Morpho-Anatomical Modulation of Seminal Roots in Response to Water Deficit in Durum Wheat (Triticum turgidum var. durum)
Abstract
1. Introduction
2. Results
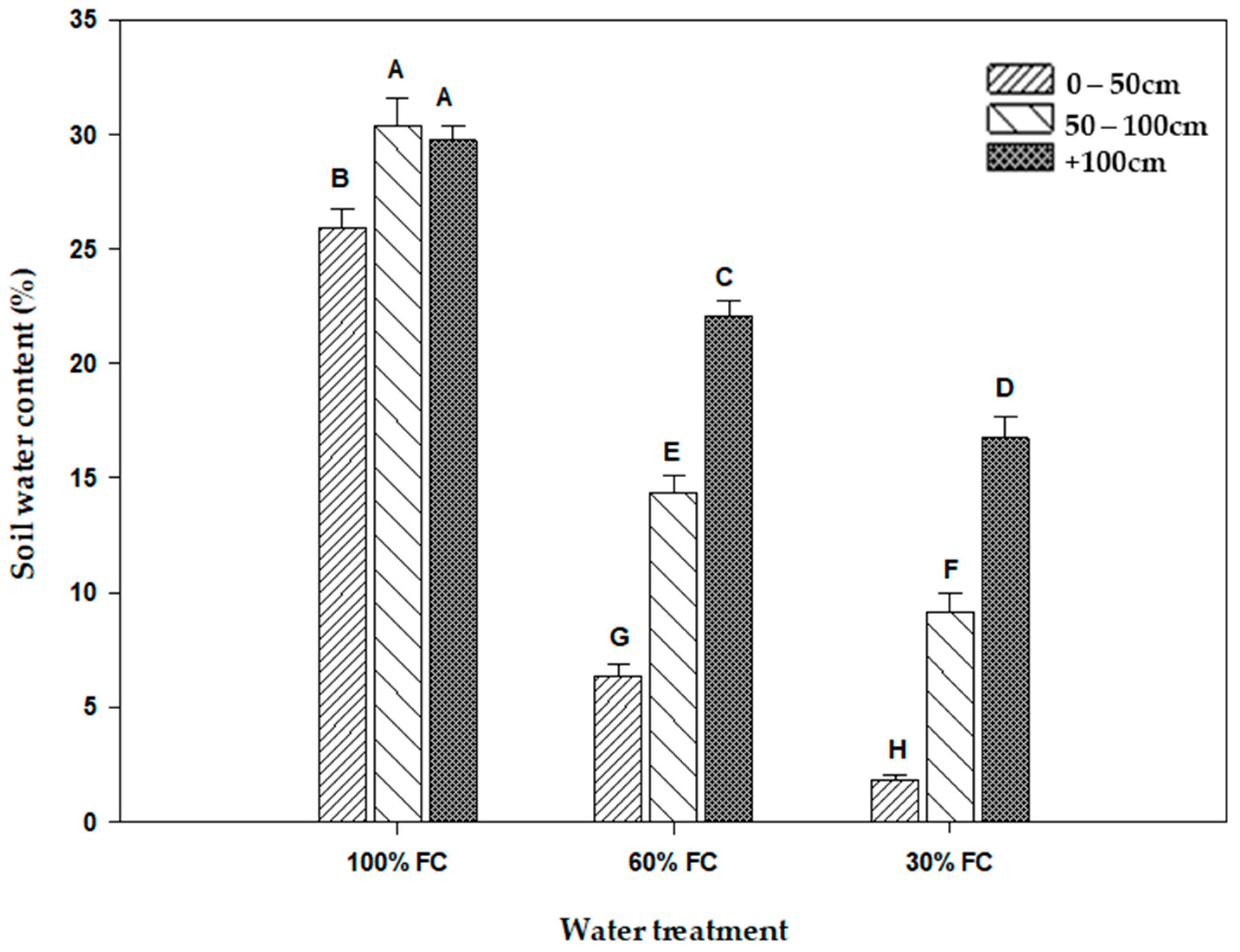
2.1. Soil Water Content
2.2. The Relative Leaf Water Content
2.3. Seminal Root Length
2.4. Anatomical Traits of Seminal Roots
2.4.1. Late (Central) Metaxylem Vessel Diameter
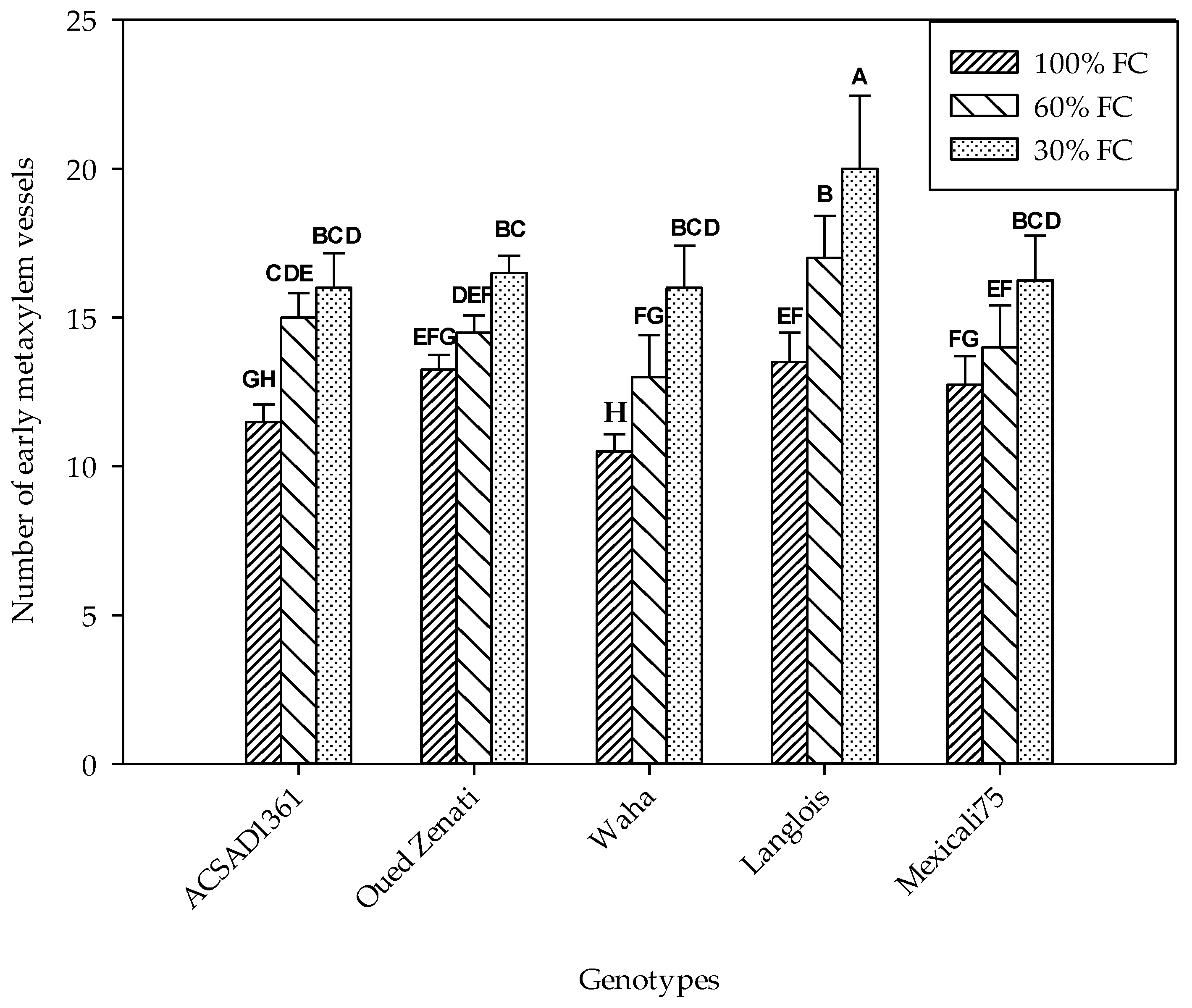
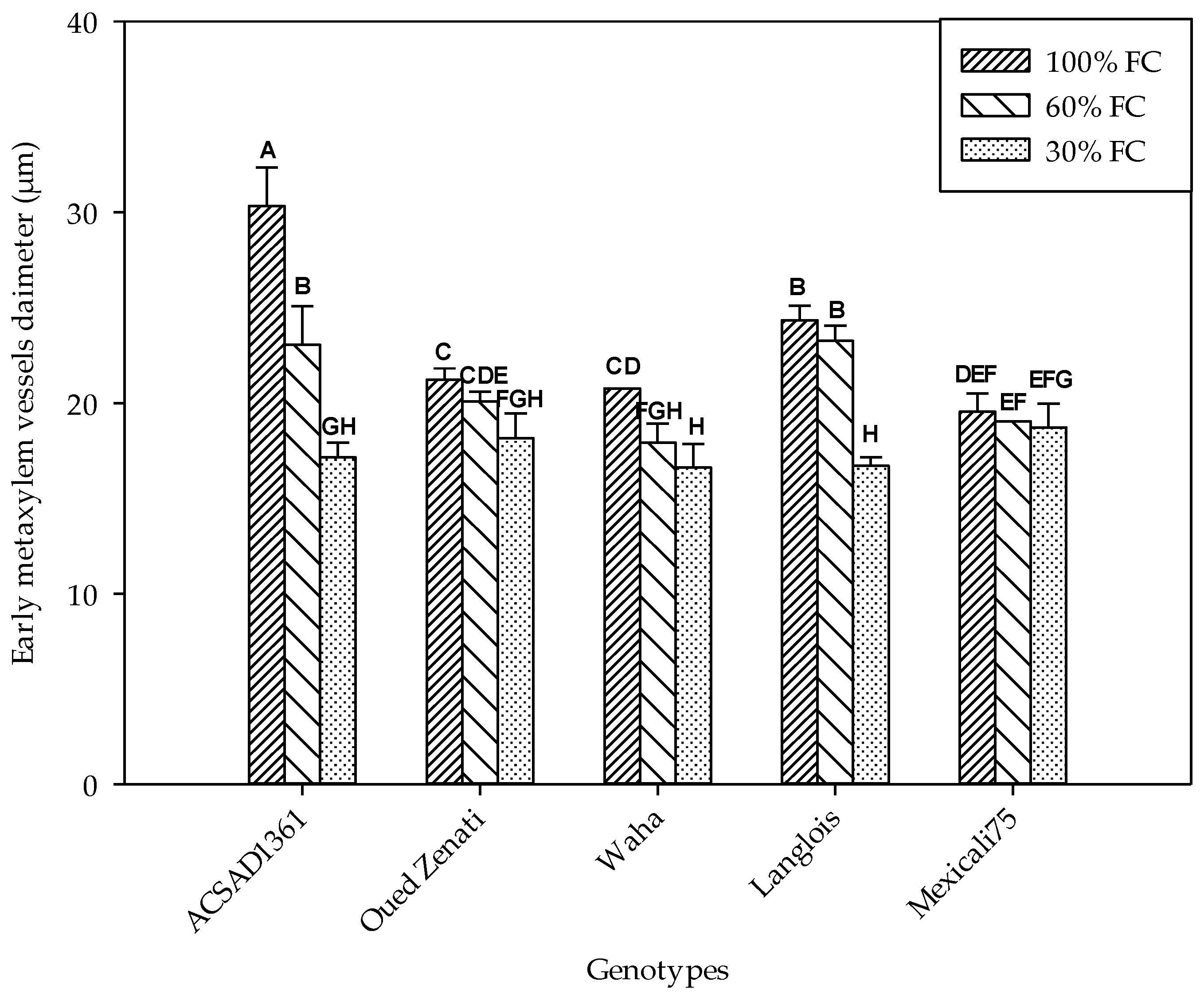
2.4.2. Number and Diameter of Early Metaxylem Vessels
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Traits Measurements
4.2.1. Relative Leaf Water Content
4.2.2. Morphological and Anatomical Traits of Seminal Roots
4.2.3. Soil Water Content
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Merah, O.; Deléens, O.; Teulat, B.; Monneveux, P. Productivity and carbon isotope discrimination in durum wheat organs under a Mediterranean climate. CR Acad. Sci. III 2001, 324, 51–57. [Google Scholar] [CrossRef]
- Boudjabi, A.F.; Abada, D.; Benbghila, N.E.H.; Ghoul, R. Experimental study of a “hybridized” photovoltaic panel. Energy Procedia 2017, 115, 290–297. [Google Scholar] [CrossRef]
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, I.; Jones, R.; Kolli, R.K.; Kwon, W.T.; Laprise, R.; et al. Regional Climate Projections; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. 926. [Google Scholar]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–444. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J. The drought environment: Physical, biological and agricultural perspectives. J. Exp. Bot. 2007, 58, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Adda, A.; Sahnoune, M.; Kaid-Harche, M.; Merah, O. Impact of water deficit intensity on durum wheat seminal roots. Comptes Rendus Biol. 2005, 328, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Benlaribi, M.; Monneveux, P.; Grignac, P. Etude des caractères d’enracinement et de leur rôle dans l’adaptation au déficit hydrique chez le blé dur (Triticum durum Desf). Agronomie 1990, 10, 305–322. [Google Scholar] [CrossRef]
- Sahnoune, M.; Adda, A.; Soualem, S.; Kaid-Harche, M.; Merah, O. Early water deficit effect on seminal root barley. Comptes Rendus Biol. 2004, 327, 389–398. [Google Scholar] [CrossRef]
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Zhang, S.; Siddique, K.H.M.; Chen, Y. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J.B. Phenotyping for drought tolerance in grain crops: When is it useful to breeders? Funct. Plant Biol. 2012, 39, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Christopher, J.; Christopher, M.; Jennings, R.; Jones, S.; Fletcher, S.; Borrell, A. QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theor. Appl. Genet. 2013, 126, 1563–1574. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, M.V.; Byrne, P.F.; Dieri, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Mohan, A.; Gill, K.S.; Prasad, P.V. Variability of root traits in spring wheat germplasm. PLoS ONE 2014, 9, e100317. [Google Scholar] [CrossRef]
- Kaur, V.; Yadav, R.; Kumarin, A.; Madaan, S. Root system characteristics in wheat for effective absorption of water under drought. Wheat Inform. Serv. 2016, 121, 21–28. [Google Scholar]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 2011, 63, 4751–4763. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Satellite Rainfall Products and Their Reliability in the Blue Nile Basin; Mesele, A., Abtew, W., Setegn, S., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2014; pp. 51–67. [Google Scholar]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Richards, R.A.; Passioura, J.B. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Aust. J. Agric. Res. 1989, 40, 943–950. [Google Scholar] [CrossRef]
- Adachi, S.; Tsuru, Y.; Kondo, M.; Yamamoto, T.; Arai-Sanoh, Y.; Ando, T.; Ookawa, T.; Yano, M.; Hirasawa, T. Characterization of a rice variety with high hydraulic conductance and identification of the chromosome region responsible using chromosome segment substitution lines. Ann. Bot. 2010, 106, 803–811. [Google Scholar] [CrossRef]
- Henry, A.; Gowda, V.R.P.; Torres, R.O.; McNally, K.L.; Serraj, R. Variation in root system architecture and drought response in rice (Oryza sativa): Phenotyping of the Oryza SNP panel in rainfed lowland fields. Field Crops Res. 2012, 120, 205–214. [Google Scholar] [CrossRef]
- Purushothaman, R.; Mainassara, Z.A.; Nalini, M.; Rajaram, P.; Lakshmanan, K.; Cholenahalli, L.L.G. Root anatomical traits and their possible contribution to drought tolerance in grain legumes. Plant Prod. Sci. 2013, 16, 1–8. [Google Scholar] [CrossRef]
- Slatyer, R.O. Plant Relationships; London Academic Press: London, UK, 1967; p. 366. [Google Scholar]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. For. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Soltys-Kalina, D.; Plich, P.; Strzelczyk-Zyta, D.; Sliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Morphology, Yield, and chlorophyll concentration of Hungarian Potato Genotypes. J. Environ. Agric. Sci. 2021, 23, 8–16. [Google Scholar]
- Keyvan, S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 2010, 8, 1051–1060. [Google Scholar]
- Meher, D.; Shivakrishna, P.; Ashok Reddy, P.; Manohar Rao, D. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, J.A.; Karim, M.A.; Khaliq, Q.A.; Ahmed, A.U.; Mondol, A.T.M.A.I. Effect of drought stress on water relation traits of four soybean genotypes. SAARC J. Agric. 2017, 15, 163–175. [Google Scholar] [CrossRef]
- Larkunthod, P.; Nounjan, N.; Siangliw, J.L.; Toojinda, T.; Sanitchon, J.; Jongdee, B.; Theerakulpisut, P. Physiological Responses under Drought Stress of Improved Drought Tolerant Rice Lines and their Parents. Not. Bot. Horti Agrobot. 2018, 4, 679–687. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Sai Prasad, S.V.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- White, C.A.; Sylvester-Bradley, R.; Berry, P.M. Root length densities of UK wheat and oilseed rape crops with implications for water capture and yield. J. Exp. Bot. 2015, 66, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Renton, M.; Poot, P. Simulation of the evolution of root water foraging strategies in dry and shallow soils. Ann. Bot. 2014, 114, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Shen, J.; Ashton, R.W.; White, R.P.; Dodd, I.C.; Parry, M.A.J.; Whalley, W.R. Wheat root growth responses to horizontal stratification of fertilizer in a water-limited environment. Plant Soil. 2015, 386, 77–88. [Google Scholar] [CrossRef]
- Asseng, S.; Ritchie, J.T.; Smucker, A.J.M.; Robertson, M.J. Root growth and water uptake during water deficit and recovering in wheat. Plant Soil. 1995, 201, 265–273. [Google Scholar] [CrossRef]
- Bchini, H.; Daaloul, A.; Sayar, R. Variabilité génétique de quelques paramètres du système racinaire du blé dur (Triticum durum Desf.) sous deux régimes hydriques. Plant Genet. Resour. Newsl. 2002, 129, 25–31. [Google Scholar]
- Fukai, T.S. Growth and yield response of barley and chickpea to water stress under three environments in southeast Queensland. II. Root growth and soil water extraction pattern. Aust. J. Agric. Res. 1995, 46, 35–48. [Google Scholar]
- El Hassouni, K.; Alahmad, S.; Belkadi, B.; Filali-Maltouf, A.; Hickey, L.T.; Bassi, F.M. Root system architecture and its association with yield under different water regimes in durum wheat. Crop. Sci. 2018, 58, 2331–2346. [Google Scholar] [CrossRef]
- Labdelli, A.; Adda, A.; Halis, Y.; Soualem, S. Effects of water regime on the structure of roots and stems of durum wheat (Triticum durum Desf.). J. Bot. 2014, 2014, 703874. [Google Scholar] [CrossRef]
- Peña-Valdivia, C.B.; Sánchez-Urdaneta, A.B.; Rangel, J.M.; Muñoz, J.J.; García-Nava, R.; Velázquez, R.C. Anatomical root variations in response to water deficit: Wild and domesticated common bean (Phaseolus vulgaris L.). Biol. Res. 2010, 43, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hort. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, Z.; Ren, Y.; Zhang, T.; Zhang, S.; Li, H.; Chen, Y.; Zhang, S. The Higher Water Absorption Capacity of Small Root System Improved the Yield and Water Use Efficiency of Maize. Plants 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, N.M.; Ciavarella, T.A.; Smith, K.F. The effects of waterlogging on growth, photosynthesis and biomass allocation in perennial ryegrass (Lolium perenne L.) genotypes with contrasting root development. J. Agric. Sci. 2003, 141, 241–248. [Google Scholar] [CrossRef]
- Slatyer, R.O. Plant–Water Relationships; Academic Press: New York, NY, USA, 1967. [Google Scholar]
| Trait | Effect | ||
|---|---|---|---|
| Genotype | Water Treatment | Genotype × Water Treatment | |
| (d.f. = 4) | (d.f. = 2) | (d.f. = 8) | |
| Relative leaf water content | 4.3 ** | 247.7 *** | 0.4 ns |
| Seminal roots length | 6.7 *** | 392.8 *** | 16.1 *** |
| Late metaxylem vessels diameter | 4370 *** | 10406 *** | 961 *** |
| Early metaxylem vessels number | 15.126 *** | 74.586 *** | 1.756 ns |
| Early metaxylem vessels diameter | 42.78 *** | 143.48 *** | 22.28 *** |
| Genotypes | Water Supply | ||||
|---|---|---|---|---|---|
| 100% FC | 60% FC | Decrease Rate (%) | 30% FC | Decrease Rate (%) | |
| ACSAD 1361 | 91.5 A ± 0.4 | 86.3 BC ± 0.41 | 5.6 | 81.7 D ± 0.82 | 10.7 |
| Oued Zenati | 91.5 A ± 0.31 | 87.0 B ± 0.26 | 4.9 | 80.7 D ± 0.73 | 11.8 |
| Waha | 89.5 A ± 0.61 | 85.3 BC ± 0.63 | 4.6 | 79.6 D ± 1.25 | 11.1 |
| Langlois | 90.8 A ± 0.98 | 84.9 BC ± 0.56 | 6.4 | 80.4 D ± 1.26 | 11.4 |
| Mexicali 75 | 89.6 A ± 0.73 | 84.6 C ± 0.29 | 5.6 | 79.9 D ± 0.47 | 10.9 |
| Means | 90.56 | 85.63 | 5.43 | 80.44 | 11.17 |
| Genotypes | Water Supply | ||||
|---|---|---|---|---|---|
| 100% FC | 60% FC | Increase Rate (%) | 30% FC | Increase Rate (%) | |
| ACSAD 1361 | 123.5 F ± 2.9 | 154.8 A ± 1.6 | 20.2 | 158.5 ABC ± 0.9 | 22.1 |
| Oued Zenati | 141.0 D ± 0.7 | 153.3 AB ± 0.6 | 8.0 | 156.3 BC ± 1.3 | 9.7 |
| Waha | 135.3 E ± 0.5 | 154.0 ABC ± 0.9 | 12.2 | 155.5 BC ± 1.2 | 13.0 |
| Langlois | 140.5 D ± 0.8 | 151.8 ABC ± 1.0 | 7.4 | 155.8 C ± 1.8 | 9.8 |
| Mexicali 75 | 140.5 D ± 0.5 | 153.3 ABC ± 1.0 | 8.3 | 155.3 BC ± 1.1 | 9.5 |
| Means | 136.15 | 153.40 | 11.2 | 156.25 | 12.8 |
| Genotypes | Water Supply | ||||
|---|---|---|---|---|---|
| 100% FC | 60% FC | Decrease Rate (%) | 30% FC | Decrease Rate (%) | |
| ACSAD 1361 | 206.6 D ± 0.5 | 184.6 H ± 1.3 | 10.6 | 169.2 K ± 0.7 | 18.1 |
| Oued Zenati | 223.6 A ± 0.3 | 214.7 B ± 0.2 | 4.0 | 171.2 J ± 0.1 | 23.4 |
| Waha | 197.0 F ± 0.7 | 162.9 L ± 0.6 | 17.3 | 102.0 M ± 0.8 | 48.2 |
| Langlois | 208.2 C ± 0.4 | 205.6 D ± 0.3 | 1.2 | 186.9 G ± 1.1 | 10.2 |
| Mexicali 75 | 200.0 E ± 0.1 | 187.0 G ± 0.4 | 6.5 | 175.9 I ± 0.4 | 12.0 |
| Means | 207.07 | 190.97 | 7.93 | 161.03 | 22.41 |
| Genotype | Origin | Drought Tolerance | Cycle Length | Stem Height |
|---|---|---|---|---|
| Oued Zenati | Algerian Landrace | Medium | Late | Tall |
| Langlois | Algerian Landrace | High | Late | Tall |
| Waha | ICARDA | High | Early | Dwarf |
| ACSAD 1361 | ACSAD | Low | Early | Dwarf |
| Mexicali 75 | CIMMYT | Medium | Early | Dwarf |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felouah, O.C.; Ammad, F.; Adda, A.; Bouzid, A.; Gharnaout, M.L.; Evon, P.; Merah, O. Morpho-Anatomical Modulation of Seminal Roots in Response to Water Deficit in Durum Wheat (Triticum turgidum var. durum). Plants 2024, 13, 487. https://doi.org/10.3390/plants13040487
Felouah OC, Ammad F, Adda A, Bouzid A, Gharnaout ML, Evon P, Merah O. Morpho-Anatomical Modulation of Seminal Roots in Response to Water Deficit in Durum Wheat (Triticum turgidum var. durum). Plants. 2024; 13(4):487. https://doi.org/10.3390/plants13040487
Chicago/Turabian StyleFelouah, Oum Cheikh, Faiza Ammad, Ahmed Adda, Assia Bouzid, Mohammed Lotfi Gharnaout, Philippe Evon, and Othmane Merah. 2024. "Morpho-Anatomical Modulation of Seminal Roots in Response to Water Deficit in Durum Wheat (Triticum turgidum var. durum)" Plants 13, no. 4: 487. https://doi.org/10.3390/plants13040487
APA StyleFelouah, O. C., Ammad, F., Adda, A., Bouzid, A., Gharnaout, M. L., Evon, P., & Merah, O. (2024). Morpho-Anatomical Modulation of Seminal Roots in Response to Water Deficit in Durum Wheat (Triticum turgidum var. durum). Plants, 13(4), 487. https://doi.org/10.3390/plants13040487







