera glycines. J. Nematol. 1998, 30, 309–312. [Google Scholar]
- Chen, J.; Lin, B.; Huang, Q.; Hu, L.; Zhuo, K.; Liao, J. A novel Meloidogyne graminicola effector, MgGPP, is secreted into host cells and undergoes glycosylation in concert with proteolysis to suppress plant defenses and promote parasitism. PLOS Pathog. 2017, 13, e1006301. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chronis, D.; Wang, X. The novel GrCEP12 peptide from the plant-parasitic nematode Globodera rostochiensis suppresses flg22-mediated PTI. Plant Signal Behav. 2013, 8, e25359. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.; Anand, A.; Kang, L.; Mysore, K.S. Agrodrench: A novel and effective agroinoculation method for virus-induced gene silencing in roots and diverse Solanaceous species. Plant J. 2004, 40, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Krichevsky, A.; Schornack, S.; Lahaye, T.; Tzfira, T.; Tang, Y.; Citovsky, V.; Mysore, K.S. Arabidopsis VIRE2 INTERACTING PROTEIN2 Is Required for Agrobacterium T-DNA Integration in Plants. Plant Cell 2007, 19, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhuo, K.; Chen, S.; Hu, L.; Sun, L.; Wang, X.; Zhang, L.H.; Liao, J. A novel nematode effector suppresses plant immunity by activating host reactive oxygen species-scavenging system. New Phytol. 2016, 209, 1159–1173. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, W.; Hu, L.; Li, Z.; Lin, B.; Zhuo, K.; Liao, J. Transcriptome Analysis of Meloidogyne javanica and the Role of a C-Type Lectin in Parasitism. Plants 2024, 13, 730. https://doi.org/10.3390/plants13050730
Chi W, Hu L, Li Z, Lin B, Zhuo K, Liao J. Transcriptome Analysis of Meloidogyne javanica and the Role of a C-Type Lectin in Parasitism. Plants. 2024; 13(5):730. https://doi.org/10.3390/plants13050730
Chicago/Turabian StyleChi, Wenwei, Lili Hu, Zhiwen Li, Borong Lin, Kan Zhuo, and Jinling Liao. 2024. "Transcriptome Analysis of Meloidogyne javanica and the Role of a C-Type Lectin in Parasitism" Plants 13, no. 5: 730. https://doi.org/10.3390/plants13050730
APA StyleChi, W., Hu, L., Li, Z., Lin, B., Zhuo, K., & Liao, J. (2024). Transcriptome Analysis of Meloidogyne javanica and the Role of a C-Type Lectin in Parasitism. Plants, 13(5), 730. https://doi.org/10.3390/plants13050730




