Hydrogen Peroxide Alleviates Salt Stress Effects on Gas Exchange, Growth, and Production of Naturally Colored Cotton
Abstract
1. Introduction
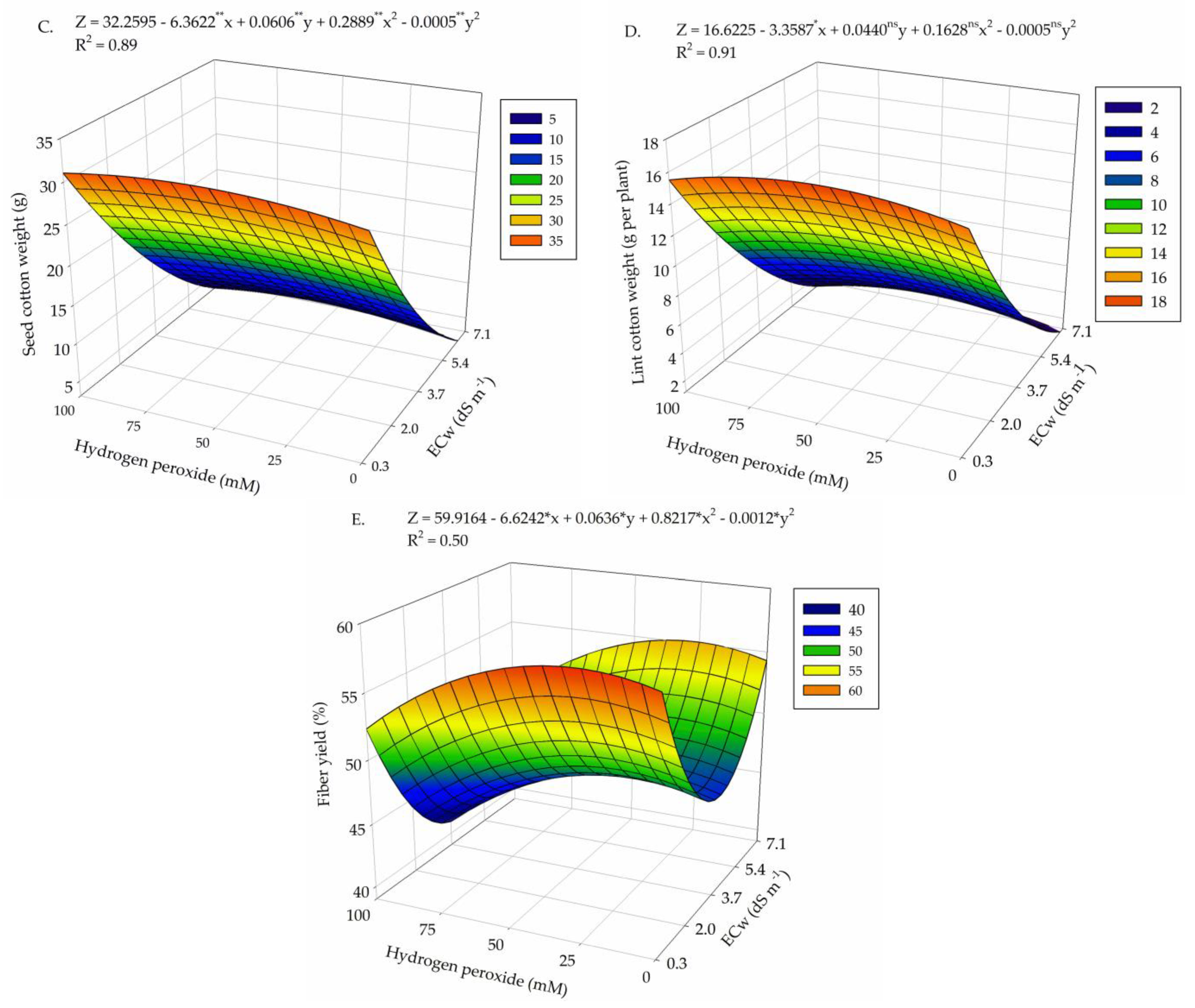
2. Results
3. Discussion
4. Materials and Methods
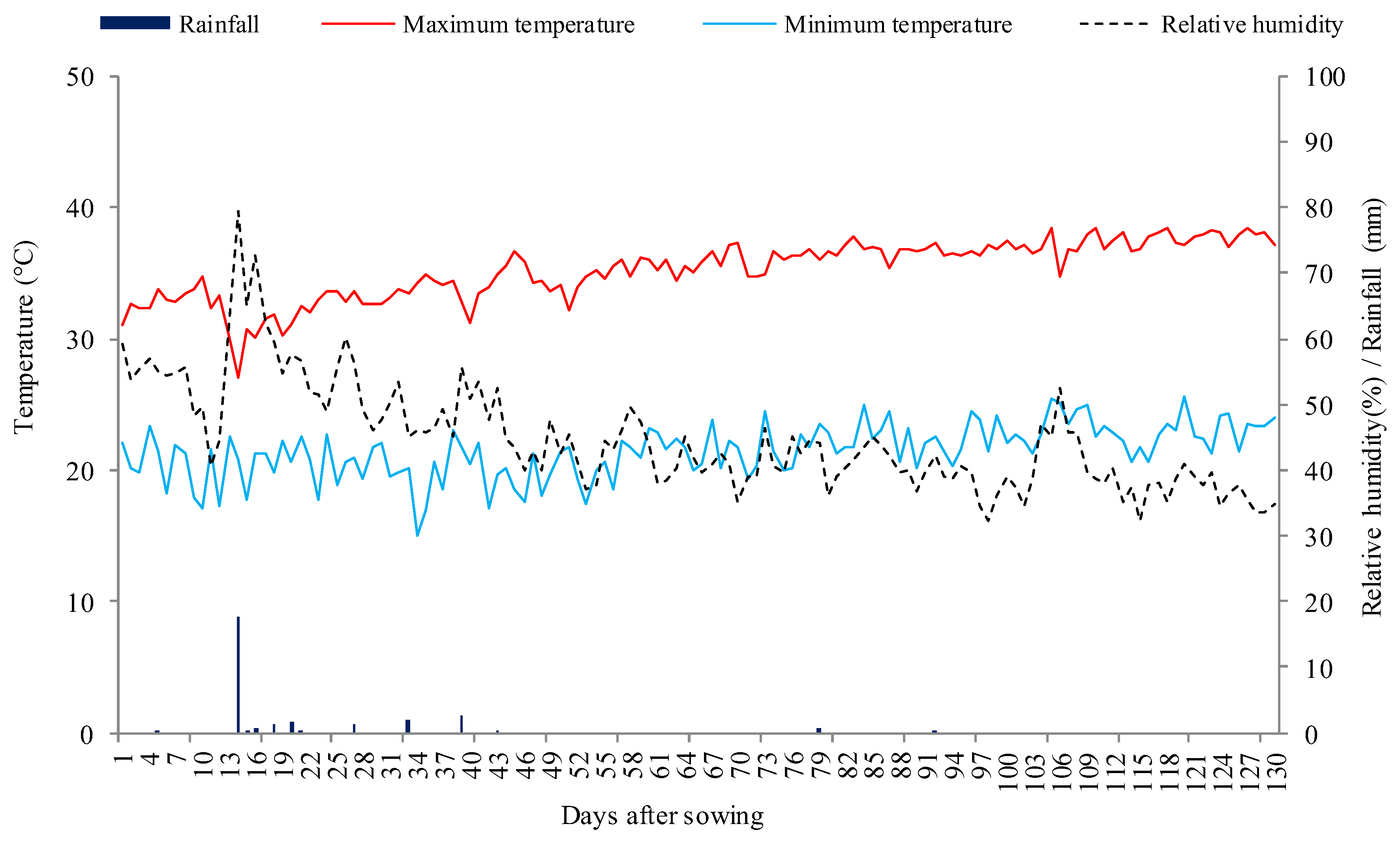
4.1. Study Location
4.2. Treatments and Statistical Design
4.3. Experiment Conduction
4.4. Water Preparation and Irrigation Management
4.5. Preparation and Application of Hydrogen Peroxide Concentrations
4.6. Variables Analyzed
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maryum, Z.; Luqman, T.; Nadeem, S.; Khan, S.M.U.D.; Wang, B.; Ditta, A.; Khan, M.K.R. An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front. Plant Sci. 2022, 13, 907937. [Google Scholar] [CrossRef] [PubMed]
- CONAB-Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira: Grãos. Safra 2022/23. 12° Levantamento. v. 9. 2022. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 12 September 2023).
- Nunes, K.G.; Costa, R.N.T.; Cavalcante, I.N.; Gondim, R.S.; Lima, S.C.R.V.; Mateos, L. Groundwater resources for agricultural purposes in the Brazilian semi-arid region. Rev. Bras. Eng. Agríc. Amb. 2022, 26, 915–923. [Google Scholar] [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.K.; Zhao, C.; Persson, S. The cell biology of primary cell walls during salt stress. Plan. Cell 2023, 35, 201–217. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Yang, W.; Qian, L.V.; Zhou, L.; Liu, S.; Li, X. Genome-wide analysis and identification of TaRING-H2 gene family and TaSDIR1 positively regulates salt stress tolerance in wheat. Int. J. Biol. Macromol. 2023, 242, 125–162. [Google Scholar] [CrossRef]
- Fu, Z.W.; Feng, Y.R.; Gao, X.; Ding, F.; Li, J.H.; Yuan, T.T.; Lu, Y.T. Salt stress-induced chloroplastic hydrogen peroxide stimulates pdTPI sulfenylation and methylglyoxal accumulation. Plan. Cell 2023, 35, 1593–1616. [Google Scholar] [CrossRef]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Cao, H.; Han, Y.; Cheng, Z.; Lv, Q.; Pompelli, M.F.; Pereira, J.D.; Araújo, W.L. Long exposure to salt stress in Jatropha curcas leads to stronger damage to the chloroplast ultrastructure and its functionality than the stomatal function. Forests 2023, 14, 1868. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Tabassum, M.; Noreen, Z.; Aslam, M.; Shah, A.N.; Usman, S.; Waqas, A.; Alsherif, E.A.; Korany, S.M.; Nazim, M. Chitosan modulated antioxidant activity, inorganic ions homeostasis and endogenous melatonin to improve yield of Pisum sativum L. accessions under salt stress. Sci. Hortic. 2024, 323, 112509. [Google Scholar] [CrossRef]
- Zafar, M.M.; Shakeel, A.; Haroon, M.; Mannan, A.; Sahar, A.; Soukat, A.; Mo, H.; Farooq, M.A.; Ren, M. Effects of salinity stress on some growth, physiological, and biochemical parameters in cotton (Gossypium hirsutum L.) germplasm. J. Nat. Fibers 2022, 19, 8854–8886. [Google Scholar] [CrossRef]
- Shalaby, O.A.E.S.; Farag, R.; Ibrahim, M.F. Effect of hydrogen sulfide and hydrogen peroxide on growth, yield and nutrient content of broccoli plants grown under saline conditions. Sci. Hortic. 2023, 316, 112–135. [Google Scholar] [CrossRef]
- Dito, S.; Gadallah, M. Hydrogen peroxide supplementation relieves the deleterious effects of cadmium on photosynthetic pigments and oxidative stress and improves the growth, yield and quality of pods in pea plants (Pisum sativum L.). Acta Physiol. Plant. 2019, 41, 113. [Google Scholar] [CrossRef]
- Khedia, J.; Agarwal, P.; Agarwal, P.K. Deciphering hydrogen peroxide-induced signalling towards stress tolerance in plants. 3 Biotech. 2019, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.K.; Gawroński, P.; Munir, S.; Jindal, S.; Kerchev, P. Hydrogen peroxide-induced stress acclimation in plants. Cell. Mol. Life Sci. 2022, 79, 129. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Kordrostami, M.; Zakir, A.; Zaki, H.; Saleh, O.M. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants 2019, 8, 303. [Google Scholar] [CrossRef]
- Limão, M.A.R.; Rodrigues, M.H.B.S.; Santos, A.S.; Barbosa, L.L.; Lopes, K.P.; Dias, D.C.F.S. Hydrogen peroxide as a mitigator of salt stress for melon seed germination. Comun. Sci. 2022, 13, e3798. [Google Scholar] [CrossRef]
- Hajivar, B.; Zare-Bavani, M.R. Alleviation of salinity stress by hydrogen peroxide and nitric oxide in tomato plants. Adv. Hortic. Sci. 2019, 33, 409–416. [Google Scholar]
- Lacerda, F.H.D.; Pereira, F.H.F.; Silva, F.A.; Queiroga, F.M.; Brito, M.E.B.; Medeiros, J.E.; Dias, M.S. Physiology and growth of maize under salinity of water and application of hydrogen peroxide. Rev. Bras. Eng. Agríc. Amb. 2022, 26, 771–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wen, W.; Shi, Z.; Gu, Q.; Ahammed, G.L.; Janhan, M.S.; Shu, S.; Wang, J.; Sun, J.; et al. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 2021, 17, 2876–2890. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Ul-Arif, M.D.T.; Brestic, M.; Afrin, S.; Sakil, M.D.A.; Hossain, M.D.T.; Hossain, M.A.; Hossain, M.D.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef]
- Nobre, R.G.; Vasconcelos, E.S.; Sales, G.S.; Linhares, E.L.R.; Souza, M.S.M.; Moreira, A.R.P.; Aviz, R.O.; Casais, L.K.N.; Neitzke, T.R. Mitigation of salt stress in passion fruit seedlings with H2O2 application. Rev. Bras. Eng. Agríc. Amb. 2024, 28, e272617. [Google Scholar] [CrossRef]
- Aragão, J.; Lima, G.S.; Lima, V.L.A.; Silva, A.A.R.; Santos, L.F.S.; Dias, M.S.; Arruda, T.F.L.; Souza, A.R.; Soares, L.A.A. Hydrogen peroxide in the mitigation of salt stress in bell pepper. Sem. Ciênc. Agrar. 2023, 44, 217–238. [Google Scholar] [CrossRef]
- Sousa, H.C.; Sousa, G.G.; Cambissa, P.B.C.; Lessa, C.I.N.; Goes, G.F.; Silva, F.D.B.; Abreu, F.S.; Viana, T.V.A. Gas exchange and growth of zucchini crop subjected to salt and water stress. Rev. Bras. Eng. Agríc. Amb. 2022, 26, 815–822. [Google Scholar] [CrossRef]
- Khan, M.N.; Al-Salami, M.A.; Siddiqui, Z.H.; Al-Omrami, M.A.M.; Kalaji, H.M. Regulation of Na+/H+ antiport system and nitrogen metabolism by melatonin and endogenous hydrogen sulfide confers resilience to drought and salt stress. S. Afr. J. Bot. 2024, 164, 152–166. [Google Scholar] [CrossRef]
- Ali, Q.; Ahmad, M.; Kamran, M.; Ashraf, S.; Shabaan, M.; Babar, B.H.; Zulkifar, U.; Haider, F.U.; Ali, M.A.; Elshikh, M.S. Synergistic Effects of Rhizobacteria and salicylic acid on maize salt-stress tolerance. Plants 2023, 12, 2519. [Google Scholar] [CrossRef] [PubMed]
- Roşca, M.; Mihalache, G.; Stoleru, V. Tomato responses to salinity stress: From morphological traits to genetic changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fricke, W. Salt Stress—Regulation of root water uptake in a whole-plant and diurnal context. Int. J. Mol. Sci. 2023, 24, 8070. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.S.; Pinheiro, F.W.A.; Souza, W.B.B.; Soares, L.A.A.; Gheyi, H.R.; Nobre, R.G.; Queiroga, R.C.F.; Fernandes, P.D. Physiological indices of sour passion fruit under brackish water irrigation strategies and potassium fertilization. Rev. Bras. Eng. Agric. Amb. 2023, 27, 383–392. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020, 20, 434. [Google Scholar] [CrossRef]
- Rodrigues, V.S.; Sousa, G.G.; Gomes, S.P.; Soares, S.C.; Silva Júnior, F.B.; Freire, M.H.C.; Santos, M.W.N.; Lima, J.M.P. Gas exchange and growth of sunflower subjected to saline stress and mineral and organic fertilization. Rev. Bras. Eng. Agríc. Amb. 2022, 11, 840–847. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Chaudhary, M.A.M.; Rehman, A. Foliage-applied sodium nitroprusside and hydrogen peroxide improves resistance against terminal drought in bread wheat. J. Agron. Crop. Sci. 2017, 203, 473–482. [Google Scholar] [CrossRef]
- Okunlolo, G.O.; Olatunji, O.A.; Obisesan, I.A.; Olowalaju, E.D.; Ogunkunle, C.O.; Rufai, A.B. Foliar application of hydrogen peroxide (H2O2) modulates growth, inorganic ion and osmolyte accumulation of soybean (Glycine max) cultivars under drought stress. S. Afr. J. Bot. 2022, 151, 425–432. [Google Scholar] [CrossRef]
- Veloso, L.L.S.; Azevedo, C.A.V.; Nobre, R.G.; Lima, G.S.; Silva, I.J.; Lacerda, C.N. Hydrogen peroxide in the acclimation of colored-fiber cotton genotypes to salt stress. Rev. Caatinga 2023, 36, 414–423. [Google Scholar] [CrossRef]
- Capitulino, J.D.; Lima, G.S.; Azevedo, C.A.V.; Silva, A.A.R.; Soares, L.A.A.; Arruda, T.F.L.; Sousa, V.D.; Gheyi, H.R. Morphophysiology of soursop under salt stress and H2O2 application in the pre-flowering phase. Rev. Bras. Eng. Agríc. Amb. 2023, 12, 948–957. [Google Scholar] [CrossRef]
- Rakkamal, K.; Priya, A.; Pandian, S.; Maharajan, T.; Rathinapriya, P.; Satish, L.; Ceasar, E.A.; Sohn, S.I.; Ramesh, M. Conventional and omics approaches for understanding the abiotic stress response in cereal crops—An updated overview. Plants 2022, 11, 2852. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Alavi, Z.; Esfandiari, A.; Panahirad, S.; Hajihoseinlou, S.; Fotopoulos, V. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol. Plant. 2020, 168, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wei, Z.; Liu, j.; Liu, X.; Liu, F. Growth and physiological responses of cotton plants to salt stress. J. Agron. Crop. Sci. 2021, 207, 565–576. [Google Scholar] [CrossRef]
- Dantas, M.V.; Lima, G.S.; Gheyi, H.R.; Silva, L.A.; Silva, P.C.C.; Soares, L.A.A.; Lopes, I.A.P.; Roque, I.A. Hydrogen peroxide and saline nutrient solution in hydroponic zucchini culture. Sem. Ciênc. Agrar. 2022, 43, 1187–1196. [Google Scholar] [CrossRef]
- Sharif, I.; Aleem, S.; Farooq, J.; Rizwan, M.; Younas, A.; Sarwar, G.; Chohan, S.M. Salinity stress in cotton: Effects, mechanism of tolerance and its management strategies. Physiol. Mol. Biol. Plants 2019, 25, 807–820. [Google Scholar] [CrossRef]
- Shaheen., S.; Baber, M.; Aslam, S.; Shasheem, M.; Waheed, R.; Seo, H.; Azhar, M.T. Effect of NaCl on Morphophysiological and Biochemical Responses in Gossypium hirsutum L. Agronomy 2023, 13, 1012. [Google Scholar] [CrossRef]
- Veloso, L.L.S.; Azevedo, C.A.V.; Nobre, R.G.; Lima, G.S.; Bezerra, J.R.C.; Silva, A.A.R.; Fátima, R.T.; Gheyi, H.R.; Soares, L.A.A.; Fernandes, P.D.; et al. Production and fiber characteristics of colored cotton cultivares under salt stress and H2O2. Plants 2023, 12, 2090. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Leonardo., J.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Lima, G.S.; Dias, A.S.; Gheyi, H.R.; Soares, L.A.A.; Andrade, E.M.G. Saline water irrigation and nitrogen fertilization on the cultivation of colored fiber cotton. Rev. Caatinga 2018, 31, 151–160. [Google Scholar] [CrossRef]
- Silva, A.A.R.; Lima, G.S.; Azevedo, C.A.V.; Veloso, L.L.S.; Capitulino, J.D.; Gheyi, H.R. Induction of tolerance to salt stress in soursop seedlings using hydrogen peroxide. Comun. Sci. 2019, 10, 484–490. [Google Scholar] [CrossRef]
- Farias, F.J.C.; de Lellis Morello, C.; Pedrosa, M.B.; Suassuna, N.D.; da Silva Filho, J.L.; de Carvalho, L.P.; Ribeiro, J.L. BRS JADE: Nova Cultivar de Algodão Colorido de Dupla Aptidão Para o Cerrado Baiano e para o Semiárido Nordestino; Congresso Brasileiro de algodão: Brasília, Brazil, 2017. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; 573p. [Google Scholar]
- Novais, R.F.; Neves, J.C.L.; Barros, N.F. Ensaio em Ambiente Controlado. In Métodos de Pesquzisa em Fertilidade do Solo; Oliveira, A.J., Ed.; Embrapa-SEA: Brasília, Brazil, 1991; pp. 189–253. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Department of Agriculture: Washington, DC, USA, 1954; 160p. [Google Scholar]
- Benincasa, M.M.P. Análise de Crescimento de Plantas, Noções Básicas, 2nd ed.; FUNEP: Jaboticabal, Brazil, 2003; 41p. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects split plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
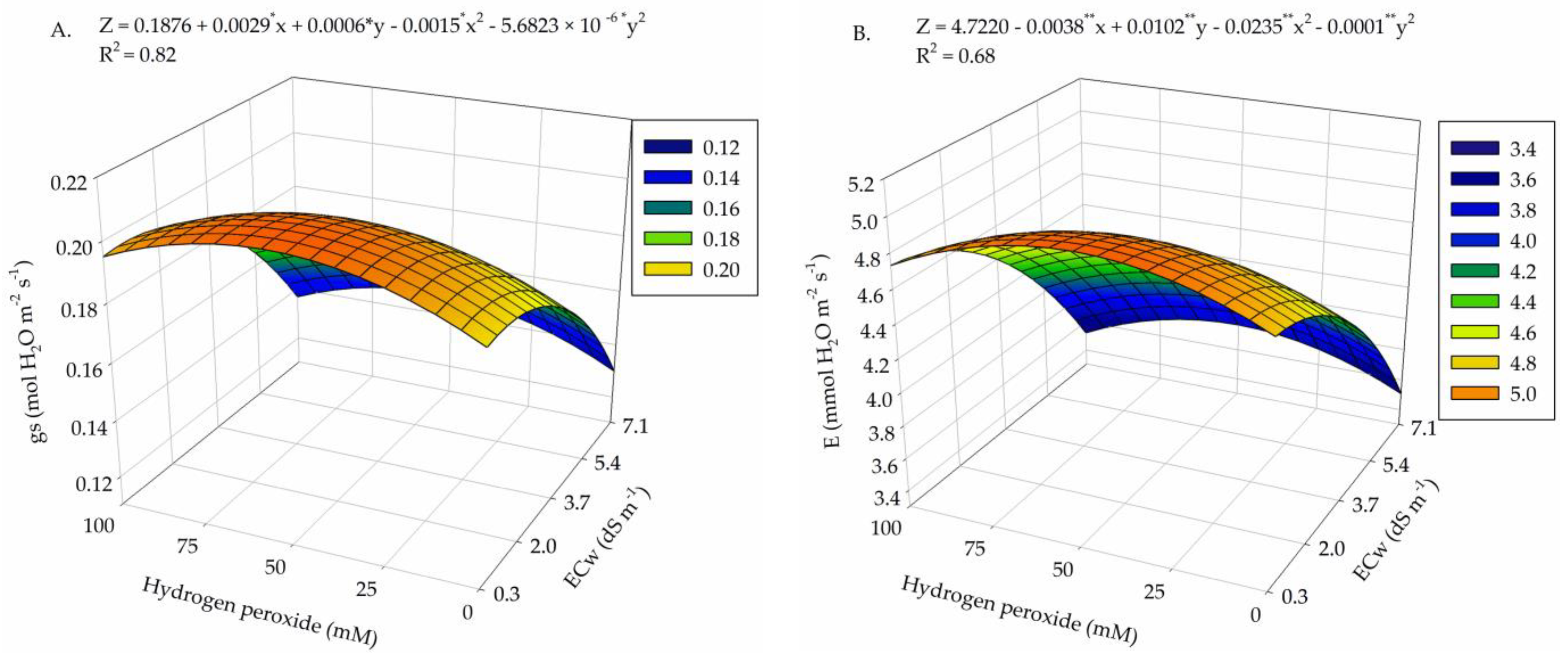
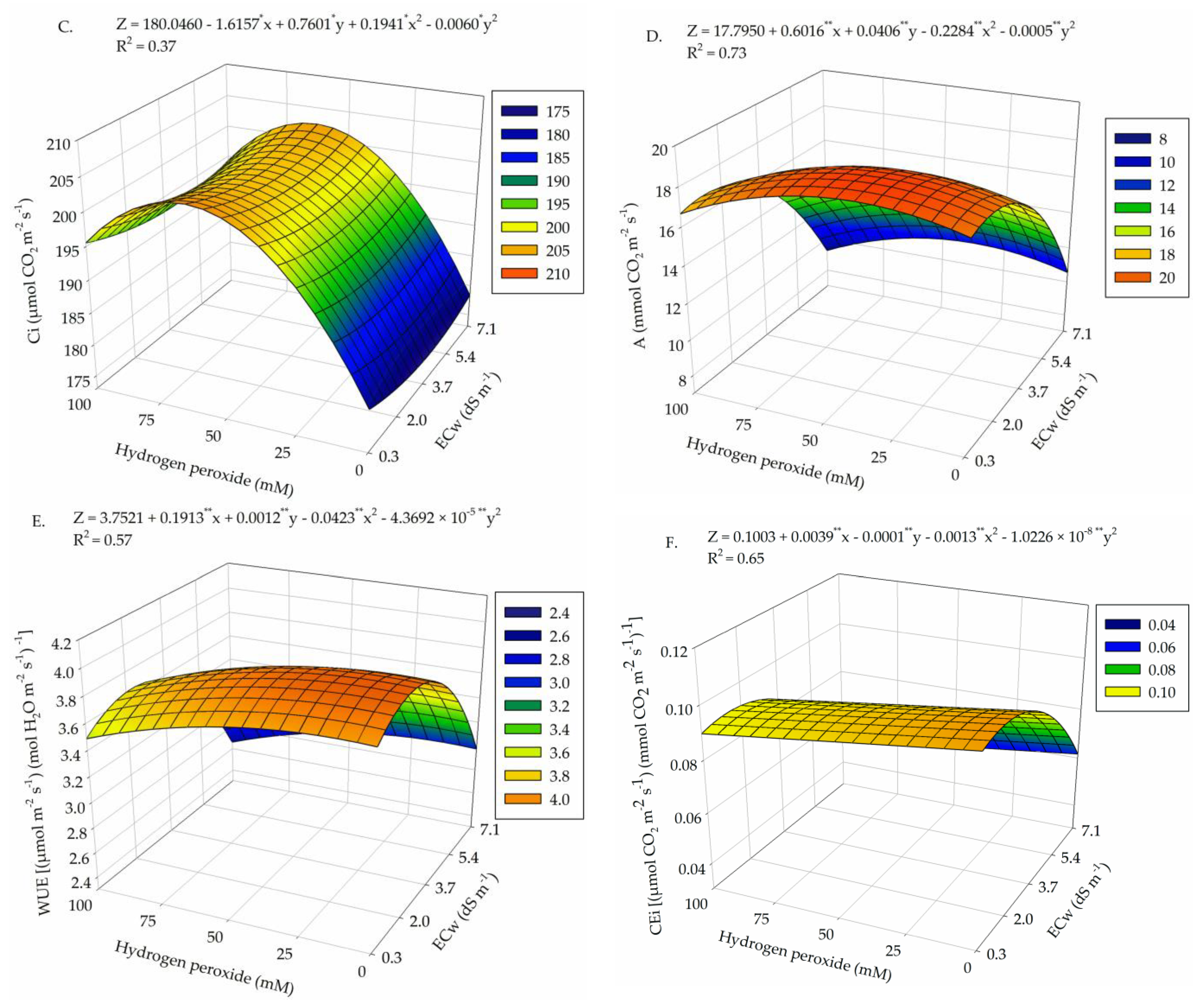
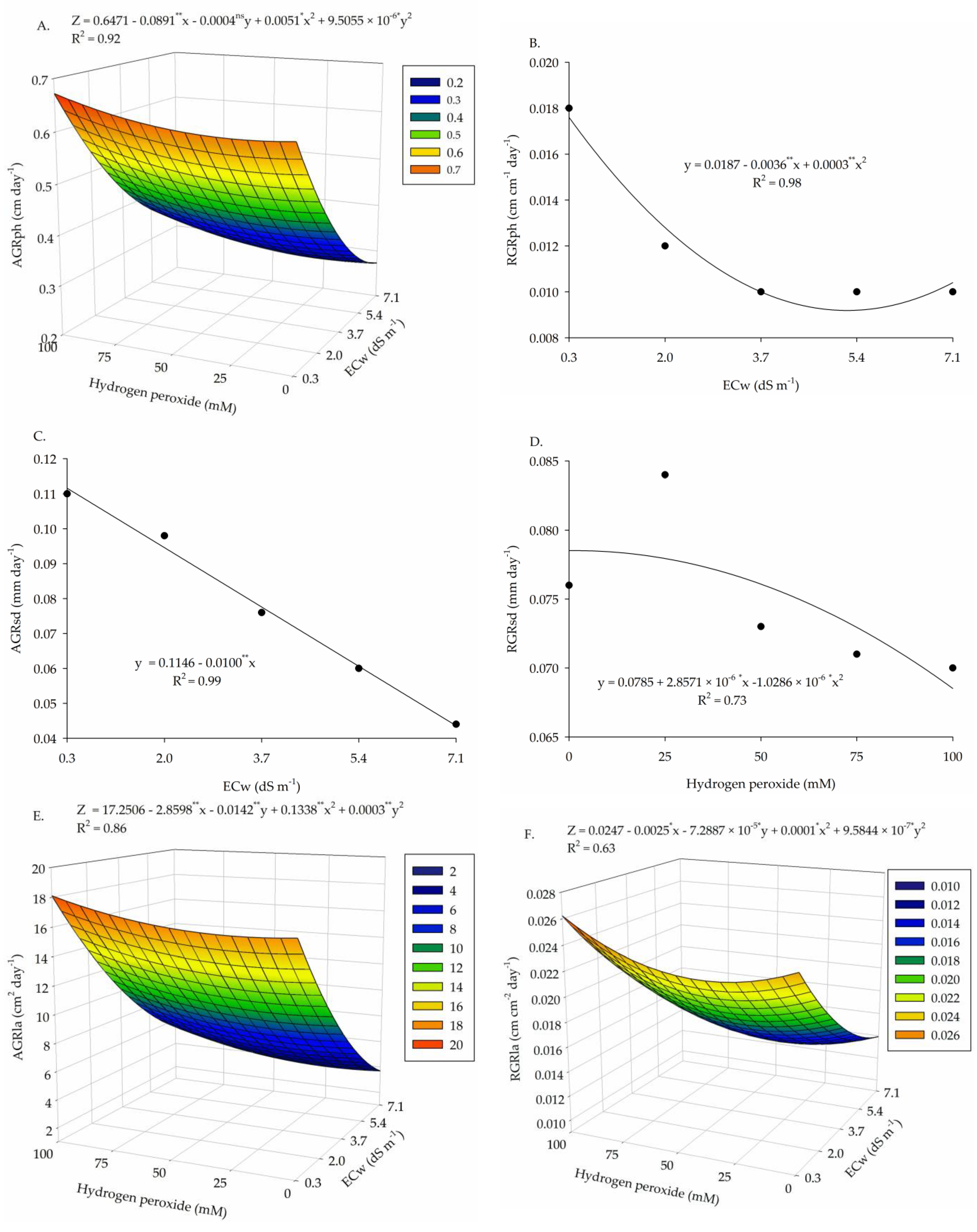
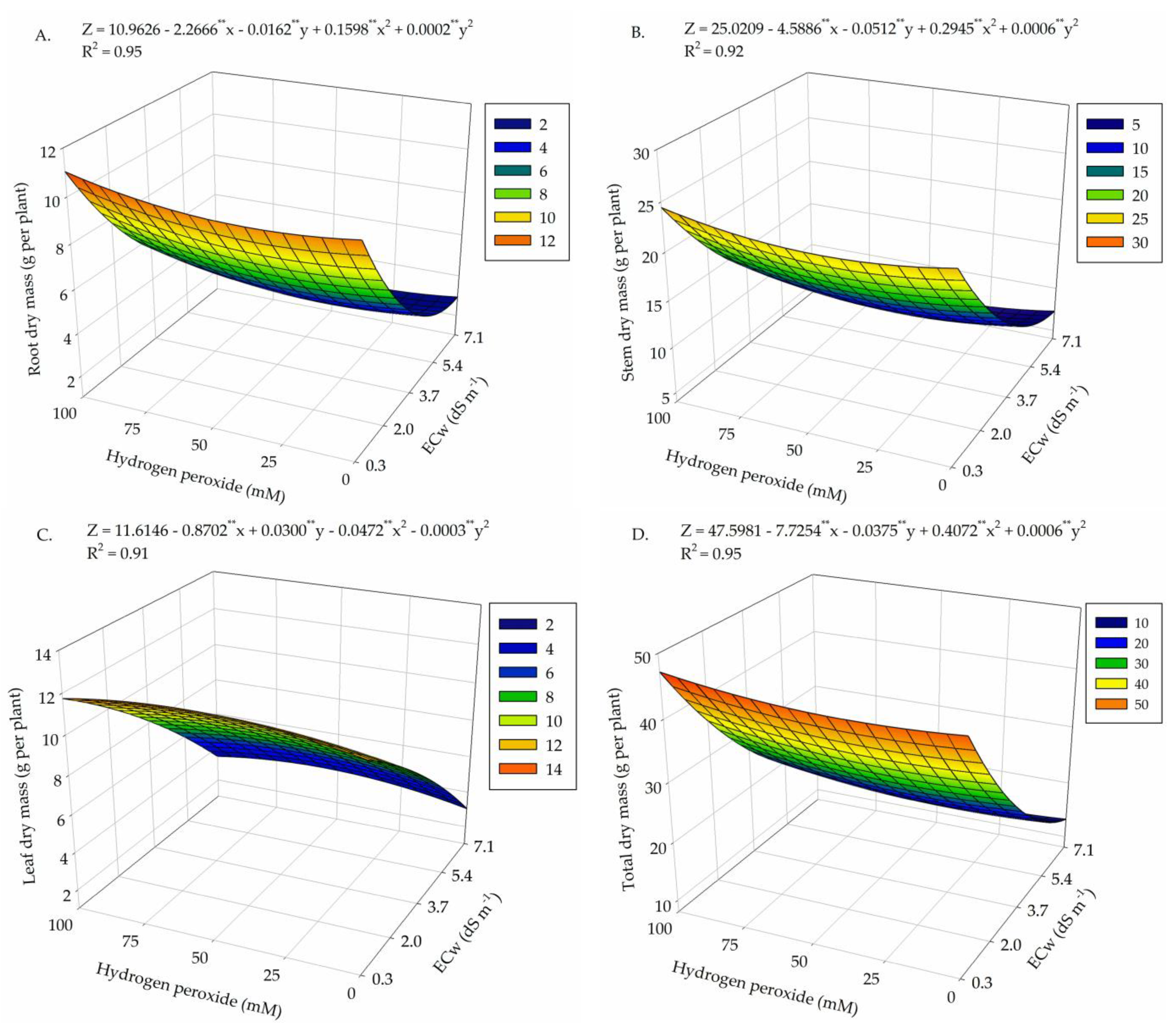

| Variation Sources | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| gs | E | Ci | A | WUE | CEi | ||
| Salinity levels (SL) | 4 | 9.1 × 10−3 ** | 5.49 ** | 348.55 ns | 158.33 ** | 2.21 ** | 4.4 × 10−3 ** |
| Linear regression | 1 | 3.2 × 10−2 ** | 15.15 ** | 305.30 ns | 525.54 ** | 5.76 ** | 1.4 × 10−2 ** |
| Quadratic regression | 1 | 3.5 × 10−3 ** | 0.98 ** | 12.87 ns | 76.50 ** | 2.30 * | 2.0 × 10−3 ** |
| Hydrogen peroxide (H2O2) | 4 | 1.3 × 10−3 * | 0.46 ** | 1124.78 ns | 39.73 ** | 1.05 ns | 1.4 × 10−3 * |
| Linear regression | 1 | 1.0 × 10−4 ns | 0.031 ns | 1485.22 ns | 22.41 ns | 0.96 ns | 1.3 × 10−3 ns |
| Quadratic regression | 1 | 3.6 × 10−3 ** | 1.077 ** | 2474.28 ns | 39.34 * | 0.43 ns | 8.0 × 10−5 ns |
| Interaction (SL × H2O2) | 16 | 1.0 × 10−3 * | 0.65 ** | 1923.74 ** | 26.51 ** | 1.15 ** | 1.2 × 10−3 ** |
| Blocks | 2 | 6.0 × 10−4 ns | 0.18 ns | 88.04 ns | 0.288 ns | 0.085 ns | 5.0 × 10−6 ns |
| Residual | 74 | 4.0 × 10−4 | 0.086 | 648.02 | 8.89 | 0.469 | 4.2 × 10−4 |
| CV (%) | 12.0 | 6.73 | 13.2 | 15.5 | 19.3 | 24.7 | |
| Variation Sources | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| AGRPH | RGRPH | AGRSD | RGRSD | AGRLA | RGRLA | ||
| Salinity levels (SL) | 4 | 0.323 ** | 2.1 × 10−4 ** | 0.011 ** | 8 × 10−6 ns | 430.53 ** | 4.4 × 10−4 ** |
| Linear regression | 1 | 1.19 ** | 6.0 × 10−4 ** | 0.039 ** | 6 × 10−6 ns | 1500.1 ** | 1.1 × 10−3 ** |
| Quadratic regression | 1 | 0.084 ** | 2.3 × 10−4 ** | 0.017 ** | 0 × 10−5 ns | 54.72 ** | 8 × 10−5 * |
| Hydrogen peroxide (H2O2) | 4 | 1.5 × 10−3 ns | 5.0 × 10−6 ns | 4.0 × 10−4 * | 3 × 10−6 ns | 22.15 ** | 8 × 10−5 ** |
| Linear regression | 1 | 5.2 × 10−3 ns | 1.7 × 10−5 ns | 1.7 × 10−4 ns | 6 × 10−6 ns | 4.40 ns | 0 × 10−5 ns |
| Quadratic regression | 1 | 2.0 × 10−4 ns | 4.0 × 10−6 ns | 9 × 10−5 ns | 0 × 10−6 ns | 0.038 ns | 9 × 10−5 * |
| Interaction (SL × H2O2) | 16 | 6.4 × 10−3 ** | 7.0 × 10−6 ns | 2.4 × 10−4 ns | 3 × 10−6 ns | 17.32 ** | 3.9 × 10−5 * |
| Blocks | 2 | 1.8 × 10−3 ns | 2.1 × 10−5 ns | 1.3 × 10−3 ** | 5 × 10−6 ns | 6.18 ns | 2.0 × 10−5 ns |
| Residual | 74 | 2.1 × 10−3 | 6.0 × 10−6 | 1.5 × 10−4 | 4 × 10−6 | 2.27 | 2.0 × 10−5 |
| CV (%) | 11.0 | 20.0 | 16.0 | 20.1 | 15.9 | 24.5 | |
| Variation Sources | DF | Mean Squares | |||
|---|---|---|---|---|---|
| RDM | SDM | LDM | TDM | ||
| Salinity levels (SL) | 4 | 143.38 ** | 719.95 ** | 179.04 ** | 2648.41 ** |
| Linear regression | 1 | 522.59 ** | 2597.33 ** | 584.54 ** | 9998.61 ** |
| Quadratic regression | 1 | 49.23 ** | 168.69 ** | 2.96 ns | 334.50 ** |
| Hydrogen peroxide (H2O2) | 4 | 1.94 ** | 29.45 ** | 5.94 ** | 57.99 ** |
| Linear regression | 1 | 2.50 * | 0.47 ns | 0.019 ns | 5.80 ns |
| Quadratic regression | 1 | 2.46 * | 15.01 ** | 9.87 ns | 5.31 ns |
| Interaction (SL × H2O2) | 16 | 2.90 ** | 13.01 ** | 5.08 ** | 29.89 ** |
| Blocks | 2 | 0.16 ns | 2.09 ns | 0.16 ns | 4.23 ns |
| Residual | 74 | 0.48 | 1.07 | 1.17 | 3.61 |
| CV (%) | 12.1 | 7.79 | 13.7 | 7.06 | |
| Variation Sources | DF | Mean Squares | ||||
|---|---|---|---|---|---|---|
| NB | ABW | SCW | LCW | RF | ||
| Salinity levels (SL) | 4 | 445.53 ** | 2.33 ** | 2128.83 ** | 577.45 ** | 367.93 ** |
| Linear regression | 1 | 1706.30 ** | 3.07 ** | 7935.93 ** | 2082.08 ** | 141.67 ns |
| Quadratic regression | 1 | 35.21 ** | 1.03 ** | 191.71 ** | 55.94 ** | 1110.62 ** |
| Hydrogen peroxide (H2O2) | 4 | 26.25 ** | 1.03 ** | 71.56 ** | 19.74 ** | 258.16 * |
| Linear regression | 1 | 34.56 ** | 0.0094 ns | 1.22 ns | 3.82 * | 275.07 * |
| Quadratic regression | 1 | 1.24 ns | 3.25 ** | 55.56 ** | 29.41 ** | 105.59 ns |
| Interaction (SL × H2O2) | 16 | 14.60 ** | 0.46 ** | 92.17 ** | 11.99 ** | 280.39 ** |
| Blocks | 2 | 3.0 ns | 0.56 ns | 0.30 ns | 0.45 ns | 116.77 ns |
| Residual | 74 | 1.16 | 0.13 | 2.79 | 0.63 | 70.65 |
| CV (%) | 14.5 | 19.3 | 11.0 | 10.2 | 16.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nóbrega, J.S.; Gomes, V.R.; Soares, L.A.d.A.; Lima, G.S.d.; Silva, A.A.R.d.; Gheyi, H.R.; Torres, R.A.F.; Silva, F.J.L.d.; Silva, T.I.d.; Costa, F.B.d.; et al. Hydrogen Peroxide Alleviates Salt Stress Effects on Gas Exchange, Growth, and Production of Naturally Colored Cotton. Plants 2024, 13, 390. https://doi.org/10.3390/plants13030390
Nóbrega JS, Gomes VR, Soares LAdA, Lima GSd, Silva AARd, Gheyi HR, Torres RAF, Silva FJLd, Silva TId, Costa FBd, et al. Hydrogen Peroxide Alleviates Salt Stress Effects on Gas Exchange, Growth, and Production of Naturally Colored Cotton. Plants. 2024; 13(3):390. https://doi.org/10.3390/plants13030390
Chicago/Turabian StyleNóbrega, Jackson Silva, Valéria Ribeiro Gomes, Lauriane Almeida dos Anjos Soares, Geovani Soares de Lima, André Alisson Rodrigues da Silva, Hans Raj Gheyi, Rafaela Aparecida Frazão Torres, Fellype Jonathar Lemos da Silva, Toshik Iarley da Silva, Franciscleudo Bezerra da Costa, and et al. 2024. "Hydrogen Peroxide Alleviates Salt Stress Effects on Gas Exchange, Growth, and Production of Naturally Colored Cotton" Plants 13, no. 3: 390. https://doi.org/10.3390/plants13030390
APA StyleNóbrega, J. S., Gomes, V. R., Soares, L. A. d. A., Lima, G. S. d., Silva, A. A. R. d., Gheyi, H. R., Torres, R. A. F., Silva, F. J. L. d., Silva, T. I. d., Costa, F. B. d., Dantas, M. V., Bruno, R. d. L. A., Nobre, R. G., & Sá, F. V. d. S. (2024). Hydrogen Peroxide Alleviates Salt Stress Effects on Gas Exchange, Growth, and Production of Naturally Colored Cotton. Plants, 13(3), 390. https://doi.org/10.3390/plants13030390









