ZmADF5, a Maize Actin-Depolymerizing Factor Conferring Enhanced Drought Tolerance in Maize
Abstract
1. Introduction
2. Results
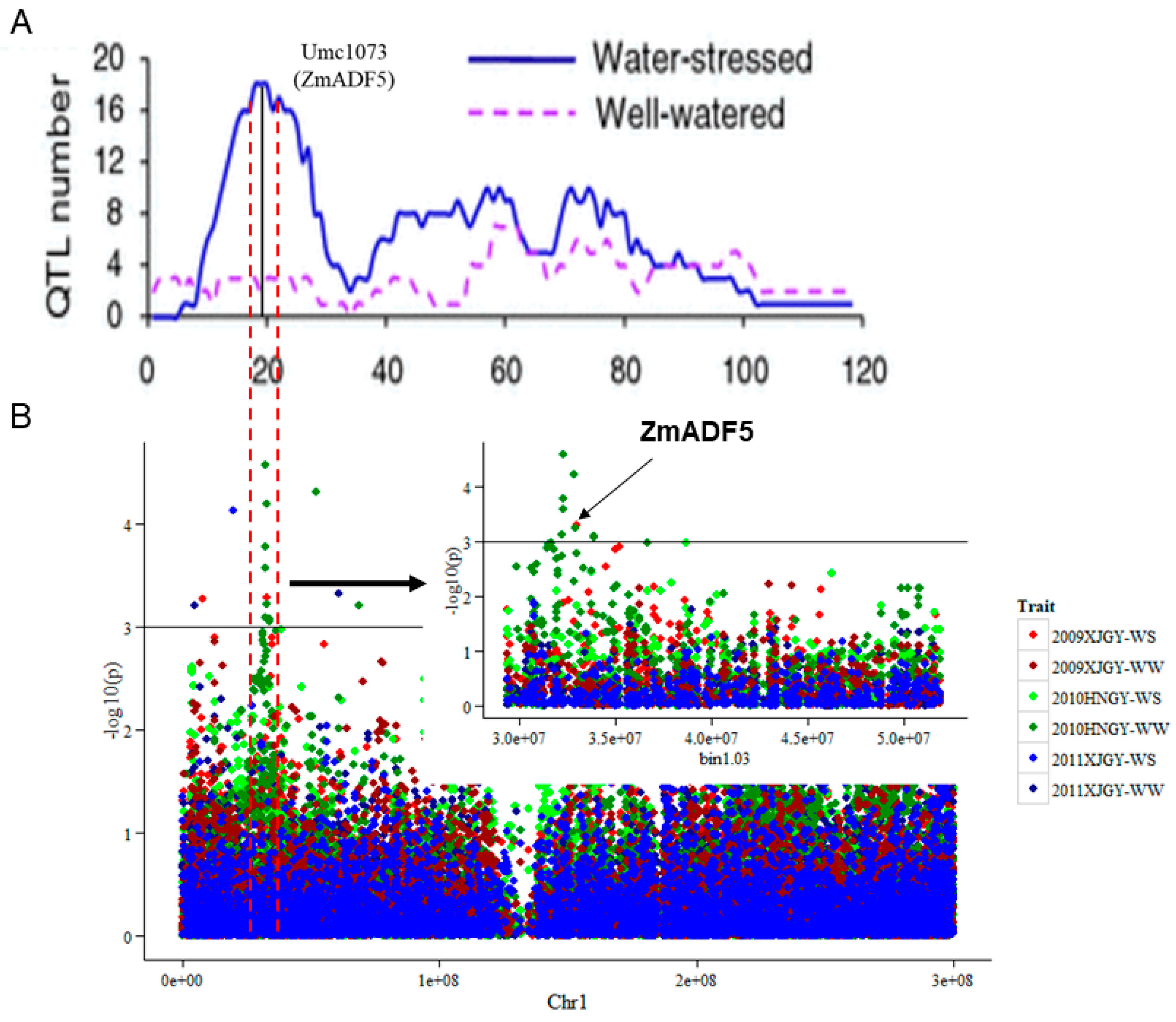
2.1. Identification of the Relationship between ZmADF5 and Two Linked Polymorphic Markers
2.2. Phylogenetic Analysis of the ADF Gene Family in Maize and Other Plant Species
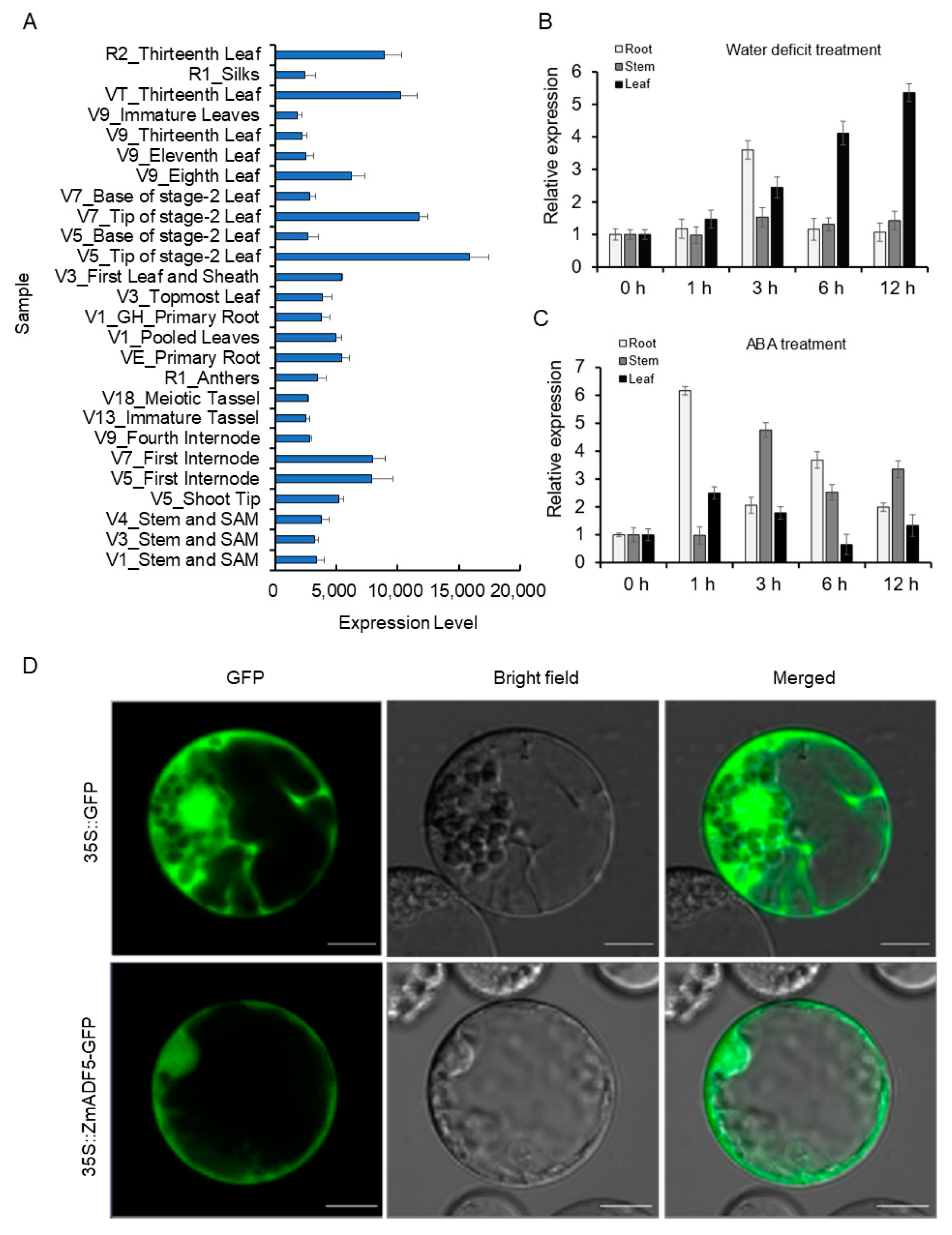
2.3. ZmADF5 Expression Is Induced by Drought Stress
2.4. Subcellular Localization of ZmADF5
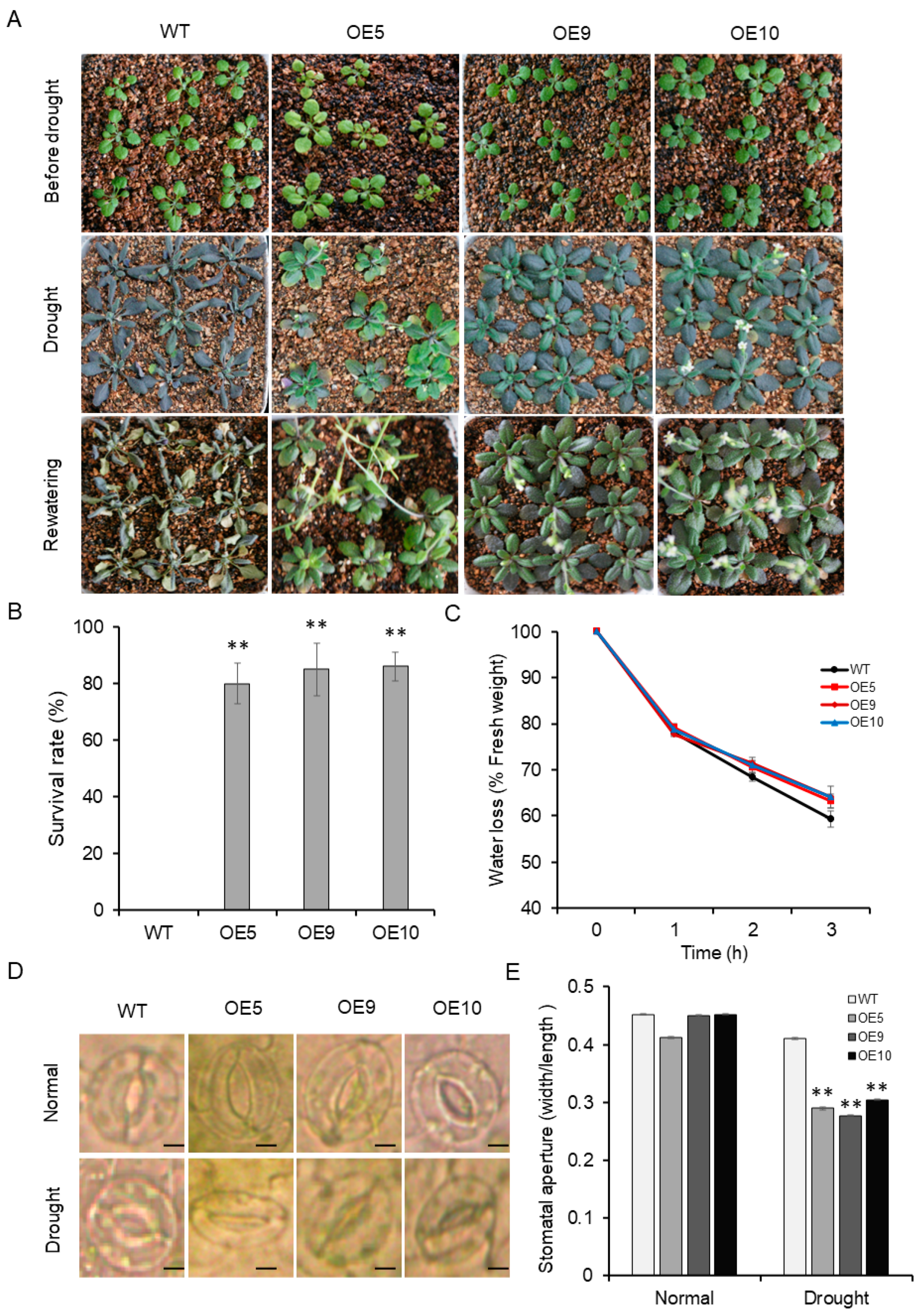
2.5. Drought Tolerance Is Enhanced in Arabidopsis Transgenic Plants through Overexpression of ZmADF5
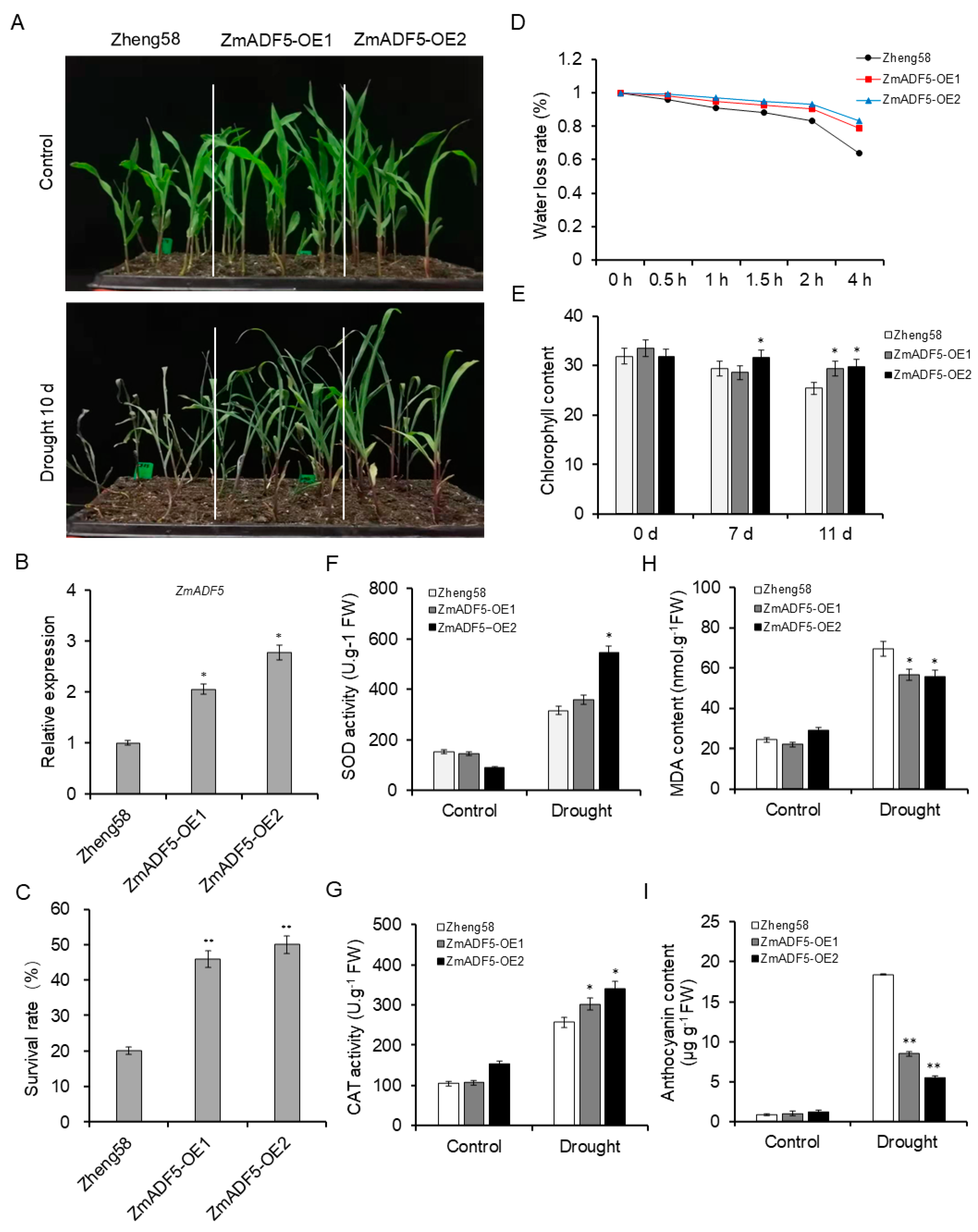
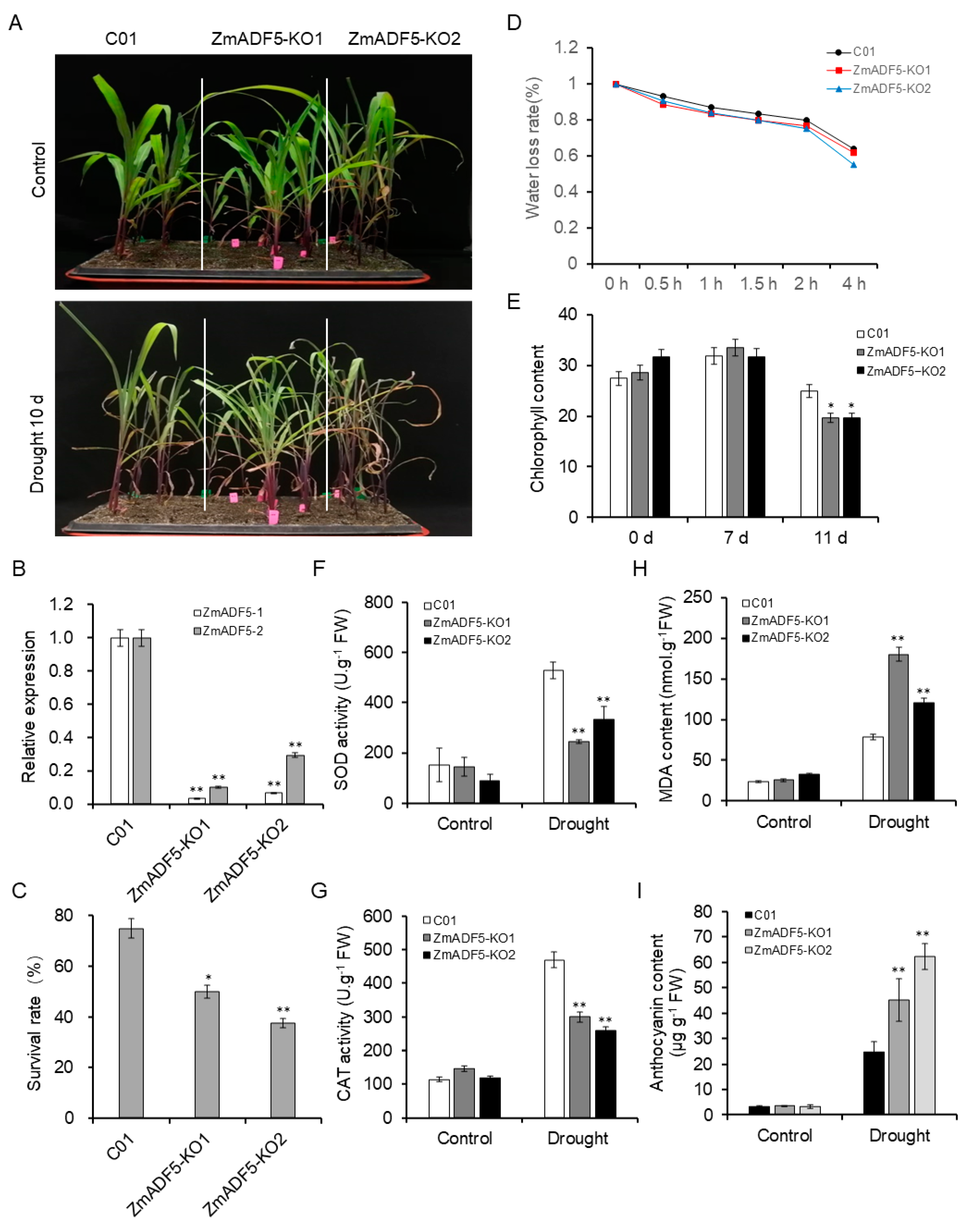
2.6. Overexpression of ZmADF5 Enhances Drought Tolerance in Maize
2.7. ABA Dependence of Enhanced Drought Tolerance in Transgenic Plants
3. Discussion
3.1. The Drought-Tolerant Candidate Gene ZmADF5 belongs to the Maize ADF Gene Family
3.2. ZmADF5 Contributes to the Regulation of Drought Tolerance through an ABA-Dependent Pathway Involving ROS Scavenging in Maize
4. Materials and Methods
4.1. Association Analysis of Grain Yield in Maize under Drought Stress
4.2. Phylogenetic Analysis of ADF Genes
4.3. Expression Profiles of Maize ZmADF5
4.4. Subcellular Localization of ZmADF5
4.5. Transgenic-Positive Plant Construction and Screening
4.6. Stress Treatment and Phenotyping of Transgenic Arabidopsis
4.7. Germination Assay and Root Growth Measurement
4.8. Phenotypic Analyses in Transgenic Maize
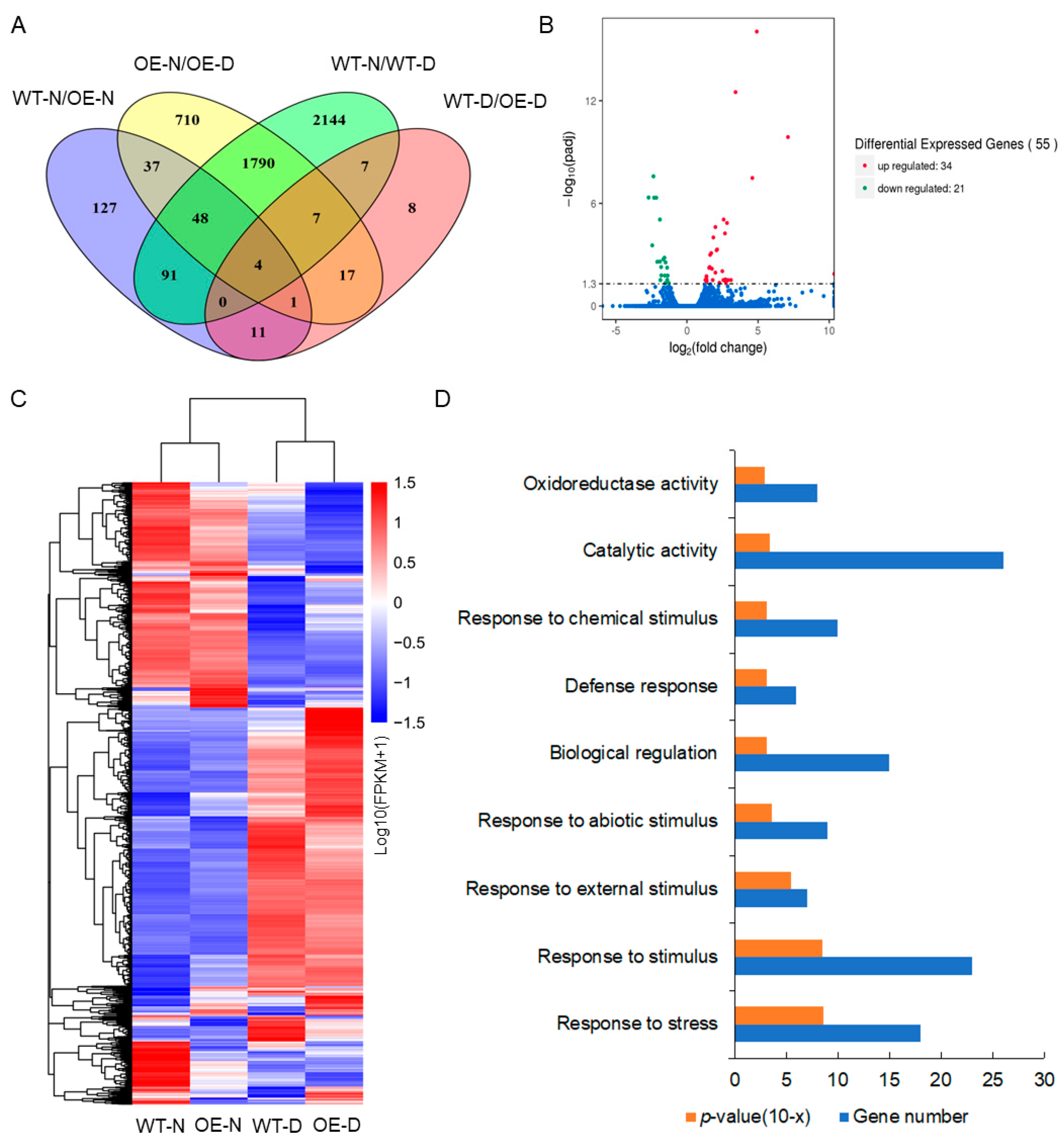
4.9. RNA-Seq Analysis of Transgenic Plants
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Almeida, G.D.; Nair, S.; Borem, A.; Cairns, J.; Trachsel, S.; Ribaut, J.M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. Molecular mapping across three populations reveals a QTL hotspot region on chromosome 3 for secondary traits associated with drought tolerance in tropical maize. Mol. Breed. 2014, 34, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.F.; Li, X.H.; Liu, X.L.; Xie, C.X.; Li, M.S.; Zhang, D.G.; Zhang, S.H. Meta-analysis of constitutive and adaptive QTL for drought tolerance in maize. Euphytica 2010, 174, 165–177. [Google Scholar] [CrossRef]
- Almeida, G.D.; Makumbi, D.; Magorokosho, C.; Nair, S.; Borem, A.; Ribaut, J.M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. QTL mapping in three tropical maize populations reveals a set of constitutive and adaptive genomic regions for drought tolerance. Theor. Appl. Genet. 2013, 126, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Beyene, Y.; Warburton, M.L.; Tarekegne, A.; Mugo, S.; Meisel, B.; Sehabiague, P.; Prasanna, B.M. Meta-analyses of QTL for grain yield and anthesis silking interval in 18 maize populations evaluated under water-stressed and well-watered environments. BMC Genom. 2013, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, S.; Sun, D.P.; SanVicente, F.M.; Zheng, H.J.; Atlin, G.N.; Suarez, E.A.; Babu, R.; Zhang, X.C. Identification of QTL for early vigor and stay-green conferring tolerance to drought in two connected advanced backcross populations in tropical maize (Zea mays L.). PLoS ONE 2016, 11, e0149636. [Google Scholar] [CrossRef]
- Goffinet, B.; Gerber, S. Quantitative trait loci: A meta-analysis. Genetics 2000, 155, 463–473. [Google Scholar] [CrossRef]
- Lu, Y.L.; Zhang, S.H.; Shah, T.; Xie, C.X.; Hao, Z.F.; Li, X.H.; Farkhari, M.; Ribaut, J.-M.; Cao, M.J.; Rong, T.Z.; et al. Joint linkage-linkage disequilibrium mapping is a powerful approach to detecting quantitative trait loci underlying drought tolerance in maize. Proc. Natl. Acad. Sci. USA 2010, 107, 19585–19590. [Google Scholar] [CrossRef]
- Aranzana, M.J.; Kim, S.; Zhao, K.Y.; Bakker, E.; Horton, M.; Jakob, K.; Lister, C.; Molitor, J.; Shindo, C.; Tang, C.L.; et al. Genome-wide association mapping in Arabidopsis identifies previously known flowering time and pathogen resistance genes. PLoS Genet. 2005, 1, 531–539. [Google Scholar] [CrossRef]
- Zhao, K.Y.; Aranzana, M.J.; Kim, S.; Lister, C.; Shindo, C.; Tang, C.L.; Toomajian, C.; Zheng, H.G.; Dean, C.; Marjoram, P.; et al. An Arabidopsis example of association mapping in structured samples. PLoS Genet. 2007, 3, e4. [Google Scholar] [CrossRef]
- Li, X.P.; Zhou, Z.J.; Ding, J.Q.; Wu, Y.B.; Zhou, B.; Wang, R.X.; Ma, J.L.; Wang, S.W.; Zhang, X.C.; Xia, Z.L.; et al. Combined linkage and association mapping reveals QTL and candidate genes for plant and ear height in maize. Front. Plant Sci. 2016, 7, 833. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.X.; Shi, Y.S.; Song, Y.C.; Zhang, D.F.; Li, C.H.; Buckler, E.S.; Li, Y.; Zhang, Z.W.; Wang, T.Y. Joint-linkage mapping and GWAS reveal extensive genetic loci that regulate male inflorescence size in maize. Plant Biotechnol. J. 2016, 14, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, J.; Sun, X.C.; Gao, Y.X.; Ochsenfeld, C.; Bakker, E.; Ren, R.H.; Flora, J.; Wang, X.J.; Kumpatla, S.; Meyer, D.; et al. Combining powers of linkage and association mapping for precise dissection of QTL controlling resistance to gray leaf spot disease in maize (Zea mays L.). BMC Genom. 2015, 16, 916. [Google Scholar] [CrossRef]
- Zhang, X.B.; Tang, B.; Yu, F.; Li, L.; Wang, M.; Xue, Y.D.; Zhang, Z.X.; Yan, J.B.; Yue, B.; Zheng, Y.L.; et al. Identification of major QTL for waterlogging tolerance using genome-wide association and linkage mapping of maize seedlings. Plant Mol. Biol. Report. 2013, 31, 594–606. [Google Scholar] [CrossRef]
- Bamburg, J.R. Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 1999, 15, 185–230. [Google Scholar] [CrossRef]
- Maciver, S.K.; Hussey, P.J. The ADF/cofilin family: Actin-remodeling proteins. Genome Biol. 2002, 3, reviews3007.1. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.L.; Deng, L.; Chang, D.; Chen, S.T.; Wang, X.J.; Kang, Z.S. TaADF3, an actin-depolymerizing factor, negatively modulates wheat resistance against Puccinia striiformis. Front. Plant Sci. 2016, 6, 1214. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.W.; Painter, W.B.; Chen, H.; Minamide, L.S.; Abe, H.; Bamburg, J.R. Intracellular pH modulation of ADF/cofilin proteins. Cell Motil. Cytoskelet. 2000, 47, 319–336. [Google Scholar] [CrossRef]
- Allwood, E.G.; Smertenko, A.P.; Hussey, P.J. Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett. 2001, 499, 97–100. [Google Scholar] [CrossRef]
- Nan, Q.; Qian, D.; Niu, Y.; He, Y.X.; Tong, S.F.; Niu, Z.M.; Ma, J.C.; Yang, Y.; An, L.Z.; Wan, D.S.; et al. Plant actin-depolymerizing factors possess opposing biochemical properties arising from key amino acid changes throughout evolution. Plant Cell 2017, 29, 395–408. [Google Scholar] [CrossRef]
- Henty, J.L.; Bledsoe, S.W.; Khurana, P.; Meagher, R.B.; Day, B.; Blanchoin, L.; Staiger, C.J. Arabidopsis actin depolymerizing factor 4 modulates the stochastic dynamic behavior of actin filaments in the cortical array of epidermal cells. Plant Cell 2011, 23, 3711–3726. [Google Scholar] [CrossRef]
- Zhu, J.G.; Nan, Q.; Qin, T.; Qian, D.; Mao, T.L.; Yuan, S.J.; Wu, X.R.; Niu, Y.; Bai, Q.F.; An, L.Z.; et al. Higher-ordered actin structures remodeled by Arabidopsis ACTIN-DEPOLYMERIZING FACTOR5 are important for pollen germination and pollen tube growth. Mol. Plant 2017, 10, 1065–1081. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Xie, Y.R.; Jiang, Y.X.; Qu, X.L.; Huang, S.J. Arabidopsis actin depolymerizing factor7 severs actin filaments and regulates actin cable turnover to promote normal pollen tube growth. Plant Cell 2013, 25, 3405–3423. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Xia, G.X.; Hong, Y.; Ramachandran, S.; Kost, B.; Chua, N.H. ADF proteins are involved in the control of flowering and regulate F-actin organization, cell expansion, and organ growth in Arabidopsis. Plant Cell 2001, 13, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Wang, J.; Gao, P.; Jiao, G.L.; Zhao, P.M.; Li, Y.; Wang, G.L.; Xia, G.X. Down regulation of GhADF1 gene expression affects cotton fibre properties. Plant Biotechnol. J. 2009, 7, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Ketelaar, T.; Rodiuc, N.; Banora, M.Y.; Smertenko, A.; Engler, G.; Abad, P.; Hussey, P.J.; Engler, J.d.A. Actin-depolymerizing factor 2 mediated actin dynamics are essential for root-knot nematode infection of Arabidopsis. Plant Cell 2009, 21, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Shimono, M.; Tian, M.Y.; Day, B. Arabidopsis actin-depolymerizing factor-4 links pathogen perception, defense activation and transcription to cytoskeletal dynamics. PLoS Pathog. 2012, 8, e1003006. [Google Scholar] [CrossRef]
- Zhao, S.S.; Jiang, Y.X.; Zhao, Y.; Huang, S.J.; Yuan, M.; Zhao, Y.X.; Guo, Y. CASEIN KINASE1-LIKE PROTEIN2 regulates actin filament stability and stomatal closure via phosphorylation of actin depolymerizing factor. Plant Cell 2016, 28, 1422–1439. [Google Scholar] [CrossRef]
- Qian, D.; Zhang, Z.; He, J.X.; Zhang, P.; Ou, X.B.; Li, T.; Niu, L.P.; Nan, Q.; Niu, Y.; He, W.L.; et al. Arabidopsis ADF5 promotes stomatal closure by regulating actin cytoskeleton remodeling in response to ABA and drought stress. J. Exp. Bot. 2019, 70, 435–446. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, W.L.; Hong, C.Y.; Lur, H.S.; Chang, M.C. Comprehensive analysis of differentially expressed rice actin depolymerizing factor gene family and heterologous overexpression of OsADF3 confers Arabidopsis thaliana drought tolerance. Rice 2012, 5, 33. [Google Scholar] [CrossRef]
- Zhang, B.; Hua, Y.; Wang, J.; Huo, Y.; Shimono, M.; Day, B.; Ma, Q. TaADF4, an actin-depolymerizing factor from wheat, is required for resistance to the stripe rust pathogen Puccinia striiformis f. sp tritici. Plant J. 2017, 89, 1210–1224. [Google Scholar] [CrossRef]
- Fu, Y.P.; Duan, X.Y.; Tang, C.L.; Li, X.R.; Voegele, R.T.; Wang, X.J.; Wei, G.R.; Kang, Z.S. TaADF7, an actin-depolymerizing factor, contributes to wheat resistance against Puccinia striiformis f. sp. tritici. Plant J. 2014, 78, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, D.R.; Kandasamy, M.K.; McKinney, E.C.; Meagher, R.B. Arabidopsis actin depolymerizing factor 5 functions in multicellular development and is a novel repressor of the CBF cold response transcription factors. Dev. Biol. 2008, 319, 565. [Google Scholar] [CrossRef][Green Version]
- Burgos-Rivera, B.; Ruzicka, D.R.; Deal, R.B.; McKinney, E.C.; King-Reid, L.; Meagher, R.B. ACTIN DEPOLYMERIZING FACTOR9 controls development and gene expression in Arabidopsis. Plant Mol. Biol. 2008, 68, 619–632. [Google Scholar] [CrossRef] [PubMed]
- González, C.V.; Ibarra, S.E.; Piccoli, P.N.; Botto, J.F.; Boccalandro, H.E. Phytochrome B increases drought tolerance by enhancing ABA sensitivity in Arabidopsis thaliana. Plant Cell Environ. 2012, 35, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.D.; Wang, H.W.; Liu, S.X.; Li, Z.G.; Yang, X.H.; Yan, J.B.; Li, J.S.; Tran, L.-S.P.; Qin, F. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 2015, 6, 8326. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, H.W.; Liu, S.X.; Ferjani, A.; Li, J.S.; Yan, J.B.; Yang, X.H.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Mi, Y.; Mao, H.D.; Liu, S.X.; Chen, L.M.; Qin, F. Genetic variation in ZmTIP1 contributes to root hair elongation and drought tolerance in maize. Plant Biotechnol. J. 2020, 18, 1271–1283. [Google Scholar] [CrossRef]
- Liu, J.X.; Raveendran, M.; Mushtaq, R.; Ji, X.M.; Yang, X.; Bruskiewich, R.M.; Katiyar, S.; Cheng, S.H.; Bennett, J. Proteomic analysis of drought-responsiveness in rice: OsADF5. In Proceedings of the International Congress ‘In the Wake of the Double Helix: From the Green Revolution to the Gene Revolution’, Bologna, Italy, 27–31 May 2003; Tuberosa, R., Phillips, R.L., Gale, M., Eds.; pp. 491–505. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, J.T.; Gao, X.S.; Tong, J.H.; Xiao, L.T.; Li, W.B.; Zhang, H.X. The Arabidopsis AP2/ERF transcription factor RAP2.6 participates in ABA, salt and osmotic stress responses. Gene 2010, 457, 1–12. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Verma, S.; Rahman, M.H.; Kav, N.N. Functional characterization of four APETALA2-family genes (RAP2.6, RAP2.6L, DREB19 and DREB26) in Arabidopsis. Plant Mol. Biol. 2011, 75, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Basu, M.R.; Bhaskara, G.B.; Verslues, P.E.; Haswell, E.S. Plastid osmotic stress activates cellular stress responses in Arabidopsis. Plant Physiol. 2014, 165, 119–128. [Google Scholar] [CrossRef]
- Umezawa, T.; Okamoto, M.; Kushiro, T.; Nambara, E.; Oono, Y.; Seki, M.; Kobayashi, M.; Koshiba, T.; Kamiya, Y.; Shinozaki, K. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 2006, 46, 171–182. [Google Scholar] [CrossRef]
- Hao, Z.F.; Li, X.H.; Su, Z.J.; Xie, C.X.; Li, M.S.; Liang, X.L.; Weng, J.F.; Zhang, D.G.; Li, L.; Zhang, S.H. A proposed selection criterion for drought resistance across multiple environments in maize. Breed. Sci. 2011, 61, 101–108. [Google Scholar] [CrossRef]
- Liu, C.L.; Hao, Z.F.; Zhang, D.G.; Xie, C.X.; Li, M.S.; Zhang, X.C.; Yong, H.J.; Zhang, S.H.; Weng, J.F.; Li, X.H. Genetic properties of 240 maize inbred lines and identity-by-descent segments revealed by high-density SNP markers. Mol. Breed. 2015, 35, 146. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.W.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef]
- Thomas, R.H. Molecular evolution and phylogenetics. Heredity 2000, 86, 385. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Drumm-Herrel, H.; Mohr, H. Effect of blue/UV light on anthocyanin synthesis in tomato seedlings in the absence of bulk carotenoids. Photochem. Photobiol. 1982, 36, 229–233. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.; Yan, H.Y.; You, Q.; Yi, X.; Du, Z.; Xu, W.Y.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef]
| Gene_ID | Change 1 | Gene Name | Gene Description | |
|---|---|---|---|---|
| Upregulated | AT1G43160 | 4.91 | RAP2-6 | Ethylene-responsive transcription factor RAP2-6 |
| AT1G51780 | 7.10 | ILL5 | IAA-amino acid hydrolase ILR1-like 5 | |
| AT1G58200 | 1.30 | MSL3 | MSCS-like 3 | |
| AT2G36590 | 2.49 | PROT3 | Proline transporter 3 | |
| AT2G38240 | 4.60 | ANS | 2-oxoglutarate (2OG) and Fe (II)-dependent oxygenase superfamily protein | |
| AT3G57460 | 3.42 | Catalytic/metal ion binding/metalloendopeptidase/zinc ion binding protein | ||
| AT4G23060 | 1.56 | IQD22 | IQ-domain 22 | |
| AT5G27060 | 2.75 | AtRLP53 | Receptor-like protein 53 | |
| AT5G62360 | 1.86 | Plant invertase/pectin methylesterase inhibitor superfamily protein | ||
| AT1G30320 | 1.81 | Remorin family protein | ||
| AT1G52720 | 1.32 | Hypothetical protein | ||
| AT2G18790 | 1.37 | PHYB | Phytochrome B | |
| AT3G05640 | 1.63 | Protein phosphatase 2C family protein | ||
| AT4G35110 | 1.39 | Phospholipase-like protein (PEARLI 4) family protein | ||
| Downregulated | AT5G45340 | −2.36 | CYP707A3 | Cytochrome p450 family, family 707, subfamily A, polypeptide 3 |
| AT5G61600 | −2.16 | ERF104 | Ethylene response factor 104 | |
| AT1G07135 | −1.36 | Glycine-rich protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Wang, N.; Yang, R.; Wang, X.; Luo, P.; Chen, Y.; Wang, F.; Li, M.; Weng, J.; Zhang, D.; et al. ZmADF5, a Maize Actin-Depolymerizing Factor Conferring Enhanced Drought Tolerance in Maize. Plants 2024, 13, 619. https://doi.org/10.3390/plants13050619
Liu B, Wang N, Yang R, Wang X, Luo P, Chen Y, Wang F, Li M, Weng J, Zhang D, et al. ZmADF5, a Maize Actin-Depolymerizing Factor Conferring Enhanced Drought Tolerance in Maize. Plants. 2024; 13(5):619. https://doi.org/10.3390/plants13050619
Chicago/Turabian StyleLiu, Bojuan, Nan Wang, Ruisi Yang, Xiaonan Wang, Ping Luo, Yong Chen, Fei Wang, Mingshun Li, Jianfeng Weng, Degui Zhang, and et al. 2024. "ZmADF5, a Maize Actin-Depolymerizing Factor Conferring Enhanced Drought Tolerance in Maize" Plants 13, no. 5: 619. https://doi.org/10.3390/plants13050619
APA StyleLiu, B., Wang, N., Yang, R., Wang, X., Luo, P., Chen, Y., Wang, F., Li, M., Weng, J., Zhang, D., Yong, H., Han, J., Zhou, Z., Zhang, X., Hao, Z., & Li, X. (2024). ZmADF5, a Maize Actin-Depolymerizing Factor Conferring Enhanced Drought Tolerance in Maize. Plants, 13(5), 619. https://doi.org/10.3390/plants13050619







