Abstract
In this study, an extensive exploration survey of wild progeny was conducted which yielded 18 candidate plus trees (CPTs) of Terminalia bellerica. Seeds of these CPTs were collected from diverse locations between 10°54′ and 28°07′ E longitude, and 76°27′ and 95°32′ N latitude, covering 18 different locations across 5 states of the Indian subcontinent. The objective of the progeny trial was to assess genetic associations and variability in growth and physio-chemical characteristics. Significant variations (p < 0.05) were observed among the growth traits, encompassing plant height, basal diameter, girth at breast height and volume, as well as physio-chemical characteristics such as leaf length, width, area and chlorophyll content, carotenoids, and protein in the progeny trial. Broad-sense heritability (h2b) estimates were consistently high, exceeding 80% for all growth and physiological related traits under investigation except for plant height, leaf length, and girth at breast height. A correlation study revealed that selecting based on plant height, leaf area, and girth at breast height effectively enhances T. bellerica volume. A moderate genetic advance in percent of the mean (GAM) was observed for most traits, except leaf length, leaf width, girth at breast height, and plant height. Across all 13 traits, phenotypic coefficient of variation (PCV) surpassed genotypic coefficient of variation (GCV). Utilizing principal component analysis (PCA) and dendrogram construction categorized the genotypes into seven distinct groups. In conclusion, the study has demonstrated that targeting girth at breast height and plant height would be a highly effective strategy for the establishment of elite seedling nurseries and clonal seed nurseries for varietal and hybridization programs in the future.
1. Introduction
T. bellerica (Gaertn.) Roxb. is an impressive deciduous tree known for its rapid growth and substantial size. With its expansive and spherical crown, it can reach remarkable heights of up to 50 m in its native environment, although it generally grows smaller when nurtured. This tree often showcases prominent buttresses and keeps its branches absent for the initial 20 m of its trunk. The bark of Terminalia exhibits a distinctive ashy grey color, highlighted by delicate longitudinal cracks. Additionally, the inner bark showcases a subtle yellowish tint. Terminalia can be commonly found in native woodlands across several regions of the Indian subcontinent, including West Bengal, Madhya Pradesh, Uttar Pradesh, Maharashtra, Assam, Tamil Nadu, Rajasthan, Karnataka, Kerala, and Punjab [1].
The wood derived from T. bellerica is known for its exceptional hardness and can be utilized in various applications. These include minor construction, the creation of grain measurement tools, boat side planks, fodder production, providing food for Tasar silkworms, soap manufacturing, as well as the extraction of gum with demulcent and purgative properties. Additionally, this wood is also used in the production of ayurvedic medicines such as Triphala. Hence, T. bellerica has been selected for the ongoing study. Candidate plus trees (CPTs) have been carefully chosen based on their exceptional morphometric characteristics from diverse geographical regions. The objective is to identify offspring with enhanced productivity through systematic tree improvement initiatives.
The establishment and productivity of forest tree plantations heavily depend on the selection of species and seed sources within those species [2]. Thus, it is crucial to grasp the variation within these seed sources to ensure effective tree improvement programs [3]. In order to obtain superior genetic material, tree breeders must evaluate the traits that require enhancement while taking into account their variability in terms of both morphological and biochemical characteristics [4]. Conducting variability studies is an essential requirement for any tree improvement program [5], however, in the case of T. bellerica, such studies are still in the early stages of development.
Besides variability, the role of heritability in estimating potential gains from selection programs is of the utmost importance [6]. Understanding the heritability of selected traits is essential [7], hence it is valuable to assess the genetic analysis to determine the heritable components. At present, there is a lack of information about T. bellerica in this field.
Evaluating the extent and type of variation in the initial population is essential for enhancing both qualitative and quantitative progress. Historically, assessing genetic diversity in trees involved conducting provenance/progeny tests and utilizing the Mahalanobis D2 statistic [8]. By clustering genotypes, distantly related clusters can be identified for hybridization, leading to improved segregation to facilitate selection of superior groups or individuals. The individuals or groups that demonstrate enhanced energy and enthusiasm can be effectively utilized in planting programs to enhance productivity [9,10,11,12,13].
Previously, the evaluation of genetic diversity relied on investigations that focused on comparative anatomy, morphology, physiology, and biochemistry [3]. However, the introduction of molecular marker techniques has revolutionized our comprehension of tropical tree population genetics. These techniques enable the analysis of protein or DNA polymorphism and have been instrumental in advancing our understanding in this field [4,14,15,16]. The utilization of DNA marker studies in tropical trees has proven to be effective in various applications. These include understanding variations in origin, determining genotypic identity, characterizing germplasm at a molecular level [17], identifying quantitative trait loci [18,19], studying molecular systematics [20,21], and evaluating genetic diversity [12,13,22]. However, there is a noticeable lack of research on these aspects specifically related to T. bellerica. In light of the aforementioned information, this document presents a research study that seeks to explore the potential of T. bellerica as a feasible alternative to secondary timber genetic resources. The main aim is to tackle the growing demand for raw wood materials within industries that heavily depend on forest resources.
2. Results
2.1. Growth Traits
The analysis of variances indicated significant variation among all the 18 accessions studied in the measured growth traits at a significance level of p < 0.05 (Figure S1). Significant variation in plant height was observed during the growth of progeny from 18 accessions in the T. bellerica (Table 1). Compared to other accessions, FCRITB17 (7.19 m) and FCRITB16 (7.17 m) exhibited significantly higher values for plant height. Conversely, a group of seven accessions exhibited lower heights, measuring less than 6.50 m which included FCRITB18 (6.41 m), FCRITB06 (6.39 m), FCRITB04 (6.29 m), and FCRITB112 (6.23 m).

Table 1.
Mean performance of selected genotypes for growth and physiological traits in T. bellerica.
Significant variation was noted among the accessions in terms of the basal diameter, with an observed mean of 43.9 cm occurring. The accession with the highest basal diameter (50.9 cm) was FCRITB03, followed by FCRITB06 (49.5 cm). The FCRITB14 with a basal diameter of 39.2 cm was apparently distinct, and its measurement was significantly lower than all other accessions.
The range of volumes observed in this study varied from 0.1653 m3 to 0.2251 m3. Among the nine progenies analyzed, namely FCRITB07 (0.2088 m3), FCITB 10 (0.1791 m3), FCRITB14 (0.1765 m3), FCRITB16 (0.2251 m3), FCRITB17 (0.1829 m3), and FCRITB18 (0.2013 m3) exhibited higher volumes compared to the average value obtained from the overall sample. It is worth mentioning that among the identified progeny, FCRITB16 exhibited the highest recorded volume of 0.2251 m3, whereas progeny FCRITB12 achieved the lowest volume of 0.1585 m3. The length of the leaves was observed to range from 25.83 cm to 28.30 cm, with an overall average of 26.75 cm. Among the 18 progenies, progeny FCRITB15 and FCRITB17 recorded the maximum leaf length at 28.30 cm, while progeny FCRITB11 exhibited the minimum leaf length at 25.83 cm. The leaf area value exhibited variation ranging from 236.63 cm2 to 185.99 cm2, with an overall mean value of 208.46 cm2. Out of the 18 progenies of T. bellerica, 7 progenies—FCRITB04, FCRITB07, FCRITB13, FCRITB15, FCRI TB 16, FCRITB17, and FCRITB18—displayed higher leaf areas compared to the general mean. The progeny FCRITB15 recorded the maximum leaf area of (236.63 cm2), while the progeny FCRITB06 exhibited the smallest leaf area of (185.99 cm2) (Table 1).
2.2. Biochemical Traits
The ANOVA revealed prominent variation (p < 0.05) among the 18 studied accessions across all the biochemical traits (Figure S2). Among the 18 progeny, FCRITB05 exhibited higher chlorophyll ‘a’ content with a measurement of 1.127 mg/g, while progeny FCRITB18 had the lowest chlorophyll ‘a’ content at 0.489 mg/g. The overall average for chlorophyll ‘a’ content was recorded as 0.743 mg/g. In terms of chlorophyll ‘b’ content, the general mean was determined to be 0.471 mg/g, ranging from 0.213 mg/g to 0.979 mg/g. Seven specific progenies—FCRITB05 (0.879 mg/g), FCRITB06 (0.613 mg/g), FCRITB07 (0.701 mg/g), FCRITB09 (0.594 mg/g), FCRITB10 (0.534 mg/g), FCR IT B11 (0.497 mg/g), and FCRITB13 (0.293 mg/g)—exhibited higher levels of chlorophyll ‘b’ content compared to the overall average value. The total chlorophyll content varied between 0.501 mg/g and 1.943 mg/g. Among the seven progenies—FCRITB05 (1.943 mg/g), FCRITB06 (0.891 mg/g), FCRITB07 (1.012 mg/g), FCRITB09 (0.904 mg/g), FCRITB10 (0.897 mg/g), FCRITB11 (0.919 mg/g), and FCRITB13 (1.009 mg/g)—the maximum total chlorophyll content was observed when compared to the overall average value. Carotenoid content varied from 0.886 mg/g to 0.372 mg/g, with an average value of 0.581 mg/g. Specifically, the progenies FCRITB02 (0.645 mg/g), FCRITB06 (0.663 mg/g), FCRITB08 (0.604 mg/g), FCRITB10 (0.831 mg/g), FCRITB14 (0.618 mg/g), FCRITB15 (0.818 mg/g), and FCRITB17 (0.886 mg/g) exhibited higher carotenoid content compared to the overall average value. Out of the 18 progenies of T. bellerica, 10 specific progenies, including FCRITB03, FCRITB04, FCRITB06, FCRITB10, FCRITB11, FCRITB12, FCRITB13, FCRITB14, and FCRITB17, have demonstrated a higher crude protein content in comparison to the overall average (Table 2).

Table 2.
Mean performance of selected genotypes for biochemical traits in T. bellerica.
2.3. Heritability
In the conducted study, it was observed that all 13 traits demonstrated a significant level of heritability, ranging from 68.11% to 99.94%, as indicated in Table 3. Among the studied traits, crude protein exhibited the highest level of heritability at 99.94%. This was closely followed by chlorophyll a, b, ratio to chlorophyll a, b (99. 76%, 99. 74%, and 99.31% respectively), as well as carotenoid (99.36%).

Table 3.
Genetic estimates of selected progeny traits in T. bellerica.
2.4. Genotypic and Phenotypic Variation
The presence of high variability in chlorophyll a, chlorophyll b, chl a/chl b, total chlorophyll, carotenoid, and crude protein is indicated by the highest values of GCV and PCV. The magnitude of phenotypic coefficient of variation (PCV) was greater than the respective genotypic coefficient of variation (GCV) for all the studied traits, albeit with only a slight difference.
In Table 3, the analysis of the genotypic coefficient of variance (GCV) and phenotypic coefficient of variance (PCV) for multiple traits is presented. The findings reveal that, in the majority of cases, the phenotypic coefficient variances (PCVs) are slightly higher than the genotypic coefficient variances (GCVs). This indicates that the traits being studied are relatively less affected by environmental factors. Notably, the highest values for both GCV and PCV were observed in chlorophyll b (47.80% and 47.74%), crude protein (41.49% and 41.48%), total chlorophyll (37.95% and 37.88%), Chl a/Chl b (35. 65% and 35.60%), chlorophyll a (28.55% and 28.45%), and carotenoid (25.36% and 25.28%). On the other hand, the traits of plant height, basal diameter, girth at breast height, volume, leaf length, leaf width, and leaf area showed limited variability as indicated by their low GCV and PCV values.
2.5. Genetic Advance
The findings of this investigation revealed all three kinds of genetic advances (low, moderate, and high). Some traits, including leaf length, leaf width, girth at breast height, and plant height displayed genetic advances of less than 10% in Table 3. In contrast, basal diameter, leaf area, and volume exhibited moderate genetic advances ranging from 13.35% to 17.54%. Particularly, biochemical traits demonstrated a high genetic advance, exceeding 20% with a maximum of 98.24% by chlorophyll b. This study uncovers biochemical traits with both high heritability and genetic advance as a percentage of the mean (>50).
2.6. Correlation among the Traits
Correlation analysis revealed several significant relationships between mean progenies traits (Table 4). Plant height of provinces was significantly correlated (r = 0.545 *) with carotenoid (Figure S2) and positively correlated with basal diameter (r = 0.194), girth at breast height (r = 0.158), leaf area (r = 0.248), volume (r = 0.413), leaf length (r = 0.205), leaf width (r = 0.194), and crude protein (r = 0.025). Progenies heights were negatively correlated with chlorophyll content.

Table 4.
Pearson Correlation Coefficient among different characters studied.
2.7. Principle Component Analysis
Principle Component Analysis (PCA) was executed to facilitate the visualization of the entire dataset through a condensed dimension plot. The application of PCA was for determining genetic relationships among progenies and exploring correlations among growth, physiological, and biochemical traits. In this study, the performed PCA revealed that over 99% of the observed variances could be accounted for by the initial three principal components (Figure S3). Specifically, PC1, PC2, and PC3 contributed 73.1%, 24.1% and 2.6% to the total variability, respectively (Figure 1. PC1 predominantly represents leaf area and crude protein, PC2 explains the same, and PC3 primarily contributes to basal diameter.
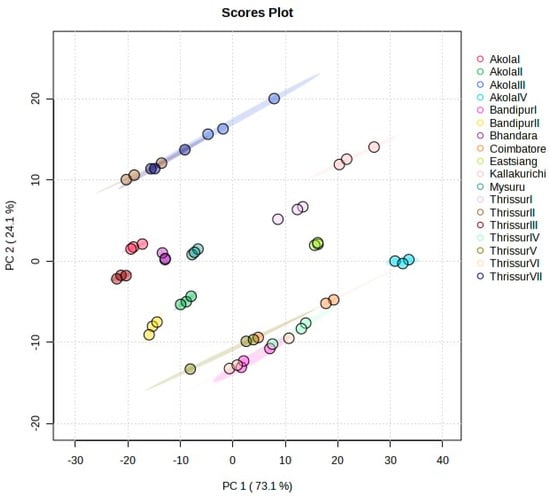
Figure 1.
PCA showed the variation in the 18 provinces.
Component scores for the 18 studied progenies are shown in Table S1. Positive values for PC1 indicate progenies with plant height, girth at breast height, leaf area, volume, leaf length, leaf width, and carotenoids in general. FCRITB10, FCRITB16, and FCRITB17 belong to this group. The lowest values for PC1 indicate basal diameter such as FCRITB12 and FCRITB14. The highest values for PC2 indicate all the parameters except for basal diameter and volume. The scatter biplot in Figure S4 shows the relationship between studied genotypes and depicts a clear pattern of the grouping of provincesprovinces. All the provincesprovinces were scattered widely in different quarters.
2.8. Heatmap Clustering
Figure 2 illustrates K-Means hierarchical clustering for growth characteristics, physiological traits, and biochemical traits in T. bellerica accessions. A total of 18 Terminalia progenies were categorized into 7 clusters using K-Means clustering, with cluster V and VII holding the highest number of accessions (4) and others sharing 2 progenies under each.
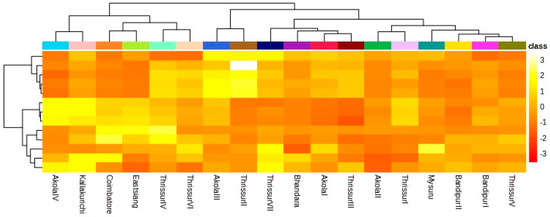
Figure 2.
Heatmap dendrogram showed the variations among the 18 progenies.
Interestingly, cluster VII consisting of FCRITB01, FCRITB02, FCRITB03, and FCRITB08 exhibited similar mean values for the growth and biophysiological characters. FCRITB15nd FCRITB17 were placed under cluster I and showed similar values for the traits. Despite having the highest number of clustering, progenies did not exhibit high mean values for any of the measured traits amidst the different climatic provincesprovinces.
3. Discussion
After a thorough analysis of 18 progenies of T. bellerica, it was observed that progenies FCRITB02 and FCRITB13 showed initial superiority based on their biometric attributes. However, progenies FCRITB05, FCRITB07, and FCRITB09 demonstrated significant superiority in more than three biometric traits including plant height, basal diameter, leaf length, leaf width, and leaf area. This highlights the potential of these specific progenies for further study or utilization in future breeding programs.
The progenies, specifically FCRITB03, FCRITB10, FCRITB16, and FCRITB17 displayed significantly higher measurements in various biometric characteristics including height, basal diameter, leaf length, leaf width, and leaf area. The outcomes are consistent with Neolamarckia cadamba, Casuarina clones, Ailanthus excelsa, Santalum album, Dalbergia sissoo, Pongamia pinnata, Acacia species, Salix species, Aquilaria malaccensis, Melia azedarach, Leucaena leucocephala, and Toona ciliata [7,9,11,23,24,25,26,27,28,29,30,31,32]. Furthermore, the comparable height observed in FCRITB16 and FCRITB17 can be attributed to the similarity in weather parameters, with both provincesprovinces experiencing an average temperature of 17.3 °C and annual rainfall of 2036 mm.
Leaf area is the most essential characteristic when it comes to biomass production. The progenies displayed a significant amount of variation in terms of leaf traits, indicating that these traits can be effectively utilized for selection purposes. The conducted study revealed notable variations in the investigated leaf-related characteristics, including leaf length, leaf breadth, and leaf area. It was observed that among the 18 progenies examined, FCRITB17 demonstrated superiority in all of the analyzed leaf parameters. This could be attributed to its remarkable growth and volume, potentially explaining its exceptional performance. The current investigation is supported by prior evidence showing variation in leaf features and their correlation to production in various plant species such as Toona ciliata [33], N. cadamba [34,35], Ficus carica [36], Acacia species [7], Pongamia pinnata [37], Aquilaria malaccensis [29], Poplar [38], Dalbergia sissoo [27], and Acacia catechu [39].
Following the analysis of biochemical data from a study on 18 offspring of T. bellerica, it was determined that one particular offspring—FCRITB10—consistently displayed significantly elevated levels for all six studied biochemical parameters. Additionally, two other offspring, namely FCRITB12 and FCRITB13, demonstrated superiority in five parameters: chlorophyll ‘a’, chlorophyll ‘b’, chlorophyll a/b ratio, total chlorophyll, and carotenoid levels.
In the present study, the biochemical characteristics of 18 progenies of T. bellerica were boserved. Out of the six examined biochemical properties, it is noteworthy that only one progeny, namely FCRITB05, consistently demonstrated superior performance compared to the other progenies. This superiority was observed to be significantly remarkable. Three progenies—FCRITB07, FCRITB10, and FCRITB13—have exhibited their superiority in five biochemical characteristics. These characteristics include chlorophyll ‘a’, chlorophyll ‘b’, chlorophyll a/b ratio, total chlorophyll, and carotenoids. Previous studies have been conducted on various plant species such as L. leucocephala, N. cadamba, Ailanthus excelsa, Albizia lebbeck, Acacia catechu, Bassia latifolia, Mangifera indica, and Ulmis pumila [40,41,42,43,44,45]. These investigations have shown that these plants exhibit similar variations in terms of their biochemical attributes. Therefore, the findings from previous studies provide support for the conclusions made in the current investigation. Due to its superior performance in a wide range of biometric and biochemical characteristics, FCRITB05 outperformed other progenies of T. bellerica. As a result, it is currently being evaluated for prompt integration into future breeding programs.
Heritability serves as a reliable indicator of the transmission of traits from parents to their progeny, categorized as low (below 30%), medium (30–60%), and high (above 60%). The concept of heritability plays a pivotal role in the field of plant breeding, assisting breeders in the selection of genotypes from a wide range of genetic populations. High heritability values are particularly valuable as they enable the effective selection of specific traits. In the conducted study, it was observed that all 13 traits demonstrated a significant level of heritability, ranging from 68.11% to 99.94% as indicated in Table 5. Among the studied traits, crude protein exhibited the highest level of heritability at 99.94%.

Table 5.
Provinces selected from the Indian subcontinent and the geographical location details.
The presence of high variability in chlorophyll a, chlorophyll b, chl a/chl b, total chlorophyll, carotenoid, and crude protein is indicated by the highest values of GCV and PCV. The magnitude of phenotypic coefficient of variation (PCV) was greater than the respective genotypic coefficient of variation (GCV) for all the studied traits, albeit with only a slight difference. Similar findings were reported by Rao et al. [46]. In the current study, there was minimal disparity between genotypic and phenotypic coefficients of variation for all the studied traits except plant height. This implies that these traits are less susceptible to environmental influences. Comparable results were reported in Populus deltoids [47] and in willow trees [48]. The marginal difference between PCV and GCV of almost all the characters studied in all the traits suggested that there was high heritability of variation among the characters.
Heritability and genetic advancement are pivotal metrics in unraveling the genetic intricacies of various agricultural traits. This study delves into the interplay between heritability and genetic advance as a percentage of mean, shedding light on the potential for effective selection strategies. The analysis reveals that traits exhibiting both high heritability and a high genetic advance as a percentage of the mean primarily operate under the influence of additive gene action. These findings signify the suitability of direct selection to enhance the performance of these traits, promising progress through selective breeding.
Conversely, traits characterized by moderate heritability and low genetic advance as a percentage of the mean are predominantly influenced by non-additive gene action. For such traits, direct selection may pose challenges, as a substantial portion of the variation is attributed to environmental factors. These environmental effects may arise from soil fertility disparities and other unpredictable variables, as suggested by Reddy et al. [49]. Researchers have proposed that traits governed by non-additive gene action may benefit more from management practices than direct selection for trait improvement. This perspective aligns with the recommendations to emphasizing the importance of tailored management approaches [50,51].
The findings of this investigation revealed all three kinds of genetic advances (low, moderate, and high) and uncovers biochemical traits with both high heritability and genetic advance as a percentage of the mean (>50). These high values indicate the prevalence of additive gene action for these specific traits, signifying the potential for effective trait enhancement through selective breeding.
Conversely, the study identifies traits (leaf length, leaf width, girth at breast height, and plant height) characterized by high heritability but low genetic advance as a percentage of the mean. Additionally, traits such as basal diameter, leaf area, and volume exhibit high heritability with moderate genetic advance as a percentage of the mean.
Hierarchical clustering, based on Ward’s minimum variance cluster analysis, revealed phylogeographic patterns of genetic diversity. Length of the horizontal branches between clusters indicates that there is a high degree of dissimilarity between clusters. K-means clustering analysis demonstrated that trees from different geographic regions were grouped together in clusters. Interestingly, trees from the same geographical area were placed in different clusters, suggesting that geographical diversity did not necessarily correlate with genetic diversity and implying that it may have undergone divergent changes in various traits due to different selection pressures. This type of genetic diversity may arise from variations in adoption methods, selection criteria, natural selection pressures, and environmental factors [52]. This suggests that genetic drift has played a more significant role in generating diversity compared to geographic diversity [53]. The absence of any relationships between genetic diversity and geographical distribution in the current study is consistent with the findings of [54,55].
Furthermore, this clustering approach identified promising accessions with favorable traits, paving the way for the establishment of elite seedling nurseries and clonal seed nurseries for varietal and hybridization programs in the future.
The growth of a plant, as indicated by volume, basal diameter, and plant height is considered highly significant for improvement in the current study. Similarly, growth traits in black poplar are the most crucial based on principal component analysis [56]. In a study on the morphological characters of P. deltoides hybrid clones in a nursery, Ozel et al. [57] applied factor analysis, explaining 71.46% of the total variance with the first five components and captured 90% cumulative variability for the first two principal components to differentiate leaf characters of Populus nigra similar to the present study (0.948) [58]. Tunctaner [59] reported five principal components based on the study of fourteen traits in willow clones, a pattern also observed by Singh et al. [53] in Salix clones. The growth characters are attributed to distinct genetic constitution of the clones as highlighted in this study [60]. The promising clones selected for this study must undergo multi location trials to investigate the relationship between genotype and environment at various sites. This will allow for an analysis of the suitability of the clones and allow for the use of the clones for intra- and inter-specific control breeding (hybridization) aimed at producing more productive clones.
4. Materials and Methods
4.1. Genetic Material
A comprehensive and extensive survey of wild germplasm was conducted with the aim of identifying promising candidate plus trees (CPTs) of T. bellerica. This survey originated from five distinct states of the Indian subcontinent: Tamil Nadu, Maharashtra, Kerala, Karnataka, and Arunachal Pradesh, and examined the ecological impact on genetic diversity, growth, and eco-physiological traits (Figure 3, Table 5). These provinces were selected due to their inherent adaptability to the growing conditions suitable for T. bellerica. As all of the selected origins are distributed across the Indian subcontinent, they exhibit both commonalities and variations in their climatic origins. The selection process of CPTs involved utilizing the single-tree selection method, which relied on assessing the phenotypic traits with economic significance viz. total height, girth at breast height, bole height, and volume [50] (Figure S5, Table 6). Precautions were taken to ensure that the selected trees were free from pest and disease infestations and excluded isolated or poorly performing trees, commonly referred to as wolf trees. A total of 18 CPTs were collected from diverse locations between 10°54′ and 28°07′ E longitude, and 76°27′ and 95°32′ N latitude, across five states of the Indian subcontinent (Figures S6 and S7). Three kilograms of mature pods were harvested from each CPT by following a random sampling procedure. These pods were collected from all four directions of the crown of each selected tree during the fruiting season between September and November in the year 2019. The gathering of potential CPTs was achieved through collaboration with officials from the respective forest departments while strictly adhering to required permissions and regulations.
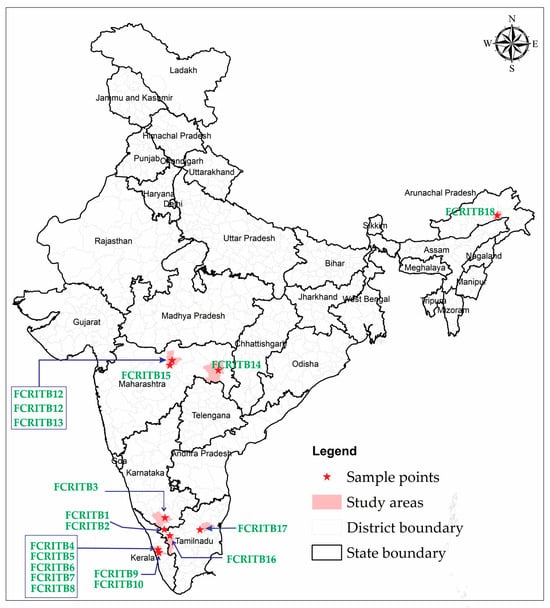
Figure 3.
Map showing the progenies collection point.

Table 6.
Morphometric attributes of selected Candidate Plus Trees of T. bellerica.
4.2. Study Site
After the collection process, the progenies were brought to the Forest College and Research Institute (FC&RI), TNAU, located in Mettupalayam, Tamil Nadu, India (geographical coordinate of 11.32° N latitude and 76.93° E longitude, 320 m MSL). Mettupalayam experiences a semi-arid climate characterized by a mean annual rainfall of 945 mm, along with an average of 73.6 rainy days per year. The annual temperature range varies from a minimum of 15.4 °C to a maximum of 34.9 °C. Typically, the lowest temperatures are recorded in January, while the highest temperatures occur in May each year. For the purpose of identifying elite progeny, a trial was initiated in the year 2020 at the FC&RI with three replications.
4.3. Progenies Planting
The plus trees’ seeds were planted in raised beds, utilizing a mixture of red soil, sand, and farmyard manure (FYM) in a 2:1:1 ratio. These beds were consistently watered and meticulously tended to for a duration of two months. Following this period, the saplings with a collar region thickness exceeding 3–4 cm were carefully chosen and transplanted into polybags containing a blend of red soil, sand, and FYM in the same 2:1:1 ratio. Approximately one month after transplantation, these young seedlings were finally transferred and planted in the main field. No treatments or fertilizers were applied during the nursery stage. The establishment of the progeny evaluation trial in the field adhered to a randomized block design (RBD), with plants spaced at intervals of 4 × 4 m. Within each replication, four progenies per CPT were included for comprehensive evaluation. During the planting process, each seedling received additional nutrients in the form of 250 g of farmyard manure (FYM), 25 g of vermicompost and 5 g of di-ammonium phosphate (DAP). The subsequent data was acquired from the trees that were planted and observed at different time intervals.
4.4. Morphological
Data were meticulously recorded for all 18 progenies within each replication when the plants were 24 months old for the morphological traits. Field measurements were taken for each individual, including tree height (H) and basal diameter (BD). The plant’s height was measured in meters (m) from the base of the stem to the tip using a measuring tape. The basal diameter of the trees at their base (in centimeters) was measured using a digital caliper from the Large SDN series. In cases where a tree had multiple basal stems, the diameters of all individual trunks were measured, and a single equivalent basal diameter (BD) value was calculated following the method outlined by Alvarez et al. [61].
4.5. Biochemical Parameters
Chlorophyll was extracted from fresh leaves using 80% acetone and 0.25 g leaf samples. The resulting extract was then measured spectrophotometrically at wavelengths of 475 nm, 645 nm, and 663 nm. The determination of total chlorophyll and carotenoid contents was carried out using established methodologies [62]. The Lowrey’s method [63] was employed to evaluate the protein content of the leaves.
4.6. Genetic Estimates
Heritability, genetic advancement as a percentage of the mean, phenotypic, and genotypic coefficients of variation (PCV and GCV), were calculated for volume as well as growth traits, following the methodologies proposed by various researchers [64,65,66].
Broad-sense heritability in all the progenies was estimated by dividing the variance in measurements into two components: between-accessions and within-accessions [67].
4.7. Statistical Analysis
The initial dataset was created by calculating the averages for each trait across four CPTS within each replication and between replication in the experiment. These calculated means were then subjected to subsequent statistical and genetic analyses. Correlation between traits to reveal possible associations was calculated with raw data based on single plant estimates, using the Pearson correlation coefficient at p ≤ 0.05. PCA was performed with progeny means to determine the relationships among progenies and to obtain an overview of correlation among traits. Various statistical analysis was conducted using the SPSS Windows software package (IBM SPSS version 26).
5. Conclusions
The ultimate objective of tree improvement is to enhance the growth and yield traits of tree species. These traits are intricate and are influenced by the interaction of various physiological and morphological characteristics. Therefore, solely relying on the performance of individual tree species for improvement might prove to be less effective. Hence, it can be concluded that for tree improvement of T. bellerica through the phenotypic selection process, the number of plus trees selected from a population should be sufficiently large in order to exploit the large intra-population genetic variation. Besides, significant differences were found between the features in the progeny study, which evaluated genetic correlations and variability in growth and physio-chemical parameters. For the majority of variables, estimates of broad-sense heritability were high, suggesting significant genetic control. Plant height, leaf area, and girth at breast height were found to be important characteristics for increasing T. bellerica volume through correlation studies. The study demonstrated the effectiveness of targeting girth at breast height and plant height for establishing elite seedling nurseries and clonal seed nurseries for future varietal and hybridization programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13040470/s1, Figure S1: One way ANOVA plot showing the significant differences among the studied physiochemical properties amidst the eighteen progenies; Figure S2: Correlation map showing the relationship between 13 characters in the study; Figure S3: PCA variance explained; Figure S4: Segregation of the 18 Terminalia progenies according to their growth, physiological and biochemical characteristics determined by principal component analysis; Figure S5: Selection of candidate plus; Figure S6: Walter-Leith diagram of the monthly rainfall and daily average temperature of Bandipur (Karnataka), Thrissur (Kerala), Kallakurichi (Tamil Nadu) and Jognari (Tamil Nadu); Figure S7: Walter-Leith diagram of the monthly rainfall and daily average temperature of Maharashtra, Mysuru (Karnataka), Pasighat (Arunachal Pradesh) and Vellanikkara (Kerala); Table S1: Component scores and loadings of T. bellerica traits.
Author Contributions
Conceptualization, S.U.K., K.T.P., K.S., S.V. and D.U.N.; methodology, S.U.K., K.T.P., K.S., S.V., D.U.N. and M.J.S.; software, S.U.K., K.S., S.V. and D.U.N.; validation, S.U.K., K.S., S.V., D.U.N., S.M.K., M.D., M.R. and V.G.; formal analysis, S.V and D.U.N.; investigation, S.U.K., K.T.P., K.S., S.V., D.U.N., S.M.K., M.D., M.R. and V.G.; resources, S.U.K., K.T.P., K.S., S.V., D.U.N., S.M.K., M.D., M.R. and V.G.; data curation, S.U.K., K.S., S.V., D.U.N., S.M.K., M.D., M.R. and V.G.; writing—original draft preparation, S.U.K., K.T.P., K.S., S.V. and D.U.N.; writing—review and editing, S.U.K., K.T.P., K.S., S.V., D.U.N., S.M.K., M.D., P.K., M.R. and V.G.; visualization, S.U.K., K.T.P., K.S., S.V., D.U.N., S.M.K., M.D., P.K., M.R. and V.G.; supervision, S.U.K., K.T.P., K.S., S.V., D.U.N., S.M.K., M.D., M.R. and V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data is available from corresponding author upon reasonable request.
Acknowledgments
The authors express their gratitude to the Forest College and Research Institute, Mettupalayam, Tamil Nadu, India. The contribution of their essential laboratory and instrumentation facilities has played a vital role in providing support for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Patil, U.H.; Gaikwad, D.K. Phytochemical evaluation and bactericidal potential of Terminalia arjuna stem bark. Int. J. Pharm. Sci. Res. 2011, 2, 614. [Google Scholar]
- Hein, P.R.G.; Chaix, G. NIR spectral heritability: A promising tool for wood breeders? J. Near Infrared Spectrosc. 2014, 22, 141–147. [Google Scholar] [CrossRef]
- Gray, L.K.; Rweyongeza, D.; Hamann, A.; John, S.; Thomas, B.R. Developing management strategies for tree improvement programs under climate change: Insights gained from long-term field trials with lodgepole pine. For. Ecol. Manag. 2016, 377, 128–138. [Google Scholar] [CrossRef]
- Sæbø, A.; Borzan, Ž.; Ducatillion, C.; Hatzistathis, A.; Lagerstrom, T.; Supuka, J.; García-Valdecantos, J.L.; Rego, F.; Van Slycken, J. The selection of plant materials for street trees, park trees and urban woodland. In Urban Forests and Trees: A Reference Book; Springer: Berlin/Heidelberg, Germany, 2005; pp. 257–280. [Google Scholar]
- Zobel, B.; Talbert, J. Applied Forest Tree Improvement; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Schmidt, P.; Hartung, J.; Bennewitz, J.; Piepho, H.P. Heritability in plant breeding on a genotype-difference basis. Genetics 2019, 212, 991–1008. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Parameswari, N.; Chin, C.F.; Baharum, Z.; Olalekan, K.K.; Aini, A.N. Selection and Screening of Superior Genotypes for Quality Planting Stock Based on Vegetative Growth Performance of Some Selected 12-Year-Old Acacia Species. Open J. For. 2016, 6, 217–229. [Google Scholar] [CrossRef]
- ICES. Herring Assessment Working Group for the Area South of 62°N (HAWG). ICES Scientific Reports. 2023. Available online: https://doi.org/10.17895/ices.pub.22182034.v2 (accessed on 15 August 2023).
- Kanna, S.U.; Krishnakumar, N.; Kather, M.M.A.; Jailani, K. Growth performance of Ailanthus excelsa through progeny test. Pharma Innov. J. 2019, 8, 204–210. [Google Scholar]
- Krishnakumar, N.; Parthiban, K.T.; Jayamani, P.; Revathi, R.; Umeshkanna, S. Genetic variability of growth parameters among different progenies of Santalum album L. J. Indian Soc. Coast. Agric. Res. 2017, 35, 56–63. [Google Scholar]
- Prakash, G.M. Genetic Evaluation and Wood Characterization Studies in Neolamarckia cadamba (Roxb.) Bosser. Ph.D. Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2017. [Google Scholar]
- Mohanraj, K.; Umesh Kanna, S.; Parthiban, K.T.; Kumaran, K. Variability, Broad Sense Heritability, Genetic Advance of Toona ciliata M. Roem., Progenies. Pharma Innov. J. 2021, 10, 1247–1251. [Google Scholar]
- Jawahar Vishnu, M.V.; Parthiban, K.T.; Umadevi, M.; Sudhagar, R.J.; Fernandaz, C.C.; Javed, T.; Alotaibi, S.S. Genetic evaluation of Jatropha backcross hybrid clones (BC4F1) for yield and oil quality. Front. Genet. 2022, 13, 953486. [Google Scholar] [CrossRef]
- Migliore, J.; Lezine, A.M.; Hardy, O.J. The recent colonization history of the most widespread Podocarpus tree species in Afromontane forests. Ann. Bot. 2020, 126, 73–83. [Google Scholar] [CrossRef]
- Dhivya, S.; Ashutosh, S.; Gowtham, I.; Baskar, V.; Harini, A.B.; Mukunthakumar, S.; Sathishkumar, R. Molecular identification and evolutionary relationships between the subspecies of Musa by DNA barcodes. BMC Genom. 2020, 21, 659. [Google Scholar] [CrossRef] [PubMed]
- Donkpegan, A.S.; Doucet, J.L.; Migliore, J.; Duminil, J.; Dainou, K.; Pineiro, R.; Hardy, O.J. Evolution in African tropical trees displaying ploidy-habitat association: The genus Afzelia (Leguminosae). Mol. Phylogenet. Evol. 2017, 107, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Doğan, Y.; Comertpay, G.; Yıldız, M.; Baloch, F.S. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef]
- Ye, M.; Zhu, X.; Gao, P.; Jiang, L.; Wu, R. Identification of quantitative trait loci for altitude adaptation of tree leaf shape with Populus szechuanica in the Qinghai Tibetan Plateau. Front. Plant Sci. 2020, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xiao, L.; Quan, M.; Wang, Q.; El-Kassaby, Y.A.; Du, Q.; Zhang, D. Linkage-linkage disequilibrium dissection of the epigenetic quantitative trait loci (epiQTLs) underlying growth and wood properties in Populus. New Phytol. 2020, 225, 1218–1233. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Shinwari, Z.K.; Zahra, N.B.; Jan, S.A.; Shinwari, S.; Najeebullah, S. DNA barcoding and molecular systematics of selected species of family Acanthaceae. Pak. J. Bot. 2020, 52, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.; Doyle, J.J. Molecular Systematics of Plants II: DNA Sequencing; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Bhandari, H.R.; Bhanu, A.N.; Srivastava, K.; Singh, M.N.; Shreya, H.A. Assessment of genetic diversity in crop plants-an overview. Adv. Plants Agric. Res. 2017, 7, 279–286. [Google Scholar]
- Parthiban, K.T.; Thirunirai-Selvan, R.; Palanikumaran, B.; Krishnakumar, N. Variability and genetic diversity studies on Neolamarckia cadamba genetic resources. J. Trop. For. Sci. 2019, 31, 90–98. [Google Scholar] [CrossRef]
- Parthiban, K.T.; Kanagaraj, N.; Palanikumaran, B.; Krishnakumar, N. Development of DUS descriptor for casuarina genetic resources. Int. J. Genet. 2018, 10, 333–338. [Google Scholar] [CrossRef]
- Daneva, V.; Dhillon, R.S.; Johar, V. Plus tree selection and progeny testing of superior candidate plus trees (CPTs) of Ailanthus excelsa. J. Pharmacogn. Phytochem. 2018, 7, 543–545. [Google Scholar]
- Krishnakumar, N.; Parthiban, K.T.; Kanna, S.U. Production, management and utilization technology for sandal wood (Santalum album L.). In Forest Technol: Complete Value Chain Approach; Scientific Publishers: New Delhi, India, 2017; pp. 372–383. [Google Scholar]
- Sharma, A.; Bakshi, M. Growth and heritability estimates among clones of Dalbergia sissoo Roxb. in a clonal seed orchard. For. Sci. Pract. 2011, 13, 211. [Google Scholar] [CrossRef]
- Sharma, J.P.; Singh, N.B.; Benal, V.; Gupta, D. Cultivation of shiitake mushroom on selected clones of willow (Salix species): A Case Study Under PPP Mode. In Proceedings of the 2nd International Conference on Bio-resource and Stress Management, Hyderabad, India, 7–10 January 2015; Volume 225. [Google Scholar]
- Mohamed, M.N.; Parthiban, K.T.; Ravi, R.; Kumar, P. Provenance variation in growth and genetic potential of Aquilaria malaccens is under nursery condition. Afr. J. Biotechnol. 2015, 14, 2005–2013. [Google Scholar]
- Meena, H.; Kumar, A.; Sharma, R.; Chauhan, S.K.; Bhargava, K.M. Genetic variation for growth and yield parameters in half-sib progenies of Melia azedarach (Linn.). Turk. J. Agric. For. 2014, 38, 531–539. [Google Scholar] [CrossRef]
- Sangram, C.; Keerthika, A. Genetic variability and association studies among morphological traits of Leucaena leucocephala (Lam.) de Wit. genetic resources. Res. J. Agric. For. Sci. 2013, 1, 23–29. [Google Scholar]
- Kundal, M.; Thakur, S.; Dhillon, G. Evaluation of growth performance of half sib progenies of Toona ciliata M. Roem under field conditions. Genetika 2020, 52, 651–660. [Google Scholar] [CrossRef]
- Vijay, R.; Atul, R. Progeny performance of plus trees of Toona ciliata M. Roem. under nursery and field conditions. Indian For. 2009, 135, 92–98. [Google Scholar]
- Selvan, R.T.; Parthiban, K.T. Clonal evaluation and genetic divergence studies in Neolamarckia cadamba roxb. Electron. J. Plant Breed. 2018, 9, 692–704. [Google Scholar] [CrossRef]
- ThiruniraIselvan. Improvement and Utilization of Neolamarckia cadamba (Roxb.) Bosser Genetic Resources for Feed Quality and Utility. Ph.D. Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2017. [Google Scholar]
- Mohamed, Z.; Abdelsalam, N.; Abdel Latif, K.; Abdelhady, R. Genetic Diversity of fig (Ficus carica L.) Based on Morphological Characters and Two-Way Hierarchical Cluster Analysis. Alex. Sci. Exch. J. 2017, 38, 168–174. [Google Scholar] [CrossRef]
- Garima, G.; Handa, A.K.; Deepak, M. Variation in seed and seedling traits of Pongamia pinnata. Indian For. 2016, 142, 852–857. [Google Scholar]
- Monclus, R.; Dreyer, E.; Delmotte, F.M.; Villar, M.; Delay, D.; Boudouresque, E.; Brignolas, F. Productivity, leaf traits and carbon isotope discrimination in 29 Populus deltoids × P. nigra clones. New Phytol. 2005, 167, 53–62. [Google Scholar] [CrossRef]
- Mohit, G.; Neelu, G. Genetic variability and character association in Acacia catechu Willd. Indian For. 2006, 132, 785–794. [Google Scholar]
- Mishra, S.; Bhatt, R. Varietal differences in photosynthetic pigments and biochemical constituents in Leucaena leucocephala. Indian J. Plant Physiol. 2004, 9, 86–89. [Google Scholar]
- Zayed, M.Z.; Zaki, M.A.; Ahmad, F.B.; Ho, W.S.; Pang, S.L. Comparison of mimosine content and nutritive values of Neolamarckia cadamba and Leucaena leucocephala with medicago sativa as forage quality index. Int. J. Sci. Technol. Res. 2014, 3, 146–150. [Google Scholar]
- Radhakrishnan, S. Genetic Divergence and DNA Based Molecular Characterization in Albizia lebbeck (L.) Benth. Ph.D. Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2001. [Google Scholar]
- Ramachandra, N.G.; Nautiyal, S.; Negi, D.S.; Thapliyal, R.C. Seed source variation in chlorophyll contents of leaves of Acacia catechu Willd. under different water stress conditions. Ann. For. 1997, 5, 88–96. [Google Scholar]
- Malik, A.B.; Makbdoom, M.I.; Hag, A. Investigations on the efficiency of exogenous synthetic growth regulators on fruit drop in mango (Mangifera indica L.). Egypt. J. Hort. 1993, 20, 1–14. [Google Scholar]
- Cai, Y.C.; Ma, G.H.; Wang, Z.Q. Physiological characters of different geoprovenant populations of Siberian elm (Ulmus pumila L.). Ningxia J. Agro-For. Sci. Technol. 1990, 2, 20–23. [Google Scholar]
- Rao, G.R.; Shanker, A.K.; Srinivas, I.; Korwar, G.R.; Venkateswarlu, B. Diversity and variability in seed characters and growth of Pongamia pinnata (L.) Pierre accessions. Trees 2011, 25, 725–734. [Google Scholar] [CrossRef]
- Kadam, S.K. Evaluation of Full-Sib Progenies of Selected Clones of Poplar (Populus deltoids Bartr.). Ph.D. Thesis, Forest Research Institute, Dehradun, India, 2002. [Google Scholar]
- Choudhary, P.; Singh, N.B.; Sharma, J.P.; Verma, A. Estimation of genetic parameters among intra and interspecific progenies of tree willows. Indian For. 2016, 142, 1157–1163. [Google Scholar]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Vashistha, R.; Dangi, A.K.; Kumar, A.; Chhabra, D.; Shukla, P. Futuristic biosensors for cardiac health care: An artificial intelligence approach. 3 Biotech 2018, 358, 1–11. [Google Scholar] [CrossRef]
- Wondimu, T.; Gizaw, A.; Tusiime, F.M.; Masao, C.A.; Abdi, A.A.; Gussarova, G.; Brochmann, C. Crossing barriers in an extremely fragmented system: Two case studies in the afro-alpine sky island flora. Plant Syst. Evol. 2014, 300, 415–430. [Google Scholar] [CrossRef]
- Vivekananda, P.; Subramaninan, S. Genetic divergence in rainfed rice. Oryza 1993, 39, 60–62. [Google Scholar]
- Singh, N.B.; Sharma, J.P.; Huse, S.A.; Thakur, I.K.; Gupta, R.K.; Sankhyan, H.P. Heritability, genetic gain, correlation and principal component analysis in introduced willow (Salix spp.) clones. Indian For. 2012, 138, 1100–1109. [Google Scholar]
- Kaushik, N.; Kumar, S.; Kumar, K.; Beniwal, R.S.; Kaushik, N.; Roy, S. Genetic variability and association studies in pod and seed traits of Pongamia pinnata (L.) Pierre in Haryana, India. Genet. Resour. Crop Evol. 2007, 54, 1827–1832. [Google Scholar] [CrossRef]
- Divakara, B.N.; Das, R. Variability and divergence in Pongamia pinnata for further use in tree improvement. J. For. Res. 2011, 22, 193–200. [Google Scholar] [CrossRef]
- Isik, F.; Toplu, F. Variation in juvenile traits of natural black poplar (Populus nigra L.) clones from Turkey. New For. 2004, 27, 175–182. [Google Scholar] [CrossRef]
- Ozel, H.B.; Ertekin, M.; Tunctaner, K. Genetic variationin growth traits and morphological characteristics ofeastern cottonwood (Populus deltoides Bartr.) hybridsat nursery stage. Sci. Res. Essays 2010, 5, 962–969. [Google Scholar]
- Kajba, D.; Ballian, D.; Idžojtić, M.; Poljak, I. Leaf morphology variation of Populus nigra L. in natural populations along the rivers in Croatia and Bosnia and Herzegovina. South-East Eur. For. Seefor 2015, 6, 39–51. [Google Scholar] [CrossRef]
- Tunctaner, K. Primary selection of willow clones for multi-purpose use in short rotation plantation. Silvae Genet. 2002, 51, 105–112. [Google Scholar]
- Singh, N.B.; Sharma, J.P.; Choudhary, P.; Gupta, R.K. Genotype x environment interaction and growth stability of exotic tree willow (Salix spp.) clones. Indian J. Genet. 2014, 74, 222–228. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Villagra, P.E.; Villalba, R.; Debandi, G. Effects of the pruning intensity and tree size on multi-stemmed Prosopis flexuosa trees in the Central Monte, Argentina. For. Ecol. Manag. 2013, 310, 857–864. [Google Scholar] [CrossRef]
- Yoshida, S.; Coronel, V. Nitrogen nutrition, leaf resistance, and leaf photosynthetic rate of the rice plant. Soil Sci. Plant Nutr. 1976, 22, 207–211. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbough, H.I.; Fair, A.L.; Randall, R.I. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Baenziger, S.P.; Gregory, S.; McMaster, R.; Wilhelm, W.W.; Weiss, A.; Hays, C.J. Putting genes into genetic coefficients. Field Crops Res. 2004, 90, 133–143. [Google Scholar] [CrossRef][Green Version]
- Pliura, A.; Zhang, S.Y.; MacKay, J.; Bousquet, J. Genotypic variation in wood density and growth traits of poplar hybrids at four clonal trials. For. Ecol. Manag. 2007, 238, 92–106. [Google Scholar] [CrossRef]
- Yoshida, H.; Takeshi, H.; Katsura, K.; Tatsuhiko, S. A model explaining genotypic and environmental variation in leaf area development of rice based on biomass growth and leaf N accumulation. Field Crops Res. 2007, 102, 228–238. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longmans Green: Harlow, UK, 1996. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).