Citrus limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Citrus limon Essential Oil (CLEO)
2.2. Antimicrobial Activity of CLEO In Vitro
2.3. In Situ Antimicrobial Activity of CLEO in Vapour Phase
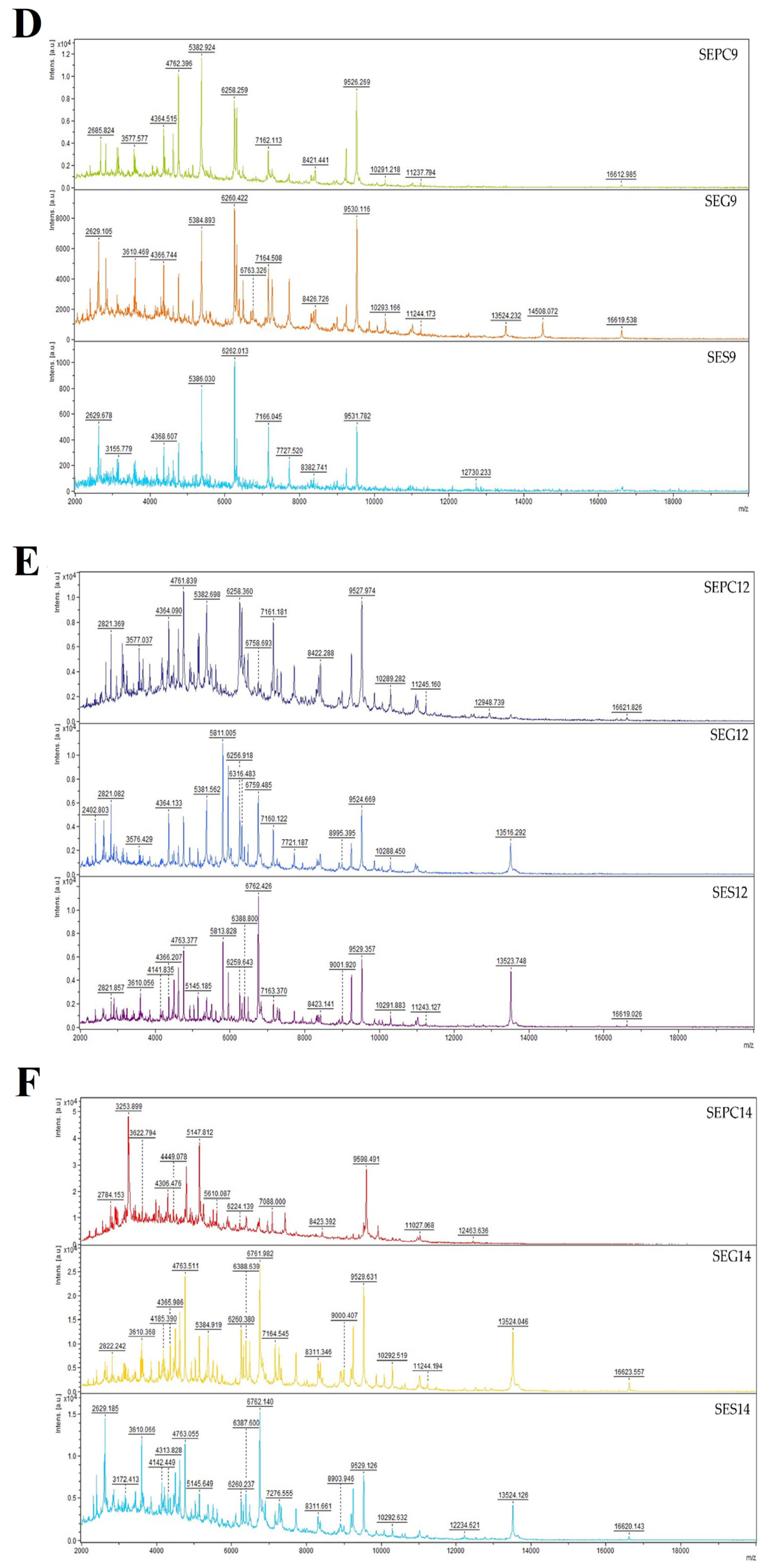
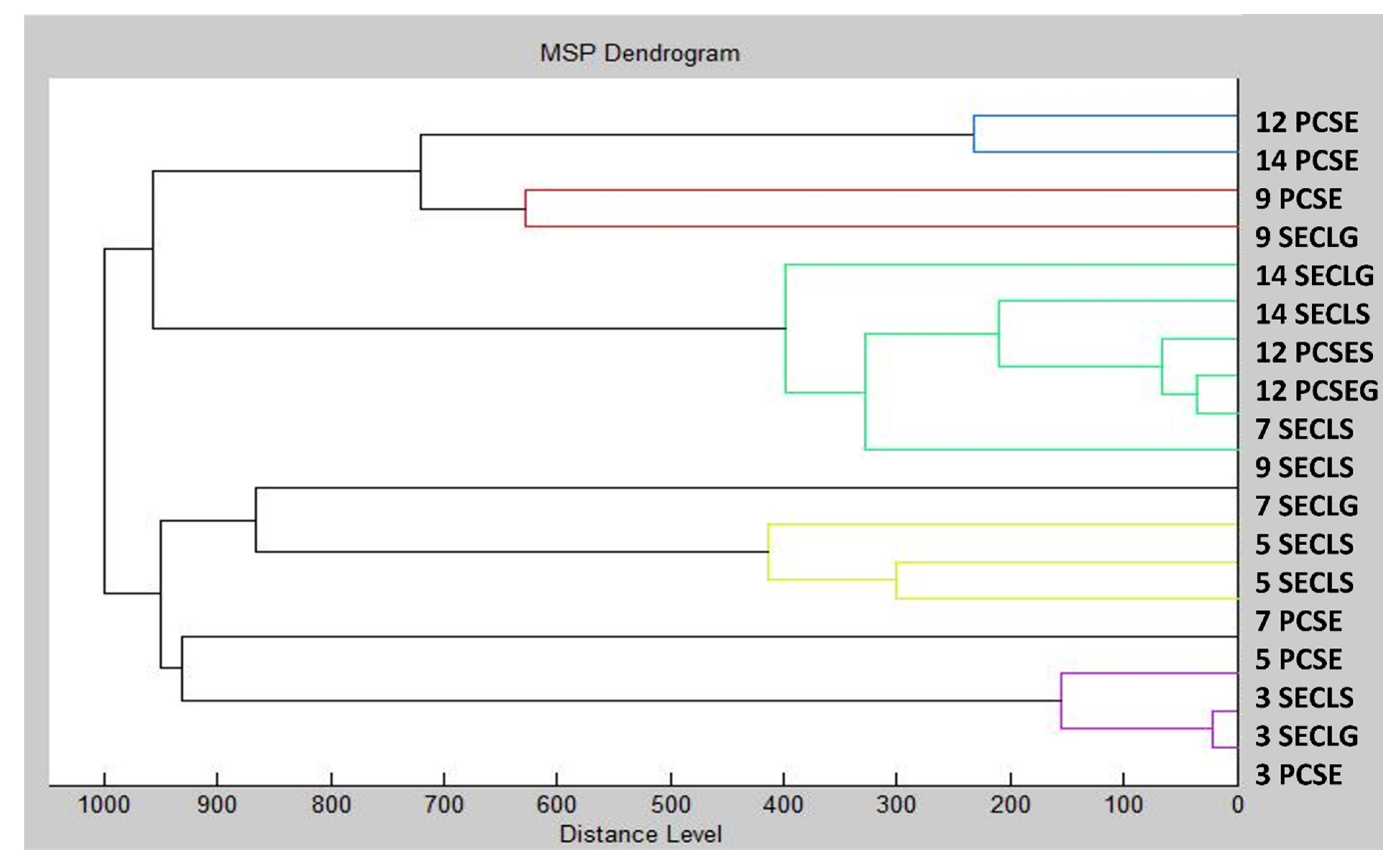
2.4. Antibiofilm Activity of CLEO against Biofilm Forming S. enterica
2.5. Insecticidal Activity of CLEO
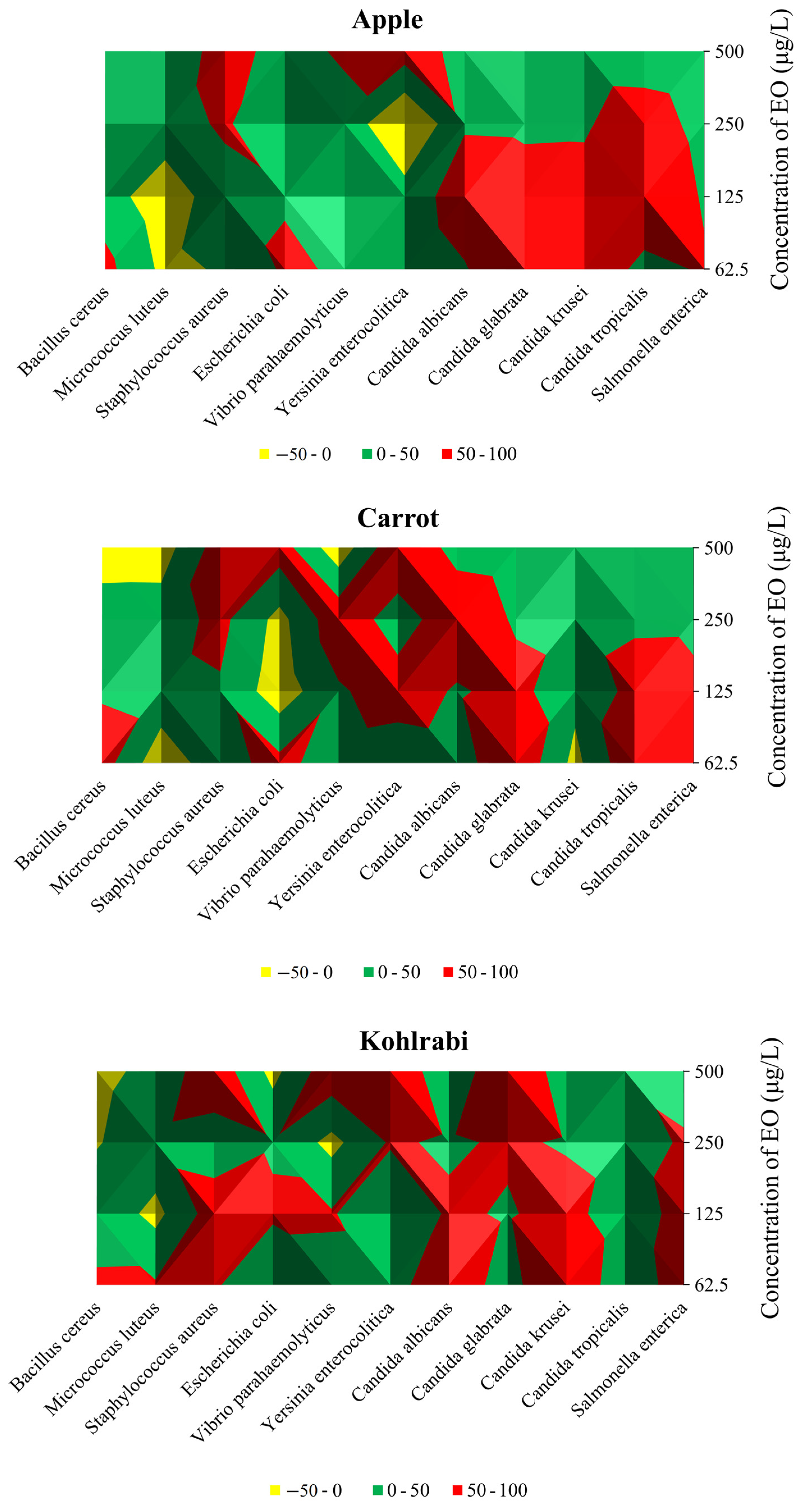
2.6. Microbiological Analyses of Carrot in Sous Vide Application with S. enterica and CLEO
3. Materials and Methods
3.1. Citrus limon Essential Oil
3.2. GC and GC/MS Chemical Analysis of CLEO Sample
3.3. Antimicrobial Assay
3.3.1. Tested Microorganisms
3.3.2. Disc Diffusion Method
3.3.3. Minimal Inhibition Concentration
3.4. In Situ Analyses on the Fruit and Vegetables
3.5. Biofilm Development Study
3.5.1. Crystal Violet Assay
3.5.2. Biofilm Formation Detection by MALDI-TOF MS Biotyper
3.6. Insecticidal Activity
3.7. Sous Vide Vegetable Analyses
3.7.1. Sample Preparation
- Control: Fresh carrot sample was treated at 50–65 °C for 5 to 25 min after being packed in polyethylene bags and kept at 4 °C.
- Control + vacuum: Fresh carrot sample was treated at 50–65 °C for 5 to 25 min after being vacuum-packed in polyethylene bags and kept at 4 °C.
- EO: vacuum-packed fresh carrot treated with 1% CLEO was kept at 4 °C and treated for 5–25 min at 50–65 °C.
- Salmonella: vacuum-packed fresh carrot treated with S. enterica was kept at 4 °C and treated for 5–25 min at 50–65 °C.
- Salmonella + EO: vacuum-packed fresh carrot treated with S. enterica and 1% CLEO was kept at 4 °C and treated for 5–25 min at 50–65 °C.
3.7.2. Microbiological Analyses
3.7.3. Identification of Bacteria
3.8. Statistically Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mabberley, D.J. Citrus (Rutaceae): A Review of Recent Advances in Etymology, Systematics and Medical Applications. Blumea Biodivers. Evol. Biogeogr. Plants 2004, 49, 481–498. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef]
- Robards, K.; Antolovich, M. Analytical Chemistry of Fruit BioflavonoidsA Review. Analyst 1997, 122, 11R–34R. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Kaskoos, R.A. Essential Oil Analysis by GC-MS and Analgesic Activity of Lippia citriodora and Citrus limon. J. Essent. Oil Bear. Plants 2019, 22, 273–281. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Costa, R.; Trozzi, A.; Dugo, P.; Mondello, L. Reduced Time HPLC Analyses for Fast Quality Control of Citrus Essential Oils. J. Essent. Oil Res. 2015, 27, 307–315. [Google Scholar] [CrossRef]
- Ghoorchibeigi, M.; Larijani, K.; Aberoomand Azar, P.; Zare, K.; Mehregan, I. Chemical Composition and Radical Scavenging Activity of Citrus Limon Peel Essential Oil. Orient. J. Chem. 2017, 33, 458–461. [Google Scholar] [CrossRef]
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Guarrasi, V.; Vargas, M.; Germanà, M.A.; Moschetti, G. Antilisterial Effect of Citrus Essential Oils and Their Performance in Edible Film Formulations. Food Control 2016, 59, 750–758. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Allen, S.C.H.; Phillips, C.A. The Anti-Biofilm Activity of Lemongrass (Cymbopogon flexuosus) and Grapefruit (Citrus paradisi) Essential Oils against Five Strains of Staphylococcus aureus. J. Appl. Microbiol. 2012, 113, 1217–1227. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Cartagena, E.; Bardón, A.; Arena, M.E. Mandarin Essential Oils Inhibit Quorum Sensing and Virulence Factors of Pseudomonas aeruginosa. LWT Food Sci. Technol. 2016, 68, 373–380. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Mah, T.-F. Biofilm-Specific Antibiotic Resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Cheng, P.; Xiong, J.; Li, H.; Wang, S.; Zhang, Y.; Mei, C.; Wu, X.; He, Y.; Chen, H. Using Plant Extracts and Their Active Ingredients to Inhibit Bacterial Biofilms. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2022, 38, 1753–1767. [Google Scholar]
- Kalbassi, S.; Yarahmadi, M.; Mohammadifard, H.; Ahmadi, F. The Antibiofilm and Antibacterial Effects of Medicinal Plant Extracts on Isolated Sulfate-Reducing Bacteria from Orthodontic Appliances. Food Sci. Technol. 2022, 42, e38322. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing Natural Products as Potential Anti-Biofilm Agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Silva, E.; Teixeira, J.A.; Pereira, M.O.; Rocha, C.M.R.; Sousa, A.M. Evolving Biofilm Inhibition and Eradication in Clinical Settings through Plant-Based Antibiofilm Agents. Phytomedicine 2023, 119, 154973. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef]
- Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon By-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsi, F. Novel Edible Coating Containing Essential Oil Nanoemulsions to Prolong the Shelf Life of Vegetable Products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar] [CrossRef]
- Perdones, Á.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of Chitosan–Lemon Essential Oil Coatings on Volatile Profile of Strawberries during Storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Olumuyiwa, T.A.; Olasehinde, T.A.; Ademiluyi, A.O.; Adeyemo, A.C. Insecticidal Activity of Essential Oil from Orange Peels (Citrus sinensis) against Tribolium confusum, Callosobruchus maculatus and Sitophilus oryzae and Its Inhibitory Effects on Acetylcholinesterase and Na+/K+-ATPase Activities. Phytoparasitica 2017, 45, 501–508. [Google Scholar] [CrossRef]
- Pourbafrani, M.; McKechnie, J.; MacLean, H.L.; Saville, B.A. Life Cycle Greenhouse Gas Impacts of Ethanol, Biomethane and Limonene Production from Citrus Waste. Environ. Res. Lett. 2013, 8, 015007. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Bonaccorsi, I.; Dugo, P.; Mondello, L.; Dugo, G. Analysis of Citrus Essential Oils: State of the Art and Future Perspectives. A Review. Flavour Fragr. J. 2012, 27, 98–123. [Google Scholar] [CrossRef]
- Suwannayod, S.; Sukontason, K.L.; Somboon, P.; Junkum, A.; Leksomboon, R.; Chaiwong, T.; Jones, M.K.; Sripa, B.; Balthaisong, S.; Phuyao, C. Activity of Kaffirlime (Citrus hystrix) Essential Oil against Blow Flies and House Fly. Southeast Asian J. Trop. Med. Public Health 2018, 49, 32–45. [Google Scholar]
- Abad, M.K.R.; Besheli, B.A. Insecticidal Potential of Essential Oil from the Leaves of Citrus aurantium L. against Oryzaephilus surinamensis (F.), Lasioderma serricorne (L.) and Sitophilus oryzae (L.). J. Entomol. Zool. Stud. 2016, 4, 865–869. [Google Scholar]
- Sarma, R.; Khanikor, B.; Mahanta, S. Essential Oil from Citrus Grandis (Sapindales: Rutaceae) as Insecticide against Aedes aegypti (L.) (Diptera: Culicidae). Int. J. Mosq. Res. 2017, 4, 88–92. [Google Scholar]
- Ruiz, J.; Calvarro, J.; Sánchez Del Pulgar, J.; Roldán, M. Science and Technology for New Culinary Techniques. J. Culin. Sci. Technol. 2013, 11, 66–79. [Google Scholar] [CrossRef]
- Kilibarda, N.; Brdar, I.; Baltic, B.; Markovic, V.; Mahmutovic, H.; Karabasil, N.; Stanisic, S. The Safety and Quality of Sous Vide Food. Meat Technol. 2018, 59, 38–45. [Google Scholar] [CrossRef]
- Zavadlav, S.; Blažić, M.; Van De Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus Lemon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect against Listeria Monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Saïdani Tounsi, M. Changes of Peel Essential Oil Composition of Four Tunisian Citrus during Fruit Maturation. Sci. World J. 2012, 2012, 528593. [Google Scholar] [CrossRef]
- Golmakani, M.; Moayyedi, M. Comparison of Heat and Mass Transfer of Different Microwave-assisted Extraction Methods of Essential Oil from Citrus Limon (Lisbon Variety) Peel. Food Sci. Nutr. 2015, 3, 506–518. [Google Scholar] [CrossRef]
- Himed, L.; Merniz, S.; Monteagudo-Olivan, R.; Barkat, M.; Coronas, J. Antioxidant Activity of the Essential Oil of Citrus limon before and after Its Encapsulation in Amorphous SiO2. Sci. Afr. 2019, 6, e00181. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Zhao, L.; Li, S.; Zhou, Z. Citrus Lemon (Citrus limon (L.) Burm. f. Cv. Eureka) Essential Oil Controls Blue Mold in Citrus by Damaging the Cell Membrane of Penicillium italicum. LWT 2023, 188, 115456. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical Composition of Commercial Citrus Fruit Essential Oils and Evaluation of Their Antimicrobial Activity Acting Alone or in Combined Processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Nannapaneni, R.; Muthaiyan, A.; Crandall, P.G.; Johnson, M.G.; O’Bryan, C.A.; Chalova, V.I.; Callaway, T.R.; Carroll, J.A.; Arthington, J.D.; Nisbet, D.J.; et al. Antimicrobial Activity of Commercial Citrus-Based Natural Extracts Against Escherichia coli O157:H7 Isolates and Mutant Strains. Foodborne Pathog. Dis. 2008, 5, 695–699. [Google Scholar] [CrossRef]
- Yazgan, H.; Ozogul, Y.; Kuley, E. Antimicrobial Influence of Nanoemulsified Lemon Essential Oil and Pure Lemon Essential Oil on Food-Borne Pathogens and Fish Spoilage Bacteria. Int. J. Food Microbiol. 2019, 306, 108266. [Google Scholar] [CrossRef]
- Kehal, F.; Chemache, L.; Himed, L.; Barkat, M. Antimicrobial and Antioxidant Activities of Citrus limon Peel Essential Oils and Their Application as a Natural Preservative in Fresh Cream: Effects on Oxidative and Sensory Properties. Acta Univ. Cibiniensis Ser. E Food Technol. 2023, 27, 1–14. [Google Scholar] [CrossRef]
- Hayes, A.J.; Markovic, B. Toxicity of Australian Essential Oil Backhousia Citriodora (Lemon myrtle). Part 1. Antimicrobial Activity and in vitro Cytotoxicity. Food Chem. Toxicol. 2002, 40, 535–543. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial Activity of Plant Essential Oils Using Food Model Media: Efficacy, Synergistic Potential and Interactions with Food Components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef]
- Hojjati, M.; Barzegar, H. Chemical Composition and Biological Activities of Lemon (Citrus limon) Leaf Essential Oil. Nutr. Food Sci. Res. 2017, 4, 15–24. [Google Scholar] [CrossRef]
- Yi, F.; Jin, R.; Sun, J.; Ma, B.; Bao, X. Evaluation of Mechanical-Pressed Essential Oil from Nanfeng Mandarin (Citrus reticulata Blanco Cv. Kinokuni) as a Food Preservative Based on Antimicrobial and Antioxidant Activities. LWT 2018, 95, 346–353. [Google Scholar] [CrossRef]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.; Tian, G.; Ren, W.; Li, J.; Xiao, H.; Zheng, J. Effects of Molecular Distillation on the Chemical Components, Cleaning, and Antibacterial Abilities of Four Different Citrus Oils. Front. Nutr. 2021, 8, 731724. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal Activity of Lemon (Citrus lemon L.), Mandarin (Citrus reticulata L.), Grapefruit (Citrus paradisi L.) and Orange (Citrus sinensis L.) Essential Oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; De Alencar, S.M.; De Sousa, R.L.M.; Moreno, A.M.; Da Gloria, E.M. Antimicrobial Activity of Several Essential Oils on Pathogenic and Beneficial Bacteria. Ind. Crops Prod. 2017, 97, 128–136. [Google Scholar] [CrossRef]
- Rajapaksha, L.; Gunathilake, D.C.; Pathirana, S.; Fernando, T. Reducing Post-Harvest Losses in Fruits and Vegetables for Ensuring Food Security–Case of Sri Lanka. MOJ Food Process. Technol. 2021, 9, 7–16. [Google Scholar] [CrossRef]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Solid- and Vapor-Phase Antimicrobial Activities of Six Essential Oils: Susceptibility of Selected Foodborne Bacterial and Fungal Strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coïsson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical Composition, Plant Genetic Differences, Antimicrobial and Antifungal Activity Investigation of the Essential Oil of Rosmarinus officinalis L. J. Agric. Food Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial Activity of Essential Oil Extracts of Various Onions (Allium cepa) and Garlic (Allium sativum). LWT Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in Shelf-Life and Safety of Perishable Foods by Plant Essential Oils and Smoke Antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Bajpai, R.; Prokop, A.; Zappi, M. Algal Biorefineries: Volume 1. Cultivation of Cells and Products; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Cakir, A.; Kordali, S.; Zengin, H.; Izumi, S.; Hirata, T. Composition and Antifungal Activity of Essential Oils Isolated from Hypericum hyssopifolium and Hypericum heterophyllum. Flavour Fragr. J. 2004, 19, 62–68. [Google Scholar] [CrossRef]
- Bosquez-Molina, E.; Jesús, E.R.; Bautista-Baños, S.; Verde-Calvo, J.R.; Morales-López, J. Inhibitory Effect of Essential Oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in Stored Papaya Fruit and Their Possible Application in Coatings. Postharvest Biol. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Mehdi, M.; Asgar, A.; Alderson, P.G. Effect of Cinnamon Oil on Incidence of Anthracnose Disease and Postharvest Quality of Bananas during Storage. Int. J. Agric. Biol. 2010, 12, 516–520. [Google Scholar]
- Singh, P.; Pandey, A.K.; Sonker, N.; Tripathi, N. Preservation of Buchnania Lanzan Spreng. Seeds by Ocimum canum Sims. Essential Oil. Ann. Plant Prot. Sci. 2011, 19, 407–410. [Google Scholar]
- Pandey, A.K.; Palni, U.T.; Tripathi, N.N. Repellent Activity of Some Essential Oils against Two Stored Product Beetles Callosobruchus chinensis L. and C. maculatus F. (Coleoptera: Bruchidae) with Reference to Chenopodium ambrosioides L. Oil for the Safety of Pigeon Pea Seeds. J. Food Sci. Technol. 2014, 51, 4066–4071. [Google Scholar] [CrossRef]
- Pandey, A.K.; Palni, U.T.; Tripathi, N.N. Evaluation of Clausena pentaphylla (Roxb.) DC Oil as a Fungitoxicant against Storage Mycoflora of Pigeon Pea Seeds. J. Sci. Food Agric. 2013, 93, 1680–1686. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Palni, U.T.; Tripathi, N.N. In Vivo Evaluation of Two Essential Oil Based Botanical Formulations (EOBBFs) for the Use against Stored Product Pathogens and Pests, Aspergillus Species and Callosobruchus Species (Coleoptera: Bruchidae). J. Stored Prod. Res. 2014, 59, 285–291. [Google Scholar] [CrossRef]
- Sonker, N.; Pandey, A.K.; Singh, P. Efficiency of Artemisia nilagirica (Clarke) Pamp. Essential Oil as a Mycotoxicant against Postharvest Mycobiota of Table Grapes. J. Sci. Food Agric. 2015, 95, 1932–1939. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sonker, N.; Singh, P. Efficacy of Some Essential Oils Against Aspergillus flavus with Special Reference to Lippia alba Oil an Inhibitor of Fungal Proliferation and Aflatoxin B 1 Production in Green Gram Seeds during Storage. J. Food Sci. 2016, 81, M928–M934. [Google Scholar] [CrossRef]
- Zubair, M. Antimicrobial and Anti-Biofilm Activities of Citrus sinensis and Moringa oleifera Against the Pathogenic Pseudomonas aeruginosa and Staphylococcus aureus. Cureus 2020, 12, e12337. [Google Scholar] [CrossRef]
- Zygadlo, J.A.; Zunino, M.P.; Pizzolitto, R.P.; Merlo, C.; Omarini, A.; Dambolena, J.S. Antibacterial and Anti-Biofilm Activities of Essential Oils and Their Components Including Modes of Action. In Essential Oils and Nanotechnology for Treatment of Microbial Diseases; Rai, M., Zacchino, S., Derita, M.G., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 99–126. ISBN 978-1-315-20924-1. [Google Scholar]
- Sun, Y.; Chen, S.; Zhang, C.; Liu, Y.; Ma, L.; Zhang, X. Effects of Sub-Minimum Inhibitory Concentrations of Lemon Essential Oil on the Acid Tolerance and Biofilm Formation of Streptococcus Mutans. Arch. Oral Biol. 2018, 87, 235–241. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Grapefruit Essential Oils Inhibit Quorum Sensing of Pseudomonas aeruginosa. Food Sci. Technol. Int. 2020, 26, 231–241. [Google Scholar] [CrossRef]
- Oliveira, S.A.C.; Zambrana, J.R.M.; Di Iorio, F.B.R.; Pereira, C.A.; Jorge, A.O.C. The Antimicrobial Effects of Citrus limonum and Citrus aurantium Essential Oils on Multi-Species Biofilms. Braz. Oral Res. 2014, 28, 22–27. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Lemon Oils Attenuate the Pathogenicity of Pseudomonas aeruginosa by Quorum Sensing Inhibition. Molecules 2021, 26, 2863. [Google Scholar] [CrossRef]
- Ahmedi, S.; Pant, P.; Raj, N.; Manzoor, N. Limonene Inhibits Virulence Associated Traits in Candida albicans: In-vitro and in-silico Studies. Phytomed. Plus 2022, 2, 100285. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of Limonene as an Antimicrobial Agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Sathiya Deepika, M.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined Effect of a Natural Flavonoid Rutin from Citrus Sinensis and Conventional Antibiotic Gentamicin on Pseudomonas aeruginosa Biofilm Formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Galovičová, L.; Ivanišová, E.; Štefániková, J.; Valková, V.; Borotová, P.; Kowalczewski, P.Ł.; Kunová, S.; Felšöciová, S.; et al. Biological Activity and Antibiofilm Molecular Profile of Citrus aurantium Essential Oil and Its Application in a Food Model. Molecules 2020, 25, 3956. [Google Scholar] [CrossRef]
- Kristensen, M.; Knorr, M.; Spencer, A.G.; Jespersen, J.B. Selection and Reversion of Azamethiphos-Resistance in a Field Population of the Housefly Musca domestica (Diptera: Muscidae), and the Underlying Biochemical Mechanisms. J. Econ. Entomol. 2000, 93, 1788–1795. [Google Scholar] [CrossRef]
- Kristensen, M.; Jespersen, J.B. Larvicide Resistance in Musca domestica (Diptera: Muscidae) Populations in Denmark and Establishment of Resistant Laboratory Strains. J. Econ. Entomol. 2003, 96, 1300–1306. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal Evaluation of Essential Oils of Citrus sinensis L. (Myrtales: Myrtaceae) against Housefly, Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 2012, 110, 1929–1936. [Google Scholar] [CrossRef]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Insecticidal Activities of Citrus aurantifolia Essential Oil against Aedes aegypti (Diptera: Culicidae). Toxicol. Rep. 2019, 6, 1091–1096. [Google Scholar] [CrossRef]
- Jain, P.; Satapathy, T.; Pandey, R.K. Acaricidal Activity and Biochemical Analysis of Citrus limetta Seed Oil for Controlling Ixodid Tick Rhipicephalus Microplus Infesting Cattle. Syst. Appl. Acarol. 2021, 26, 1350–1360. [Google Scholar] [CrossRef]
- Palazzolo, E.; Armando Laudicina, V.; Germanà, A.M. Current and Potential Use of Citrus Essential Oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Guillén, S.; Mir-Bel, J.; Oria, R.; Salvador, M.L. Influence of Cooking Conditions on Organoleptic and Health-Related Properties of Artichokes, Green Beans, Broccoli and Carrots. Food Chem. 2017, 217, 209–216. [Google Scholar] [CrossRef]
- Iborra-Bernad, C.; Tárrega, A.; García-Segovia, P.; Martínez-Monzó, J. Advantages of Sous-Vide Cooked Red Cabbage: Structural, Nutritional and Sensory Aspects. LWT Food Sci. Technol. 2014, 56, 451–460. [Google Scholar] [CrossRef]
- Kosewski, G.; Górna, I.; Bolesławska, I.; Kowalówka, M.; Więckowska, B.; Główka, A.K.; Morawska, A.; Jakubowski, K.; Dobrzyńska, M.; Miszczuk, P.; et al. Comparison of Antioxidative Properties of Raw Vegetables and Thermally Processed Ones Using the Conventional and Sous-Vide Methods. Food Chem. 2018, 240, 1092–1096. [Google Scholar] [CrossRef]
- Sila, D.N.; Smout, C.; Elliot, F.; Loey, A.V.; Hendrickx, M. Non-enzymatic Depolymerization of Carrot Pectin: Toward a Better Understanding of Carrot Texture During Thermal Processing. J. Food Sci. 2006, 71, E1–E9. [Google Scholar] [CrossRef]
- Rude, R.A.; Jackson, G.J.; Bier, J.W.; Sawyer, T.K.; Risty, N.G. Survey of Fresh Vegetables for Nematodes, Amoebae, and Salmonella. J. AOAC Int. 1984, 67, 613–615. [Google Scholar] [CrossRef]
- Mattson, T.E.; Johny, A.K.; Amalaradjou, M.A.R.; More, K.; Schreiber, D.T.; Patel, J.; Venkitanarayanan, K. Inactivation of Salmonella Spp. on Tomatoes by Plant Molecules. Int. J. Food Microbiol. 2011, 144, 464–468. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.-J.E. Development and Evaluation of a Model Predicting the Survival of Escherichia coli O157:H7 NCTC 12900 in Homemade Eggplant Salad at Various Temperatures, pHs, and Oregano Essential Oil Concentrations. Appl. Environ. Microbiol. 2000, 66, 1646–1653. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.K.; Bhunia, A.K.; Stroshine, R.L. Efficacy of Chlorine Dioxide, Ozone, and Thyme Essential Oil or a Sequential Washing in Killing Escherichia coli O157:H7 on Lettuce and Baby Carrots. LWT 2002, 35, 720–729. [Google Scholar] [CrossRef]
- Weissinger, W.R.; Mcwatters, K.H.; Beuchat, L.R. Evaluation of Volatile Chemical Treatments for Lethality to Salmonella on Alfalfa Seeds and Sprouts. J. Food Prot. 2001, 64, 442–450. [Google Scholar] [CrossRef]
- Gündüz, G.T.; Gönül, Ş.A.; Karapinar, M. Efficacy of Myrtle Oil against Salmonella typhimurium on Fresh Produce. Int. J. Food Microbiol. 2009, 130, 147–150. [Google Scholar] [CrossRef]
- Roller, S.; Seedhar, P. Carvacrol and Cinnamic Acid Inhibit Microbial Growth in Fresh-Cut Melon and Kiwifruit at 4° and 8° C. Lett. Appl. Microbiol. 2002, 35, 390–394. [Google Scholar] [CrossRef]
- Gündüz, G.T.; Gönül, Ş.A.; Karapınar, M. Efficacy of Oregano Oil in the Inactivation of Salmonella typhimurium on Lettuce. Food Control 2010, 21, 513–517. [Google Scholar] [CrossRef]
- Gündüz, G.T.; Gönül, Ş.A.; Karapinar, M. Efficacy of Sumac and Oregano in the Inactivation of Salmonella typhimurium on Tomatoes. Int. J. Food Microbiol. 2010, 141, 39–44. [Google Scholar] [CrossRef]
- Rinaldi, M.; Caligiani, A.; Borgese, R.; Palla, G.; Barbanti, D.; Massini, R. The Effect of Fruit Processing and Enzymatic Treatments on Pomegranate Juice Composition, Antioxidant Activity and Polyphenols Content. LWT Food Sci. Technol. 2013, 53, 355–359. [Google Scholar] [CrossRef]
- Sebastiá, C.; Soriano, J.M.; Iranzo, M.; Rico, H. Microbiological Quality Of Sous Vide Cook-Chill Preserved Food at Different Shelf Life: Microbiological Quality Of Sous Vide Cook-Chill Preserved Food. J. Food Process. Preserv. 2010, 34, 964–974. [Google Scholar] [CrossRef]
- Azi, F.; Odo, M.O.; Okorie, P.A.; Njoku, H.A.; Nwobasi, V.N.; David, E.; Onu, T.C. Heavy Metal and Microbial Safety Assessment of Raw and Cooked Pumpkin and Amaranthus Viridis Leaves Grown in Abakaliki, Nigeria. Food Sci. Nutr. 2018, 6, 1537–1544. [Google Scholar] [CrossRef]
- Balali, G.I.; Yar, D.D.; Afua Dela, V.G.; Adjei-Kusi, P. Microbial Contamination, an Increasing Threat to the Consumption of Fresh Fruits and Vegetables in Today’s World. Int. J. Microbiol. 2020, 2020, 3029295. [Google Scholar] [CrossRef]
- Hyytiä-Trees, E.; Skyttä, E.; Mokkila, M.; Kinnunen, A.; Lindström, M.; Lähteenmäki, L.; Ahvenainen, R.; Korkeala, H. Safety Evaluation of Sous Vide-Processed Products with Respect to Nonproteolytic Clostridium botulinum by Use of Challenge Studies and Predictive Microbiological Models. Appl. Environ. Microbiol. 2000, 66, 223–229. [Google Scholar] [CrossRef]
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative Hurdle Technologies for the Preservation of Functional Fruit Juices. Foods 2020, 9, 699. [Google Scholar] [CrossRef]
- Rizzo, V.; Amoroso, L.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Restuccia, C.; Lombardo, S.; Pandino, G.; Strano, M.G.; Mauromicale, G. The Effect of Sous Vide Packaging with Rosemary Essential Oil on Storage Quality of Fresh-Cut Potato. LWT 2018, 94, 111–118. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Liu, X.-L. Chemical Composition and Antimicrobial Activity of the Essential Oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Adams, D.H. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Gut 2007, 56, 1175. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2023, 24, 5179. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, Antimicrobial and Antibiofilm Activity of Coriander (Coriandrum Sativum L.) Essential Oil for Its Application in Foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Valková, V.; Ďuranová, H.; Borotová, P.; Štefániková, J.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Felsöciová, S.; et al. Chemical Composition and Biological Activity of Salvia Officinalis Essential Oil. Acta Hortic. Regiotect. 2021, 24, 81–88. [Google Scholar] [CrossRef]
- Kačániová, M.; Čmiková, N.; Kluz, M.I.; Akacha, B.B.; Saad, R.B.; Mnif, W.; Waszkiewicz-Robak, B.; Garzoli, S.; Hsouna, A.B. Anti-Salmonella Activity of Thymus serpyllum Essential Oil in Sous Vide Cook–Chill Rabbit Meat. Foods 2024, 13, 200. [Google Scholar] [CrossRef]
- Sokołowicz, Z.; Augustyńska-Prejsnar, A.; Krawczyk, J.; Kačániová, M.; Kluz, M.; Hanus, P.; Topczewska, J. Technological and Sensory Quality and Microbiological Safety of RIR Chicken Breast Meat Marinated with Fermented Milk Products. Animals 2021, 11, 3282. [Google Scholar] [CrossRef]




| No | RI (Lit) a | RI (Cal) b | Compound c | % d |
|---|---|---|---|---|
| non-Terpenic Compounds | 0.1 | |||
| alkanes | tr e | |||
| 1 | 1400 | 1400 | n-tetradecane | tr |
| alcohols | tr | |||
| 2 | 1068 | 1077 | n-octanol | tr |
| 3 | 1169 | 1176 | n-nonanol | tr |
| aldehydes | 0.1 | |||
| 4 | 998 | 1004 | n-octanal | tr |
| 5 | 1100 | 1106 | n-nonanal | 0.1 |
| 6 | 1201 | 1206 | n-decanal | tr |
| 7 | 1306 | 1309 | n-undecanal | tr |
| esters | tr | |||
| 8 | 1312 | 1311 | n-nonyl acetate | tr |
| monoterpenes | 97.8 | |||
| monoterpene hydrocarbons | 92.4 | |||
| 9 | 930 | 927 | α-thujene | 0.5 |
| 10 | 939 | 935 | α-pinene | 2.6 |
| 11 | 954 | 951 | camphene | 0.1 |
| 12 | 975 | 974 | sabinene | 2.4 |
| 13 | 979 | 980 | β-pinene | 12.6 |
| 14 | 990 | 989 | β-myrcene | 1.9 |
| 15 | 1002 | 1007 | α-phellandrene | tr |
| 16 | 1017 | 1019 | α-terpinene | 0.2 |
| 17 | 1024 | 1028 | p-cymene | 0.5 |
| 18 | 1029 | 1039 | limonene | 60.7 |
| 19 | 1050 | 1053 | (E)-β-ocimene | 0.1 |
| 20 | 1059 | 1066 | γ-terpinene | 10.3 |
| 21 | 1088 | 1088 | α-terpinolene | 0.5 |
| oxygenated monoterpenes | 5.4 | |||
| monoterpene alcohols | 0.9 | |||
| 22 | 1096 | 1100 | linalool | 0.2 |
| 23 | 1177 | 1183 | terpinen-4-ol | 0.1 |
| 24 | 1188 | 1195 | α-terpineol | 0.3 |
| 25 | 1229 | 1224 | nerol | 0.1 |
| 26 | 1252 | 1249 | geraniol | 0.2 |
| monoterpene aldehydes | 3.4 | |||
| 27 | 1153 | 1158 | citronellal | tr |
| 28 | 1238 | 1238 | neral | 1.2 |
| 29 | 1267 | 1267 | geranial | 2.2 |
| 30 | 1271 | 1274 | perilla aldehyde | tr |
| monoterpene ketones | tr | |||
| 31 | 1146 | 1151 | camphor | tr |
| 32 | 1243 | 1243 | carvone | tr |
| monoterpene epoxides | tr | |||
| 33 | 1136 | 1137 | cis-limonene oxide | tr |
| 34 | 1142 | 1142 | trans-limonene oxide | tr |
| monoterpene esters | 1.1 | |||
| 35 | 1352 | 1352 | citronellyl acetate | tr |
| 36 | 1361 | 1361 | neryl acetate | 0.6 |
| 37 | 1379 | 1380 | geranyl acetate | 0.5 |
| sesquiterpenes | 1.8 | |||
| sesquiterpene hydrocarbons | 1.8 | |||
| 38 | 1419 | 1420 | (E)-caryophyllene | 0.3 |
| 39 | 1434 | 1434 | α-trans-bergamotene | 0.6 |
| 40 | 1442 | 1438 | (Z)-β-farnesene | tr |
| 41 | 1456 | 1454 | (E)-β-farnesene | tr |
| 42 | 1496 | 1492 | valencene | tr |
| 43 | 1500 | 1495 | bicyclogermacrene | tr |
| 44 | 1505 | 1506 | β-bisabolene | 0.9 |
| 45 | 1507 | 1530 | (Z)-α-bisabolene | tr |
| total | 99.7 |
| Microorganism | Inhibition Zone | ATB * |
|---|---|---|
| G+ | ||
| Bacillus cereus CCM 2010 | 5.67 ± 0.58 bcd | 27.76 ± 0.47 |
| Micrococcus luteus CCM 732 | 7.67 ± 0.58 a | 29.33 ± 0.94 |
| Staphylococcus aureus CCM 3953 | 6.33 ± 0.58 abc | 30.33 ± 0.48 |
| G− | ||
| Escherichia coli CCM 3953 | 4.67 ± 0.58 cd | 29.67 ± 0.48 |
| Vibrio parahaemolyticus CCM 5937 | 5.00 ± 1.00 bcd | 30.33 ± 1.25 |
| Yersinia enterocolitica CCM 7204T | 4.67 ± 0.58 cd | 28.67 ± 0.48 |
| Yeasts | ||
| Candida albicans CCM 8186 | 6.33 ± 0.58 abc | 29.33 ± 0.48 |
| Candida glabrata CCM 8270 | 6.00 ± 1.00 abcd | 28.67 ± 0.47 |
| Candida krusei CCM 8271 | 6.67 ± 0.58 ab | 28.00 ± 0.82 |
| Candida tropicalis CCM 8223 | 6.33 ± 0.58 abc | 29.67 ± 0.94 |
| Biofilm forming bacteria (BFB) | ||
| Salmonella enterica | 4.33 ± 0.58 d | 30.33 ± 0.48 |
| Microorganism | MIC50 | MIC90 |
|---|---|---|
| G+ | ||
| Bacillus cereus CCM 2010 | 3.28 ± 0.17 d | 3.62 ± 0.17 d |
| Micrococcus luteus CCM 732 | 2.33 ± 0.39 de | 2.52 ± 0.43 de |
| Staphylococcus aureus CCM 3953 | 6.19 ± 0.25 c | 6.37 ± 0.28 c |
| G− | ||
| Escherichia coli CCM 3953 | 22.61 ± 1.05 a | 22.80 ± 1.10 a |
| Vibrio parahaemolyticus CCM 5937 | 6.23 ± 0.34 c | 6.35 ± 0.17 c |
| Yersinia enterocolitica CCM 7204T | 12.36 ± 0.52 b | 12.58 ± 0.54 b |
| Yeasts | ||
| Candida albicans CCM 8186 | 23.33 ± 0.51 a | 24.03 ± 0.79 a |
| Candida glabrata CCM 8270 | 3.33 ± 0.10 d | 3.48 ± 0.06 d |
| Candida krusei CCM 8271 | 12.09 ± 0.41 b | 12.28 ± 0.35 b |
| Candida tropicalis CCM 8223 | 6.30 ± 0.16 c | 6.41 ± 0.16 c |
| Biofilm forming bacteria (BFB) | ||
| Salmonella enterica | 1.37 ± 0.42 e | 1.47 ± 0.50 e |
| Food Model | Microorganisms | Concentration of EO (μg/L) | |||
|---|---|---|---|---|---|
| Apple | 62.5 | 125 | 250 | 500 | |
| G+ | Bacillus cereus | 65.17 ± 2.31 b | 23.88 ± 2.40 f | 43.62 ± 2.00 c | 34.72 ± 3.13 d |
| Micrococcus luteus | −13.46 ± 0.53 h | −33.17 ± 1.40 g | 33.12 ± 2.10 d | 25.00 ± 1.60 e | |
| Staphylococcus aureus | 6.26 ± 1.14 g | 34.76 ± 2.03 e | 55.68 ± 2.02 b | 65.41 ± 2.07 b | |
| G− | Escherichia coli | 57.32 ± 2.21 c | 46.35 ± 2.67 d | 12.07 ± 1.04 e | 35.54 ± 1.54 d |
| Vibrio parahaemolyticus | 44.08 ± 1.47 d | 23.31 ± 2.29 f | 34.80 ± 3.06 d | 55.91 ± 2.60 c | |
| Yersinia enterocolitica | 16.71 ± 2.30 f | 23.00 ± 1.17 f | −56.40 ± 2.26 f | 74.63 ± 1.59 a | |
| Yeast | Candida albicans | 46.57 ± 2.11 d | 75.40 ± 2.83 b | 45.44 ± 3.09 c | 35.19 ± 2.52 d |
| Candida glabrata | 95.54 ± 1.59 a | 64.91 ± 2.62 c | 44.20 ± 1.98 c | 26.11 ± 2.24 e | |
| Candida krusei | 94.81 ± 3.59 a | 64.17 ± 1.43 c | 45.17 ± 3.27 c | 26.78 ± 1.63 e | |
| Candida tropicalis | 33.77 ± 1.64 e | 95.67 ± 1.85 a | 75.17 ± 2.43 a | 24.47 ± 3.18 e | |
| BFB | Salmonella enterica | 55.39 ± 3.59 c | 45.17 ± 2.07 d | 34.21 ± 2.07 d | 15.11 ± 1.62 f |
| Carrot | |||||
| G+ | Bacillus cereus | 74.04 ± 1.66 b | 43.61 ± 1.51 de | 24.36 ± 1.69 e | −23.89 ± 2.37 e |
| Micrococcus luteus | −34.47 ± 1.51 h | 35.50 ± 3.11 e | 25.63 ± 2.76 e | −24.51 ± 2.42 ef | |
| Staphylococcus aureus | 34.80 ± 2.76 d | 44.93 ± 2.26 d | 63.59 ± 1.80 b | 75.90 ± 2.67 b | |
| G− | Escherichia coli | 63.88 ± 1.05 c | −28.57 ± 6.11 g | −15.87 ± 1.76 f | 75.02 ± 3.08 b |
| Vibrio parahaemolyticus | 25.82 ± 2.02 e | 43.83 ± 1.96 de | 86.34 ± 3.09 a | −31.91 ± 3.55 f | |
| Yersinia enterocolitica | 16.72 ± 1.95 f | 75.43 ± 2.93 a | 25.16 ± 3.30 e | 93.34 ± 1.62 a | |
| Yeast | Candida albicans | 26.23 ± 2.91 e | 45.10 ± 3.12 d | 84.85 ± 1.85 a | 33.81 ± 3.51 c |
| Candida glabrata | 94.44 ± 1.45 a | 64.85 ± 2.67 b | 44.36 ± 2.35 c | 26.82 ± 2.77 cd | |
| Candida krusei | −14.18 ± 1.44 g | 14.95 ± 2.52 f | 35.47 ± 2.51 d | 26.51 ± 2.51 cd | |
| Candida tropicalis | 94.41 ± 2.10 a | 63.92 ± 3.09 b | 44.99 ± 1.24 c | 25.77 ± 2.95 d | |
| BFB | Salmonella enterica | 65.98 ± 3.94 c | 54.51 ± 2.09 c | 45.50 ± 2.49 c | 25.73 ± 1.17 d |
| Kohlrabi | |||||
| G+ | Bacillus cereus | 54.57 ± 2.93 c | 36.12 ± 1.28 e | −3.93 ± 0.30 e | −13.52 ± 1.75 g |
| Micrococcus luteus | 54.95 ± 2.59 c | −14.21 ± 1.67 h | 35.51 ± 2.61 c | 13.62 ± 2.55 f | |
| Staphylococcus aureus | 54.51 ± 2.03 c | 75.29 ± 2.74 a | 24.46 ± 2.61 d | 85.53 ± 2.08 b | |
| G− | Escherichia coli | 19.24 ± 2.50 e | 55.51 ± 1.56 c | 45.62 ± 2.48 b | −15.14 ± 2.02 g |
| Vibrio parahaemolyticus | 36.91 ± 3.26 d | 54.56 ± 2.47 c | −14.42 ± 2.07 f | 84.19 ± 3.07 b | |
| Yersinia enterocolitica | 35.77 ± 3.37 d | 6.37 ± 2.05 g | 55.17 ± 2.43 a | 93.48 ± 2.34 a | |
| Yeast | Candida albicans | 76.63 ± 4.29 b | 56.47 ± 3.26 c | 44.52 ± 3.20 b | 13.76 ± 2.79 f |
| Candida glabrata | 33.23 ± 2.60 d | 46.48 ± 2.45 d | 55.18 ± 1.97 a | 94.41 ± 1.19 a | |
| Candida krusei | 97.56 ± 2.53 a | 64.74 ± 2.71 b | 46.49 ± 2.18 b | 25.87 ± 1.63 e | |
| Candida tropicalis | 15.76 ± 1.06 e | 24.65 ± 1.55 f | 35.18 ± 3.57 c | 46.07 ± 3.39 c | |
| BFB | Salmonella enterica | 76.32 ± 3.30 b | 65.48 ± 2.12 b | 53.48 ± 1.04 a | 36.82 ± 1.86 d |
| Concentration (%) | Number of Living Individuals | Number of Dead Individuals | Insecticidal Activity (%) |
|---|---|---|---|
| 100 | 0 | 100 | 100.00 ± 0.00 |
| 50 | 10 | 90 | 90.00 ± 0.00 |
| 25 | 25 | 75 | 75.00 ± 0.00 |
| 12.5 | 50 | 50 | 50.00 ± 0.00 |
| 6.25 | 75 | 25 | 25.00 ± 0.00 |
| 3.125 | 90 | 10 | 10.00 ± 0.00 |
| Control group | 100 | 0 | 0.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kačániová, M.; Čmiková, N.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Vukic, M.D. Citrus limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot. Plants 2024, 13, 524. https://doi.org/10.3390/plants13040524
Kačániová M, Čmiková N, Vukovic NL, Verešová A, Bianchi A, Garzoli S, Ben Saad R, Ben Hsouna A, Ban Z, Vukic MD. Citrus limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot. Plants. 2024; 13(4):524. https://doi.org/10.3390/plants13040524
Chicago/Turabian StyleKačániová, Miroslava, Natália Čmiková, Nenad L. Vukovic, Andrea Verešová, Alessandro Bianchi, Stefania Garzoli, Rania Ben Saad, Anis Ben Hsouna, Zhaojun Ban, and Milena D. Vukic. 2024. "Citrus limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot" Plants 13, no. 4: 524. https://doi.org/10.3390/plants13040524
APA StyleKačániová, M., Čmiková, N., Vukovic, N. L., Verešová, A., Bianchi, A., Garzoli, S., Ben Saad, R., Ben Hsouna, A., Ban, Z., & Vukic, M. D. (2024). Citrus limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot. Plants, 13(4), 524. https://doi.org/10.3390/plants13040524










