Spatial and Temporal Disparity Analyses of Glycosylated Benzaldehyde and Identification and Expression Pattern Analyses of Uridine Diphosphate Glycosyltransferase Genes in Prunus mume
Abstract
1. Introduction
2. Results
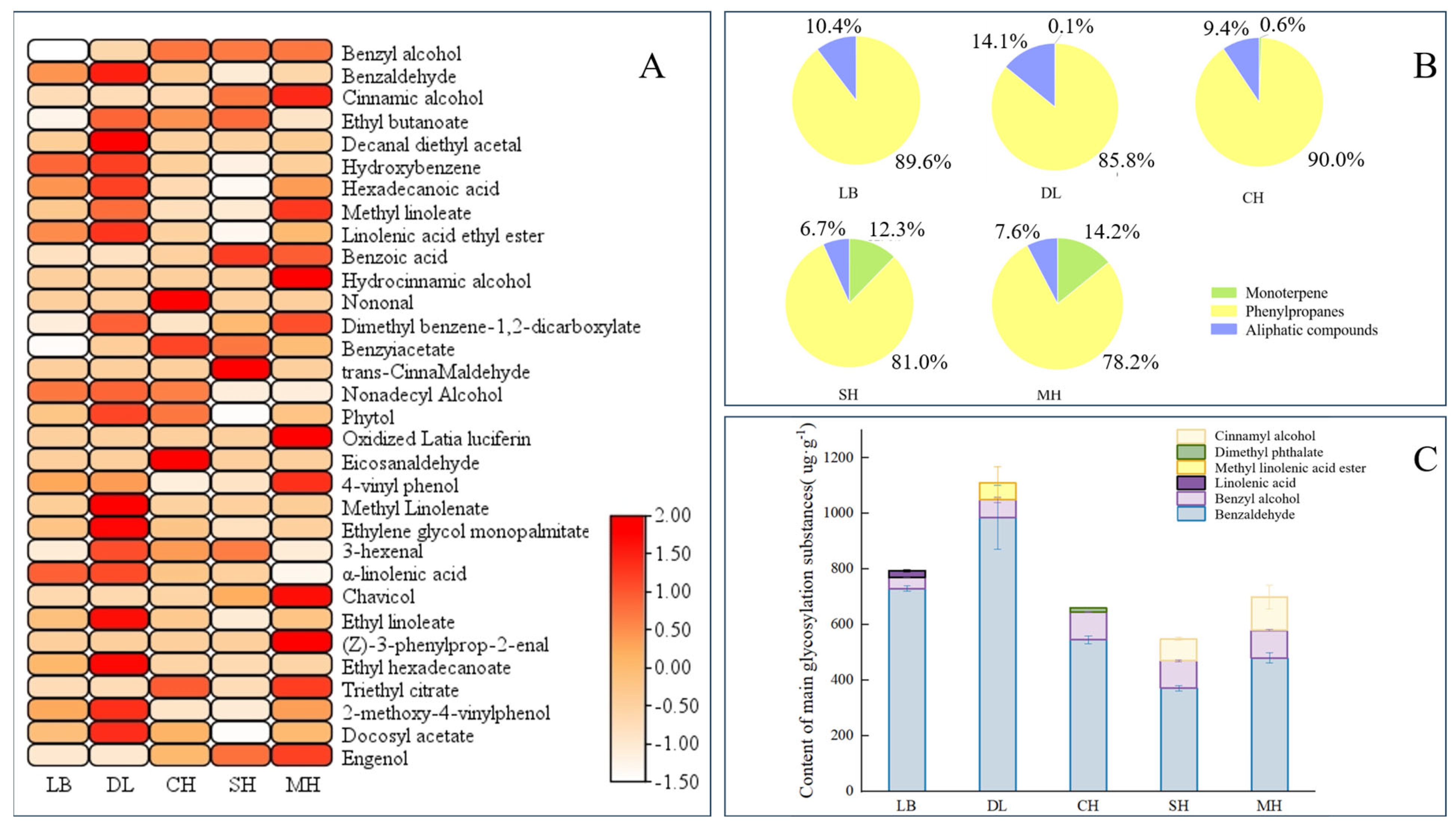
2.1. Glycosidic Aromatic Substances of P. mume in Different Anthesis Stages
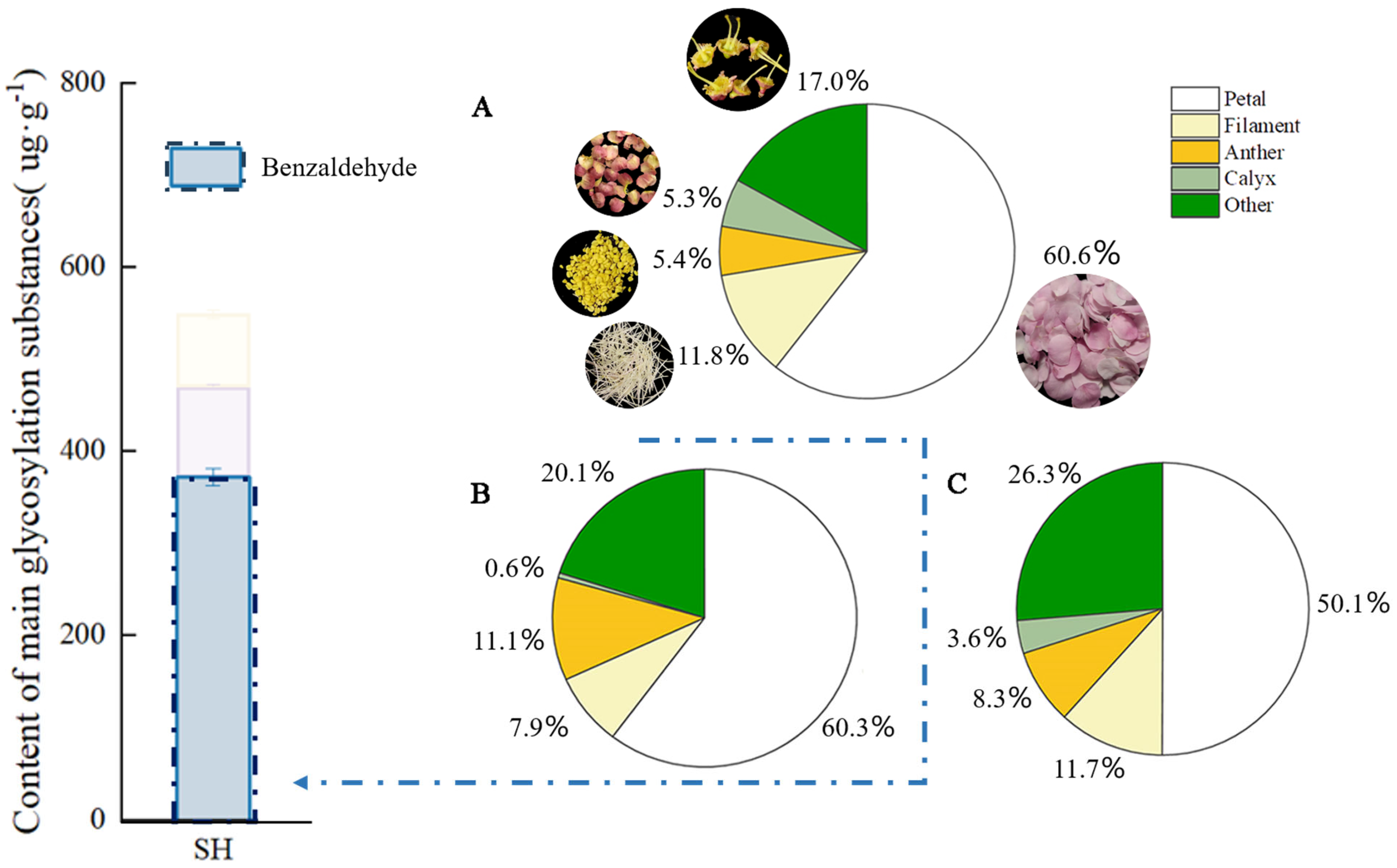
2.2. Quantitative Analysis of Endogenous Benzaldehyde in Different Floral Parts of P. mume
2.3. Quantitative Analysis of Glycosidic State of Key Components of P. mume in Different Anthesis Stages
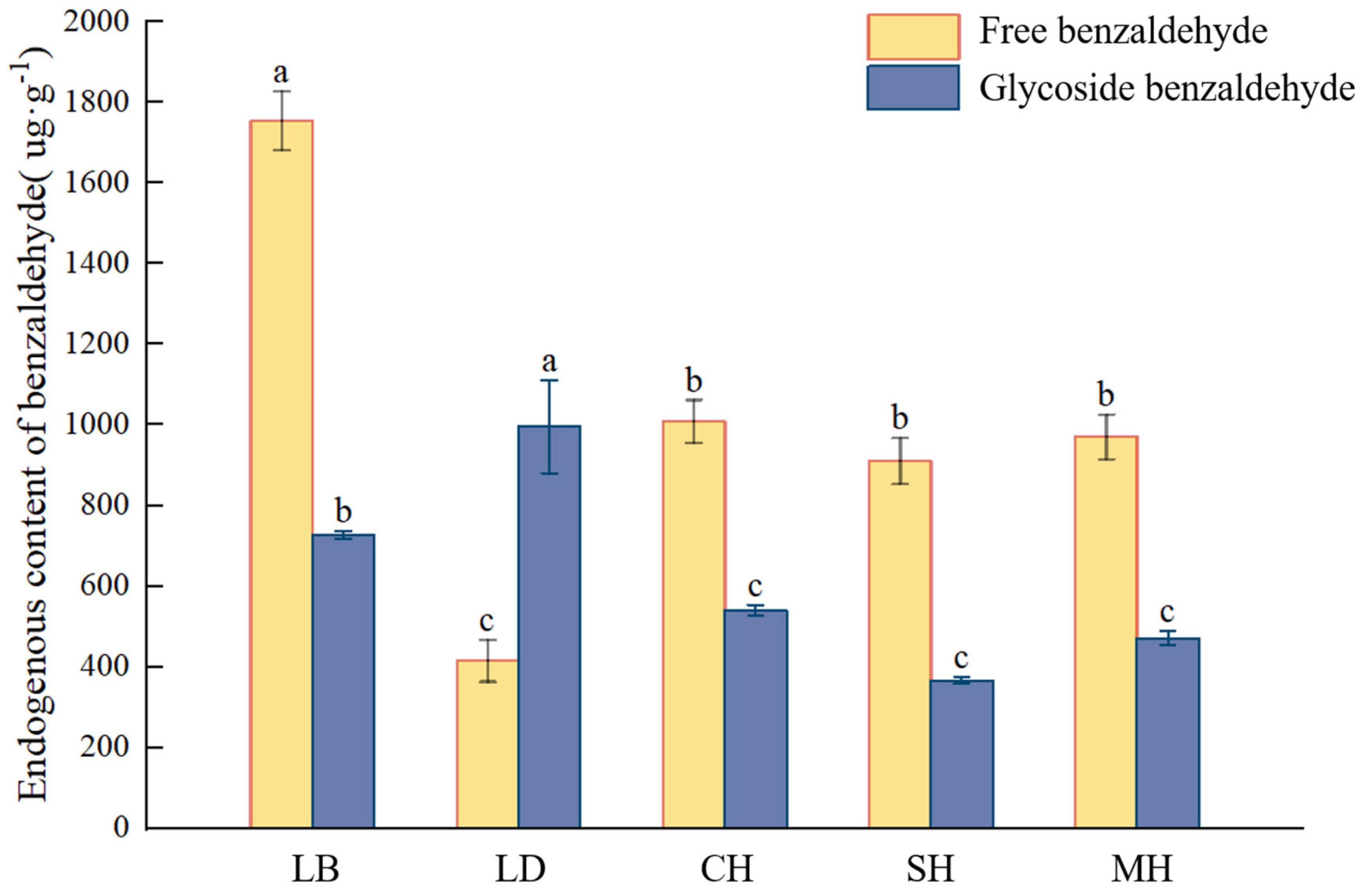
2.4. Quantitative Analysis of Endogenous Benzaldehyde at Different Anthesis Stages of P. mume
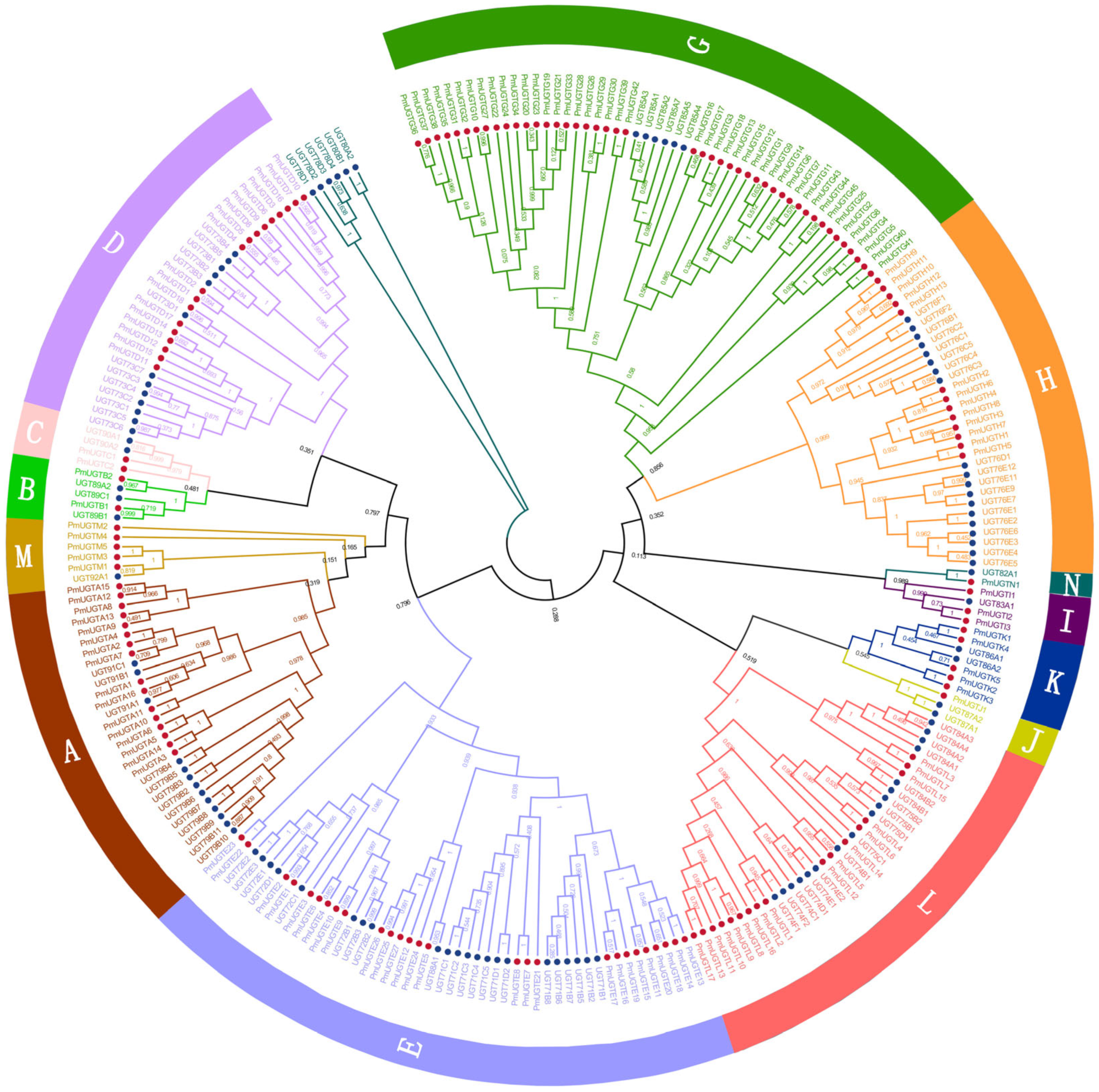
2.5. Identification and Subfamily Classification of PmUGT Family Members
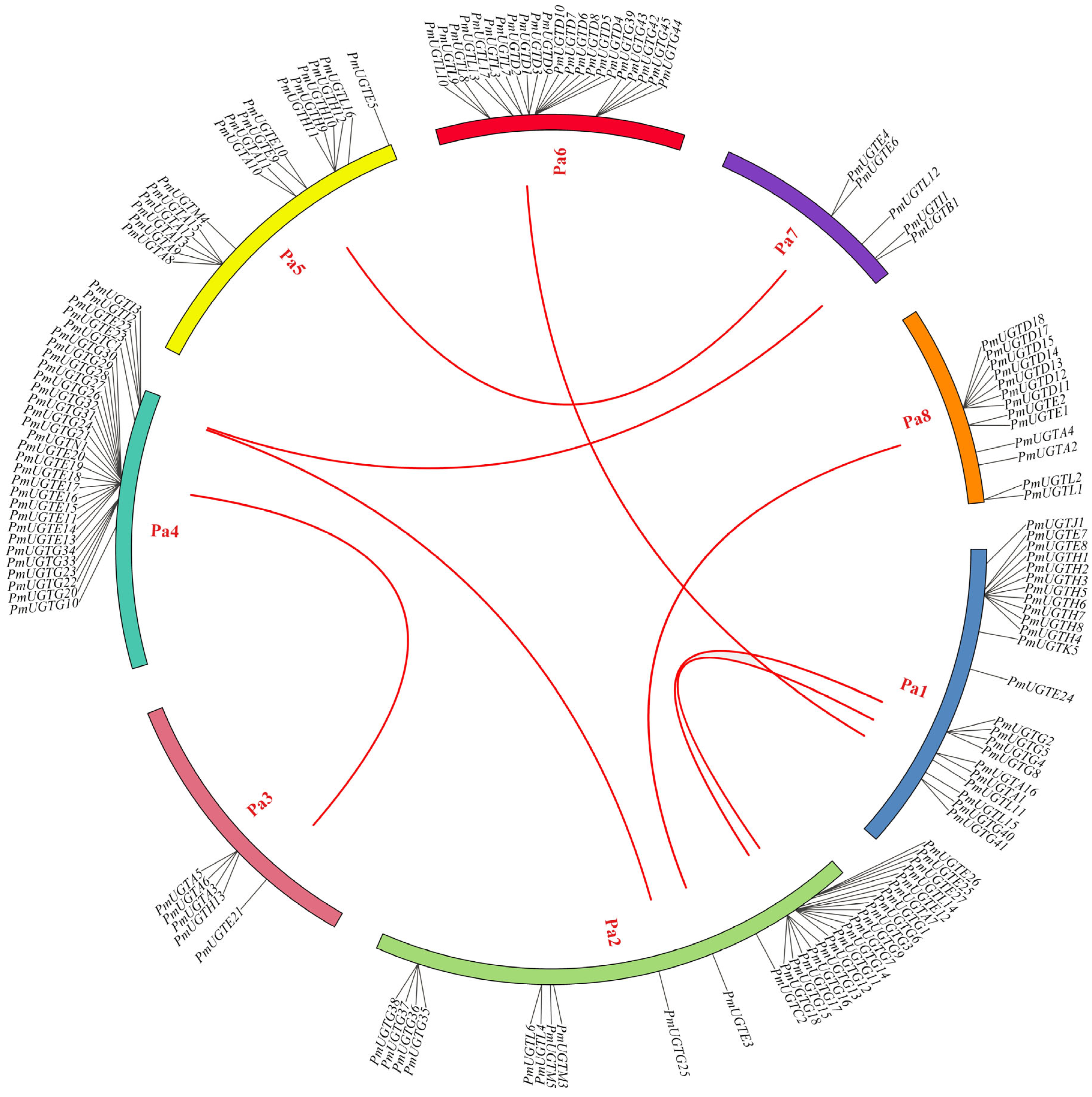
2.6. Chromosome Localization and Colinearity Analysis of the PmUGT Family
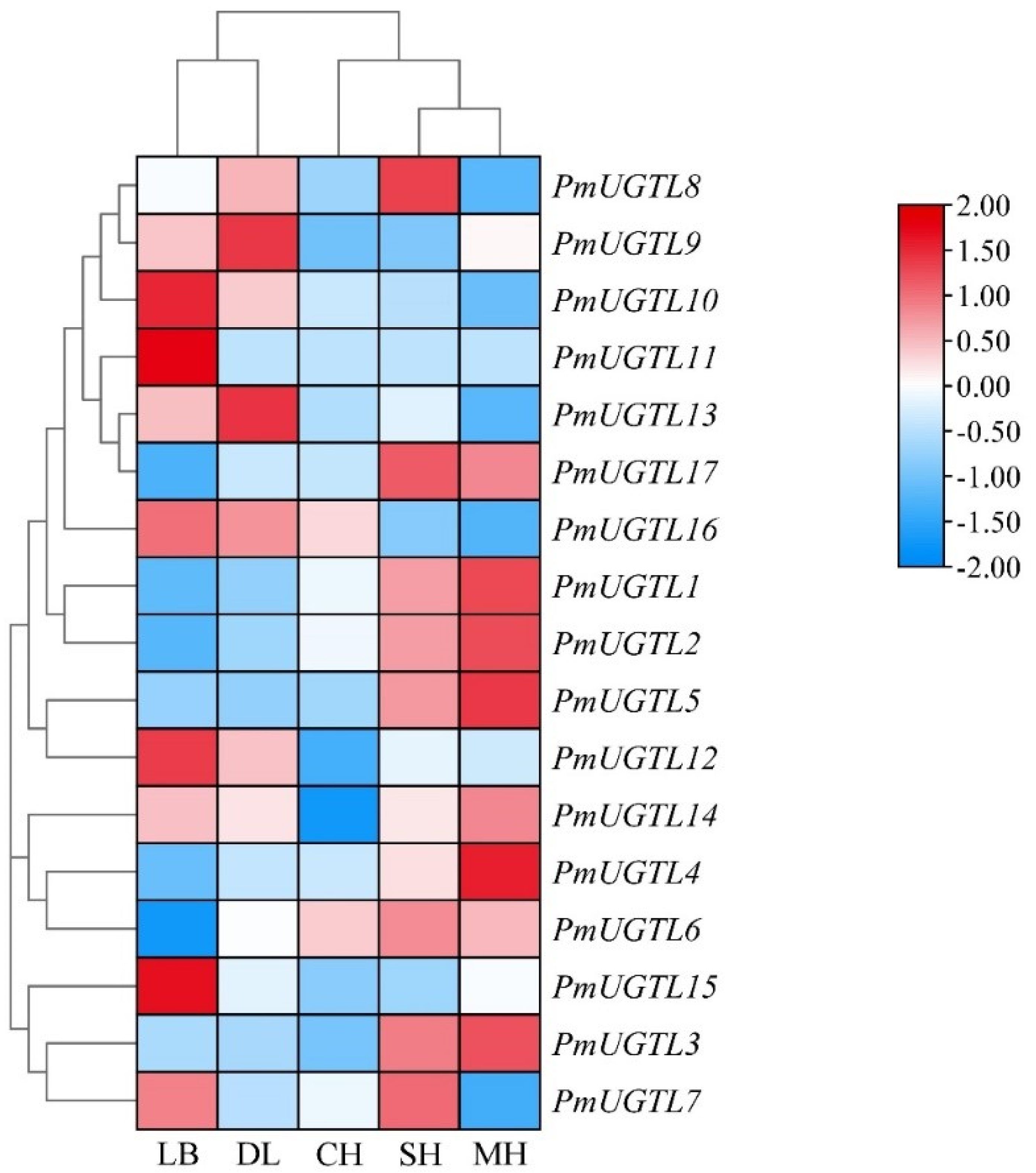
2.7. Expression Analysis of PmUGTL Genes at Different Anthesis Stages of P. mume
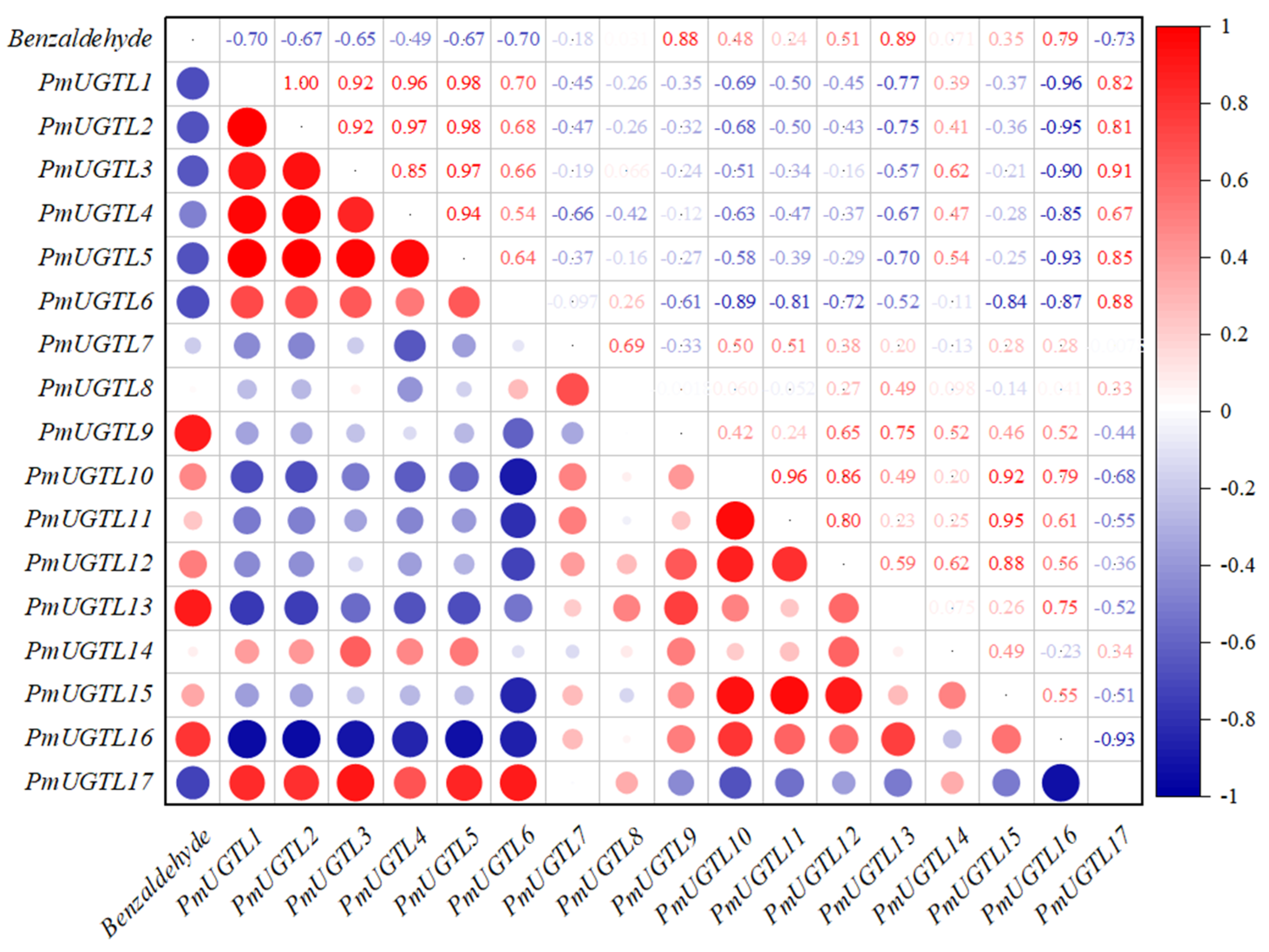
2.8. Correlation Analysis between Benzaldehyde Glycosidation and PmUGTL Gene Expression in Prunus mume
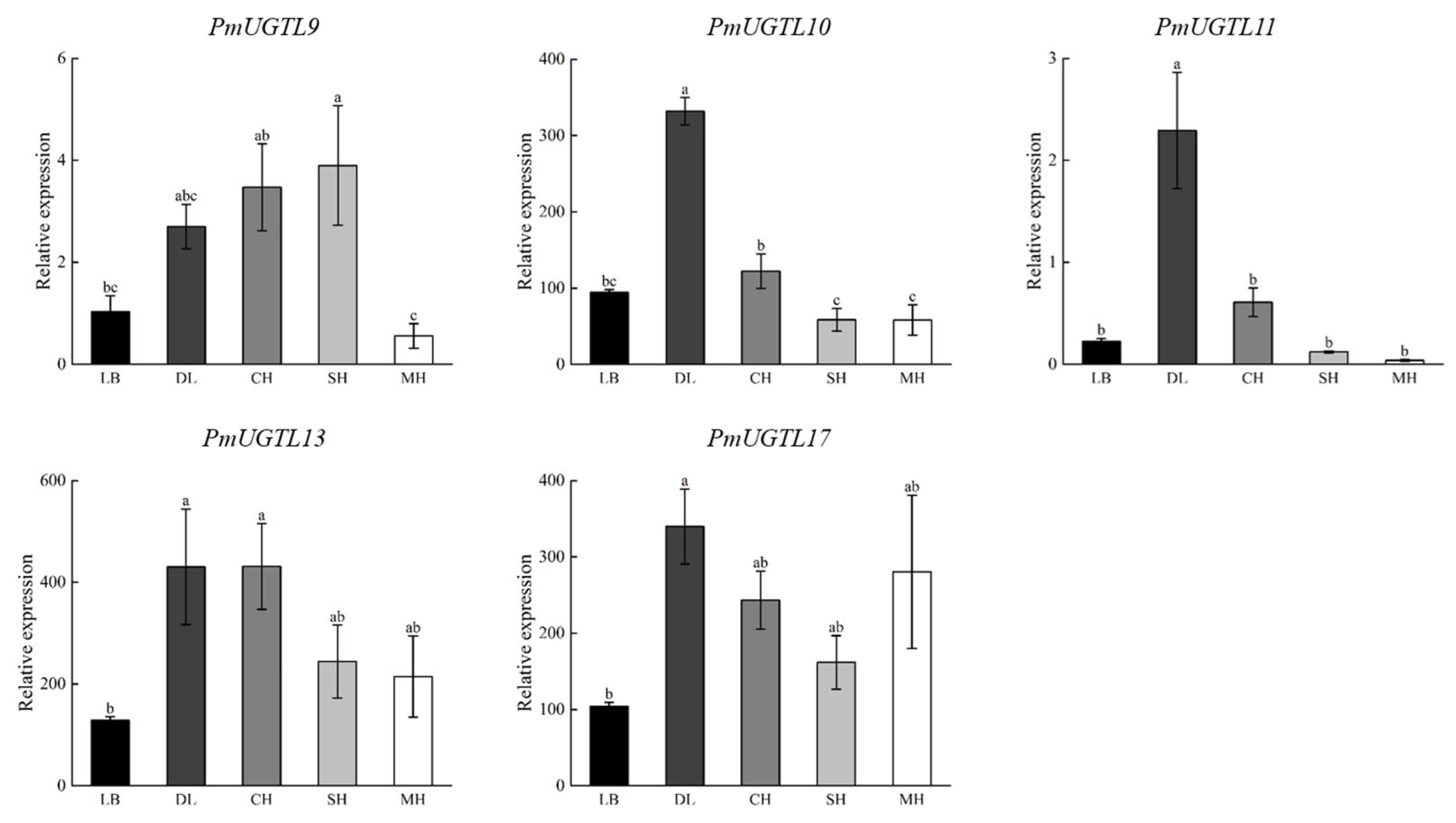
2.9. Validation of qRT-RCR of Key Genes of PmUGTLs
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Instruments and Reagents
4.3. Extraction of Glycosidic Aromatic Substances
4.4. Hydrolysis of Glycosidic Aromatic Substances
4.5. GC-MS Analysis
4.6. Statistics and Analysis of Data
4.7. Transcriptome Measurement of P. mume at Different Nthesis Stages
4.8. Identification of UGT Gene Family Members
4.9. Multiple Sequence Alignment and Subfamily Classification of the UGT Gene Family
4.10. Chromosomal Localization and Colinearity Analysis of the UGT Gene
4.11. Analysis of the Expression of UGTLs Genes in P. mume at Different Anthesis Stages with Time
4.12. Quantitative Expression Analysis of Candidate Genes for UGTLs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Fang, X.; Yang, C.; Li, J.; Chen, X. Biosynthesis and regulation of secondary terpenoid metabolism in plants. Sci. Sin. 2013, 43, 1030–1046. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Shi, L.; Liu, Y. Application of functional genomic and metabolomic techniques to the studies on biosynthesis and regulation of plant secondary metabolites. J. Beijing For. Univ. 2007, 29, 153–159. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, J.; Shentu, X.; Hao, P.; Pang, K.; Yu, X. Review of how secondary metabolites defend plants against herbivorous insects. Chin. J. Appl. Entomol. 2022, 59, 969–978. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, W.; Tang, K. Research progress of jasmonates’ regulation on the plant secondary metabolis. J. Shanghai Jiaotong Univ. (Agric. Sci.) 2011, 29, 87–91. [Google Scholar] [CrossRef]
- Davide, S.; Stéphanie, M.M.; Tristan, R.; Pierre, W.T.; Jonathan, B.; Jean-Pierre, M.; Jean-Michel, M.; de Gilles, R. Centrifugal partition chromatography applied to the isolation of oak wood aroma precursors. Food Chem. 2013, 141, 2238–2245. [Google Scholar] [CrossRef]
- Shoji, O.; Eiichiro, O.; Manabu, H.; Jun, M.; Koujirou, T.; Hiromi, T.; Yukie, O.; Hideo, D.; Tatsuo, A.; Kenji, M.; et al. Volatile glycosylation in tea plants: Sequential glycosylations for the biosynthesis of aroma β-Primeverosides are catalyzed by two camellia sinensis glycosyltransferases. Plant Physiol. 2015, 168, 464–477. [Google Scholar] [CrossRef]
- Qin, J.; Sun, C.; Zhang, M.; Wang, Y. Classification, function and evolution of plant UDP-glycosyltransferase. Genom. Appl. Biol. 2018, 37, 440–450. [Google Scholar] [CrossRef]
- Keiko, Y.S.; Kousuke, H. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 2011, 66, 182–193. [Google Scholar] [CrossRef]
- Alexandra, L.; Denise, H.; Rosamond, G.J.; Gilu, L.G.; Luisa, E.; Eng-Kiat, L.; Fabián, E.V.; Dianna, J.B. The glucosyltransferase UGT72E2 is responsible for monolignol 4-O-glucoside production in Arabidopsis thaliana. Plant J. 2006, 48, 286–295. [Google Scholar] [CrossRef]
- Florian, P.S. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef]
- Tang, F.; Chen, Y.; Li, D.; Huang, N. Allelopathic effects of volatiles from Eucalyptus grandis × E. urophylla’s leaves on seed germination and seedling growth of three kinds of plants. Med. Plant. 2014, 5, 33. [Google Scholar]
- Cai, J.; Liu, B.; Ling, P.; Su, Q. Analysis of free and bound volatiles by gas chromatography and gas chromatography–mass spectrometry in uncased and cased tobaccos. J. Chromatogr. A 2002, 947, 267–275. [Google Scholar] [CrossRef]
- Francis, M.; Allcock, C. Geraniol β-D-glucoside; occurrence and synthesis in rose flowers. Phytochemistry 1969, 8, 1339–1347. [Google Scholar] [CrossRef]
- Williams, P.J.; Strauss, C.R.; Wilson, B.; Massy-Westropp, R.A. Novel monoterpene disaccharide glycosides of vitis vinifera grapes and wines. Phytochemistry 1982, 21, 2013–2020. [Google Scholar] [CrossRef]
- Guo, W.; Sakata, K.; Watanabe, N.; Nakajima, R.; Yagi, A.; Ina, K.; Luo, S. Geranyl 6-O-β-d-xylopyranosyl-β-d-glucopyranoside isolated as an aroma precursor from tea leaves for oolong tea. Phytochemistry 1993, 33, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, Y.; Chemardin, P.; Janbon, G.; Arnaud, A.; Galzy, P. A very efficient β-glucosidase catalyst for the hydrolysis of flavor precursors of wines and fruit juices. J. Agric. Food Chem. 1996, 44, 2336–2340. [Google Scholar] [CrossRef]
- Yuan, J.; Luo, J.; Zhu, L.; Xi, W.; Zeng, X.; Xiong, K.; Wang, C.; Zheng, R. Free and glycosylated aroma components in petals of three Osmanthus fragrans cultivars. Sci. Silvae Sin. 2021, 57, 33–42. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, L.; Gui, J.; Liao, Y.; Li, J.; Tang, J.; Meng, Q.; Dong, F.; Yang, Z. Functional characterizations of β-glucosidases involved in aroma compound formation in tea (Camellia sinensis). Food Res. Int. 2017, 96, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Jiang, Q.; Lin, Q.; Ma, Q.; Wang, L.; Weng, S.; Huang, G.; Li, L.; Chen, F. Enzymatic hydrolysis and auto-isomerization during β-glucosidase treatment improve the aroma of instant white tea infusion. Food Chem. 2021, 342, 128565. [Google Scholar] [CrossRef] [PubMed]
- Tikunov, Y.M.; Molthoff, J.; de Vos, R.C.; Beekwilder, J.; van Houwelingen, A.; van der Hooft, J.J.; Nijenhuis-de Vries, M.; Labrie, C.W.; Verkerke, W.; van de Geest, H. Non-smoky glycosyltransferase1 prevents the release of smoky aroma from tomato fruit. Plant Cell 2013, 25, 3067–3078. [Google Scholar] [CrossRef]
- Zhang, T.; Huo, T.; Ding, A.; Hao, R.; Wang, J.; Cheng, T.; Bao, F.; Zhang, Q. Genome-wide identification, characterization, expression and enzyme activity analysis of coniferyl alcohol acetyltransferase genes involved in eugenol biosynthesis in Prunus mume. PLoS ONE 2019, 10, e0223974. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Zhang, Q.; Sun, M.; Pan, C. Studies on the volatile constituents from cultivars of Prunus mume. J. Trop. Subtrop. Bot. 2010, 18, 310–315. [Google Scholar] [CrossRef]
- Hao, R.; Zhang, Q.; Yang, W.; Wang, J.; Pan, H.; Cheng, T. Study on the difference in characteristic scent between Prunus mume and its interspecific hybrids. J. Nucl. Agric. Sci. 2014, 28, 808–816. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, Y.; Luo, Y.; Shen, F.; Gao, H.; Gao, R. Aldehyde volatiles emitted in succession from mechanically damaged leaves of poplar cuttings. J. Plant Biol. 2008, 51, 269–275. [Google Scholar] [CrossRef]
- Liu, T.; Zou, L.; Tian, D.; Can, Q.; Zhu, M.; Mo, M.; Zhang, K. Proteomic changes in Arthrobotrys oligospora conidia in response to benzaldehyde-induced fungistatic stress. J. Proteom. 2019, 192, 358–365. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.J.; Shin, J.H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Yang, S.; Zhang, Z.; Zhang, Y.; Chang, J.; Qiu, C. Identification and specific expression patterns in flower organs of ABCG genes related to floral scent from Prunus mume. Sci. Hortic. 2021, 288, 110218. [Google Scholar] [CrossRef]
- János, V. Plant cyanogenic glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Qi, L.; Wan, X. Antifungal activities of major tea leaf volatile constituents toward Colletorichum camelliae Massea. J. Agric. Food Chem. 2006, 54, 3936–3940. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, L.; Han, X.; Yang, Y. Research advances in allelopathy of volatile organic compounds (VOCs) of plants. Horticulturae 2021, 7, 278. [Google Scholar] [CrossRef]
- Nicholas, J.B.; Steven, J.R. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998, 16, 561–569. [Google Scholar] [CrossRef]
- Axel, M.; Wilhelm, B. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Ilker, D.; Murat, K. Prulaurasin content of leaves, kernels and pulps of Prunus lauracerasus L. (Cherry Laurel) during ripening. J. Res. Pharm. 2019, 23, 69–75. [Google Scholar] [CrossRef]
- Morten, E.J.; Hussam, H.N.; Barbara, A.H. Transport of defense compounds from source to sink: Lessons learned from glucosinolates. Trends Plant Sci. 2015, 20, 508–514. [Google Scholar] [CrossRef]
- Paul, D.B.; Jim, G.T.; Michael, R.; Jonathan, G. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Doyle, M. Adaptive patterns in alkaloid physiology. Am. Nat. 1974, 108, 305–320. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Yang, J.; Kang, C.; Huang, L.; Guo, L. Glycosylation of plant secondary metabolites: Regulating from chaos to harmony. Environ. Exp. Bot. 2022, 194, 104703. [Google Scholar] [CrossRef]
- John, H.L.; Thomas, R.H.; Harold, R.B.; Roger, A.A.; David, F.H. Glycosidically bound volatile components of Nicotiana sylvestris and N. Suaveolens flowers. Phytochemistry 1992, 31, 1537–1540. [Google Scholar] [CrossRef]
- Hao, R.; Chang, J.; Qiu, C.; Yang, S. An identification and expression analysis of the ABCG genes related to benzaldehyde transportation among three Prunus species. Horticulturae 2022, 8, 475. [Google Scholar] [CrossRef]
- Eng-Kiat, L.; Dianna, J.B. A class of plant glycosyltransferases involved in cellular homeostasis. EMBO J. 2004, 23, 2915–2922. [Google Scholar] [CrossRef]
- Yi, L.; Sandie, B.; Eng-Kiat, L.; Dianna, J.B. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana* 210. J. Biol. Chem. 2001, 276, 4338–4343. [Google Scholar] [CrossRef]
- Wu, B.; Gao, L.; Gao, J.; Xu, Y.; Liu, H.; Cao, X.; Zhang, B.; Chen, K. Genome-wide identification, expression patterns, and functional analysis of UDP glycosyltransferase family in peach (Prunus persica L. Batsch). Front. Plant Sci. 2017, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Tricia, K.F.; Abbas, Y.; Michelle, G.W.; Jennifer, R.G.; Brent, N.K.; Margaret, S.; Christopher, M.F. A seed coat cyanohydrin glucosyltransferase is associated with bitterness in almond (Prunus dulcis) kernels. Funct. Plant Biol. 2008, 35, 236–246. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R. Pfam: A domain-centric method for analyzing proteins and proteomes. Comp. Genom. 2007, 396, 43–58. [Google Scholar] [CrossRef]
- Wang, T.; Lu, J.; Xu, Z.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Selection of suitable reference genes for miRNA expression normalization by qRT-PCR during flower development and different genotypes of Prunus mume. Sci. Hortic. 2014, 169, 130–137. [Google Scholar] [CrossRef]
- Mohd, A.; Glyn, M.; Sibte, H. Analysis of rpoS and bolA gene expression under various stress-induced environments in planktonic and biofilm phase using 2−ΔΔCT method. Mol. Cell. Biochem. 2011, 357, 275–282. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Geng, X.; Fan, L.; Li, X.; Wang, J.; Hao, R. Spatial and Temporal Disparity Analyses of Glycosylated Benzaldehyde and Identification and Expression Pattern Analyses of Uridine Diphosphate Glycosyltransferase Genes in Prunus mume. Plants 2024, 13, 703. https://doi.org/10.3390/plants13050703
Jia H, Geng X, Fan L, Li X, Wang J, Hao R. Spatial and Temporal Disparity Analyses of Glycosylated Benzaldehyde and Identification and Expression Pattern Analyses of Uridine Diphosphate Glycosyltransferase Genes in Prunus mume. Plants. 2024; 13(5):703. https://doi.org/10.3390/plants13050703
Chicago/Turabian StyleJia, Haotian, Xiaoyun Geng, Lina Fan, Xin Li, Jiao Wang, and Ruijie Hao. 2024. "Spatial and Temporal Disparity Analyses of Glycosylated Benzaldehyde and Identification and Expression Pattern Analyses of Uridine Diphosphate Glycosyltransferase Genes in Prunus mume" Plants 13, no. 5: 703. https://doi.org/10.3390/plants13050703
APA StyleJia, H., Geng, X., Fan, L., Li, X., Wang, J., & Hao, R. (2024). Spatial and Temporal Disparity Analyses of Glycosylated Benzaldehyde and Identification and Expression Pattern Analyses of Uridine Diphosphate Glycosyltransferase Genes in Prunus mume. Plants, 13(5), 703. https://doi.org/10.3390/plants13050703




