Spatial Distribution and Sustainable Development of Living Woody and Coarse Woody Debris in Warm-Temperate Deciduous Broadleaved Secondary Forests in China
Abstract
1. Introduction
2. Materials and Methods
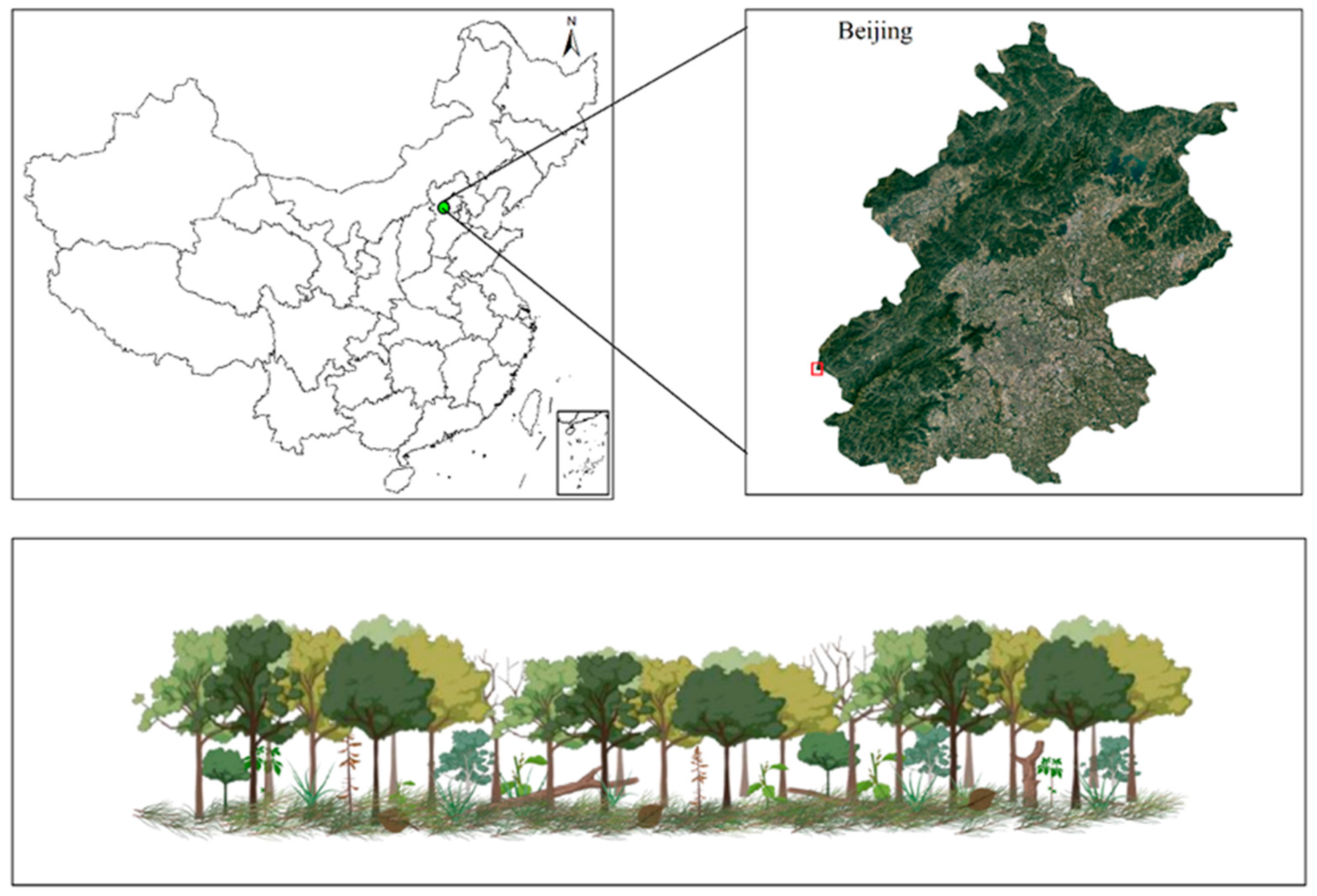
2.1. Study Site
2.2. Data Collection
2.3. Data Analyses
3. Results
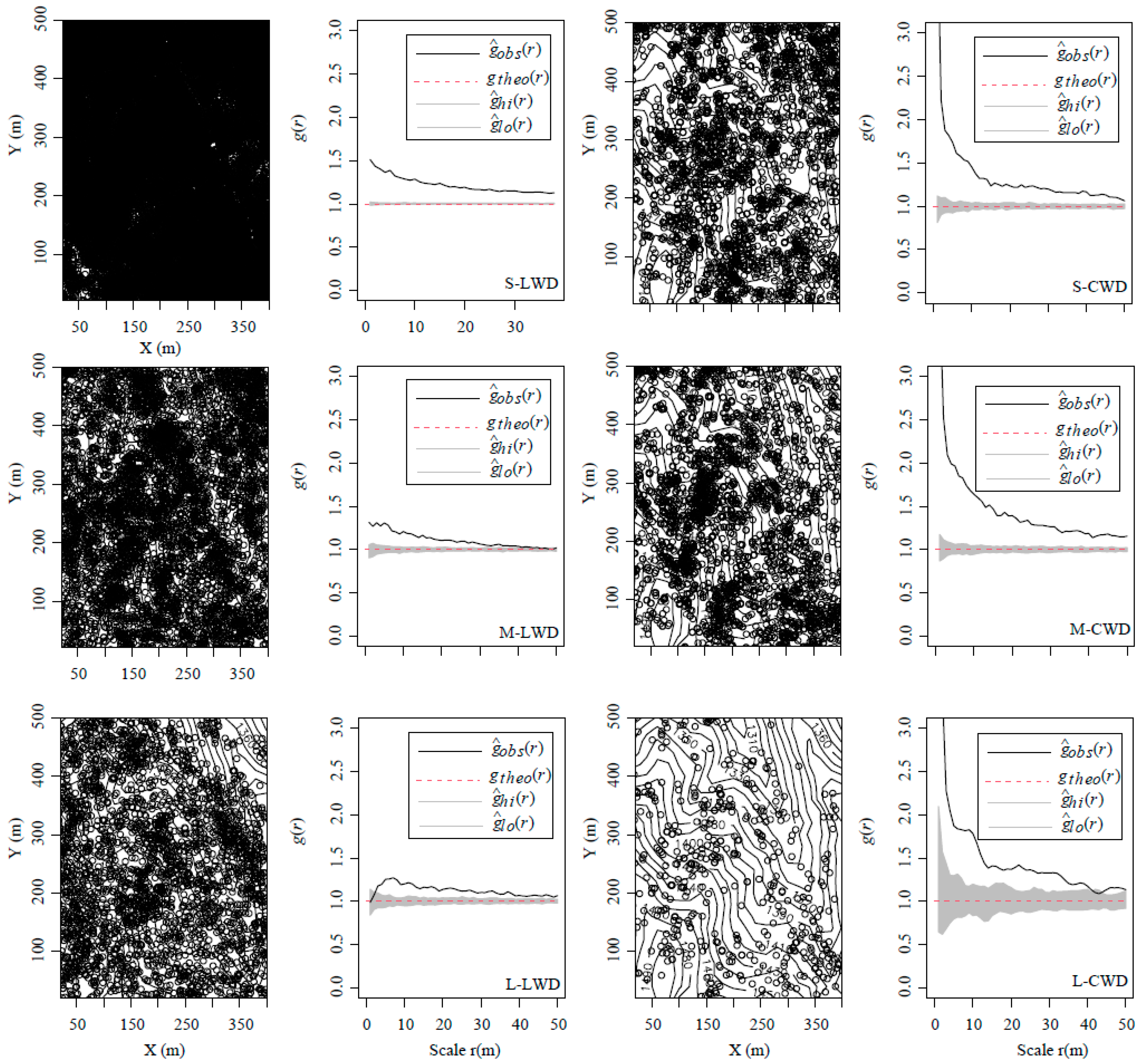
3.1. Spatial Distribution Pattern of LWD and CWD in Each Size Class
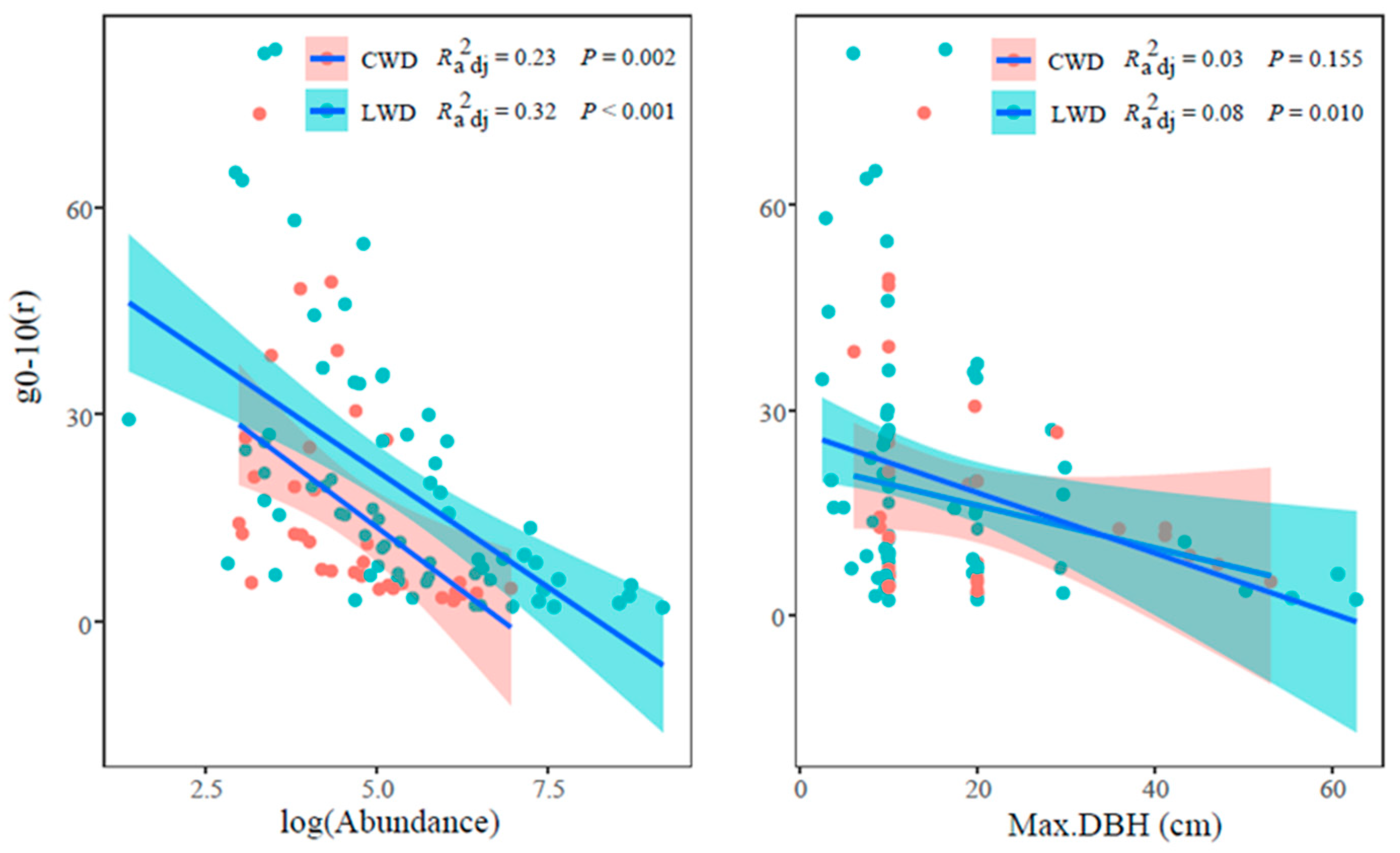
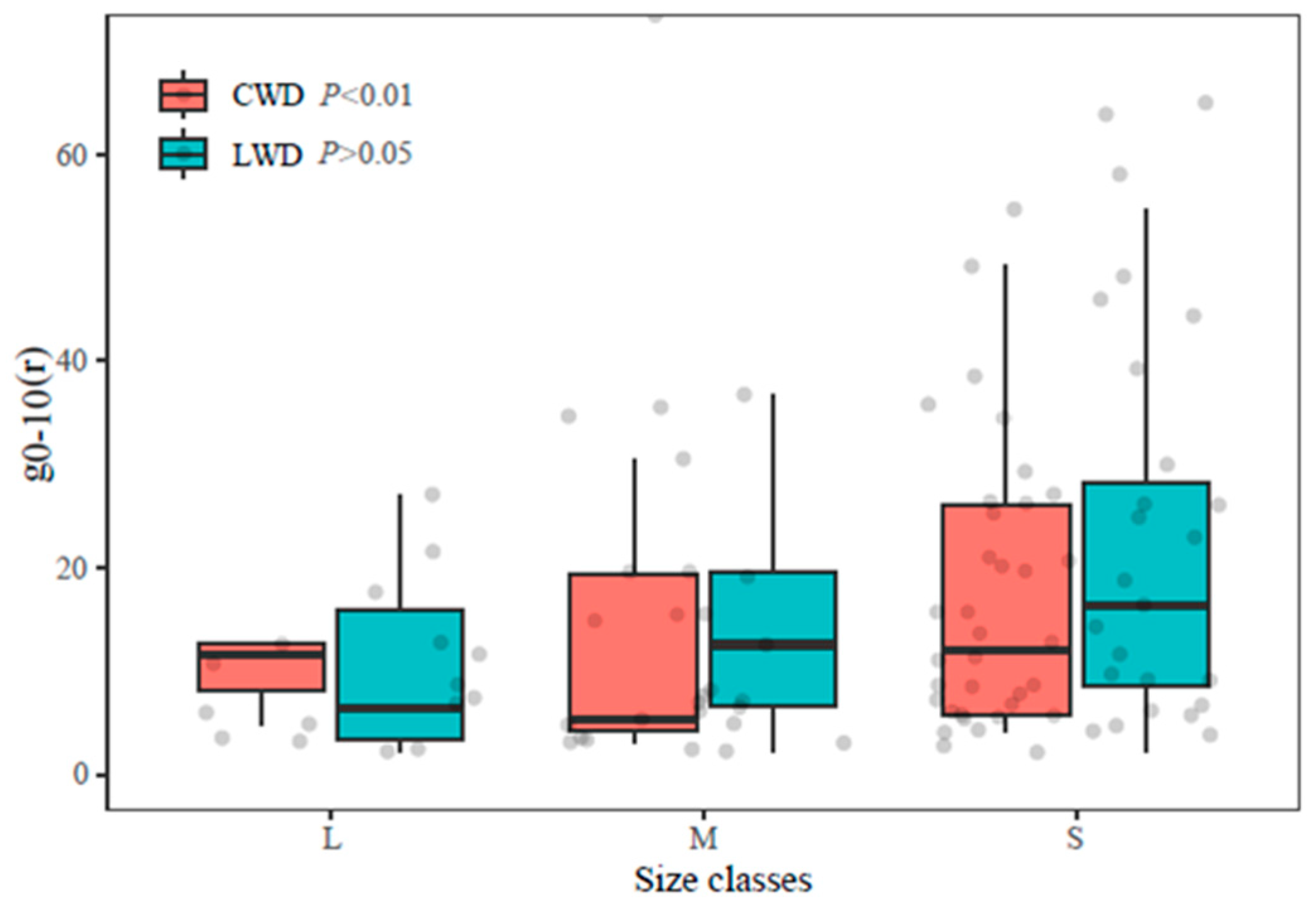
3.2. Aggregation Intensity g0–10 and Attributes of LWD and CWD in Each Size Class
3.3. Spatial Correlation Analysis of LWD and CWD
3.4. Microtopographic Effect on LWD and CWD
4. Discussion
4.1. General Spatial Pattern
4.2. Functional Traits on Spatial Pattern of LWD and CWD
4.3. Spatial Correlation Analysis of LWD and CWD
4.4. Microtopographic Variables on LWD and CWD
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wiegand, T.; Gunatilleke, S.; Gunatilleke, N.; Okuda, T. Analyzing the spatial structure of a Sri Lankan tree species with multiple scales of clustering. Ecology 2007, 88, 3088–3102. [Google Scholar] [CrossRef]
- Song, H.; Xu, Y.; Hao, J.; Zhao, B.; Guo, D.; Shao, H. Investigating distribution pattern of species in a warm-temperate conifer-broadleaved-mixed forest in China for sustainably utilizing forest and soils. Sci. Total Environ. 2017, 578, 81–89. [Google Scholar] [CrossRef]
- Legendre, P.; Fortin, M.J. Spatial pattern and ecological analysis. Vegetatio 1989, 80, 107–138. [Google Scholar] [CrossRef]
- Martínez, I.; Taboada, F.G.; Wiegand, T.; Obeso, J.R. Spatial patterns of seedling-adult associations in a temperate forest community. For. Ecol. Manag. 2013, 296, 74–80. [Google Scholar] [CrossRef]
- Li, Y.; Hui, G.; Zhao, Z.; Hu, Y.; Ye, S. Spatial structural characteristics of three hardwood species in Korean pine broad-leaved forest validating the bivariate distribution of structural parameters from the point of tree population. For. Ecol. Manag. 2014, 314, 17–25. [Google Scholar] [CrossRef]
- Rockwell, C.A.; Guariguata, M.R.; Menton, M.; Quispe, E.A.; Quaedvlieg, J.; Warren-Thomas, E.; Silva, H.F.; Rojas, E.E.J.; Arrunátegui, J.A.H.K.; Vega, L.A.M.; et al. Spatial distribution of Bertholletia excelsa in selectively logged forests of the Peruvian Amazon. J. Trop. Ecol. 2017, 33, 114–127. [Google Scholar] [CrossRef]
- McIntire, E.J.; Fajardo, A. Beyond description: The active and effective way to infer processes from spatial patterns. Ecology 2009, 90, 46–56. [Google Scholar] [CrossRef]
- McDonald, R.I.; Motzkin, G.; Bank, M.S.; Kittredge, D.B.; Burk, J.; Foster, D.R. Forest harvesting and land-use conversion over two decades in Massachusetts. For. Ecol. Manag. 2006, 227, 31–41. [Google Scholar] [CrossRef]
- Wang, X.; Ye, J.; Li, B.; Zhang, J.; Lin, F.; Hao, Z. Spatial distributions of species in an old-growth temperate forest, northeastern China. Can. J. For. Res. 2010, 40, 1011–1019. [Google Scholar] [CrossRef]
- Romero, L.M.; Smith, T.J.I.; Fourqurean, J.W. Changes in mass and nutrient content of wood during decomposition in a south Florida mangrove forest. J. Ecol. 2005, 93, 618–631. [Google Scholar] [CrossRef]
- Kim, R.; Son, Y.; Lim, J.H.; Lee, I.K.; Seo, K.W.; Koo, J.W.; Noh, N.J.; Ryu, S.R.; Hong, S.K.; Ihm, B.S. Coarse woody debris mass and nutrients in forest ecosystems of Korea. Ecol. Res. 2006, 21, 819–827. [Google Scholar] [CrossRef]
- Wang, X.; Comita, L.S.; Hao, Z.; Davies, S.J.; Ye, J.; Lin, F.; Yuan, Z. Local-scale drivers of tree survival in a temperate forest. PLoS ONE 2012, 7, e29469. [Google Scholar] [CrossRef]
- Woldendorp, G.; Keenan, R.J. Coarse woody debris in Australian forest ecosystems: A review. Austral. Ecol. 2005, 30, 834–843. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Z.; Sun, I.; Song, B.; Ye, J.; Li, B.; Wang, X. Density dependence on tree survival in an old-growth temperate forest in northeastern China. Ann. For. Sci. 2009, 66, 204. [Google Scholar] [CrossRef]
- Iida, Y.; Poorter, L.; Sterck, F.; Kassim, A.R.; Potts, M.D.; Kubo, T.; Kohyama, T.S. Linking size–dependent growth and mortality with architectural traits across 145 co–occurring tropical tree species. Ecology 2014, 95, 353–363. [Google Scholar] [CrossRef]
- Bigler, C.; Veblen, T.T. Changes in litter and dead wood loads following tree death beneath subalpine conifer species in northern Colorado. Can. J. For. Res. 2011, 41, 331–340. [Google Scholar] [CrossRef]
- Sterner, R.W.; Ribic, C.A.; Schatz, G.E. Testing for life historical changes in spatial patterns of four tropical tree species. J. Ecol. 1986, 74, 621–633. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50–ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Wright, J.S. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Erfanifard, Y.; Pham, V.D.; Le, X.T.; Bui, T.D.; Petritan, I.C. Spatial patterns and interactions of dominant tree species in an Amazon tropical rainforest. Rev. Biol. Trop. 2018, 66, 1009–1017. [Google Scholar]
- Hubbell, S.P.; Ahumada, J.A.; Condit, R.; Foster, R.B. Local neighborhood effects on long–term survival of individual trees in a neotropical forest. Ecol. Res. 2001, 16, 859–875. [Google Scholar] [CrossRef]
- Zhu, Y.; Mi, X.; Ren, H.; Ma, K. Density dependence is prevalent in a heterogeneous subtropical forest. Oikos 2010, 119, 109–119. [Google Scholar] [CrossRef]
- Greig-Smith, P. The use of random and contiguous quadrats in the study of the structure of plant communities. Ann. Bot. 1952, 16, 293–316. [Google Scholar] [CrossRef]
- Salas, C.; LeMay, V.; Núñez, P.; Pacheco, P.; Espinosa, A. Spatial patterns in an old-growth Nothofagus obliqua forest in south-central Chile. For. Ecol. Manag. 2006, 231, 38–46. [Google Scholar] [CrossRef]
- Plotkin, J.B.; Chave, J.; Ashton, P.S. Cluster analysis of spatial patterns in Malaysian tree species. Am. Nat. 2002, 160, 629–644. [Google Scholar] [CrossRef]
- Comita, L.S.; Condit, R.; Hubbell, S.P. Developmental changes in habitat associations of tropical trees. J. Ecol. 2007, 95, 482–492. [Google Scholar] [CrossRef]
- Lai, J.; Mi, X.; Ren, H.; Ma, K. Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 2009, 20, 415–423. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Campbell, J.J.N. Effects of tree size, shade tolerance, and spatial pattern on the mortality of woody plants in a seminatural forest in Central Kentucky. Prof. Geogr. 2016, 68, 436–450. [Google Scholar] [CrossRef]
- Kubota, Y.; Kubo, H.; Shimatani, K. Spatial pattern dynamics over 10 years in a conifer/broadleaved forest, northern Japan. Plant Ecol. 2007, 190, 143–157. [Google Scholar] [CrossRef]
- Lan, G.; Getzin, S.; Wiegand, T.; Hu, Y.; Xie, G.; Zhu, H.; Cao, M. Spatial distribution and interspecific associations of tree species in a tropical seasonal rain forest of China. PLoS ONE 2012, 7, e46074. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ding, B.; Mi, X.; Yu, J.; Wu, Y. Distribution patterns of tree species in an evergreen broadleaved forest in eastern China. Front. Biol. China 2009, 4, 531–538. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Ye, W.; Cao, H.; Wei, S.; Wang, Z.; Lian, J.; Sun, I.F.; Ma, K.; He, F. Spatial distributions of tree species in a subtropical forest of China. Oikos 2009, 118, 495–502. [Google Scholar] [CrossRef]
- Piao, T.; Comita, L.S.; Jin, G.; Kim, J.H. Density dependence across multiple life stages in a temperate old-growth forest of northeast China. Oecologia 2013, 172, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; He, F.; Waagepetersen, R.; Sun, I.; Hao, Z.; Chen, Z.; Yu, M. Quantifying effects of habitat heterogeneity and other clustering processes on spatial distributions of tree species. Ecology 2013, 94, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhuang, L.; Wang, S.; Sang, W. Coarse woody debris features of a warm temperate deciduous broad-leaved forest, northern China. J. For. Res. 2021, 32, 1105–1114. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Guo, Y.; Lu, J.; Franklin, S.B.; Wang, Q.; Xu, Y.; Zhang, K.; Bao, D.; Qiao, X.; Huang, H.; Lu, Z.; et al. Spatial distribution of tree species in a species-rich subtropical mountain forest in central China. Can. J. For. Res. 2013, 43, 826–835. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Illian, J.; Penttinen, A.; Stoyan, H.; Stoyan, D. Statistical Analysis and Modelling of Spatial Point Patterns; John Wiley: Chichester, UK, 2008; pp. 493–513. [Google Scholar]
- Fibich, P.; Lepš, J.; Novotný, V.; Klimeš, P.; Těšitel, J.; Molem, K.; Damas, K.; Weiblen, G.D. Spatial patterns of tree species distribution in New Guinea primary and secondary lowland rain forest. J. Veg. Sci. 2016, 27, 328–339. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Uria Diez, J.; Wiegand, K. Spatial distribution and association patterns in a tropical evergreen broad-leaved forest of north-central Vietnam. J. Veg. Sci. 2016, 27, 318–327. [Google Scholar] [CrossRef]
- Gómez Aparicio, L. The role of plant interactions in the restoration of degraded ecosystems: A meta-analysis across life-forms and ecosystems. J. Ecol. 2009, 97, 1202–1214. [Google Scholar] [CrossRef]
- Akhavan, R.; Sagheb Talebi, K.; Zenner, E.K.; Safavimanesh, F. Spatial patterns in different forest development stages of an intact old-growth Oriental beech forest in the Caspian region of Iran. Eur. J. For. Res. 2012, 131, 1355–1366. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Jin, G. Spatial patterns and associations of four species in an old-growth temperate forest. J. Plant Interact. 2014, 9, 745–753. [Google Scholar] [CrossRef]
- Boyden, S.; Binkley, D.; Shepperd, W. Spatial and temporal patterns in structure, regeneration, and mortality of an old-growth ponderosa pine forest in the Colorado Front Range. For. Ecol. Manag. 2005, 219, 43–55. [Google Scholar] [CrossRef]
- Zhu, Y.; Queenborough, S.A.; Condit, R.; Hubbell, S.P.; Ma, K.P.; Comita, L.S. Density-dependent survival varies with species life-history strategy in a tropical forest. Ecol. Lett. 2018, 21, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Klaus, V.H.; Hölzel, N.; Prati, D.; Schmitt, B.; Schöning, I.; Schrumpf, M.; Solly, E.F.; Hänsel, F.; Fischer, M.; Kleinebecker, T. Plant diversity moderates drought stress in grasslands: Implications from a large real-world study on 13C natural abundances. Sci. Total Environ. 2016, 566–567, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Comita, L.S.; Muller-Landau, H.C.; Aguilar, S.; Hubbell, S.P. Asymmetric density dependence shapes species abundances in a tropical tree community. Science 2010, 329, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Jin, G. Conspecific and phylogenetic density-dependent survival differs across life stages in two temperate old-growth forests in Northeast China. For. Ecol. Manag. 2018, 424, 95–104. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Jin, G.Z. Spatial distribution patterns and dynamics of four dominant tree species in a typical mixed broadleaved-Korean pine forest. In Advances in Biodiversity Conservation and Research in China IX; China Meteorolical Press: Beijing, China, 2012; pp. 278–296. [Google Scholar]
- He, C.; Jia, S.; Luo, Y.; Hao, Z.; Yin, Q. Spatial distribution and species association of dominant tree species in Huangguan Plot of Qinling Mountains, China. Forests 2022, 13, 866. [Google Scholar] [CrossRef]
- Taylor, A.H.; Wei, J.S.; Jun, Z.L.; Ping, L.C.; Jin, M.C.; Jinyan, H. Regeneration patterns and tree species coexistence in old-growth Abies–Picea forests in southwestern China. For. Ecol. Manag. 2006, 223, 303–317. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.; Peng, C.; Peng, Y.; Zhang, C.; Huang, C.; Tan, Y.; Wu, F. Foliar litter decomposition in an alpine forest meta-ecosystem on the eastern Tibetan Plateau. Sci. Total Environ. 2016, 566, 279–287. [Google Scholar] [CrossRef]
- Queenborough, S.A.; Burslem, D.F.; Garwood, N.C.; Valencia, R. Habitat niche partitioning by 16 species of Myristicaceae in Amazonian Ecuador. Plant Ecol. 2007, 192, 193–207. [Google Scholar] [CrossRef]
- Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N.; Esufali, S.; Harms, K.E.; Ashton, P.M.S.; Burslem, D.F.R.P. Species-habitat associations in a Sri Lankan dipterocarp forest. J. Trop. Ecol. 2006, 22, 371–384. [Google Scholar] [CrossRef]
- Ye, J.; Hao, Z.; Xie, P.; Li, J. Habitat associations of saplings and adults in an old-growth temperate forest in the Changbai mountains, northeastern China. For. Stud. China 2011, 13, 13–22. [Google Scholar] [CrossRef]
- Svenning, J. Environmental heterogeneity, recruitment limitation and the mesoscale distribution of palms in a tropical montane rain forest (Maquipucuna, Ecuador). J. Trop. Ecol. 2001, 17, 97–113. [Google Scholar] [CrossRef]
- Punchi Manage, R.; Getzin, S.; Wiegand, T.; Kanagaraj, R.; Savitri Gunatilleke, C.V.; Nimal Gunatilleke, I.A.U.; Wiegand, K.; Huth, A. Effects of topography on structuring local species assemblages in a Sri Lankan mixed dipterocarp forest. J. Ecol. 2013, 101, 149–160. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.; Harms, K.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef]
- Hu, Y.H.; Sha, L.Q.; Blanchet, F.G.; Zhang, J.L.; Tang, Y.; Lan, G.Y.; Cao, M. Dominant species and dispersal limitation regulate tree species distributions in a 20-ha plot in Xishuangbanna, southwest China. Oikos 2012, 121, 952–960. [Google Scholar] [CrossRef]
- Lan, G.; Hu, Y.; Cao, M.; Zhu, H. Topography related spatial distribution of dominant tree species in a tropical seasonal rain forest in China. For. Ecol. Manag. 2011, 262, 1507–1513. [Google Scholar] [CrossRef]
- Larsary, M.K.; Abkenar, K.T.; Pourbabaei, H.; Pothier, D.; Amanzadeh, B. Spatial patterns of trees from different development stages in mixed temperate forest in the Hyrcanian region of Iran. J. For. Sci. 2018, 64, 260–270. [Google Scholar] [CrossRef]

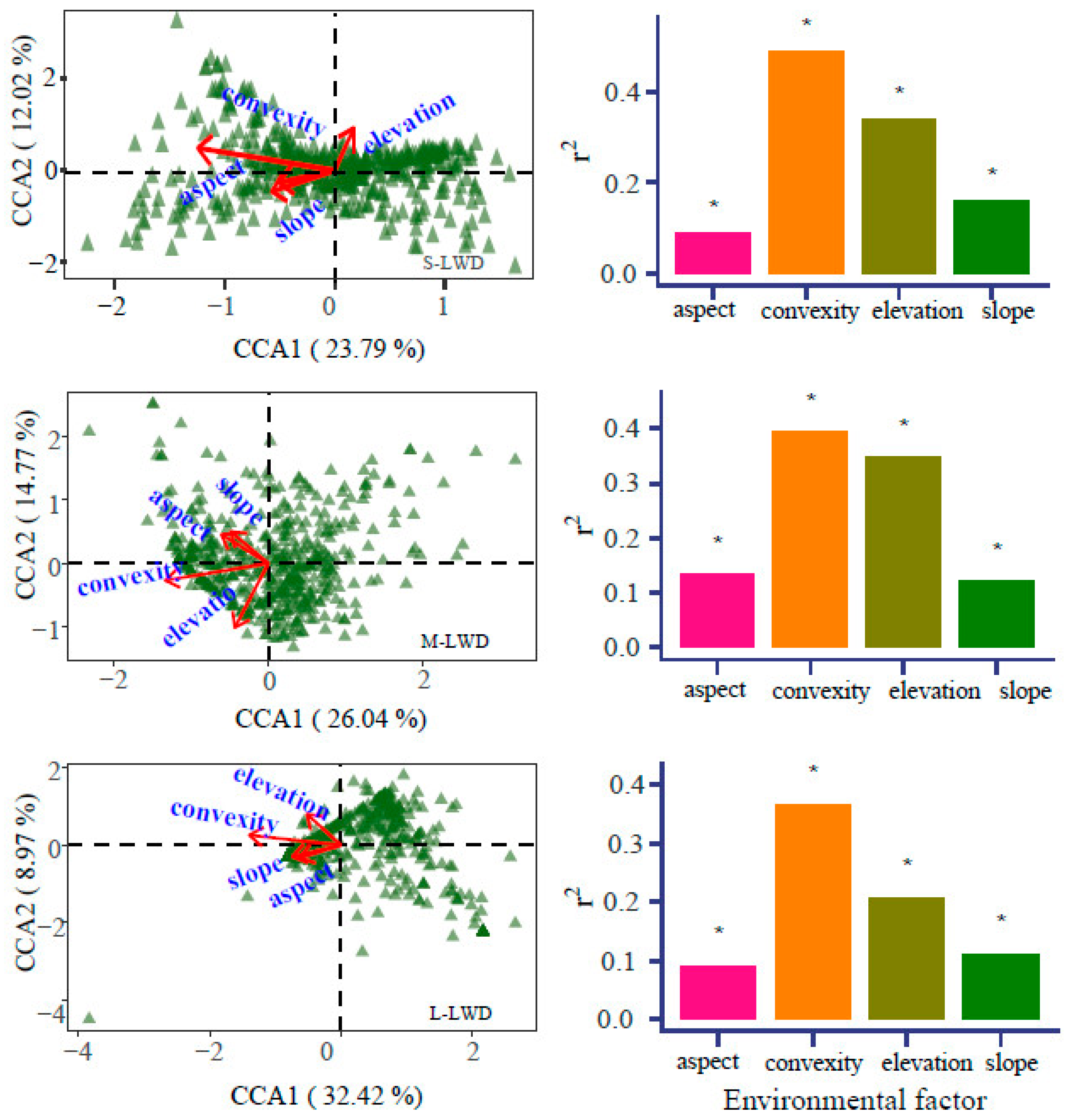

| LWD | Topographic Factor | CCA1 | CCA2 | R2 | Pr (>r) |
| Small size | Elevation | 0.131 | 0.991 | 0.342 | 0.001 *** |
| Convexity | −0.905 | 0.425 | 0.489 | 0.001 *** | |
| Slope | −0.735 | −0.679 | 0.160 | 0.001 *** | |
| Aspect | −0.862 | −0.508 | 0.087 | 0.001 *** | |
| Middle size | Topographic factor | CCA1 | CCA2 | R2 | Pr (>r) |
| Elevation | −0.389 | −0.921 | 0.348 | 0.001 *** | |
| Convexity | −0.973 | −0.229 | 0.395 | 0.001 *** | |
| Slope | −0.666 | 0.746 | 0.121 | 0.001 *** | |
| Aspect | −0.778 | 0.628 | 0.135 | 0.001 *** | |
| Large size | Topographic factor | CCA1 | CCA2 | R2 | Pr (>r) |
| Elevation | −0.698 | 0.717 | 0.206 | 0.001 *** | |
| Convexity | −0.987 | 0.159 | 0.366 | 0.001 *** | |
| Slope | −0.969 | −0.248 | 0.112 | 0.001 *** | |
| Aspect | −0.944 | −0.329 | 0.091 | 0.001 *** | |
| CWD | Topographic factor | CCA1 | CCA2 | R2 | Pr (>r) |
| Small size | Elevation | −0.999 | 0.005 | 0.117 | 0.001 *** |
| Convexity | 0.747 | 0.665 | 0.100 | 0.001 *** | |
| Slope | 0.974 | 0.228 | 0.237 | 0.001 *** | |
| Aspect | 0.882 | −0.471 | 0.217 | 0.001 *** | |
| Middle size | Topographic factor | CCA1 | CCA2 | R2 | Pr (>r) |
| Elevation | 0.939 | 0.342 | 0.245 | 0.001 *** | |
| Convexity | 0.399 | 0.917 | 0.102 | 0.001 *** | |
| Slope | −0.929 | 0.368 | 0.113 | 0.001 *** | |
| Aspect | −0.723 | 0.691 | 0.064 | 0.001 *** | |
| Large size | Topographic factor | CCA1 | CCA2 | R2 | Pr (>r) |
| Elevation | 0.956 | −0.294 | 0.189 | 0.001 *** | |
| Convexity | 0.957 | 0.290 | 0.172 | 0.001 *** | |
| Slope | −0.572 | 0.821 | 0.010 | 0.421 | |
| Aspect | 0.864 | 0.503 | 0.004 | 0.754 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Wang, S.; Sang, W.; Zhang, S.; Ma, K. Spatial Distribution and Sustainable Development of Living Woody and Coarse Woody Debris in Warm-Temperate Deciduous Broadleaved Secondary Forests in China. Plants 2024, 13, 638. https://doi.org/10.3390/plants13050638
Ma F, Wang S, Sang W, Zhang S, Ma K. Spatial Distribution and Sustainable Development of Living Woody and Coarse Woody Debris in Warm-Temperate Deciduous Broadleaved Secondary Forests in China. Plants. 2024; 13(5):638. https://doi.org/10.3390/plants13050638
Chicago/Turabian StyleMa, Fang, Shunzhong Wang, Weiguo Sang, Shuang Zhang, and Keming Ma. 2024. "Spatial Distribution and Sustainable Development of Living Woody and Coarse Woody Debris in Warm-Temperate Deciduous Broadleaved Secondary Forests in China" Plants 13, no. 5: 638. https://doi.org/10.3390/plants13050638
APA StyleMa, F., Wang, S., Sang, W., Zhang, S., & Ma, K. (2024). Spatial Distribution and Sustainable Development of Living Woody and Coarse Woody Debris in Warm-Temperate Deciduous Broadleaved Secondary Forests in China. Plants, 13(5), 638. https://doi.org/10.3390/plants13050638





