Physiological and Biochemical Analysis Revealing the Key Factors Influencing 2-Phenylethanol and Benzyl Alcohol Production in Crabapple Flowers
Abstract
1. Introduction
2. Results
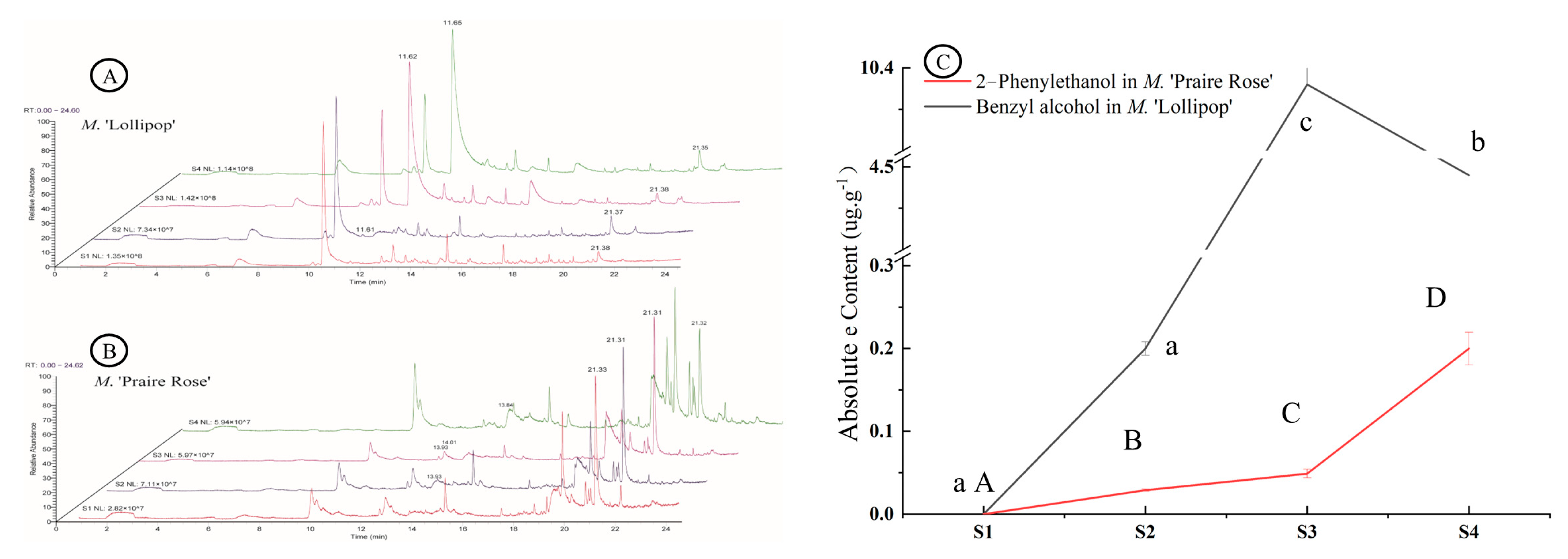
2.1. Analysis of Volatile Compounds
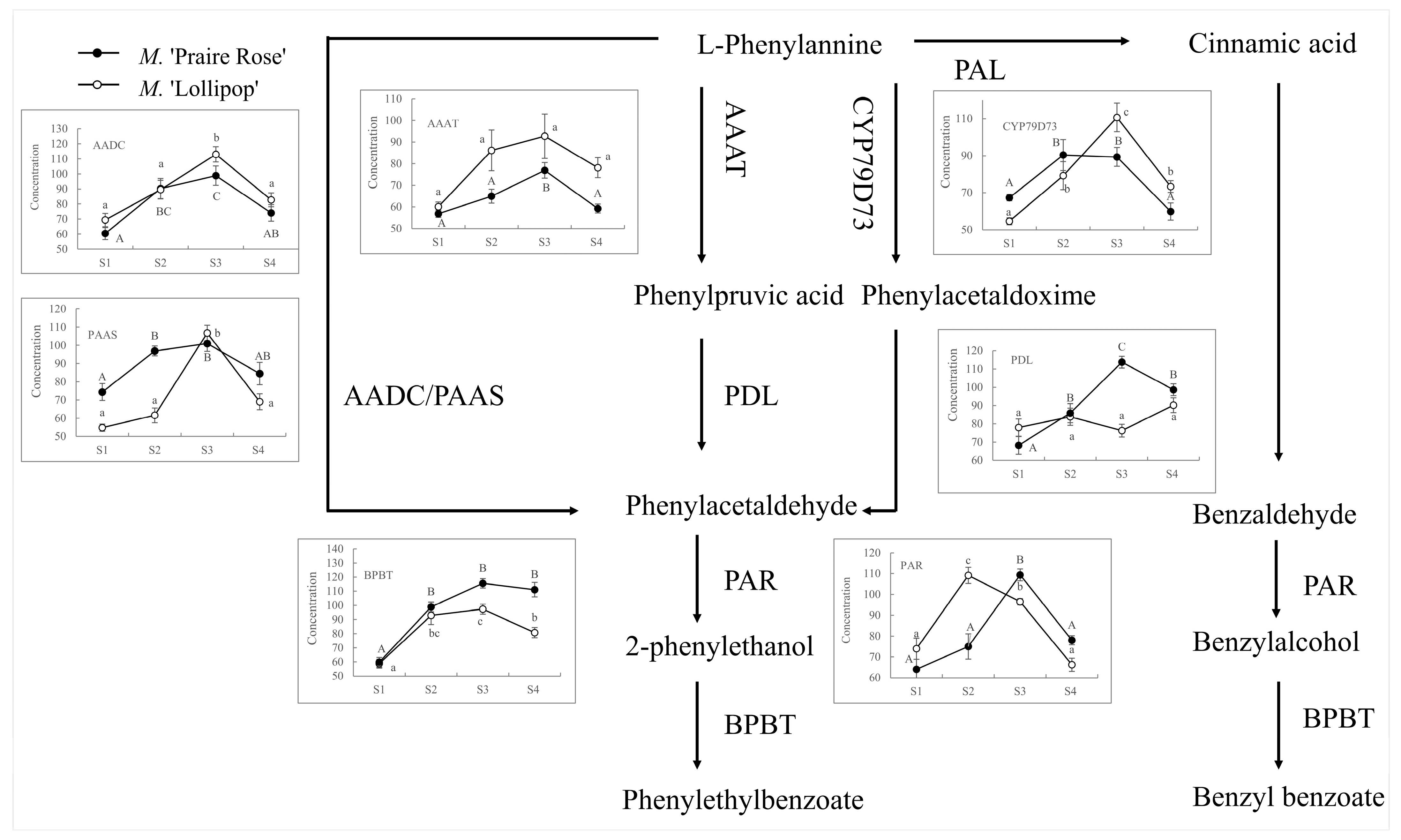
2.2. Analysis of BA/2-PE Related Enzyme Concentrations
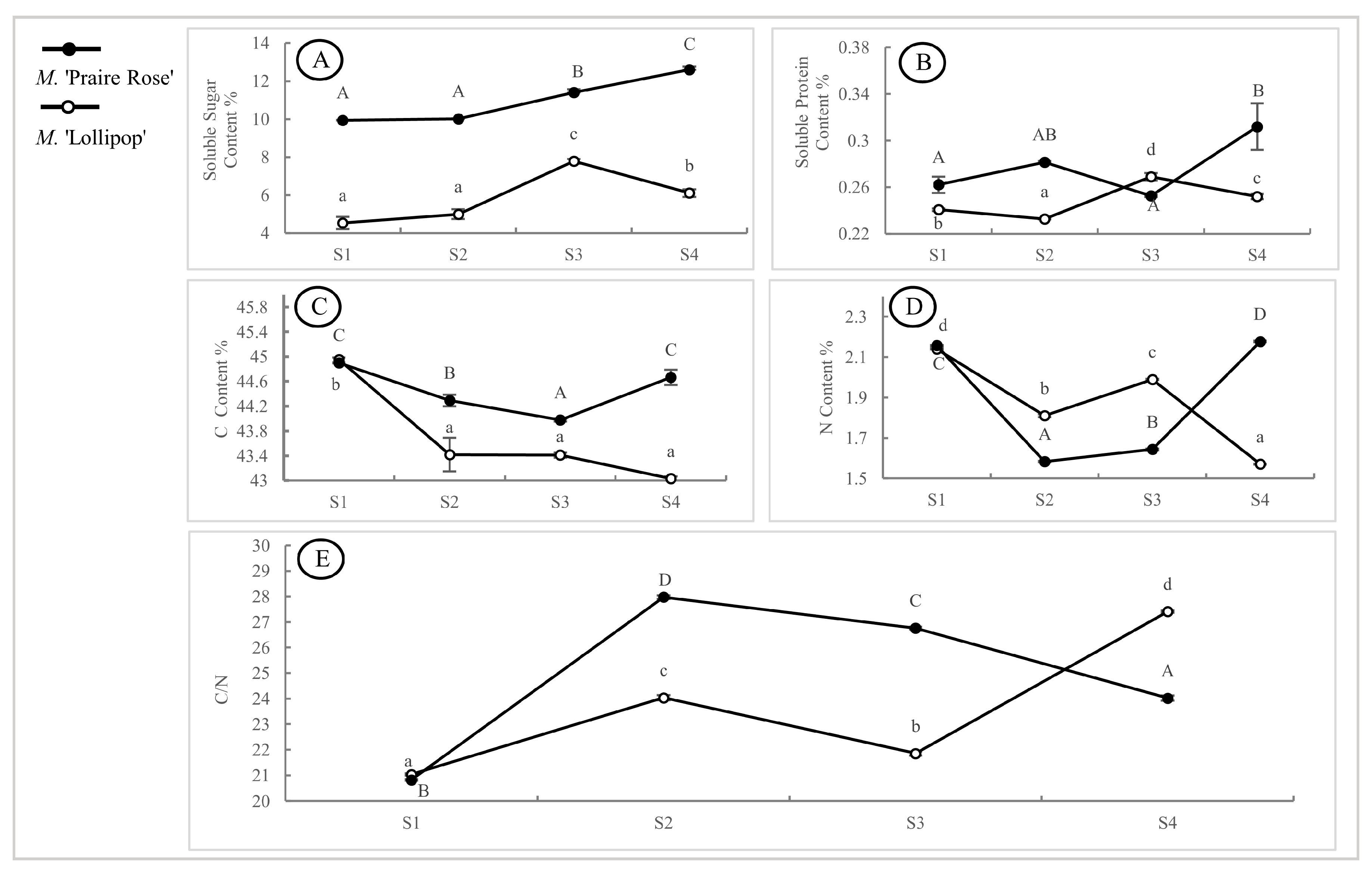
2.3. Analysis of Other Physiological Indicators in Two Species
2.4. Correlation Analysis of Key Compounds with Physiological Indicators
2.5. PCA Analysis of Key Compounds with Physiological Indicators
3. Discussion
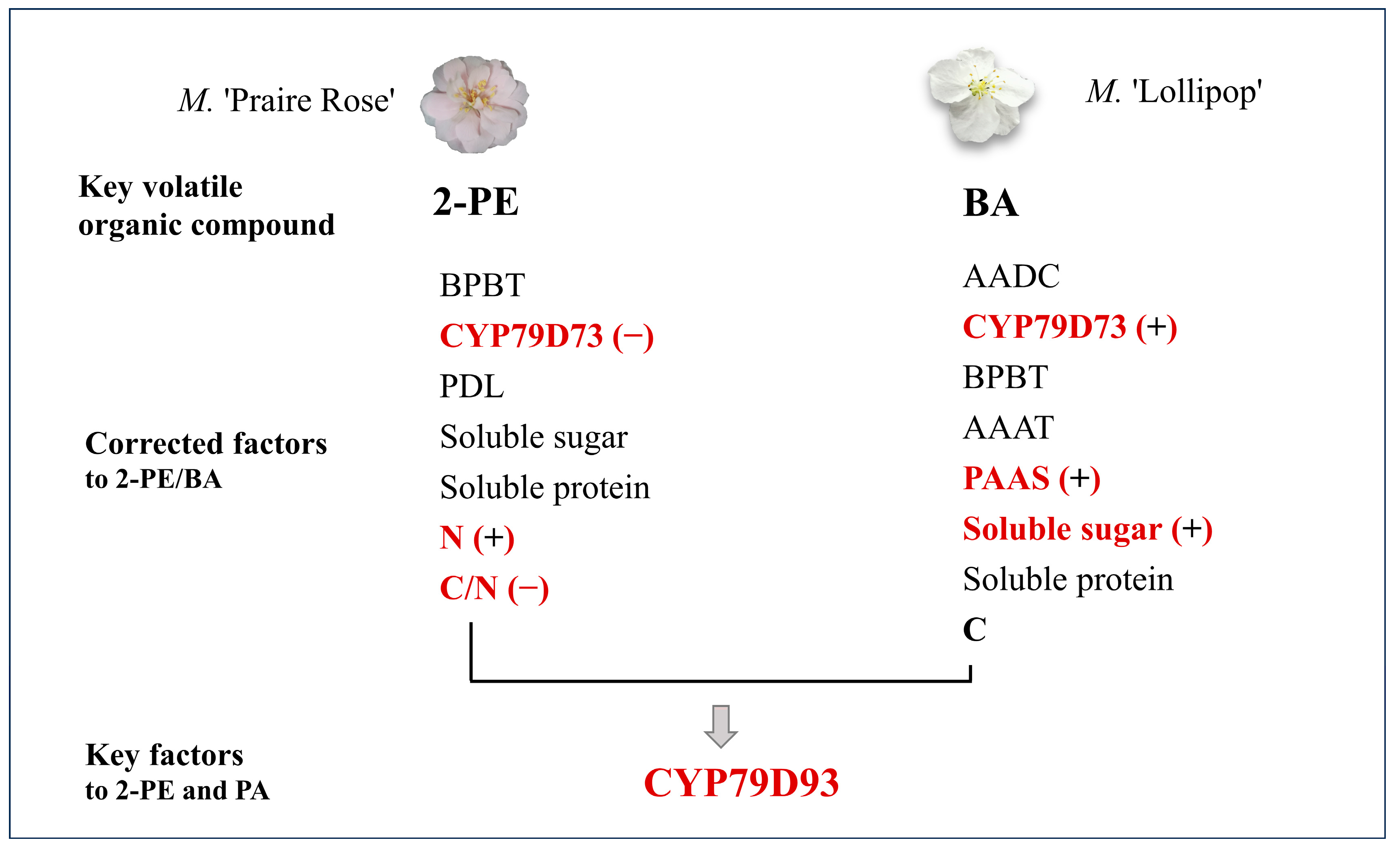
3.1. 2-PE and BA Become the Key Differencial Compounds between M. ‘Praire Rose’ and M. ‘Lollipop’
3.2. CYP79D73 Becomes the Key Regulator for the Synthesis of BA/2-PE in Two Species
3.3. N Sources and Soluable Sugar Content Become Key Factors Influencing in 2-PE and BA Synthesis, Respectively
4. Materials and Methods
5. HS-SPME-GC-MS
6. Determination of Physiological Indices in Different Varieties
6.1. Determination of Key Enzyme Concentrations
6.2. Determination of Soluble Sugar Content
6.3. Determination of Soluble Protein Content
6.4. Determination of Carbon and Nitrogen Contents
6.5. Data Analysis
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghissing, U.; Mitra, A. Biology of Floral Scent Volatiles in Ornamental Plants. In Floriculture and Ornamental Plants; Datta, S.K., Gupta, Y.C., Eds.; Springer: Singapore, 2021; pp. 1–41. ISBN 9789811515545. [Google Scholar]
- Dudareva, N.; Pichersky, E. Biology of Floral Scent; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-1-4200-0400-7. [Google Scholar]
- Aros, D.; Garrido, N.; Rivas, C.; Medel, M.; Müller, C.; Rogers, H.; Úbeda, C. Floral Scent Evaluation of Three Cut Flowers Through Sensorial and Gas Chromatography Analysis. Agronomy 2020, 10, 131. [Google Scholar] [CrossRef]
- Barman, M.; Tenhaken, R.; Dötterl, S. A Review on Floral Scents and Pigments in Cucurbits: Their Biosynthesis and Role in Flower Visitor Interactions. Sci. Hortic. 2023, 322, 112402. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Yu, F.; Li, X.; Jin, J.; Zhou, Y.; Wang, Q.; Jing, T.; Wan, X.; Schwab, W.; et al. A Geraniol Synthase Regulates Plant Defense via Alternative Splicing in Tea Plants. Hortic. Res. 2023, 10, uhad184. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Gershenzon, J. The Chemical Diversity of Floral Scent. In Biology of Plant Volatiles, 2nd ed; CRC Press: Boca Raton, FL, USA, 2020; pp. 57–58. ISBN 978-0-429-45561-2. [Google Scholar]
- Fan, J.; Zhang, W.; Zhang, D.; Wang, G.; Cao, F. Flowering Stage and Daytime Affect Scent Emission of Malus Ioensis “Prairie Rose. Molecules 2019, 24, 2356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, R.; Huang, C.; Mao, Z.; Guo, L.; Shen, X. Taxonomic Analysis of Volatiles Emitted by Ornamental Crabapple Flowers. Acta Ecol. Sin. 2014, 34, 213–218. [Google Scholar] [CrossRef]
- Sakai, M.; Hirata, H.; Sayama, H.; Sekiguchi, K.; Itano, H.; Asai, T.; Dohra, H.; Hara, M.; Watanabe, N. Production of 2-Phenylethanol in Roses as the Dominant Floral Scent Compound from L-Phenylalanine by Two Key Enzymes, a PLP-Dependent Decarboxylase and a Phenylacetaldehyde Reductase. Biosci. Biotechnol. Biochem. 2007, 71, 2408–2419. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, J.C.; Ric De Vos, C.H.; Verhoeven, H.A.; Haring, M.A.; Van Tunen, A.J.; Schuurink, R.C. Regulation of Floral Scent Production in Petunia Revealed by Targeted Metabolomics. Phytochemistry 2003, 62, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, S.; Jin, J.; Sridhar, V.; Sarojam, R.; Chua, N.-H.; Jang, I.-C. Integrated Metabolome and Transcriptome Analysis of Magnolia Champaca Identifies Biosynthetic Pathways for Floral Volatile Organic Compounds. BMC Genom. 2017, 18, 463. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-Phenylethanol (Rose Aroma) Production Potential of an Isolated Pichia Kudriavzevii through Solid-State Fermentation. Process Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Phenylethyl Alcohol. Food Chem. Toxicol. 2012, 50, S224–S239. [Google Scholar] [CrossRef]
- Sirilun, S.; Chaiyasut, C.; Sivamaruthi, B.S.; Peerajan, S.; Kumar, N.; Periyanaina, K. Phenethyl Alcohol Is an Effective Non-Traditional Preservative Agent for Cosmetic Preparations. Asian J. Pharm. Clin. Res. 2017, 10, 129. [Google Scholar] [CrossRef]
- Gibernau, M.; Buser, H.R.; Frey, J.E.; Hossaert-McKey, M. Volatile Compounds from Extracts of Figs of Ficus carica. Phytochemistry 1997, 46, 241–244. [Google Scholar] [CrossRef]
- Raguso, R.A.; Roy, B.A. ‘Floral’ Scent Production by Puccinia Rust Fungi That Mimic Flowers. Mol. Ecol. 1998, 7, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Wang, D.; Yu, L.; Tang, X.; Chai, G.; He, G.; Ma, W.; Li, S.; Kong, Y.; Fu, C.; et al. Metabolic Engineering of 2-Phenylethanol Pathway Producing Fragrance Chemical and Reducing Lignin in Arabidopsis. Plant Cell Rep. 2015, 34, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Dötterl, S.; Gershenzon, J. Chemistry, Biosynthesis and Biology of Floral Volatiles: Roles in Pollination and Other Functions. Nat. Prod. Rep. 2023, 40, 1901–1937. [Google Scholar] [CrossRef] [PubMed]
- Kaminaga, Y.; Schnepp, J.; Peel, G.; Kish, C.M.; Ben-Nissan, G.; Weiss, D.; Orlova, I.; Lavie, O.; Rhodes, D.; Wood, K.; et al. Plant Phenylacetaldehyde Synthase Is a Bifunctional Homotetrameric Enzyme That Catalyzes Phenylalanine Decarboxylation and Oxidation. J. Biol. Chem. 2006, 281, 23357–23366. [Google Scholar] [CrossRef] [PubMed]
- Functional Characterization of Aromatic Amino Acid Aminotransferase Involved in 2-Phenylethanol Biosynthesis in Isolated Rose Petal Protoplasts—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0176161711004986 (accessed on 6 November 2023).
- Dhandapani, S.; Jin, J.; Sridhar, V.; Chua, N.-H.; Jang, I.-C. CYP79D73 Participates in Biosynthesis of Floral Scent Compound 2-Phenylethanol in Plumeria Rubra. Plant Physiol. 2019, 180, 171–184. [Google Scholar] [CrossRef]
- Huang, X.-Q.; Li, R.; Fu, J.; Dudareva, N. A Peroxisomal Heterodimeric Enzyme Is Involved in Benzaldehyde Synthesis in Plants. Nat. Commun. 2022, 13, 1352. [Google Scholar] [CrossRef]
- Boatright, J.; Negre, F.; Chen, X.; Kish, C.M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N. Understanding in Vivo Benzenoid Metabolism in Petunia Petal Tissue. Plant Physiol. 2004, 135, 1993–2011. [Google Scholar] [CrossRef]
- Bao, F.; Zhang, T.; Ding, A.; Ding, A.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Metabolic, Enzymatic Activity, and Transcriptomic Analysis Reveals the Mechanism Underlying the Lack of Characteristic Floral Scent in Apricot Mei Varieties. Front. Plant Sci. 2020, 11, 574982. [Google Scholar] [CrossRef]
- D’Auria, J.C. Acyltransferases in Plants: A Good Time to Be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef]
- Biosynthesis and Regulation of Floral Scent in Snapdragon and Petunia Flowers—ProQuest. Available online: https://www.proquest.com/openview/192a500c58b46aead50ff00e5092b53c/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 6 November 2023).
- Chen, X.-M.; Kobayashi, H.; Sakai, M.; Hirata, H.; Asai, T.; Ohnishi, T.; Baldermann, S.; Watanabe, N. Functional Characterization of Rose Phenylacetaldehyde Reductase (PAR), an Enzyme Involved in the Biosynthesis of the Scent Compound 2-Phenylethanol. J. Plant Physiol. 2011, 168, 88–95. [Google Scholar] [CrossRef]
- Barman, M.; Mitra, A. Floral Maturation and Changing Air Temperatures Influence Scent Volatiles Biosynthesis and Emission in Jasminum auriculatum Vahl. Environ. Exp. Bot. 2021, 181, 104296. [Google Scholar] [CrossRef]
- Li, Y.; Jia, W.; Wang, Q.; Wang, B.; Wang, S. Comparative Analysis of Floral Scent Profiles between Two Chimonanthus Praecox Plants under Different Rhythms and Blooming Stages. Sci. Hortic. 2022, 301, 111129. [Google Scholar] [CrossRef]
- Maiti, S.; Mitra, A. Morphological, Physiological and Ultrastructural Changes in Flowers Explain the Spatio-Temporal Emission of Scent Volatiles in Polianthes Tuberosa L. Plant Cell Physiol. 2017, 58, 2095–2111. [Google Scholar] [CrossRef]
- Chowdhary, V.A.; Tank, J.G. Biomolecules Regulating Defense Mechanism in Plants. Proc. Natl. Acad. Sci. India B Biol. Sci. 2023, 93, 17–25. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, L.; Liao, Y.; Li, J.; Zhou, B.; Yang, Z.; Tang, J. Enzymatic Reaction-Related Protein Degradation and Proteinaceous Amino Acid Metabolism during the Black Tea (Camellia sinensis) Manufacturing Process. Foods 2020, 9, 66. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble Sugars. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, S.; Liu, R.; Jin, J. Effects of Nitrogen Application on Flavor Compounds of Cherry Tomato Fruits. J. Plant Nutr. Soil Sci. 2007, 170, 461–468. [Google Scholar] [CrossRef]
- Majetic, C.J.; Fetters, A.M.; Beck, O.M.; Stachnik, E.F.; Beam, K.M. Petunia Floral Trait Plasticity in Response to Soil Nitrogen Content and Subsequent Impacts on Insect Visitation. Flora 2017, 232, 183–193. [Google Scholar] [CrossRef]
- Massad, T.J.; Dyer, L.A.; Vega, C.G. Costs of Defense and a Test of the Carbon-Nutrient Balance and Growth-Differentiation Balance Hypotheses for Two Co-Occurring Classes of Plant Defense. PLoS ONE 2012, 7, e47554. [Google Scholar] [CrossRef] [PubMed]
- Royer, M.; Larbat, R.; Le Bot, J.; Adamowicz, S.; Robin, C. Is the C:N Ratio a Reliable Indicator of C Allocation to Primary and Defence-Related Metabolisms in Tomato? Phytochemistry 2013, 88, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, J.; Li, Y.; Luo, G.; Li, L.; Yuan, H.; Mur, L.A.J.; Guo, S. Negative Effects of the Simulated Nitrogen Deposition on Plant Phenolic Metabolism: A Meta-Analysis. Sci. Total Environ. 2020, 719, 137442. [Google Scholar] [CrossRef]
- Abbas, F.; Nian, X.; Zhou, Y.; Ke, Y.; Liu, L.; Yu, R.; Fan, Y. Putative Regulatory Role of Hexokinase and Fructokinase in Terpenoid Aroma Biosynthesis in Lilium ‘Siberia’. Plant Physiol. Biochem. 2021, 167, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chen, Y.; Zhang, W.; Zhang, D. ‘Luokeke Nüshi’ Crabapple. Hortscience 2021, 56, 1289–1290. [Google Scholar] [CrossRef]
- Iles, J. Crabapples… with no Apologies. Mag. Arnold Arbor. 2009, 2. Available online: https://arboretum.harvard.edu/wp-content/uploads/2020/06/2009-67-2-Arnoldia.pdf (accessed on 6 November 2023).
- Ramya, M.; Kwon, O.K.; An, H.R.; Park, P.M.; Baek, Y.S.; Park, P.H. Floral Scent: Regulation and Role of MYB Transcription Factors. Phytochem. Lett. 2017, 19, 114–120. [Google Scholar] [CrossRef]
- Ramya, M.; An, H.R.; Baek, Y.S.; Reddy, K.E.; Park, P.H. Orchid Floral Volatiles: Biosynthesis Genes and Transcriptional Regulations. Sci. Hortic. 2018, 235, 62–69. [Google Scholar] [CrossRef]
- Wong, D.C.J.; Pichersky, E.; Peakall, R. Many Different Flowers Make a Bouquet: Lessons from Specialized Metabolite Diversity in Plant–Pollinator Interactions. Curr. Opin. Plant Biol. 2023, 73, 102332. [Google Scholar] [CrossRef]
- Oyama-Okubo, N.; Tsuji, T. Analysis of Floral Scent Compounds and Classification by Scent Quality in Tulip Cultivars. J. Jpn. Soc. Hortic. Sci. 2013, 82, 344–353. [Google Scholar] [CrossRef]
- Han, Y.; Wang, H.; Wang, X.; Li, K.; Dong, M.; Li, Y.; Zhu, Q.; Shang, F. Mechanism of Floral Scent Production in Osmanthus Fragrans and the Production and Regulation of Its Key Floral Constituents, β-Ionone and Linalool. Hortic. Res. 2019, 6, 106. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Shan, Q.; Shi, Z.; Li, J.; Zhao, X.; Chang, C.; Yu, J. Integrated Volatile Metabolomic and Transcriptomic Analysis Provides Insights into the Regulation of Floral Scents between Two Contrasting Varieties of Lonicera Japonica. Front. Plant Sci. 2022, 13, 989036. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, F.; Yang, Y.; Hu, L.; Ding, A.; Ding, A.; Wang, J.; Cheng, T.; Zhang, Q. A Comparative Analysis of Floral Scent Compounds in Intraspecific Cultivars of Prunus Mume with Different Corolla Colours. Molecules 2019, 25, 145. [Google Scholar] [CrossRef]
- Zhou, Y.; Abbas, F.; Wang, Z.; Yu, Y.; Yue, Y.; Li, X.; Yu, R.; Fan, Y. HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules 2021, 26, 5425. [Google Scholar] [CrossRef]
- Feng, H.; Wang, X.; Luo, Y.; Huang, S. Floral Scent Emission Is the Highest at the Second Night of Anthesis in Lonicera japonica (Caprifoliaceae). J. Syst. Evol. 2023, 61, 530–537. [Google Scholar] [CrossRef]
- Bisrat, D.; Jung, C. Roles of Flower Scent in Bee–Flower Mediations: A Review. J. Ecol. Environ. 2022, 46, 3. [Google Scholar] [CrossRef]
- Sun, W.-X.; Hu, K.; Zhang, J.-X.; Zhu, X.-L.; Tao, Y.-S. Aroma Modulation of Cabernet Gernischt Dry Red Wine by Optimal Enzyme Treatment Strategy in Winemaking. Food Chem. 2018, 245, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Fernández-Martínez, M.; Filella, I.; Junker, R.R.; Peñuelas, J. Deciphering the Biotic and Climatic Factors That Influence Floral Scents: A Systematic Review of Floral Volatile Emissions. Front. Plant Sci. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.; Mitra, A. Temporal Relationship between Emitted and Endogenous Floral Scent Volatiles in Summer- and Winter-Blooming Jasminum Species. Physiol. Plant. 2019, 166, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, Y.; Chen, S.; Chen, F.; Chen, F. Concentration-Dependent Emission of Floral Scent Terpenoids from Diverse Cultivars of Chrysanthemum Morifolium and Their Wild Relatives. Plant Sci. 2021, 309, 110959. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kong, X.; Zhang, Y.; Wang, S.; Zhou, H.; Fang, D.; Yue, W.; Chen, C. Metabolomic Profiling in Combination with Data Association Analysis Provide Insights about Potential Metabolic Regulation Networks among Non-Volatile and Volatile Metabolites in Camellia sinensis Cv Baijiguan. Plants 2022, 11, 2557. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, W.; Zhou, Y.; Zhang, J.; Li, Y.; Ahmad, S.; Zhang, Q. Comparative Transcriptome Reveals Benzenoid Biosynthesis Regulation as Inducer of Floral Scent in the Woody Plant Prunus Mume. Front. Plant Sci. 2017, 8, 319. [Google Scholar] [CrossRef]
- Bao, W.; Li, X.; Liu, J.; Zheng, R.; Liu, L.; Zhang, H. The Characterization of an Efficient Phenylpyruvate Decarboxylase KDC4427, Involved in 2-Phenylethanol and IAA Production from Bacterial Enterobacter sp. CGMCC 5087. Microbiol. Spectr. 2022, 10, e02660-21. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral Volatiles: From Biosynthesis to Function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Pan, H.; Li, X.; Guo, D. Metabolic Engineering of Escherichia Coli to High Efficient Synthesis Phenylacetic Acid from Phenylalanine. AMB Express 2017, 7, 105. [Google Scholar] [CrossRef]
- Siringam, K.; Juntawong, N.; Cha-Um, S.; Boriboonkaset, T.; Kirdmanee, C. Salt Tolerance Enhancement in Indica Rice (“Oryza sativa” L. spp. Indica) Seedlings Using Exogenous Sucrose Supplementation. Plant Omics 2020, 5, 52–59. [Google Scholar]
- Izumi, M.; Nakamura, S.; Li, N. Autophagic Turnover of Chloroplasts: Its Roles and Regulatory Mechanisms in Response to Sugar Starvation. Front. Plant Sci. 2019, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Ninio, R.; Lewinsohn, E.; Mizrahi, Y.; Sitrit, Y. Changes in Sugars, Acids, and Volatiles during Ripening of Koubo [Cereus peruvianus (L.) Miller] Fruits. J. Agric. Food Chem. 2003, 51, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. Effect of Different Drying Methods on the Sensory Quality and Chemical Components of Black Tea. LWT 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Ghissing, U.; Jayanthan, K.; Bera, P.; Bimolata, W.; Mitra, A. Targeted Profiling and Temporal Expression of a Few Key Genes Revealed an Apparent Coordination among the Metabolites Contributing to the Volatiles Internal Pool in Jasminum sambac (L.) Aiton Flowers. Braz. J. Bot. 2022, 45, 587–597. [Google Scholar] [CrossRef]
- Barbosa, C.; Falco, V.; Mendes-Faia, A.; Mendes-Ferreira, A. Nitrogen Addition Influences Formation of Aroma Compounds, Volatile Acidity and Ethanol in Nitrogen Deficient Media Fermented by Saccharomyces Cerevisiae Wine Strains. J. Biosci. Bioeng. 2009, 108, 99–104. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, Y.; Zhang, A.; Wang, P.; He, S.; Zhang, K.; Wang, J.; Fang, Y.; Sun, X. Using Foliar Nitrogen Application during Veraison to Improve the Flavor Components of Grape and Wine. J. Sci. Food Agric. 2021, 101, 1288–1300. [Google Scholar] [CrossRef]
- Charoimek, N.; Phusuwan, S.; Petcharak, C.; Huanhong, K.; Prasad, S.K.; Junmahasathien, T.; Khemacheewakul, J.; Sommano, S.R.; Sunanta, P. Do Abiotic Stresses Affect the Aroma of Damask Roses? Plants 2023, 12, 3428. [Google Scholar] [CrossRef]
- Fang, Q.-T.; Luo, W.-W.; Zheng, Y.-N.; Ye, Y.; Hu, M.-J.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R.; Ye, J.-H. Identification of Key Aroma Compounds Responsible for the Floral Ascents of Green and Black Teas from Different Tea Cultivars. Molecules 2022, 27, 2809. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Gao, L.; Qi, Y.; Fu, W.; Li, X.; Zhou, X.; Gao, Q.; Gao, Z.; Jia, H. Acyl-CoA Oxidase 1 Is Involved in γ-Decalactone Release from Peach (Prunus persica) Fruit. Plant Cell Rep. 2017, 36, 829–842. [Google Scholar] [CrossRef]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.R.; Hanson, A.D.; Klee, H.J. Tomato Aromatic Amino Acid Decarboxylases Participate in Synthesis of the Flavor Volatiles 2-Phenylethanol and 2-Phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, C.; Chen, J.; Yang, S. Streptomyces virginiae PPDC Is a New Type of Phenylpyruvate Decarboxylase Composed of Two Subunits. ACS Chem. Biol. 2017, 12, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Becerra, L.D.; Ruíz, R.Y.; Rodríguez Cortina, J.; Quintanilla-Carvajal, M.X.; Coy-Barrera, E.; Escobar Parra, S. Chemical Characterization of Quality-Related Compounds in Cocoa Matrices: An Overview of Analytical Methods Applied for Their Analysis. Crit. Rev. Anal. Chem. 2023, 53, 689–717. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, L.; Duan, X.; Chai, X.; Huang, R.; Kang, Y.; Yang, X. Effects of Exogenous Sucrose and Selenium on Plant Growth, Quality, and Sugar Metabolism of Pea Sprouts. J. Sci. Food Agric. 2022, 102, 2855–2863. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Tao, W.; Yu, F.; Xiong, Q.; Nong, C.; Zhang, W.; Fan, J. Physiological and Biochemical Analysis Revealing the Key Factors Influencing 2-Phenylethanol and Benzyl Alcohol Production in Crabapple Flowers. Plants 2024, 13, 631. https://doi.org/10.3390/plants13050631
Peng Q, Tao W, Yu F, Xiong Q, Nong C, Zhang W, Fan J. Physiological and Biochemical Analysis Revealing the Key Factors Influencing 2-Phenylethanol and Benzyl Alcohol Production in Crabapple Flowers. Plants. 2024; 13(5):631. https://doi.org/10.3390/plants13050631
Chicago/Turabian StylePeng, Qin, Wenkai Tao, Fangyuan Yu, Qinqin Xiong, Chunshi Nong, Wangxiang Zhang, and Junjun Fan. 2024. "Physiological and Biochemical Analysis Revealing the Key Factors Influencing 2-Phenylethanol and Benzyl Alcohol Production in Crabapple Flowers" Plants 13, no. 5: 631. https://doi.org/10.3390/plants13050631
APA StylePeng, Q., Tao, W., Yu, F., Xiong, Q., Nong, C., Zhang, W., & Fan, J. (2024). Physiological and Biochemical Analysis Revealing the Key Factors Influencing 2-Phenylethanol and Benzyl Alcohol Production in Crabapple Flowers. Plants, 13(5), 631. https://doi.org/10.3390/plants13050631







