Hybrid Rice Production: A Worldwide Review of Floral Traits and Breeding Technology, with Special Emphasis on China
Abstract
1. Introduction
2. Challenges Faced in Hybrid Rice Production
- Maintaining genetic purity: Hybrid rice breeding requires strict maintenance of genetic purity ensuring that the desired traits are consistently passed on to the next generation. Any contamination can result in reduced yield and poor performance of the hybrid [40].
- High cost of hybrid seeds: Hybrid seeds are more expensive than traditional varieties, which can make them unaffordable for small-scale farmers [29].
- Inconsistent performance: Hybrid rice can exhibit variability in performance due to genotype-by-environment interactions, making it difficult to predict yield under different growing conditions [41].
- Limited genetic diversity: Hybrid rice relies on a limited number of parental lines, which can lead to decreased genetic diversity and increased susceptibility to disease and pests [42].
- Difficulty in achieving high seed purity: The maintenance of seed purity in hybrid rice production is difficult and requires meticulous management practices [43].
- Lack of infrastructure: Lack of infrastructure for hybrid seed production and distribution can limit the adoption and availability of hybrid rice varieties [4].
- Limited understanding of genetic mechanisms: Despite advances in genetic research, knowledge of the fundamental genetic pathways that contribute to hybrid vigor in rice is still lacking [44].
- Poor adaptation to different environments: Hybrid rice varieties may perform well in some environments but may not be well adapted to others, which can limit their widespread adoption [45].
- The limited availability of high-quality hybrid parents is critical for the success of hybrid rice breeding, but the limited availability of such parents can be a bottleneck in the process [46].
- Regulatory issues: Regulations related to seed certification, intellectual property rights, and biosafety can pose challenges to the commercialization and adoption of hybrid rice varieties [47].
3. Breeding Techniques for Producing Hybrid Rice
3.1. Contribution of Classical Breeding Approaches in the Production of Hybrid Rice
3.2. Advances in Breeding Technologies for Hybrid Rice
3.3. Use of Genomics and Genetic Markers in the Development of Hybrid Rice
3.4. Germplasm Characterization
- (1)
- Analyzing the structure and morphology of spikelets are the basic parts of rice inflorescence [91].
- (2)
- Evaluate the florets’ fertility, including the number of florets that mature into fully developed seeds and the amount of floret fertility per spikelet [92].
- (3)
- Estimating the time between planting and flowering is essential for coordinating the flowering of many parental lines and enabling regulated pollination [93].
- (4)
- Determining the pollen’s viability to make sure it can successfully fertilize for hybrid rice production [94].
- (5)
- Evaluating the time when the stigma is susceptible to pollen and the amount of exsertion it receives. This information is used in the planning of controlled pollination for hybrid rice production [16]. Examining the mechanism and time of pollen release in anthers is crucial for synchronizing pollination programs [95].
- (6)
3.5. Role of QTLs for Hybrid Rice Production
| Traits | QTLs | Markers | References |
|---|---|---|---|
| Female floral traits (stigma length, style length, stigma breadth, stigma area, and pistil length) | 14 | 164 polymorphic SSR and STS markers | [77] |
| Flower morphology (filament, anther length, style length, palea, lemma) | 11 | 180 SSR markers | [101] |
| Stigma exertion | 8 | 213 SSR markers | [102] |
| Pistil, stamen, size, and shape of glume | 7, 4, 14, 6 | 147 markers, mostly RFLP | [103] |
| Stigma exertion | 11 | 171 SSR markers | [104] |
| Anther length and stigma exertion | 4 | 120 RFLP markers | [105] |
| Anther length | 4 | 181 RFLP markers | [106] |
| Glume, pistil, and stamen | 160 QTLs | 182 RFLP markers | [107] |
3.6. Contribution of GWAS towards Hybrid Rice Production
3.7. Transgenic Technology for Floral Traits for Hybrid Rice Production
3.8. Development of New Hybrid Rice Breeding Strategies
4. Hybrid Rice Production: A Way Forward to Combat Global Food Scarcity
5. Floral Traits and Their Importance towards Hybrid Rice
- Anther length
- b.
- Stigma exsertion
- Single stigma exsertion: The stigma exsertion appeared just on one side of the spikelet.
- Dual stigma exsertion: The stigma exsertion appeared on both sides of the spikelet.
- Total stigma exsertion: The stigma exsertion occurred in addition to dual and single stigma exsertion on the spikelet.
- No stigma exsertion: The stigma exsertion does not occur on the spikelet [104].
- c.
- Photoperiod sensitivity
6. Prospective Advances in Breeding Technology of Hybrid Rice
6.1. Here Are Some Future Directions in Hybrid Rice Breeding Technology
- Creating hybrid rice cultivars that possess resilience to both abiotic and biotic stresses, including diseases. Rice plants are vulnerable to a range of challenges, including pests, diseases, and unfavorable abiotic, biotic, and climatic conditions.
- Developing biofortified hybrid rice: Rice is a major staple food for a huge population of the world. However, it is deficient in several essential nutrients, including iron, zinc, and vitamins and these mineral elements are essential for the normal growth and development of human beings. Developing hybrid rice varieties with improved nutritional value can help to address these deficiencies and improve the health of rice consumers [166].
- Developing hybrid rice varieties with higher yield potential: Despite the significant yield gains achieved through hybrid rice breeding technology, there is still room for improvement. Developing hybrid rice varieties with higher yield potential can help to meet the growing demand for rice and reduce the pressure on land use. Scientists are employing progressive breeding methods, such as genome editing and CRISPR/Cas9, to identify and incorporate yield-enhancing genes [167] that confer drought, heat, and salinity tolerance into hybrid rice varieties [168].
- Developing hybrid rice varieties with enhanced resilience to climate change: Climate change significantly threatens rice production, affecting yield and quality. Developing hybrid rice varieties with enhanced resilience to climate change is critical for ensuring this [169].
- Developing hybrid rice varieties with enhanced agronomic traits: Agronomic traits, such as plant height, tillering ability, and panicle size, play a critical role in rice production. Developing hybrid rice varieties with enhanced agronomic traits can help to improve rice yield and quality. Researchers are using innovative breeding practices, such as quantitative genetics and phenomics, to identify and incorporate genes that confer desirable agronomic traits into hybrid rice varieties [56,170].
- Use of machine learning and artificial intelligence: The use of machine learning and artificial intelligence (AI) could help to identify patterns and relationships in large datasets, allowing for more effective prediction of hybrid rice performance [171]. In hybrid rice breeding, various parental lines are chosen and crossed to produce new lines with enhanced features by using this technique. AI makes it simple and easy to combine and analyze various datasets, offers models for the performance prediction of hybrid [172], and helps the breeders and researchers to make wise choices and increase the effectiveness, accuracy, and success rate of hybrid rice breeding. This technique ultimately increased agricultural yields and food security [173].
- Incorporation of genomic selection: Genomic selection could allow for the selection of desirable traits based on genetic markers, even before the phenotype is observed, leading to more efficient breeding [174].
6.2. Potential Impact of Emerging Technologies on Hybrid Rice Production
- i.
- Genomics and molecular breeding: Advances in genomics and molecular breeding technologies have greatly accelerated the identification of key floral traits in hybrid rice varieties [175]. These technologies can identify genes associated with specific floral traits, such [175] as flowering time, panicle size, and pollen viability, allowing for targeted breeding and genetic modification of hybrid rice varieties [176,177,178]. Moreover, the use of molecular markers in plant breeding to explore important traits has gradually increased in recent years. The foundation for developing these molecular markers is QTL mapping, which identifies the genetic loci that are quantitatively associated with desirable traits [179]. Marker-assisted selection is the targeted modification of a certain genomic area that affects how a desired characteristic expresses itself in a short time by using DNA markers. These developments have propelled molecular breeding technology into the research of innovations [180]. Plant breeding technology also used marker-assisted selection to uplift the biotic and abiotic stress tolerance, and, ultimately, this process increased crop production. Marker-assisted selection uses the linkage disequilibrium (LD) between QTLs and markers, which entails the non-random connection between QTLs’ alleles and markers [181]. Identifying the target trait’s genes and the markers closely associated with quantitative trait loci (QTLs) is crucial before using marker-assisted selection [182]. Marker-assisted selection is distributed into four categories: marker-assisted pyramiding, marker-assisted backcrossing, marker-based recurrent selection, and early-generation marker-assisted selection. These methods classify the early genetic material in different generations and strongly impact the breeding cycle [74]. Marker-assisted selection (MAS) in plant breeding has some restrictions despite its benefits. The poor selection of traits that are controlled by several minor effect alleles is one such restriction [183]. Moreover, marker-assisted selection also becomes limited when numerous genes with minor effects are present to control one trait. Therefore, this selection is optimum for qualitative characters, not quantitative traits [184]. New methods are being used to get around these restrictions. Predictive breeding techniques that use agro-big data, sophisticated statistical models, and basic machine learning algorithms have recently gained popularity as efficient methods for overcoming MAS’s limitations [185].
- ii.
- High-throughput phenotyping: High-throughput phenotyping technologies, such as crewless aerial vehicles (UAVs), can quickly and accurately collect data on floral traits of hybrid rice varieties. This can help breeders identify and select hybrid rice varieties with desirable floral traits, improving yield and quality [197,198,199].
- iii.
- Imaging and machine learning: Imaging and machine learning technologies can analyze large datasets of floral traits, identifying patterns and relationships between different traits. This can help breeders understand the complex interactions between different floral traits and develop hybrid rice varieties with optimized floral architecture and reproductive biology [200].
- iv.
- Metabolomics: Metabolomics technologies can analyze the chemical composition of rice flowers, identifying the compounds that contribute to aroma, flavor, and nutritional value. This can help breeders to develop hybrid rice varieties with improved sensory and nutritional characteristics [201].
- v.
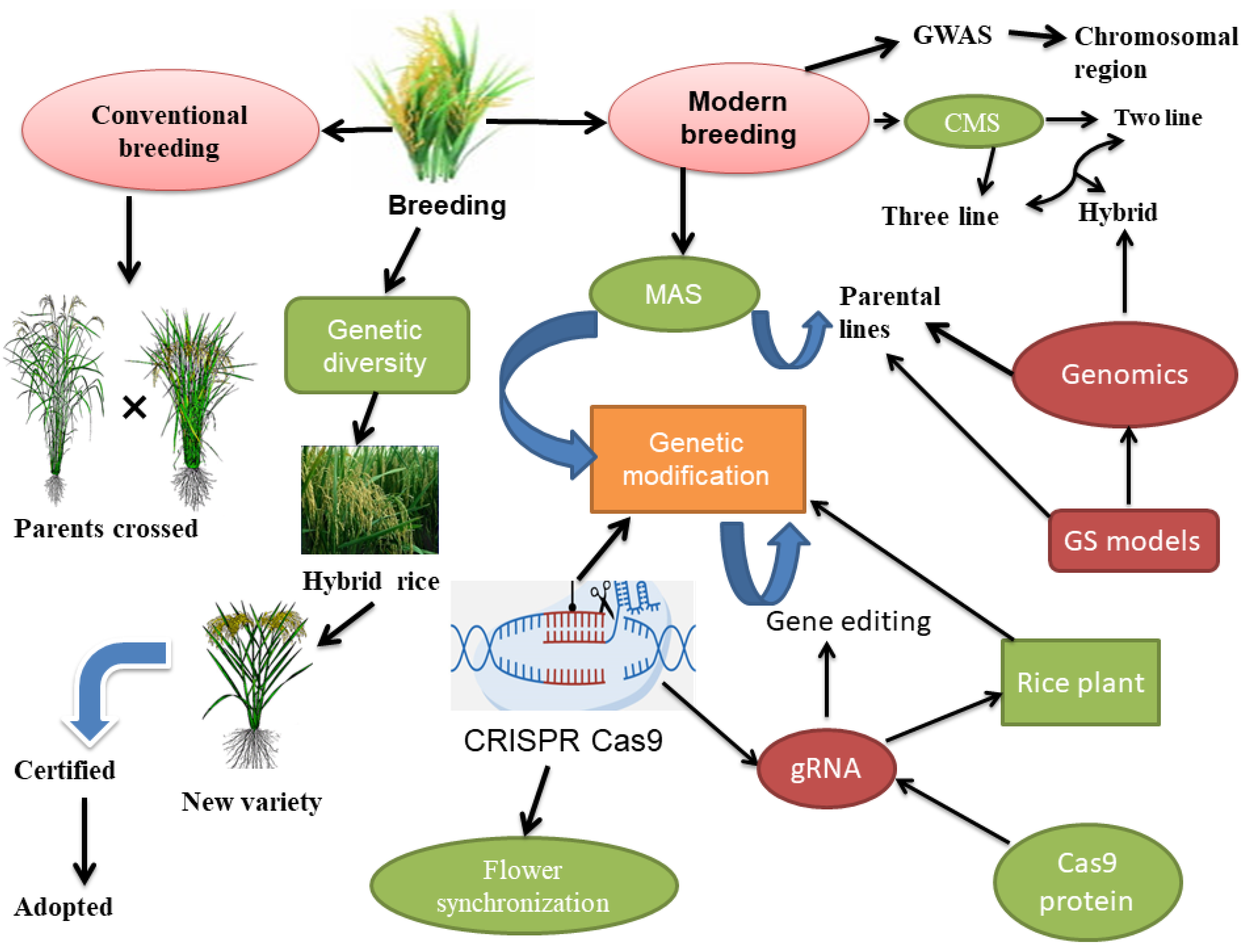
- Gene editing: CRISPR/Cas9 gene-editing technology can be used to modify genes associated with key floral traits in hybrid rice varieties precisely. This can enable breeders to develop hybrid rice varieties with optimized floral architecture and reproductive biology, improving yield and quality. To enhance desirable characters, CRISPR-Cas9 can be used to insert particular genetic alterations into parental lines [202]. It makes it possible to specifically modify the genes linked to desired traits like productivity, disease resistance, stress tolerance, and nutritional quality [203]. Breeders can increase hybrid rice production with better qualities by directly altering the DNA [126]. In rice plants, certain genes can be “knocked out” or turned off using CRISPR-Cas9. Breeders can deliberately delete genes that cause undesirable qualities or prevent the development of desired traits by using CRISPR-Cas9 and improving the hybrid rice performance [204]. Modifying floral traits can be useful in the production of hybrid rice for several reasons. For flower synchronization, CRISPR cas9 technology was used to control flowering time in plants. For aberrant floral growth or early flowering, genes including SVP, TFL1, and AP1 were addressed in Arabidopsis [205]. Other floral properties can also be altered using CRISPR besides flowering time. To produce pale blue flowers, the CRISPR/Cas9 system has been successfully applied to alter the color of flowers in Torenia fournieri [206], as well as multiplex CRISPR-Cas9, which introduce novel flowering and architectural characteristics in hexaploid Camelina sativa [207]. For instance, adjusting the flowering period can assist in synchronizing the female and male parental lines’ flowering, which is important to increase hybrid rice production [37]. Researchers often identify the genes responsible for the qualities they want to modify before using gene editing to change floral attributes in hybrid rice [60]. Next, they create a guide RNA (gRNA) specially designed for the target gene and insert it into the rice plant cells with the CRISPR-Cas9 system. The Cas9 protein makes a double-stranded break in the DNA of the target gene after being directed to it by the gRNA. After the break, the plant’s natural DNA repair system repairs gene mutations or insertions/deletions (InDels) that can change floral traits [208], as shown in Figure 1. However, CRISPPR Cas9 also has some limits, such as difficulty in obtaining higher editing efficiency, the transfer of technology to different rice populations, in vitro transcription, off-target effects, and plant lethality, as well as the use of common Cas9, can limit the editable range in plants, despite the obstacles, and CRISPR Cas9 proved useful in hybrid seed production [209,210,211]. Researchers can modify numerous genes at once using CRISPR-Cas9 and other gene editing methods, enabling the fine tuning of floral traits in the development of hybrid rice [203].
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maurya, R.K.; Dwivedi, D.; Khan, N.; Giri, S.; Dixit, S. Genetic variability studies for qualitative and quantitative traits in rice (Oryza sativa L.). Pharma Innov. J. 2022, 11, 1140–1143. [Google Scholar]
- Wang, X.; Chang, X.; Ma, L.; Bai, J.; Liang, M.; Yan, S. Global and regional trends in greenhouse gas emissions from rice production, trade, and consumption. Environ. Impact Assess. Rev. 2023, 101, 107141. [Google Scholar] [CrossRef]
- Durand-Morat, A.; Wailes, E.J.; Chavez, E.C. Hybrid rice and its impact on food security and the pattern of global production and trade. Res. Agric. Appl. Econ. 2011. [Google Scholar] [CrossRef]
- Li, J.; Xin, Y.; Yuan, L. Hybrid Rice Technology Development: Ensuring China’s Food Security; International Food Policy Research Institute: Washington, DC, USA, 2009; Volume 918. [Google Scholar]
- Horgan, F.G.; Crisol, E. Hybrid rice and insect herbivores in Asia. Entomol. Exp. Appl. 2013, 148, 1–19. [Google Scholar] [CrossRef]
- Cao, L.; Zhan, X. Chinese experiences in breeding three-line, two-line and super hybrid rice. In Rice: Germplasm, Genetics and Improvement; Yan, W.G., Bao, J.S., Eds.; InTech: Rijeka, Croatia, 2014; pp. 279–308. [Google Scholar]
- Li, D.; Huang, Z.; Song, S.; Xin, Y.; Mao, D.; Lv, Q.; Zhou, M.; Tian, D.; Tang, M.; Wu, Q. Integrated analysis of phenome, genome, and transcriptome of hybrid rice uncovered multiple heterosis-related loci for yield increase. Proc. Natl. Acad. Sci. USA 2016, 113, E6026–E6035. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Xu, Z.-J.; Chen, W.-F. Advances and prospects of super rice breeding in China. J. Integr. Agric. 2017, 16, 984–991. [Google Scholar]
- Lin, Z.; Qin, P.; Zhang, X.; Fu, C.; Deng, H.; Fu, X.; Huang, Z.; Jiang, S.; Li, C.; Tang, X. Divergent selection and genetic introgression shape the genome landscape of heterosis in hybrid rice. Proc. Natl. Acad. Sci. USA 2020, 117, 4623–4631. [Google Scholar] [CrossRef]
- Futakuchi, K.; Senthilkumar, K.; Arouna, A.; Vandamme, E.; Diagne, M.; Zhao, D.; Manneh, B.; Saito, K. History and progress in genetic improvement for enhancing rice yield in sub-Saharan Africa. Field Crops Res. 2021, 267, 108159. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, X.; Zhang, Q. Understanding the genetic and molecular constitutions of heterosis for developing hybrid rice. J. Genet. Genom. 2022, 49, 385–393. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, F.; Xin, Y. Yuan Longping and hybrid rice research. Rice 2021, 14, 101. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, J. Shanyou 63: An elite mega rice hybrid in China. Rice 2018, 11, 17. [Google Scholar] [CrossRef]
- Chen, Q.; Zeng, G.; Hao, M.; Jiang, H.; Xiao, Y. Improvement of rice blast and brown planthopper resistance of PTGMS line C815S in two-line hybrid rice through marker-assisted selection. Mol. Breed. 2020, 40, 21. [Google Scholar] [CrossRef]
- Safitri, D.A.; Suwarno, W.B.; Darmanto, A.; Aswidinnoor, H. Agronomic traits of rice hybrids (Oryza sativa L.) derived from new plant type and male sterile parents. J. Agron. Indones. 2023, 51, 181–189. [Google Scholar] [CrossRef]
- Zheng, W.; Ma, Z.; Zhao, M.; Xiao, M.; Zhao, J.; Wang, C.; Gao, H.; Bai, Y.; Wang, H.; Sui, G. Research and development strategies for hybrid japonica rice. Rice 2020, 13, 36. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, F.; Mishra, A.K.; Sha, W. An economic assessment of adoption of hybrid rice: Micro-level evidence from southern China. Fron. Sustain. Food Syst. 2022, 6, 1066657. [Google Scholar] [CrossRef]
- Yu, H.; Li, J. Producing hybrid seeds like conventional rice. Cell Res. 2022, 32, 959–960. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, T.; Li, Y.; Hu, J.; Kan, R.; Qiu, M.; Deng, Y.; Liu, P.; Zhang, L.; Dong, H. A novel strategy for creating a new system of third-generation hybrid rice technology using a cytoplasmic sterility gene and a genic male-sterile gene. Plant Biotechnol. J. 2021, 19, 251–260. [Google Scholar] [CrossRef]
- ElShamey, E.A.; Sakran, R.M.; ElSayed, M.A.; Aloufi, S.; Alharthi, B.; Alqurashi, M.; Mansour, E.; Abd El-Moneim, D. Heterosis and combining ability for floral and yield characters in rice using cytoplasmic male sterility system. Saudi J. Biol. Sci. 2022, 29, 3727–3738. [Google Scholar] [CrossRef]
- Anwar, M.; Zulfiqar, F.; Ferdous, Z.; Tsusaka, T.W.; Datta, A. Productivity, profitability, efficiency, and land utilization scenarios of rice cultivation: An assessment of hybrid rice in Bangladesh. Sustain. Prod. Consum. 2021, 26, 752–758.e752. [Google Scholar] [CrossRef]
- Pattnaik, S.S.; Dash, B.; Bhuyan, S.S.; Katara, J.L.; Parameswaran, C.; Verma, R.; Ramesh, N.; Samantaray, S. Anther culture efficiency in quality hybrid rice: A comparison between hybrid rice and its ratooned plants. Plants 2020, 9, 1306. [Google Scholar] [CrossRef]
- Kato, H.; Namai, H. Floral Characteristcs and Environmental Factors for Increasing Natural Outcrossing Rate for F1 Hybrid Seed Production of Rice Oryza sativa L. Jap. J. Breed. 1987, 37, 318–330. [Google Scholar] [CrossRef]
- Wang, H.; Deng, X.W. Development of the “third-generation” hybrid rice in China. Genom. Proteom. Bioinform. 2018, 16, 393–396. [Google Scholar] [CrossRef]
- Lenaerts, B.; Collard, B.C.; Demont, M. Global survey of rice breeders to investigate characteristics and willingness to adopt alternative breeding methods. Agric. Food Secur. 2018, 7, 40. [Google Scholar] [CrossRef]
- He, Y.; Yan, L.; Ge, C.; Yao, X.-F.; Han, X.; Wang, R.; Xiong, L.; Jiang, L.; Liu, C.-M.; Zhao, Y. PINOID is required for formation of the stigma and style in rice. Plant Physiol. 2019, 180, 926–936. [Google Scholar] [CrossRef]
- Benavente, E.; Giménez, E. Modern approaches for the genetic improvement of rice, wheat and maize for abiotic constraints-related traits: A comparative overview. Agronomy 2021, 11, 376. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, W. Hybrid rice production. In Modern Techniques of Rice Crop Production; Springer: Berlin/Heidelberg, Germany, 2022; pp. 629–645. [Google Scholar]
- Rout, D.; Jena, D.; Singh, V.; Kumar, M.; Arsode, P.; Singh, P.; Katara, J.L.; Samantaray, S.; Verma, R. Hybrid Rice Research: Current Status and Prospects; IntechOpen: London, UK, 2020; Volume 2020. [Google Scholar]
- Abebrese, S.O.; Yeboah, A. Hybrid rice in Africa: Progress, prospects, and challenges. Rec. Adv. Rice Res. 2020, 1–12. [Google Scholar] [CrossRef]
- El-Namaky, R.; Bare Coulibaly, M.M.; Alhassan, M.; Traore, K.; Nwilene, F.; Dieng, I.; Ortiz, R.; Manneh, B. Putting plant genetic diversity and variability at work for breeding: Hybrid rice suitability in West Africa. Diversity 2017, 9, 27. [Google Scholar] [CrossRef]
- He, Q.; Deng, H.; Sun, P.; Zhang, W.; Shu, F.; Xing, J.; Peng, Z. Hybrid rice. Engineering 2020, 6, 967–973. [Google Scholar] [CrossRef]
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G. Rational design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef] [PubMed]
- El-Namaky, R. The genetic variability of floral and agronomic characteristics of newly-bred cytoplasmic male sterile rice. Agriculture 2018, 8, 68. [Google Scholar] [CrossRef]
- Li, J.; Yuan, L. Hybrid rice: Genetics, breeding, and seed production. Plant Breed. Rev. 2000, 17, 15–158. [Google Scholar]
- Phukan, A.; Barua, P.; Sarma, D.; Deka, S. Study on floral traits and seed setting in parental lines of hybrid rice during early ahu and kharif seasons. Indian J. Genet. Plant Breed. 2018, 78, 285–291. [Google Scholar] [CrossRef]
- Gaballah, M.; Hamad, H.; Bamagoos, A.; Alharby, H.; Ahmed, S.; Ismail, I.A.; Sohidul Islam, M.; El Sabagh, A. Flowering synchronization in hybrid rice parental lines at different sowing dates. Sustainability 2021, 13, 3229. [Google Scholar] [CrossRef]
- Ghazy, M.I.; Hamad, H.S.; Gewaily, E.E.; Bleih, E.M.; Arafat, E.F.; El-Kallawy, W.H.; El-Naem, S.A.; Rehan, M.; Alwutayd, K.M.; Abd El Moneim, D. Impacts of kinetin implementation on leaves, floral and root-related traits during seed production in hybrid rice under water deficiency. BMC Plant Biol. 2023, 23, 398. [Google Scholar] [CrossRef]
- Liu, Q.; Han, R.; Wu, K.; Zhang, J.; Ye, Y.; Wang, S.; Chen, J.; Pan, Y.; Li, Q.; Xu, X. G-protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice. Nat. Commun. 2018, 9, 852. [Google Scholar] [CrossRef]
- Garg, A.; Singh, A.; Prabhu, K.; Mohapatra, T.; Tyagi, N.; Nandakumar, N.; Singh, R.; Zaman, F. Utility of a fertility restorer gene linked marker for testing genetic purity of hybrid seeds in rice (Oryza sativa L.). Seed Sci. Technol. 2006, 34, 9–18. [Google Scholar] [CrossRef]
- Senguttuvel, P.; Sravanraju, N.; Jaldhani, V.; Divya, B.; Beulah, P.; Nagaraju, P.; Manasa, Y.; Prasad, A.H.; Brajendra, P.; Gireesh, C. Evaluation of genotype by environment interaction and adaptability in lowland irrigated rice hybrids for grain yield under high temperature. Sci. Rep. 2021, 11, 15825. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-Z.; Xie, F.-M.; Chen, L.-Y.; Paz, M.A.D. Genetic diversity of tropical hybrid rice germplasm measured by molecular markers. Rice Sci. 2012, 19, 193–201. [Google Scholar] [CrossRef]
- Tiwari, A.; Tikoo, S.K.; Angadi, S.P.; Kadaru, S.B.; Ajanahalli, S.R.; Vasudeva Rao, M. Designing plant breeding programs for targeted deliveries. In Market-Driven Plant Breeding for Practicing Breeders; Springer: Berlin/Heidelberg, Germany, 2023; pp. 69–100. [Google Scholar]
- Song, S.; Tian, D.; Zhang, Z.; Hu, S.; Yu, J. Rice genomics: Over the past two decades and into the future. Genom. Proteom. Bioinform. 2018, 16, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gars, J.; Ward, P.S. Can differences in individual learning explain patterns of technology adoption? Evidence on heterogeneous learning patterns and hybrid rice adoption in Bihar, India. World Dev. 2019, 115, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Bohra, A.; Yu, J.; Graner, A.; Zhang, Q.; Sorrells, M.E. Designing future crops: Genomics-assisted breeding comes of age. Trends Plant Sci. 2021, 26, 631–649. [Google Scholar] [CrossRef]
- Pati, P.; Rathore, S.K. Intellectual property and rice research. In Applications of Bioinformatics in Rice Research; Springer: Singapore, 2021; pp. 277–296. [Google Scholar]
- Seck, P.A.; Diagne, A.; Mohanty, S.; Wopereis, M.C. Crops that feed the world 7: Rice. Food Secur. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Zhang, A.; Kong, D.; Bi, J.; Liu, G.; Yu, X.; Luo, L. Breeding an early maturing, blast resistance water-saving and drought-resistance rice (WDR) cultivar using marker-assisted selection coupled with rapid generation advance. Mol. Breed. 2022, 42, 46. [Google Scholar] [CrossRef]
- Kaushal, L.; Balachandran, S.; Ulaganathan, K.; Singh, A.K.; Priyadarshi, R.; Shenoy, V. Auxin to improve green plant regeneration of rice anther culture. Int. J. Agric. Crop Sci. 2015, 8, 15. [Google Scholar]
- Virmani, S. Prospects of hybrid rice in the tropics and subtropics. In Hybrid Rice Technology: New Developments and Future Prospects; International Rice Research Institute: Los Baños, Philippines, 1994; pp. 7–19. [Google Scholar]
- Shuro, A.R. Review paper on approaches in developing inbred lines in cross-pollinated crops. Biochem. Mol. Biol. 2017, 2, 40–45. [Google Scholar] [CrossRef]
- Fu, D.; Xiao, M.; Hayward, A.; Fu, Y.; Liu, G.; Jiang, G.; Zhang, H. Utilization of crop heterosis: A review. Euphytica 2014, 197, 161–173. [Google Scholar] [CrossRef]
- Casco, V.V.; Tapic, R.T.; Undan, J.R.; Latonio, A.M.L.S.; Suralta, R.R.; Manigbas, N.L. Combining ability, floral biology, and seed producibility of promising cytoplasmic male-sterile (CMS) lines for hybrid rice development. CABI Agric. Biosci. 2021, 2, 38. [Google Scholar] [CrossRef]
- Breseghello, F.; Coelho, A.S.G. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 2013, 61, 8277–8286. [Google Scholar] [CrossRef]
- Anis, G.; Hassan, H.; El-Sherif, A.; Saneoka, H.; EL Sabagh, A. Evaluation of new promising rice hybrid and its parental lines for floral, agronomic traits and genetic purity assessment. Pak. J. Agric. Sci. 2019, 56, 567–576. [Google Scholar]
- Hashim, S.; Ding, P.; Ismail, M.F.; Ramli, A. Floral traits and flowering behaviors of Malaysian rice cytoplasmic male sterile and maintainer lines and its relationship with out-crossing performance. Aust. J. Crop Sci. 2021, 15, 180–186. [Google Scholar] [CrossRef]
- Yahaya, M.A.; Shimelis, H.; Laing, M.; Mohammed, M.S.; Mathew, I. Methodologies for hybridization in predominantly self-pollinating crops: A review. J. Crop Improv. 2020, 34, 268–289. [Google Scholar] [CrossRef]
- Collard, B.C.; Gregorio, G.B.; Thomson, M.J.; Islam, R.; Vergara, G.V.; Laborte, A.G.; Nissila, E.; Kretzschmar, T.; Cobb, J.N. Transforming rice breeding: Re-designing the irrigated breeding pipeline at the International Rice Research Institute (IRRI). Crop Breed. Genet. Genom. 2019, 2019, e190008. [Google Scholar]
- Ali, Z.; Raza, Q.; Atif, R.M.; Aslam, U.; Ajmal, M.; Chung, G. Genetic and molecular control of floral organ identity in cereals. Int. J. Mol. Sci. 2019, 20, 2743. [Google Scholar] [CrossRef]
- Harfouche, A.L.; Petousi, V.; Meilan, R.; Sweet, J.; Twardowski, T.; Altman, A. Promoting ethically responsible use of agricultural biotechnology. Trends Plant Sci. 2021, 26, 546–559. [Google Scholar] [CrossRef]
- Rana, N.; Rahim, M.S.; Kaur, G.; Bansal, R.; Kumawat, S.; Roy, J.; Deshmukh, R.; Sonah, H.; Sharma, T.R. Applications and challenges for efficient exploration of omics interventions for the enhancement of nutritional quality in rice (Oryza sativa L.). Crit. Rev. Food Sci. Nutr. 2020, 60, 3304–3320. [Google Scholar] [CrossRef]
- Shariatpanahi, M.E.; Ahmadi, B. Isolated microspore culture and its applications in plant breeding and genetics. In Plant Tissue Culture: Propagation, Conservation and Crop Improvement; Springer: Singapore, 2016; pp. 487–507. [Google Scholar]
- Sharma, P.; Nair, S.A.; Sharma, P. Male sterility and its commercial exploitation in hybrid seed production of vegetable crops: A review. Agric. Rev. 2019, 40, 261–270. [Google Scholar] [CrossRef]
- Virmani, S.S. Two-Line Hybrid Rice Breeding Manual; International Rice Research Institute: Los Baños, Philippines, 2003. [Google Scholar]
- Njau, K.S. Production of Hybrid Rice Using Environment Sensitive Genic Male Sterile (Egms) and Basmati Rice Lines; Kenyatta University: Nairobi, Kenya, 2017. [Google Scholar]
- Pak, H.; Wang, H.; Kim, Y.; Song, U.; Tu, M.; Wu, D.; Jiang, L. Creation of male-sterile lines that can be restored to fertility by exogenous methyl jasmonate for the establishment of a two-line system for the hybrid production of rice (Oryza sativa L.). Plant Biotechnol. J. 2021, 19, 365–374. [Google Scholar] [CrossRef]
- Mekaroon, A.; Jompuk, C.; Kaveeta, R.; Thammasamisorn, B.-O.; Jompuk, P. Development of A, B and R Lines by gamma irradiation for hybrid rice. Agric. Nat. Resour. 2013, 47, 675–683. [Google Scholar]
- El Hanafi, S.; Cherkaoui, S.; Kehel, Z.; Sanchez-Garcia, M.; Sarazin, J.-B.; Baenziger, S.; Tadesse, W. Hybrid seed set in relation with male floral traits, estimation of heterosis and combining abilities for yield and its components in wheat (Triticum aestivum L.). Plants 2022, 11, 508. [Google Scholar] [CrossRef]
- Zajączkowska, U.; Denisow, B.; Łotocka, B.; Dołkin-Lewko, A.; Rakoczy-Trojanowska, M. Spikelet movements, anther extrusion and pollen production in wheat cultivars with contrasting tendencies to cleistogamy. BMC Plant Biol. 2021, 21, 136. [Google Scholar] [CrossRef]
- Fasahat, P.; Rajabi, A.; Rad, J.M.; Derera, J. Principles and utilization of combining ability in plant breeding. Biom. Biostat. Int. J. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Zhong, Q.; Jia, Q.; Yin, W.; Wang, Y.; Rao, Y.; Mao, Y. Advances in cloning functional genes for rice yield traits and molecular design breeding in China. Front. Plant Sci. 2023, 14, 1206165. [Google Scholar] [CrossRef]
- Hamad, H.S.; Bleih, E.M.; Gewaily, E.E.; Abou Elataa, A.E.; El Sherbiny, H.A.; Abdelhameid, N.M.; Rehan, M. Cyanobacteria Application Ameliorates Floral Traits and Outcrossing Rate in Diverse Rice Cytoplasmic Male Sterile Lines. Plants 2022, 11, 3411. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Doğan, Y.; Comertpay, G.; Yıldız, M.; Hatipoğlu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef]
- Shamshad, M.; Sharma, A. The usage of genomic selection strategy in plant breeding. Next Gener. Plant Breed. 2018, 26, 93–108. [Google Scholar]
- Awad-Allah, M.M.; Elekhtyar, N.M.; El-Abd, M.A.-E.-M.; Abdelkader, M.F.; Mahmoud, M.H.; Mohamed, A.H.; El-Diasty, M.Z.; Said, M.M.; Shamseldin, S.A.; Abdein, M.A. Development of new restorer lines carrying some restoring fertility genes with flowering, yield and grains quality characteristics in rice (Oryza sativa L.). Genes 2022, 13, 458. [Google Scholar] [CrossRef]
- Prahalada, G.; Marathi, B.; Vinarao, R.; Kim, S.-R.; Diocton, R.; Ramos, J.; Jena, K.K. QTL mapping of a novel genomic region associated with high out-crossing rate derived from Oryza longistaminata and development of new CMS lines in rice, O. sativa L. Rice 2021, 14, 80. [Google Scholar] [CrossRef]
- Lou, J.; Yue, G.; Yang, W.; Mei, H.; Luo, L.; Lu, H. Mapping QTLs influencing stigma exertion in rice. Bulg. J. Agric. Sci. 2014, 20, 1450–1456. [Google Scholar]
- Liu, Q.; Qin, J.; Li, T.; Liu, E.; Fan, D.; Edzesi, W.M.; Liu, J.; Jiang, J.; Liu, X.; Xiao, L. Fine mapping and candidate gene analysis of qSTL3, a stigma length-conditioning locus in rice (Oryza sativa L.). PLoS ONE 2015, 10, e0127938. [Google Scholar] [CrossRef]
- Jiang, G.-L. Molecular markers and marker-assisted breeding in plants. Plant Breed. Lab. Fields 2013, 3, 45–83. [Google Scholar]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A.; Ashkani, S.; Malek, M.A.; Latif, M.A. Marker-assisted backcrossing: A useful method for rice improvement. Biotechnol. Biotechnol. Equip. 2015, 29, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; De Los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y. Genomic selection in plant breeding: Methods, models, and perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef]
- Singh, C.; Kumar, S.J.; Kv, S. Characterization and identification of rice germplasm accessions using chemical tests. Seed Res. 2017, 45, 75–83. [Google Scholar]
- Angira, B.; Cerioli, T.; Famoso, A.N. Discovery and Validation of Grain Shape Loci in US Rice Germplasm Through Haplotype Characterization. Front. Genet. 2022, 13, 923078. [Google Scholar] [CrossRef]
- Surapaneni, M.; Balakrishnan, D.; Mesapogu, S.; Krishnam Raju, A.; Rao, Y.V.; Neelamraju, S. Genetic characterization and population structure of Indian rice cultivars and wild genotypes using core set markers. 3 Biotech 2016, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Sabar, M.; Akhter, M. Evaluation of rice germplasm for the development of hybrid rice. Asian J. Plant. Sci. 2003, 2, 1195–1197. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, L.; Xiao, N.; Dai, Z.; Li, Y.; Pan, C.; Zhang, X.; Liu, G.; Li, A. Characterization and evaluation of rice blast resistance of Chinese indica hybrid rice parental lines. Crop J. 2017, 5, 509–517. [Google Scholar] [CrossRef]
- Rathore, M.; Singh, R.; Kumar, B.; Chauhan, B. Characterization of functional trait diversity among Indian cultivated and weedy rice populations. Sci. Rep. 2016, 6, 24176. [Google Scholar] [CrossRef]
- Zou, T.; Xiao, Q.; Li, W.; Luo, T.; Yuan, G.; He, Z.; Liu, M.; Li, Q.; Xu, P.; Zhu, J. OsLAP6/OsPKS1, an orthologue of Arabidopsis PKSA/LAP6, is critical for proper pollen exine formation. Rice 2017, 10, 53. [Google Scholar] [CrossRef]
- Gao, X.; Liang, W.; Yin, C.; Ji, S.; Wang, H.; Su, X.; Guo, C.; Kong, H.; Xue, H.; Zhang, D. The SEPALLATA-like gene OsMADS34 is required for rice inflorescence and spikelet development. Plant Physiol. 2010, 153, 728–740. [Google Scholar] [CrossRef]
- Li, L.; Shi, F.; Wang, Y.; Yu, X.; Zhi, J.; Guan, Y.; Zhao, H.; Chang, J.; Chen, M.; Yang, G. TaSPL13 regulates inflorescence architecture and development in transgenic wheat (Triticum aestivum L.). Plant Sci. 2020, 296, 110516. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. The gymnastics of epigenomics in rice. Plant Cell Rep. 2018, 37, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, X.; Schmidt, R.C.; Struik, P.C.; Yin, X.; Jagadish, S.K. Pollen germination and in vivo fertilization in response to high-temperature during flowering in hybrid and inbred rice. Plant Cell Environ. 2018, 41, 1287–1297. [Google Scholar] [CrossRef]
- Fahad, S.; Ihsan, M.Z.; Khaliq, A.; Daur, I.; Saud, S.; Alzamanan, S.; Nasim, W.; Abdullah, M.; Khan, I.A.; Wu, C. Consequences of high temperature under changing climate optima for rice pollen characteristics-concepts and perspectives. Arch. Agron. Soil Sci. 2018, 64, 1473–1488. [Google Scholar]
- Guo, L.; Qiu, F.; Gandhi, H.; Kadaru, S.; De Asis, E.J.; Zhuang, J.; Xie, F. Genome-wide association study of outcrossing in cytoplasmic male sterile lines of rice. Sci. Rep. 2017, 7, 3223. [Google Scholar] [CrossRef]
- Ghaleb, M.A.A.; Li, C.; Shahid, M.Q.; Yu, H.; Liang, J.; Chen, R.; Wu, J.; Liu, X. Heterosis analysis and underlying molecular regulatory mechanism in a wide-compatible neo-tetraploid rice line with long panicles. BMC Plant Biol. 2020, 20, 83. [Google Scholar] [CrossRef]
- Kulkarni, S.R.; Balachandran, S.; Ulaganathan, K.; Balakrishnan, D.; Praveen, M.; Prasad, A.H.; Fiyaz, R.; Senguttuvel, P.; Sinha, P.; Kale, R.R. Molecular mapping of QTLs for yield related traits in recombinant inbred line (RIL) population derived from the popular rice hybrid KRH-2 and their validation through SNP genotyping. Sci. Rep. 2020, 10, 13695. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Wu, W.; Zhan, X.; Anis, G.B.; Rahman, M.H.; Hong, Y.; Riaz, A.; Zhu, A.; Cao, Y. qSE7 is a major quantitative trait locus (QTL) influencing stigma exsertion rate in rice (Oryza sativa L.). Sci. Rep. 2018, 8, 14523. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Bu, S.; Chen, G.; Yan, Z.; Chang, Z.; Zhu, H.; Yang, W.; Zhan, P.; Lin, S.; Xiong, L. Reconstruction of the high stigma exsertion rate trait in rice by pyramiding multiple QTLs. Front. Plant Sci. 2022, 13, 921700. [Google Scholar] [CrossRef]
- Ishikawa, R.; Watabe, T.; Nishioka, R.; Thanh, P.T.; Ishii, T. Identification of quantitative trait loci controlling floral morphology of rice using a backcross population between Common cultivated rice, Oryza sativa and Asian wild rice, O. rufipogon. Am. J. Plant Sci. 2017, 8, 734–744. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Fu, D.; Kong, D.; Ma, X.; Zhang, A.; Wang, F.; Wang, L.; Xia, H.; Liu, G.; Yu, X. Linkage mapping and association analysis to identify a reliable QTL for stigma exsertion rate in rice. Front. Plant Sci. 2022, 13, 982240. [Google Scholar] [CrossRef] [PubMed]
- Uga, Y.; Fukuta, Y.; Cai, H.; Iwata, H.; Ohsawa, R.; Morishima, H.; Fujimura, T. Mapping QTLs influencing rice floral morphology using recombinant inbred lines derived from a cross between Oryza sativa L. and Oryza rufipogon Griff. Theor. Appl. Genet. 2003, 107, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, F.; Zhang, Q.; Chao, Y.; Gao, G.; He, Y. Genetic mapping and validation of quantitative trait loci for stigma exsertion rate in rice. Mol. Breed. 2014, 34, 2131–2138. [Google Scholar] [CrossRef]
- Li, C.; Sun, C.-Q.; Mu, P.; Chen, L.; Wang, X. QTL analysis of anther length and ratio of stigma exsertion, two key traits of classification for cultivated rice (Oryza sativa L.) and common wild rice (O. rufipogon Griff.). Yi Chuan Xue Bao = Acta Genet. Sinica 2001, 28, 746–751. [Google Scholar]
- Tazib, T.; Kobayashi, Y.; Koyama, H.; Matsui, T. QTL analyses for anther length and dehiscence at flowering as traits for the tolerance of extreme temperatures in rice (Oryza sativa L.). Euphytica 2015, 203, 629–642. [Google Scholar] [CrossRef]
- Uga, Y.; Siangliw, M.; Nagamine, T.; Ohsawa, R.; Fujimura, T.; Fukuta, Y. Comparative mapping of QTLs determining glume, pistil and stamen sizes in cultivated rice (Oryza sativa L.). Plant Breed. 2010, 129, 657–669. [Google Scholar] [CrossRef]
- Hoffmann, T.J.; Kvale, M.N.; Hesselson, S.E.; Zhan, Y.; Aquino, C.; Cao, Y.; Cawley, S.; Chung, E.; Connell, S.; Eshragh, J. Next generation genome-wide association tool: Design and coverage of a high-throughput European-optimized SNP array. Genomics 2011, 98, 79–89. [Google Scholar] [CrossRef]
- Thomson, M.J. High-throughput SNP genotyping to accelerate crop improvement. Plant Breed. Biotechnol. 2014, 2, 195–212. [Google Scholar] [CrossRef]
- Huang, X.; Han, B. Genome-wide association mapping of complex traits in rice. Rice Genom. Genet. Breed. 2018, 497–510. [Google Scholar]
- Zhou, H.; Li, P.; Xie, W.; Hussain, S.; Li, Y.; Xia, D.; Zhao, H.; Sun, S.; Chen, J.; Ye, H. Genome-wide association analyses reveal the genetic basis of stigma exsertion in rice. Mol. Plant 2017, 10, 634–644. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, J.; Han, B.; Huang, X. Advances in genome-wide association studies of complex traits in rice. Theor. Appl. Genet. 2020, 133, 1415–1425. [Google Scholar] [CrossRef]
- Abhijith, K.P.; Gopala Krishnan, S.; Ravikiran, K.T.; Dhawan, G.; Kumar, P.; Vinod, K.K.; Bhowmick, P.K.; Nagarajan, M.; Seth, R.; Sharma, R. Genome-wide association study reveals novel genomic regions governing agronomic and grain quality traits and superior allelic combinations for Basmati rice improvement. Front. Plant Sci. 2022, 13, 994447. [Google Scholar] [CrossRef]
- Zhang, Z.; Ober, U.; Erbe, M.; Zhang, H.; Gao, N.; He, J.; Li, J.; Simianer, H. Improving the accuracy of whole genome prediction for complex traits using the results of genome wide association studies. PLoS ONE 2014, 9, e93017. [Google Scholar] [CrossRef]
- Wu, J.; Qiu, S.; Wang, M.; Xu, C.; Deng, X.W.; Tang, X. Construction of a weight-based seed sorting system for the third-generation hybrid rice. Rice 2021, 14, 66. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Z.; Wang, N.; Xie, G.; Lu, J.; Yan, W.; Zhou, J.; Tang, X.; Deng, X.W. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl. Acad. Sci. USA 2016, 113, 14145–14150. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-R.; Snow, A.A. Gene flow from genetically modified rice and its environmental consequences. BioScience 2005, 55, 669–678. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Ling, F.; You, A. Application and Development of Bt Insect Resistance Genes in Rice Breeding. Sustainability 2023, 15, 9779. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Arolu, F.; Anisuzzaman, M. Flooding tolerance in Rice: Adaptive mechanism and marker-assisted selection breeding approaches. Mol. Biol. Rep. 2023, 50, 2795–2812. [Google Scholar] [CrossRef] [PubMed]
- Kajala, K.; Covshoff, S.; Karki, S.; Woodfield, H.; Tolley, B.J.; Dionora, M.J.A.; Mogul, R.T.; Mabilangan, A.E.; Danila, F.R.; Hibberd, J.M. Strategies for engineering a two-celled C4 photosynthetic pathway into rice. J. Exp. Bot. 2011, 62, 3001–3010. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.N.B.L.; Kim, B.-K.; Yoon, I.-S.; Kim, K.-H.; Kwon, T.-R. Salt tolerance in rice: Focus on mechanisms and approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Spielman, D.J.; Ward, P.S.; Kolady, D.E.; Ar-Rashid, H. Public incentives, private investment, and outlooks for hybrid rice in Bangladesh and India. Appl. Econ. Perspect. Policy 2017, 39, 154–176. [Google Scholar] [CrossRef]
- Iftekharuddaula, K.M.; Ahmed, H.U.; Ghosal, S.; Moni, Z.R.; Amin, A.; Ali, M.S. Development of new submergence tolerant rice variety for Bangladesh using marker-assisted backcrossing. Rice Sci. 2015, 22, 16–26. [Google Scholar] [CrossRef]
- Anilkumar, C.; Sunitha, N.; Devate, N.B.; Ramesh, S. Advances in integrated genomic selection for rapid genetic gain in crop improvement: A review. Planta 2022, 256, 87. [Google Scholar] [CrossRef]
- Zhou, H.; He, M.; Li, J.; Chen, L.; Huang, Z.; Zheng, S.; Zhu, L.; Ni, E.; Jiang, D.; Zhao, B. Development of commercial thermo-sensitive genic male sterile rice accelerates hybrid rice breeding using the CRISPR/Cas9-mediated TMS5 editing system. Sci. Rep. 2016, 6, 37395. [Google Scholar] [CrossRef]
- McCouch, S.; Navabi, Z.K.; Abberton, M.; Anglin, N.L.; Barbieri, R.L.; Baum, M.; Bett, K.; Booker, H.; Brown, G.L.; Bryan, G.J. Mobilizing crop biodiversity. Mole. Plant 2020, 13, 1341–1344. [Google Scholar] [CrossRef]
- Wimalasekera, R. Role of seed quality in improving crop yields. Crop Prod. Global Environ. Issues 2015, 42, 153–168. [Google Scholar] [CrossRef]
- ElShamey, E.A.; Hamad, H.S.; Alshallash, K.S.; Alghuthaymi, M.A.; Ghazy, M.I.; Sakran, R.M.; Selim, M.E.; ElSayed, M.A.; Abdelmegeed, T.M.; Okasha, S.A. Growth regulators improve outcrossing rate of diverse rice cytoplasmic male sterile lines through affecting floral traits. Plants 2022, 11, 1291. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, M.M.; Attia, K.A.; Ghoneim, A.M.; Khan, N.; El-Ezz, A.F.; Yang, B.; Xiao, L.; Ibrahim, E.I.; Al-Doss, A.A. Assessment of genetic parameters and gene action associated with heterosis for enhancing yield characters in novel hybrid rice parental lines. Plants 2022, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Schmalzer, S. Yuan Longping, hybrid rice, and the meaning of science in the cultural revolution and beyond. Endeavour 2017, 41, 94–101. [Google Scholar] [CrossRef]
- Yuan, L.-P. Hybrid rice achievements, development and prospect in China. J. Integr. Agric. 2015, 14, 197–205. [Google Scholar]
- Shi, W.; Yin, X.; Struik, P.C.; Xie, F.; Schmidt, R.C.; Jagadish, K.S. Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crops Res. 2016, 190, 18–25. [Google Scholar] [CrossRef]
- Spielman, D.J.; Kolady, D.E.; Ward, P.; Rashid, H.-A.; Gulati, K. Public Expenditures, Private Incentives, and Technology Adoption: The Economics of Hybrid Rice in South Asia. 2012. Available online: https://ebrary.ifpri.org/utils/getfile/collection/p15738coll2/id/127309/filename/127520.pdf (accessed on 2 December 2023).
- Liao, C.; Yan, W.; Chen, Z.; Xie, G.; Deng, X.W.; Tang, X. Innovation and development of the third-generation hybrid rice technology. Crop J. 2021, 9, 693–701. [Google Scholar] [CrossRef]
- Bin Rahman, A.R.; Zhang, J. Trends in rice research: 2030 and beyond. Food Energy Secur. 2023, 12, e390. [Google Scholar] [CrossRef]
- Zhu, X.; Gou, Y.; Heng, Y.; Ding, W.; Li, Y.; Zhou, D.; Li, X.; Liang, C.; Wu, C.; Wang, H. Targeted manipulation of grain shape genes effectively improves outcrossing rate and hybrid seed production in rice. Plant Biotechnol. J. 2023, 21, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Landge, R.; Patil, K.; Patil, P.; Salunkhe, H. Molecular basis of heterosis: A review. Pharma Innov. J. 2022, 11, 426–434. [Google Scholar]
- Sah, S.; Joshi, B. Hybrid Rice Seed Production Manual; Nepal Agricultural Research Council: Lalitpur, Nepal, 2020. [Google Scholar]
- Mohammed, S.; Samad, A.; Rahmat, Z. Long-Day flowering regulation network in rice by RFT1 gene: A review. Cell Mol. Biol. 2016, 2, 1–14. [Google Scholar]
- Chongloi, G.L.; Prakash, S.; Vijayraghavan, U. Regulation of meristem maintenance and organ identity during rice reproductive development. J. Exp. Bot. 2019, 70, 1719–1736. [Google Scholar] [CrossRef]
- Sakuma, S.; Schnurbusch, T. Of floral fortune: Tinkering with the grain yield potential of cereal crops. New Phytol. 2020, 225, 1873–1882. [Google Scholar] [CrossRef]
- Yoshida, H.; Nagato, Y. Flower development in rice. J. Exp. Bot. 2011, 62, 4719–4730. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, D.; Fang, J.; Wang, P.; Deng, X.; Chu, C. Understanding the genetic and epigenetic architecture in complex network of rice flowering pathways. Protein Cell 2014, 5, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Maung, P.P.; Kim, B.; Jin, Z.; Jang, S.; Lee, Y.K.; Koh, H.-J. Identification and characterization of a novel gene controlling floral organ number in rice (Oryza sativa L.). PLoS ONE 2023, 18, e0280022. [Google Scholar] [CrossRef]
- Zhu, B.-S.; Zhu, Y.-X.; Zhang, Y.-F.; Zhong, X.; Pan, K.-Y.; Jiang, Y.; Wen, C.-K.; Yang, Z.-N.; Yao, X. Ethylene activates the EIN2-EIN3/EIL1 signaling pathway in tapetum and disturbs anther development in Arabidopsis. Cells 2022, 11, 3177. [Google Scholar] [CrossRef]
- Åstrand, J.; Knight, C.; Robson, J.; Talle, B.; Wilson, Z.A. Evolution and diversity of the angiosperm anther: Trends in function and development. Plant Reprod. 2021, 34, 307–319. [Google Scholar] [CrossRef]
- Matsui, T. Function of long basal dehiscence of the thecae in Rice (Oryza sativa L.) pollination under hot and humid condition. Phyton 2005, 45, 401–407. [Google Scholar]
- Xu, S.; Zheng, Y.; Liu, Y.; Guo, X.; Tan, Y.; Qian, Q.; Shu, Q.; Huang, J. Identification of a major quantitative trait locus and its candidate underlying genetic variation for rice stigma exsertion rate. Crop J. 2019, 7, 350–359. [Google Scholar] [CrossRef]
- Rahman, M.; Yu, P.; Zhang, Y.; Sun, L.; Wu, W.; Shen, X.; Zhan, X.; Chen, D.; Cao, L.; Cheng, S. Quantitative trait loci mapping of the stigma exertion rate and spikelet number per panicle in rice (Oryza sativa L.). Genet. Mol. Res. 2016, 15, gmr15048432. [Google Scholar] [CrossRef]
- Xin-Qiao, Y.; Han-Wei, M.; Li-Jun, L.; Guo-Lan, L.; Hong-Yan, L.; Gui-Hua, Z.; Song-Ping, H.; Ming-Sou, L.; Jin-Hong, W. Dissection of additive, epistatic effect and Q× E interaction of quantitative trait loci influencing stigma exsertion under water stress in rice. Acta Genet. Sinica 2006, 33, 542–550. [Google Scholar]
- Ma, X.; Zheng, Z.; Lin, F.; Ge, T.; Sun, H. Genetic analysis and gene mapping of a low stigma exposed mutant gene by high-throughput sequencing. PLoS ONE 2018, 13, e0186942. [Google Scholar] [CrossRef]
- Yan, W.G.; Li, Y.; Agrama, H.A.; Luo, D.; Gao, F.; Lu, X.; Ren, G. Association mapping of stigma and spikelet characteristics in rice (Oryza sativa L.). Mol. Breed. 2009, 24, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Goel, K.; Kundu, P.; Sharma, P.; Zinta, G. Thermosensitivity of pollen: A molecular perspective. Plant Cell Rep. 2023, 42, 843–857. [Google Scholar] [CrossRef]
- Takano-Kai, N.; Doi, K.; Yoshimura, A. GS3 participates in stigma exsertion as well as seed length in rice. Breed. Sci. 2011, 61, 244–250. [Google Scholar] [CrossRef]
- Akhilesh Singh, K.; Kemparaju, K.; Virupaxagouda Patil, R.P.; Pranitha Koradi, D.K.; Jayaramulu, K. Stigma exsertion trait in rice (Oryza sativa L.) and comparison of two phenotyping methods. Int. J. Curr. Res. 2015, 7, 13123–13135. [Google Scholar]
- Cao, S.; Luo, X.; Xu, D.; Tian, X.; Song, J.; Xia, X.; Chu, C.; He, Z. Genetic architecture underlying light and temperature mediated flowering in Arabidopsis, rice, and temperate cereals. New. Phytol. 2021, 230, 1731–1745. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, S.; Cui, S.; Hou, H.; Wu, H.; Hao, B.; Cai, L.; Xu, Z.; Liu, L.; Jiang, L. Transcriptional and post-transcriptional regulation of heading date in rice. New. Phytol. 2021, 230, 943–956. [Google Scholar] [CrossRef]
- Singh, R.; Ram, L. Ideal hybrid rice seed production package: An overview. Indian Res. J. Ext. Educ. 2012, 3, 244–251. [Google Scholar]
- Sruthi, K.; Divya, B.; Senguttuvel, P.; Revathi, P.; Kemparaju, K.; Koteswararao, P.; Sundaram, R.; Singh, V.J.; Ranjith Kumar, E.; Bhowmick, P.K. Evaluation of genetic diversity of parental lines for development of heterotic groups in hybrid rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2020, 29, 236–252. [Google Scholar] [CrossRef]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef]
- Jackson, S.D. Plant responses to photoperiod. New. Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef]
- Yano, M.; Kojima, S.; Takahashi, Y.; Lin, H.; Sasaki, T. Genetic control of flowering time in rice, a short-day plant. Plant Physiol. 2001, 127, 1425–1429. [Google Scholar] [CrossRef]
- Endo-Higashi, N.; Izawa, T. Flowering time genes Heading date 1 and Early heading date 1 together control panicle development in rice. Plant Cell Physiol. 2011, 52, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Current status, challenges, and opportunities in rice production. Rice Prod. Worldw. 2017, 1–32. [Google Scholar]
- Ashokkumar, K.; Govindaraj, M.; Karthikeyan, A.; Shobhana, V.; Warkentin, T.D. Genomics-integrated breeding for carotenoids and folates in staple cereal grains to reduce malnutrition. Front. Genet. 2020, 11, 414. [Google Scholar] [CrossRef]
- Siddiq, E.; Vemireddy, L.R. Advances in genetics and breeding of rice: An overview. Rice Improv. Physiol. Mol. Breed. Genet. Perspect. 2021, 1–29. [Google Scholar] [CrossRef]
- Younis, A.; Ramzan, F.; Ramzan, Y.; Zulfiqar, F.; Ahsan, M.; Lim, K.B. Molecular markers improve abiotic stress tolerance in crops: A review. Plants 2020, 9, 1374. [Google Scholar] [CrossRef]
- Gahatraj, S.; Jha, R.K.; Singh, O.P. Impacts of climate change on rice production and strategies for adaptation in Chitwan, Nepal. J. Agric. Nat. Resour. 2018, 1, 114–121. [Google Scholar] [CrossRef][Green Version]
- Awad-Allah, M.M.; Attia, K.A.; Omar, A.A.; Mohamed, A.H.; Habiba, R.M.; Alzuaibr, F.M.; Alshehri, M.A.; Alqurashi, M.; Aloufi, S.; Dessoky, E.S. Combining Ability and Gene Action Controlling Agronomic Traits for Cytoplasmic Male Sterile Line, Restorer Lines, and New Hybrids for Developing of New Drought-Tolerant Rice Hybrids. Genes 2022, 13, 906. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Li, H.; Zheng, H.; Zhang, J.; Olsen, M.S.; Varshney, R.K.; Prasanna, B.M.; Qian, Q. Smart breeding driven by big data, artificial intelligence, and integrated genomic-enviromic prediction. Mol. Plant 2022, 15, 1664–1695. [Google Scholar] [CrossRef]
- Sabouri, H.; Sajadi, S.J. Predicting hybrid rice performance using AIHIB model based on artificial intelligence. Sci. Rep. 2022, 12, 9709. [Google Scholar] [CrossRef]
- Rai, K.K. Integrating speed breeding with artificial intelligence for developing climate-smart crops. Mol. Biol. Rep. 2022, 49, 11385–11402. [Google Scholar] [CrossRef]
- Heslot, N.; Jannink, J.L.; Sorrells, M.E. Perspectives for genomic selection applications and research in plants. Crop Sci. 2015, 55, 1–12. [Google Scholar] [CrossRef]
- Collard, B.C.; Cruz, C.M.V.; McNally, K.L.; Virk, P.S.; Mackill, D.J. Rice molecular breeding laboratories in the genomics era: Current status and future considerations. Int. J. Plant Genom. 2008, 2008, 524847. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.; Prasad, P.V.; Siddique, K.H. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Schnurbusch, T. A genetic playground for enhancing grain number in cereals. Trends Plant Sci. 2012, 17, 91–101. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Ibrahim, A.K.; Zhang, L.; Niyitanga, S.; Afzal, M.Z.; Xu, Y.; Zhang, L.; Zhang, L.; Qi, J. Principles and approaches of association mapping in plant breeding. Trop. Plant Biol. 2020, 13, 212–224. [Google Scholar] [CrossRef]
- Kumawat, G.; Kumawat, C.K.; Chandra, K.; Pandey, S.; Chand, S.; Mishra, U.N.; Lenka, D.; Sharma, R. Insights into marker assisted selection and its applications in plant breeding. In Plant Breeding—Current and Future Views; Intechopen: London, UK, 2020. [Google Scholar]
- Hospital, F. Challenges for effective marker-assisted selection in plants. Genetica 2009, 136, 303–310. [Google Scholar] [CrossRef]
- Sanchez, A.; Brar, D.; Huang, N.; Li, Z.; Khush, G. Sequence tagged site marker-assisted selection for three bacterial blight resistance genes in rice. Crop Sci. 2000, 40, 792–797. [Google Scholar] [CrossRef]
- Xu, Y.; Crouch, J.H. Marker-assisted selection in plant breeding: From publications to practice. Crop Sci. 2008, 48, 391–407. [Google Scholar] [CrossRef]
- Bernardo, R. Molecular markers and selection for complex traits in plants: Learning from the last 20 years. Crop Sci. 2008, 48, 1649–1664. [Google Scholar] [CrossRef]
- Jeon, D.; Kang, Y.; Lee, S.; Choi, S.; Sung, Y.; Lee, T.-H.; Kim, C. Digitalizing breeding in plants: A new trend of next-generation breeding based on genomic prediction. Front. Plant Sci. 2023, 14, 1092584. [Google Scholar] [CrossRef] [PubMed]
- Ertiro, B.T.; Ogugo, V.; Worku, M.; Das, B.; Olsen, M.; Labuschagne, M.; Semagn, K. Comparison of Kompetitive Allele Specific PCR (KASP) and genotyping by sequencing (GBS) for quality control analysis in maize. BMC Genom. 2015, 16, 908. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Hasan, M.T.; Rahman, J. Kompetitive Allele-Specific PCR (KASP): An Efficient High-Throughput Genotyping Platform and Its Applications in Crop Variety Development. In Molecular Marker Techniques: A Potential Approach of Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2023; pp. 25–54. [Google Scholar]
- Yang, G.; Chen, S.; Chen, L.; Gao, W.; Huang, Y.; Huang, C.; Zhou, D.; Wang, J.; Liu, Y.; Huang, M. Development and utilization of functional KASP markers to improve rice eating and cooking quality through MAS breeding. Euphytica 2019, 215, 1–12. [Google Scholar] [CrossRef]
- Yang, G.; Chen, S.; Chen, L.; Sun, K.; Huang, C.; Zhou, D.; Huang, Y.; Wang, J.; Liu, Y.; Wang, H. Development of a core SNP arrays based on the KASP method for molecular breeding of rice. Rice 2019, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Cheon, K.-S.; Jeong, Y.-M.; Oh, H.; Oh, J.; Kang, D.-Y.; Kim, N.; Lee, E.; Baek, J.; Kim, S.L.; Choi, I. Development of 454 new Kompetitive Allele-Specific PCR (KASP) markers for temperate japonica rice varieties. Plants 2020, 9, 1531. [Google Scholar] [CrossRef] [PubMed]
- Gorjanc, G.; Cleveland, M.A.; Houston, R.D.; Hickey, J.M. Potential of genotyping-by-sequencing for genomic selection in livestock populations. Genet. Sel. Evol. 2015, 47, 12. [Google Scholar] [CrossRef] [PubMed]
- Jannink, J.-L.; Lorenz, A.J.; Iwata, H. Genomic selection in plant breeding: From theory to practice. Brief Funct Genom. 2010, 9, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Bhat, J.A.; Ali, S.; Salgotra, R.K.; Mir, Z.A.; Dutta, S.; Jadon, V.; Tyagi, A.; Mushtaq, M.; Jain, N.; Singh, P.K. Genomic selection in the era of next generation sequencing for complex traits in plant breeding. Front. Genet. 2016, 7, 221. [Google Scholar] [CrossRef]
- Budhlakoti, N.; Rai, A.; Mishra, D.C. Statistical approach for improving genomic prediction accuracy through efficient diagnostic measure of influential observation. Sci. Rep. 2020, 10, 8408. [Google Scholar] [CrossRef]
- Goddard, M. Genomic selection: Prediction of accuracy and maximisation of long term response. Genetics 2009, 136, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Hein, N.T.; Ciampitti, I.A.; Jagadish, S.K. Bottlenecks and opportunities in field-based high-throughput phenotyping for heat and drought stress. J. Exp. Bot. 2021, 72, 5102–5116. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Duan, B.; Luo, X.; Ma, Y.; Yuan, Z.; Zhu, R.; Peng, Y.; Gong, Y.; Fang, S.; Wu, X. Identification of high nitrogen use efficiency phenotype in rice (Oryza sativa L.) through entire growth duration by unmanned aerial vehicle multispectral imagery. Front. Plant Sci. 2021, 12, 740414. [Google Scholar] [CrossRef]
- Song, P.; Wang, J.; Guo, X.; Yang, W.; Zhao, C. High-throughput phenotyping: Breaking through the bottleneck in future crop breeding. Crop J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Dong, W.; Cao, X.; Wen, Z.; Xiao, R.; Wei, Y.; Zeng, H.; Ma, X. An automatic approach for detecting seedlings per hill of machine-transplanted hybrid rice utilizing machine vision. Comput. Electron. Agric. 2021, 185, 106178. [Google Scholar] [CrossRef]
- Calingacion, M.; Boualaphanh, C.; Daygon, V.; Anacleto, R.; Sackville Hamilton, R.; Biais, B.; Deborde, C.; Maucourt, M.; Moing, A.; Mumm, R. A genomics and multi-platform metabolomics approach to identify new traits of rice quality in traditional and improved varieties. Metabolomics 2012, 8, 771–783. [Google Scholar] [CrossRef]
- Rao, M.J.; Wang, L. CRISPR/Cas9 technology for improving agronomic traits and future prospective in agriculture. Planta 2021, 254, 68. [Google Scholar] [CrossRef]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for crop improvement: An update review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Patron, N.J.; Nekrasov, V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 2015, 32, 76–84. [Google Scholar] [CrossRef]
- Hodaei, A.; Werbrouck, S.P. Unlocking Nature’s Clock: CRISPR Technology in Flowering Time Engineering. Plants 2023, 12, 4020. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Higuchi, A.; Watanabe, A.; Tasaki, K. Application of the CRISPR/Cas9 system for modification of flower color in Torenia fournieri. BMC Plant Biol. 2018, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Bellec, Y.; Guyon-Debast, A.; François, T.; Gissot, L.; Biot, E.; Nogué, F.; Faure, J.-D.; Tepfer, M. New Flowering and Architecture Traits Mediated by Multiplex CRISPR-Cas9 Gene Editing in Hexaploid Camelina sativa. Agronomy 2022, 12, 1873. [Google Scholar] [CrossRef]
- Montecillo, J.A.V.; Chu, L.L.; Bae, H. CRISPR-Cas9 system for plant genome editing: Current approaches and emerging developments. Agronomy 2020, 10, 1033. [Google Scholar] [CrossRef]
- Romero, F.M.; Gatica-Arias, A. CRISPR/Cas9: Development and application in rice breeding. Rice Sci. 2019, 26, 265–281. [Google Scholar] [CrossRef]
- Zegeye, W.A.; Tsegaw, M.; Zhang, Y.; Cao, L. CRISPR-based genome editing: Advancements and opportunities for rice improvement. Int. J. Mol. Sci. 2022, 23, 4454. [Google Scholar] [CrossRef]
- Son, S.; Park, S.R. Challenges facing CRISPR/Cas9-based genome editing in plants. Front. Plant Sci. 2022, 13, 902413. [Google Scholar] [CrossRef] [PubMed]

| Hybrid Rice Cultivars | Developing Organization | Country | Reference |
|---|---|---|---|
| 117 rice hybrid varieties | Public and private sector | Brazil, the United States, Egypt, India, Bangladesh, China, Vietnam, the Philippines, Indonesia, Myanmar, and Sri Lanka | [29] |
| Two-line, three-line, and super-hybrid rice | IRRI, China, and India | China | [6] |
| Local rice hybrids | The Global Rice Research Institute and Egypt’s rice research program | Egypt | [30] |
| 50 high-yielding hybrid lines | African programme for rice | Several countries in Africa | [31] |
| Three indica hybrid rice varieties | China National seed group Co., Ltd. | China | [32] |
| Transgenic Rice | Functions | Reference |
|---|---|---|
| Bt rice | Insect resistance | [118] |
| Golden rice | Increased provitamin A content | [119] |
| Sub1 rice | Flooding tolerance | [120] |
| C4 rice | Increased photosynthetic efficiency and yield | [121] |
| Salt-tolerance rice varieties | Tolerance towards high-salinity environment | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, H.; Ghouri, F.; Baloch, F.S.; Nadeem, M.A.; Fu, X.; Shahid, M.Q. Hybrid Rice Production: A Worldwide Review of Floral Traits and Breeding Technology, with Special Emphasis on China. Plants 2024, 13, 578. https://doi.org/10.3390/plants13050578
Ashraf H, Ghouri F, Baloch FS, Nadeem MA, Fu X, Shahid MQ. Hybrid Rice Production: A Worldwide Review of Floral Traits and Breeding Technology, with Special Emphasis on China. Plants. 2024; 13(5):578. https://doi.org/10.3390/plants13050578
Chicago/Turabian StyleAshraf, Humera, Fozia Ghouri, Faheem Shehzad Baloch, Muhammad Azhar Nadeem, Xuelin Fu, and Muhammad Qasim Shahid. 2024. "Hybrid Rice Production: A Worldwide Review of Floral Traits and Breeding Technology, with Special Emphasis on China" Plants 13, no. 5: 578. https://doi.org/10.3390/plants13050578
APA StyleAshraf, H., Ghouri, F., Baloch, F. S., Nadeem, M. A., Fu, X., & Shahid, M. Q. (2024). Hybrid Rice Production: A Worldwide Review of Floral Traits and Breeding Technology, with Special Emphasis on China. Plants, 13(5), 578. https://doi.org/10.3390/plants13050578









