Exploring the Bioactive Potential of Taraxacum officinale F.H. Wigg Aerial Parts on MDA Breast Cancer Cells: Insights into Phytochemical Composition, Antioxidant Efficacy, and Gelatinase Inhibition within 3D Cellular Models
Abstract
1. Introduction
2. Results and Discussion
2.1. SPME/GC-MS: Chemical Volatile Composition of Dried T. officinale
2.2. Chemical Composition of T. officinale Aerial Parts after Derivatization Reaction
2.3. Chemical Composition of T. officinale MeOH and DCM Extracts
2.4. Cytotoxicity Assay
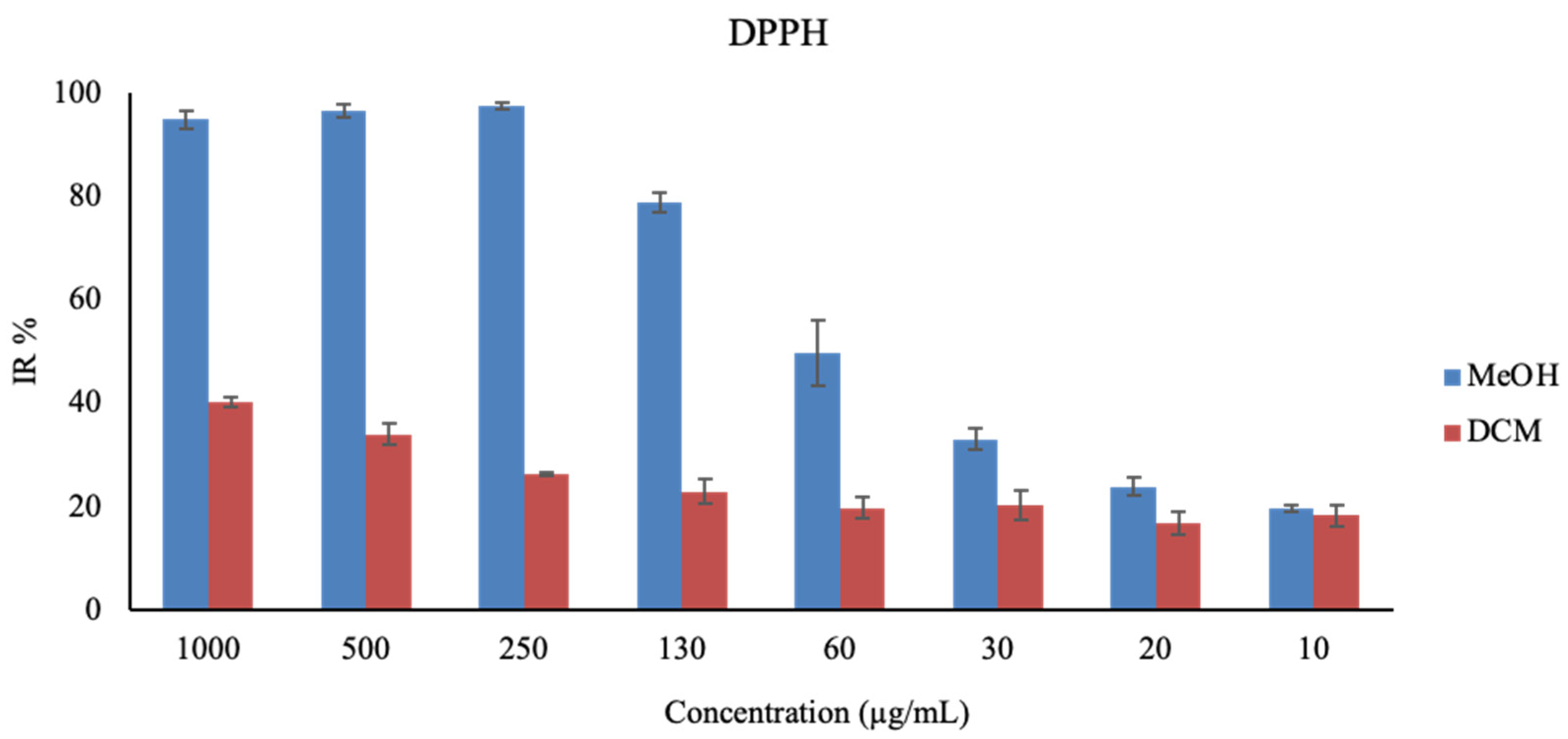
2.5. Antioxidant Activity
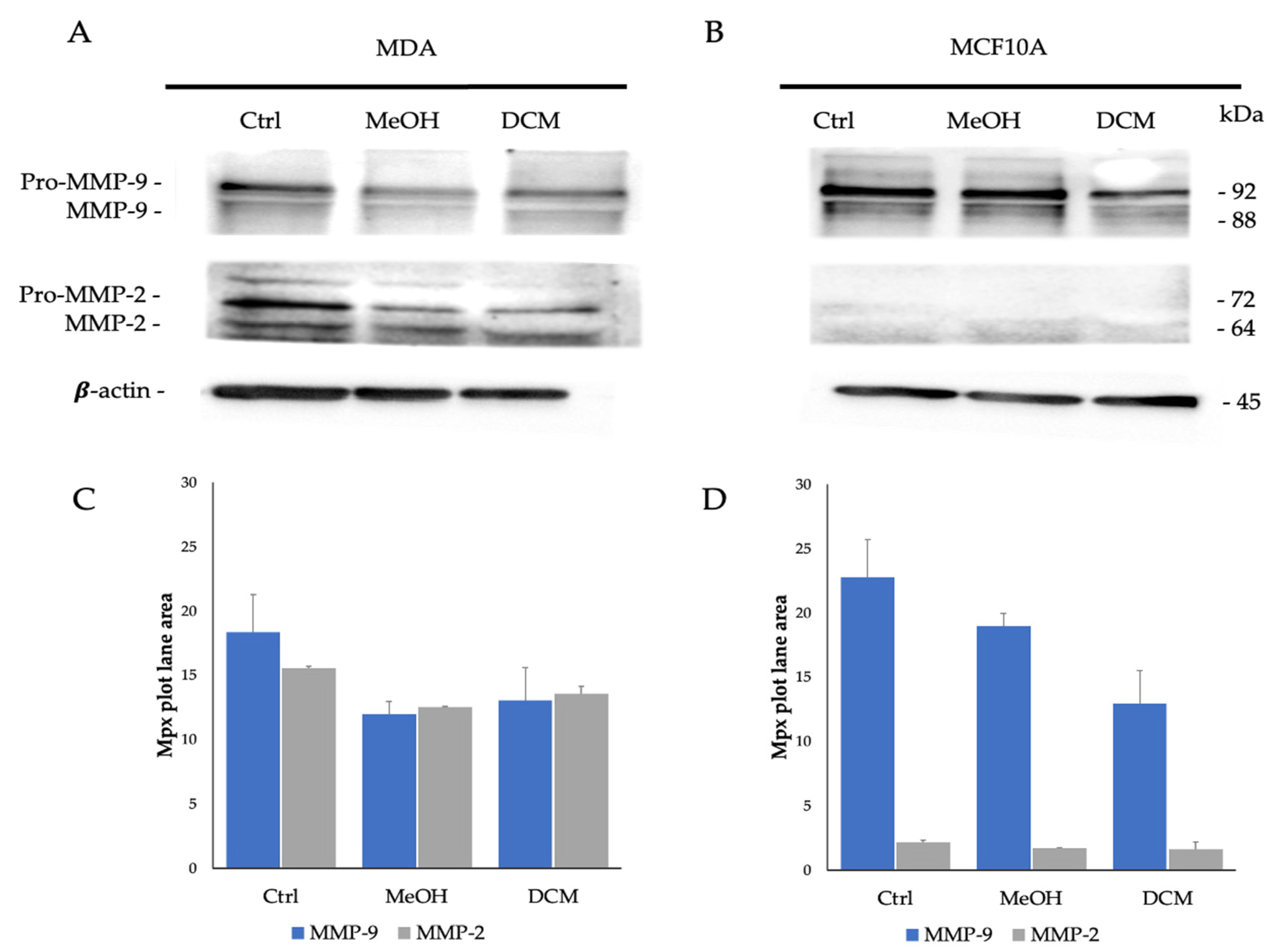
2.6. MMP-9 and MMP-2 Detection by Gelatin Zymography and Western Blot Analyses
3. Materials and Methods
3.1. Plant Material
3.2. SPME Sampling
3.3. GC-MS Analysis
3.4. GC-MS Analysis of Taraxacum officinale after the Derivatization Reaction
3.5. Extraction Process
3.6. HRGC-MS Analysis of MeOH and DCM Extracts
3.7. Cell Lines and Culture Conditions
Preparation of 3D RAFT™ Cultures
3.8. Cytotoxicity and Antiproliferative Assay
3.9. Antioxidant Assays
3.9.1. DPPH Assay
3.9.2. FRAP Assay
3.10. Gelatin Zymography
3.11. MMP-9 and MMP-2 Detection by Western Blot
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Chen, Y.; Sun-Waterhouse, D. The potential of dandelion in the fight against gastrointestinal diseases: A review. J. Ethnopharmacol. 2022, 293, 115272. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Y.; Zhao, C.; Li, S.; Wang, T.; Qiao, B.; Fu, Y. Application of fingerprint combined with quantitative analysis and multivariate chemometric methods in quality evaluation of dandelion (Taraxacum mongolicum). R. Soc. Open Sci. 2021, 8, 210614. [Google Scholar] [CrossRef]
- Jalili, C.; Taghadosi, M.; Pazhouhi, M.; Bahrehmand, F.; Miraghaee, S.S.; Pourmand, D.; Rashidi, I. An overview of therapeutic potentials of Taraxacum officinale (dandelion): A traditionally valuable herb with a reach historical background. WCRJ 2020, 7, e1679. [Google Scholar] [CrossRef]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Phamacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef]
- Tiwari, D.; Kewlani, P.; Singh, L.; Rawat, S.; Bhatt, I.D.; Sundriyal, R.C.; Pande, V. A review on natural bioactive compounds of Taraxacum officinale Weber: A potential anticancer plant. J. Pharm. Pharmacol. 2024, 3, rqae009. [Google Scholar] [CrossRef]
- Ahmadi, S.; Saberivand, A.; Jalili, C.; Asadpour, R.; Khordadmehr, M.; Saberivand, M. Hydroalcoholic extract of Taraxacum officinale induces apoptosis and autophagy in 4T1 breast cancer cells. Vet. Res. Forum. 2023, 14, 507–513. [Google Scholar] [PubMed]
- Pucci-Minafra, I.; Albanese, N.N.; Di Cara, G.; Minafra, L.; Marabeti, M.R.; Cancemi, P. Breast cancer cells exhibit selective modulation induced by different collagen substrates. Connect. Tissue Res. 2008, 49, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Sigstedt, S.C.; Hooten, C.J.; Callewaert, M.C.; Jenkins, A.R.; Romero, A.E.; Pullin, M.J.; Kornienko, A.; Lowrey, T.K.; Slambrouck, S.V.; Steelant, W.F. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008, 32, 1085–1090. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta 2010, 1803, 3–19. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Khan, Y.H.; Uttra, A.M.; Qasim, S.; Mallhi, T.H.; Alotaibi, N.H.; Rasheed, M.; Alzarea, A.I.; Iqbal, M.S.; Alruwaili, N.K.; Khan, S.U.; et al. Potential Role of Phytochemicals Against Matrix Metalloproteinase Induced Breast Cancer; An Explanatory Review. Front. Chem. 2021, 8, 592152. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Jezierska-Drutel, A.; Rosenzweig, S.A.; Neumann, C.A. Role Of Oxidative Stress And The Microenvironment In Breast Cancer Development And Progression. Adv. Cancer Res. 2013, 119, 107–125. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef] [PubMed]
- Shumbe, L.; Bott, R.; Havaux, M. Dihydroactinidiolide, a high light-induced β-carotene derivative that can regulate gene expression and photoacclimation in Arabidopsis. Mol. Plant. 2014, 7, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Rocca, J.R.; Tumlinson, J.H.; Glancey, B.M.; Lofgren, C.S. Synthesis and stereochemistry of tetrahydro-3,5-dimethyl-6-(1-methylbutyl)-2H-pyran -2-one, a component of the queen recognition pheromone of Solenopsis invicta. Tetrahedron Lett. 1983, 24, 1893–1896. [Google Scholar] [CrossRef]
- Malek, S.N.A.; Shin, S.K.; Wahab, N.A.; Yaacob, H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Saveer, A.M.; Hatano, E.; Wada-Katsumata, A.; Meagher, R.L.; Schal, C. Nonanal, a new fall armyworm sex pheromone component, significantly increases the efficacy of pheromone lures. Pest Manag. Sci. 2023, 79, 2831–2839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, B.; Lv, Y.; Wei, S.; Zhang, S.; Hu, Y. Synergistic effects of combined cinnamaldehyde and nonanal vapors against Aspergillus flavus. Int. J. Food Microbiol. 2023, 402, 110277. [Google Scholar] [CrossRef]
- Bisignano, G.; Laganà, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microb. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, T.; Ye, S.; Gao, S.; Dong, Y. Comparative Analysis with GC–MS of Fatty Acids and Volatile Compounds of Taraxacum kok-saghyz Rodin and Taraxacum officinale as Edible Resource Plants. Separations 2022, 9, 314. [Google Scholar] [CrossRef]
- Escudero, N.; De Arellano, M.; Fernández, S.; Albarracín, G.; Mucciarelli, S. Taraxacum officinale as a food source. Plant Foods Hum Nutr. 2003, 58, 1–10. [Google Scholar] [CrossRef]
- Milovanovica, S.; Grzegorczyk, A.; Świątek, Ł.; Boguszewska, A.; Kowalski, R.; Tyśkiewicz, K.; Konkol, M. Phenolic, tocopherol, and essential fatty acid-rich extracts from dandelion seeds: Chemical composition and biological activity. Food Bioprod. Process. 2023, 142, 70–81. [Google Scholar] [CrossRef]
- Savych, A.; Basaraba, R.; Muzyka, N.; Ilashchuk, P. Analysis of fatty acid composition content in the plant components of antidiabetic herbal mixture by GC-MS. Pharmacia 2021, 68, 433–439. [Google Scholar] [CrossRef]
- Furuno, T.; Kamiyama, A.; Akashi, T.; Usui, M.; Takahashi, T.; Ayabe, S. Triterpenoid Constituents of Tissue Cultures and Regenerated Organs of Taraxacum officinale. Plant Tissue Cult. Lett. 1993, 3, 275–280. [Google Scholar] [CrossRef]
- Martínez, M.E.; Jorquera, L.; Poirrier, P.; Díaz, K.; Chamy, R. Effect of Inoculum Size and Age, and Sucrose Concentration on Cell Growth to Promote Metabolites Production in Cultured Taraxacum officinale (Weber) Cells. Plants 2023, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants–rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Barros, F.W.A.; Bandeira, P.N.; Lima, D.J.B.; Meira, A.S.; de Farias, S.S.; Albuquerque, M.R.J.R.; dos Santos, H.S.; Lemos, T.L.G.; de Morais, M.O.; Costa-Lotufo, L.V. Amyrin esters induce cell death by apoptosis in HL-60 leukemia cells. Bioorg. Med. Chem. 2011, 19, 1268–1276. [Google Scholar] [CrossRef]
- Lima, E.M.; Nascimento, A.M.; Lenz, D.; Scherer, R.; Meyrelles, S.S.; Boëchat, G.A.P.; Andrade, T.U.; Endringer, D.C. Triterpenes from the Protium heptaphyllum resin-chemical composition and cytotoxicity. Rev. Bras. Farmacogn. 2014, 24, 399–407. [Google Scholar] [CrossRef]
- Viet, T.D.; Xuan, T.D.; Anh, H. α-Amyrin and β-Amyrin Isolated from Celastrus hindsii Leaves and Their Antioxidant, Anti-Xanthine Oxidase, and Anti-Tyrosinase Potentials. Molecules 2021, 29, 7248. [Google Scholar] [CrossRef]
- McGlackena, G.P.; Fairlamb, I.J.S. 2-Pyrone natural products and mimetics: Isolation, characterization and biological activity. Nat. Prod. Res. 2005, 22, 369–385. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, P.; Singh, H.; Nepali, K.; Kumar Gupta, G.; Kumar Jain, S.; Ntie-Kang, F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017, 59, 36977–36999. [Google Scholar] [CrossRef]
- Sang-Uk, C.; Chang-Hyu, B.; Sheong Chun, L. Antioxidant and Cytotoxic Potentials of Methanol Extracts from Taraxacum officinale F. H. Wigg. at Different Plant Parts. Korean J. Plant Res. 2012, 25, 232–239. [Google Scholar]
- Dedić, S.; Džaferović, A.; Jukić, H. Chemical Composition And Antioxidant Activity Of Water-Ethanol Extracts of Dandelion (Taraxacum officinale). J. Acad. Nutr. Diet. 2022, 11, 8–14. [Google Scholar]
- Amin Mir, M.; Sawhney, S.S.; Jassal, M.M.S. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. J. Pharm. Pharmacol. 2013, 2, 2315–7259. [Google Scholar]
- Sasikala, P.; Ganesan, S.; Jayaseelan, T.; Azhagumadhavan, S.; Padma, M.; Senthilkumar, S.; Mani, P. Phytochemicals and GC–MS analysis of bioactive compounds present in ethanolic leaves extract of Taraxacum officinale (L). J. Drug Deliv. Ther. 2019, 9, 90–94. [Google Scholar] [CrossRef]
- Razak, S.; Afsar, T.; Al-Disi, D.; Almajwal, A.; Arshad, M.; Alyousef, A.A.; Chowdary, R.A. GCMS fingerprinting, in vitro pharmacological activities, and in vivo anti-inflammatory and hepatoprotective effect of selected edible herbs from Kashmir valley. J. King Saud Univ. Sci. 2020, 32, 2868–2879. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar]
- Muhammed, R.A.; Hassawi, D.S.; Ibaheem, N.K. Cytotoxic activity of Taraxacum officinale ethanolic plant extract against human breast cancer (MCF-7) cells and human hepatic (WRL-68) cells. Iraqi J. Cancer Med. Genet. 2018, 11, 16–21. [Google Scholar] [CrossRef]
- Trinh, N.V.; Doan-Phuong Dang, N.; Hong Tran, D.; Van Pham, P. Taraxacum officinale dandelion extracts efficiently inhibited the breast cancer stem cell proliferation. Biomed. Res. Ther. 2016, 3, 34. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M. In vitro antioxidant activity, total phenolic and total flavonoid contents of Taraxacum officinale leaves. Int. J. Innov. Pharm. Sci. Res. 2015, 3, 697–707. [Google Scholar]
- Aremu, O.O.; Oyedeji, A.O.; Oyedeji, O.O.; Nkeh-Chungag, B.N.; Rusike, C.R.S. In Vitro and In Vivo Antioxidant Properties of Taraxacum officinale in Nω-Nitro-l-Arginine Methyl Ester (L-NAME)-Induced Hypertensive Rats. Antioxidants 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Hunter, T. The regulatory crosstalk between kinases and proteases in cancer. Nat. Rev. Cancer 2010, 10, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Natural matrix metalloproteinase inhibitors: Leads from herbal resources. Stud. Nat. Prod. Chem. 2013, 39, 91–113. [Google Scholar]
- Kumar, G.B.; Nair, B.G.; Perry, J.J.P.; Martin, D.B.C. Recent insights into natural product inhibitors of matrix metalloproteinases. Medchemcomm 2019, 10, 2024–2037. [Google Scholar] [CrossRef]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef]
- Nicolai, E.; Sinibaldi, F.; Sannino, G. Omega-3 and Omega-6 Fatty Acids Act as Inhibitors of the Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Activity. Protein J. 2017, 36, 278–285. [Google Scholar] [CrossRef]
- Taiti, C.; Di Matteo, G.; Spano, M.; Vinciguerra, V.; Masi, E.; Mannina, L.; Garzoli, S. Metabolomic Approach Based on Analytical Techniques for the Detection of Secondary Metabolites from Humulus lupulus L. Dried Leaves. Int. J. Mol. Sci. 2023, 24, 13732. [Google Scholar] [CrossRef]
- Taiti, C.; Di Vito, M.; Di Mercurio, M.; Costantini, L.; Merendino, N.; Sanguinetti, M.; Bugli, F.; Garzoli, S. Detection of Secondary Metabolites, Proximat Composition and Bioactivity of Organic Dried Spirulina (Arthrospira platensis). Appl. Sci. 2024, 14, 67. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Di Martile, M.; Del Bufalo, D.; Garzoli, S. Phytochemical characterization and cytotoxic potential of extracts from roots and inflorescences of Cannabis sativa L. cv. Eletta Campana. Sustain. Chem. Pharm. 2023, 36, 101269. [Google Scholar]
- Letaief, T.; Garzoli, S.; Laghezza Masci, V.; Mejri, J.; Abderrabba, M.; Tiezzi, A.; Ovidi, E. Chemical Composition and Biological Activities of Tunisian Ziziphus lotus Extracts: Evaluation of Drying Effect, Solvent Extraction, and Extracted Plant Parts. Plants 2021, 10, 2651. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Toth, M.; Fridman, R. Assessment of Gelatinases (MMP-2 and MMP-9 by Gelatin Zymography. Met. Mol. Med. 2001, 57, 163–174. [Google Scholar]

| N° | Component 1 | LRI 2 | LRI 3 | Content (%) |
|---|---|---|---|---|
| 1 | α-pinene | 928 | 932 | 2.1 ± 0.03 |
| 2 | β-pinene | 978 | 981 | 0.8 ± 0.02 |
| 3 | β-myrcene | 982 | 987 | 1.8 ± 0.02 |
| 4 | limonene | 1020 | 1022 | 31.9 ± 0.15 |
| 5 | 1,8-cineole | 1031 | 1033 | 38.3 ± 0.20 |
| 6 | linalool | 1087 | 1089 | 1.2 ± 0.02 |
| 7 | nonanal | 1100 | 1104 | 6.1 ± 0.04 |
| 8 | isothujol | 1162 | 1165 | 1.6 ± 0.03 |
| 9 | terpinen-4-ol | 1178 | 1182 | 0.6 ± 0.02 |
| 10 | 1-octanol, 2-butyl- | 1270 | 1277 | 1.0 ± 0.01 |
| 11 | isobornyl acetate | 1281 | 1286 | 0.2 ± 0.02 |
| 12 | β-elemene | 1395 | 1393 | 1.3 ± 0.02 |
| 13 | tetradecane | 1410 | 1413 | 1.5 ± 0.03 |
| 14 | β-caryophyllene | 1430 | 1435 | 1.0 ± 0.01 |
| 15 | humulene | 1468 | 1473 | 0.5 ± 0.02 |
| 16 | β-eudesmene | 1479 | 1485 | 0.4 ± 0.02 |
| 17 | dihydroactinidiolide | 1520 | 1525 | 7.5 ± 0.04 |
| 18 | hexadecane | 1609 | 1612 | 0.9 ± 0.03 |
| 19 | 2,6-diisopropylnaphthalene | 1725 | 1728 | 0.7 ± 0.03 |
| 20 | hexahydrofarnesyl acetone | 1840 | 1846 | 0.6 ± 0.02 |
| monoterpenes | 78.5 | |||
| sesquiterpenes | 3.2 | |||
| others | 18.3 | |||
| SUM | 100.0 |
| N° | Components | (%) |
|---|---|---|
| Fatty acids | ||
| 1 | palmitic acid | 24.8 ± 0.15 |
| 2 | oleic acid | tr |
| 3 | linoleic acid | 9.1 ± 0.08 |
| 4 | α-linolenic acid | 33.1 ± 0.22 |
| 5 | stearic acid | 6.9 ± 0.07 |
| Triterpenes | ||
| 6 | lupeol | 8.1 ± 0.10 |
| 7 | α-amyrin | 1.5 ± 0.02 |
| 8 | β-amyrin | 1.3 ± 0.04 |
| Diterpenes | ||
| 9 | neophytadiene | 6.3 ± 0.08 |
| 10 | phytol | 4.1 ± 0.03 |
| Others | ||
| 11 | 2-pyranone | 4.8 ± 0.05 |
| N° | Component 1 | LRI 2 | LRI 3 | Content (%) | Content (%) |
|---|---|---|---|---|---|
| MeOH Extract | DCM Extract | ||||
| 1 | 2-pyranone | 1110 | 1107 | 22.4 ± 0.12 | tr |
| 2 | tetradecane, 2,6,10-trimethyl- | 1552 | 1557 | - | 0.5 ± 0.01 |
| 3 | hexadecane | 1609 | 1612 | - | 0.5 ± 0.00 |
| 4 | hydroxylauric acid | 1810 | 1813 | 0.9 ± 0.03 | - |
| 5 | neophytadiene | 1832 | 1836 | 4.9 ± 0.05 | 8.7 ± 0.05 |
| 6 | palmitic acid | 1958 | 1962 | 28.9 ± 2.15 | 33.2 ± 4.30 |
| 7 | thunbergol | 2028 | 2032 | - | 0.4 ± 0.01 |
| 8 | phytol | 2101 | 2105 | - | 0.6 ± 0.03 |
| 9 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 2112 | 2116 | - | 2.1 ± 0.02 |
| 10 | oleic acid | 2145 | 2147 | - | 0.3 ± 0.01 |
| 11 | linoleic acid | 2148 | 2152 | 6.6 ± 0.07 | 14.8 ± 0.03 |
| 12 | α-linolenic acid | 2162 | 2159 | 28.2 ± 0.11 | 26.5 ± 0.08 |
| 13 | stearic acid | 2170 | 2166 | 6.0 ± 0.06 | 3.0 ± 0.03 |
| 14 | erucic acid | 2568 | 2572 | - | 0.2 ± 0.01 |
| 15 | squalene | 2541 | 2847 | - | 0.9 ± 0.02 |
| 16 | lupeol | 3272 | 3270 | 0.6 ± 0.02 | 4.7 ± 0.05 |
| 17 | β-amyrin | 3342 | 3337 | 0.8 ± 0.03 | 1.0 ± 0.02 |
| 18 | α-amyrin | 3381 | 3376 | 0.6 ± 0.02 | 0.8 ± 0.02 |
| fatty acids | 70.6 | 78.0 | |||
| triterpenes | 2.0 | 7.4 | |||
| diterpenes | 4.9 | 9.7 | |||
| others | - | 3.1 | |||
| SUM | 99.9 | 98.2 |
| DPPH | FRAP | |
|---|---|---|
| MeOH | 0.04 × 103 ± 0.01 × 103 | 220.65 ± 8.68 |
| DCM | 2.47 × 103 ± 0.19 × 103 | 208.06 ± 1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laghezza Masci, V.; Ovidi, E.; Tomassi, W.; De Vita, D.; Garzoli, S. Exploring the Bioactive Potential of Taraxacum officinale F.H. Wigg Aerial Parts on MDA Breast Cancer Cells: Insights into Phytochemical Composition, Antioxidant Efficacy, and Gelatinase Inhibition within 3D Cellular Models. Plants 2024, 13, 2829. https://doi.org/10.3390/plants13192829
Laghezza Masci V, Ovidi E, Tomassi W, De Vita D, Garzoli S. Exploring the Bioactive Potential of Taraxacum officinale F.H. Wigg Aerial Parts on MDA Breast Cancer Cells: Insights into Phytochemical Composition, Antioxidant Efficacy, and Gelatinase Inhibition within 3D Cellular Models. Plants. 2024; 13(19):2829. https://doi.org/10.3390/plants13192829
Chicago/Turabian StyleLaghezza Masci, Valentina, Elisa Ovidi, William Tomassi, Daniela De Vita, and Stefania Garzoli. 2024. "Exploring the Bioactive Potential of Taraxacum officinale F.H. Wigg Aerial Parts on MDA Breast Cancer Cells: Insights into Phytochemical Composition, Antioxidant Efficacy, and Gelatinase Inhibition within 3D Cellular Models" Plants 13, no. 19: 2829. https://doi.org/10.3390/plants13192829
APA StyleLaghezza Masci, V., Ovidi, E., Tomassi, W., De Vita, D., & Garzoli, S. (2024). Exploring the Bioactive Potential of Taraxacum officinale F.H. Wigg Aerial Parts on MDA Breast Cancer Cells: Insights into Phytochemical Composition, Antioxidant Efficacy, and Gelatinase Inhibition within 3D Cellular Models. Plants, 13(19), 2829. https://doi.org/10.3390/plants13192829










