Abstract
The tomato is a convenient object for studying reproductive processes, which has become a classic. Such complex processes as flowering and fruit setting require an understanding of the fundamental principles of molecular interaction, the structures of genes and proteins, the construction of signaling pathways for transcription regulation, including the synchronous actions of cis-regulatory elements (promoter and enhancer), trans-regulatory elements (transcription factors and regulatory RNAs), and transposable elements and epigenetic regulators (DNA methylation and acetylation, chromatin structure). Here, we discuss the current state of research on tomatoes (2017–2023) devoted to studying the function of genes that regulate flowering and signal regulation systems using genome-editing technologies, RNA interference gene silencing, and gene overexpression, including heterologous expression. Although the central candidate genes for these regulatory components have been identified, a complete picture of their relationship has yet to be formed. Therefore, this review summarizes the latest achievements related to studying the processes of flowering and fruit set. This work attempts to display the gene interaction scheme to better understand the events under consideration.
1. Introduction
Flowering plants have morphological diversity and can grow in different ecological niches. The transition of plants from the vegetative phase to the reproductive phase is a significant switch in their life cycle since the reproduction of offspring is the most essential function of all living things. The optimal timing of this event from a physiological point of view is a prerequisite for successful reproduction. Flowering and fruit set are initiated and regulated via the combined action of various genetic factors in response to endo- [1,2] and exogenous [3,4] stimuli. Processes, including male and female organogenesis, meiosis, gametogenesis, pollination, and fertilization, occur during the diploid and haploid phases of reproductive development, which are necessary to maintain genetic variability [5]. After successful pollination and fertilization of the ovary, the coordinated action of growth signals removes the inhibition of ovary development [6,7]. Concurrently, pistil senescence and flower abscission occur in the absence of positive stimuli. [8,9]. The functions of the flowering and development regulators are often conserved in different angiosperms; in this regard, tomato is a convenient object for studying the mechanisms of regulation at the gene level in climacteric fruits [4].
Tomato (Solanum lycopersicum L.) is a commercially important crop grown for fresh or processed consumption [10,11]. Self-compatibility and short life cycles (90–120 days) are appealing factors for agricultural producers [12], while high taste quality [13,14,15] and nutritional value [16,17,18] are important for consumers.
Genetic engineering methods have been used to study genetic factors regulating tomato reproduction for several decades [19]. A vast amount of knowledge has been accumulated, which gives an idea of the general picture of molecular interactions. However, creating new methodological tools makes it possible to significantly expand the knowledge concerning flowering, setting, and fruit development processes.
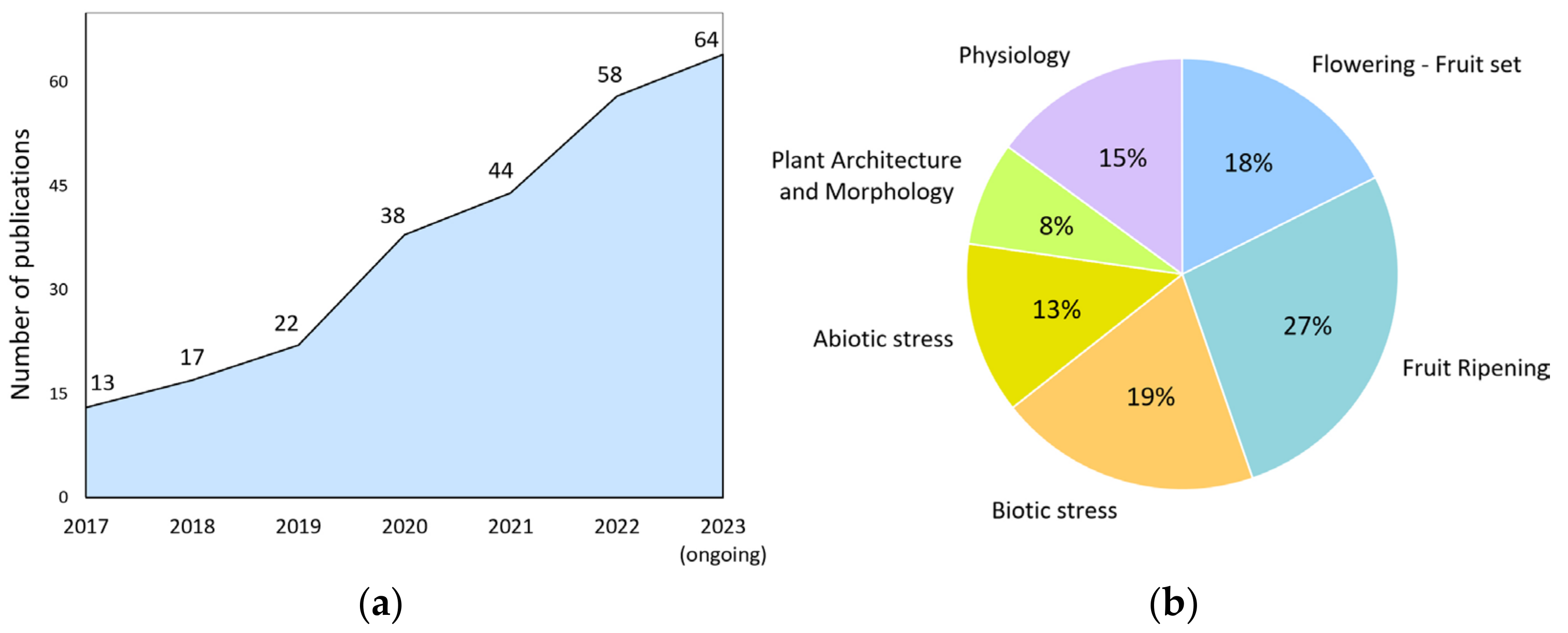
For example, precise genome editing by the CRISPR/Cas9 system allows researchers to create knockout alleles, make subtle changes to gene sequences, and even replace entire genes [20]. This, in turn, helps to study gene function, interactions, and regulation. Despite the 10-year history of using this powerful technology, the number of publications using it, in which tomato is the subject, is steadily increasing year by year (Figure 1a). Its research potential for scientists is far from being exhausted.
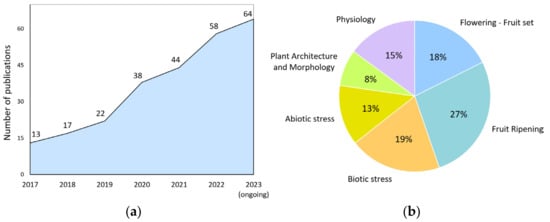
Figure 1.
Publication activity of the world scientific community. (a) CRISPR/Cas9-related studies on tomato by year; (b) research topics focused on exploring various physiological processes in tomato (2017–2023) using CRISPR/Cas9 technology.
We analyzed the publications where the CRISPR/Cas9 system was applied on tomatoes for the last six years. This allowed us to identify the most exciting topics for the scientific community (Figure 1b). It turned out that almost half (45%) of the studies are devoted to the study of genes involved in tomato reproductive processes in general. Publications revealing narrower topics—flowering, fertilization, and fruit development—occupy 40% of this share (18% of the total publications). It turned out that many of the gene-regulatory genes encode transcription factors and co-regulators of transcription, miRNAs, or proteins involved in the epigenetic control of gene expression. The high research interest in this area is that, in many cases, these regulators’ molecular mechanisms of action still need to be fully understood. The stably relevant topic of stress (abiotic and biotic) occupies one-third of the total publications. The remaining publications cover fields devoted to other physiological processes (15%), plant architecture, and morphology (8%).
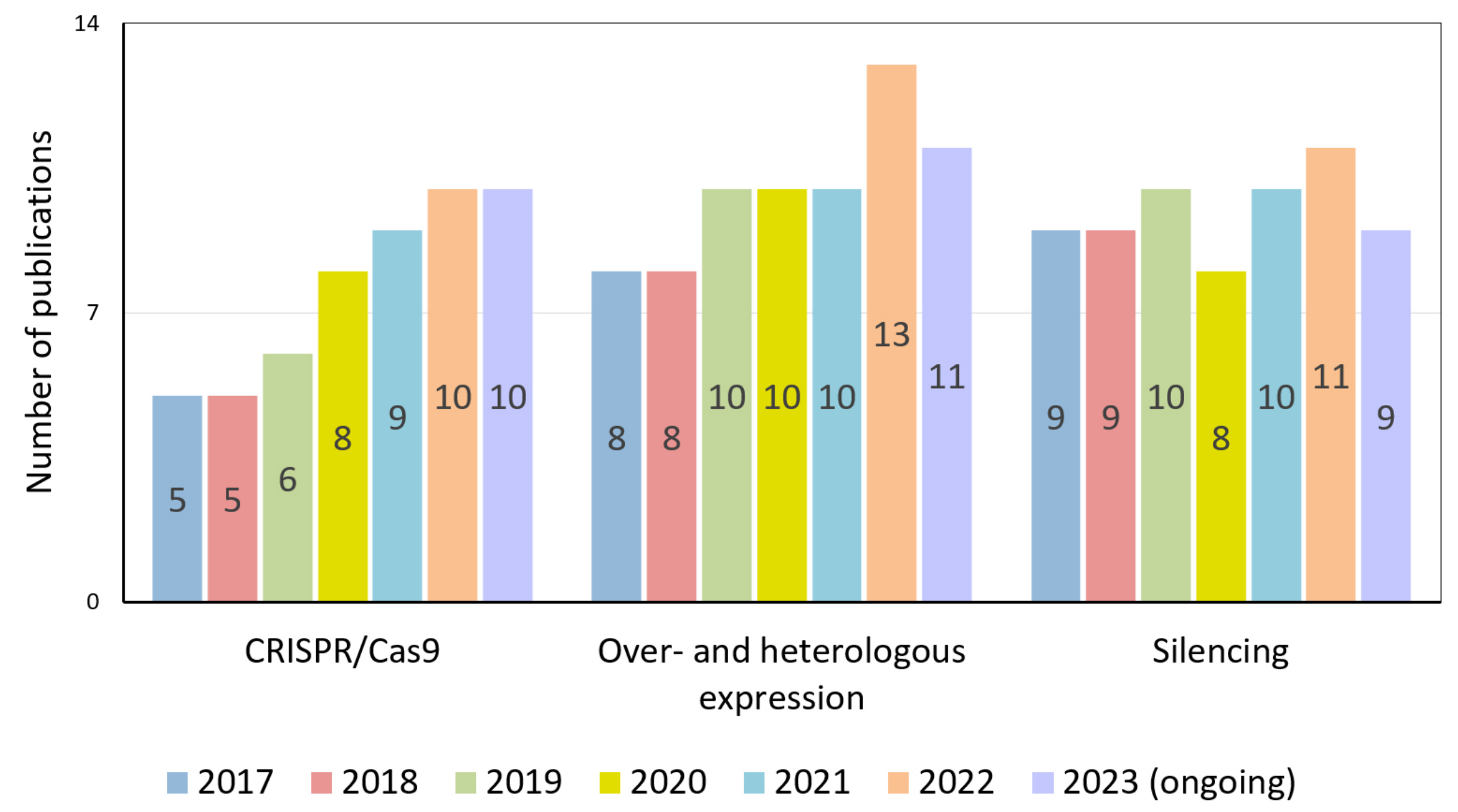
Among the genetic engineering methods used to study the regulation of reproductive processes, the use of the CRISPR/Cas9 system is expected to increase (Figure 2).
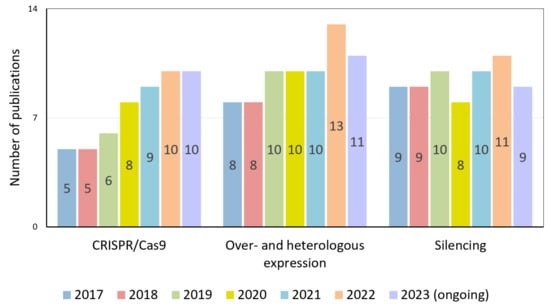
Figure 2.
Number of publications by year devoted to studying tomato flowering processes using CRISPR/Cas9 technology, over- and heterologous expression approaches, and gene-silencing technologies.
At the same time, the approaches that have already become classical, such as the RNA interference (RNAi) silencing of gene expression and gene overexpression (OE), have not lost their relevance and the quantity of related publications has remained stably high over the last six years. Thus, despite the large chunk of accumulated scientific data, regulating reproductive processes still needs to be explained.
Thus, this review highlights the significant advances in the study of genes regulating tomato flowering obtained using directed genome-editing, silencing, and gene overexpression methods. Firstly, genes associated with the meristem transition from the vegetative to the reproductive phase are described. Then, the consideration of genes involved in flower formation is proposed, and, finally, genes involved in pollen maturation and fertility are outlined. In addition, based on the collected data, we propose a model of signaling regulation of tomato flowering.
2. Meristem Transition
Tomato shoot architecture is shaped by determinate and indeterminate meristems [21]. Indeterminate meristems are pools of undifferentiated plant cells capable of unlimited division and growth. In contrast, determinate meristems represent finite structures. Determinate reproductive meristems do not form de novo but originate from indeterminate meristems [22].
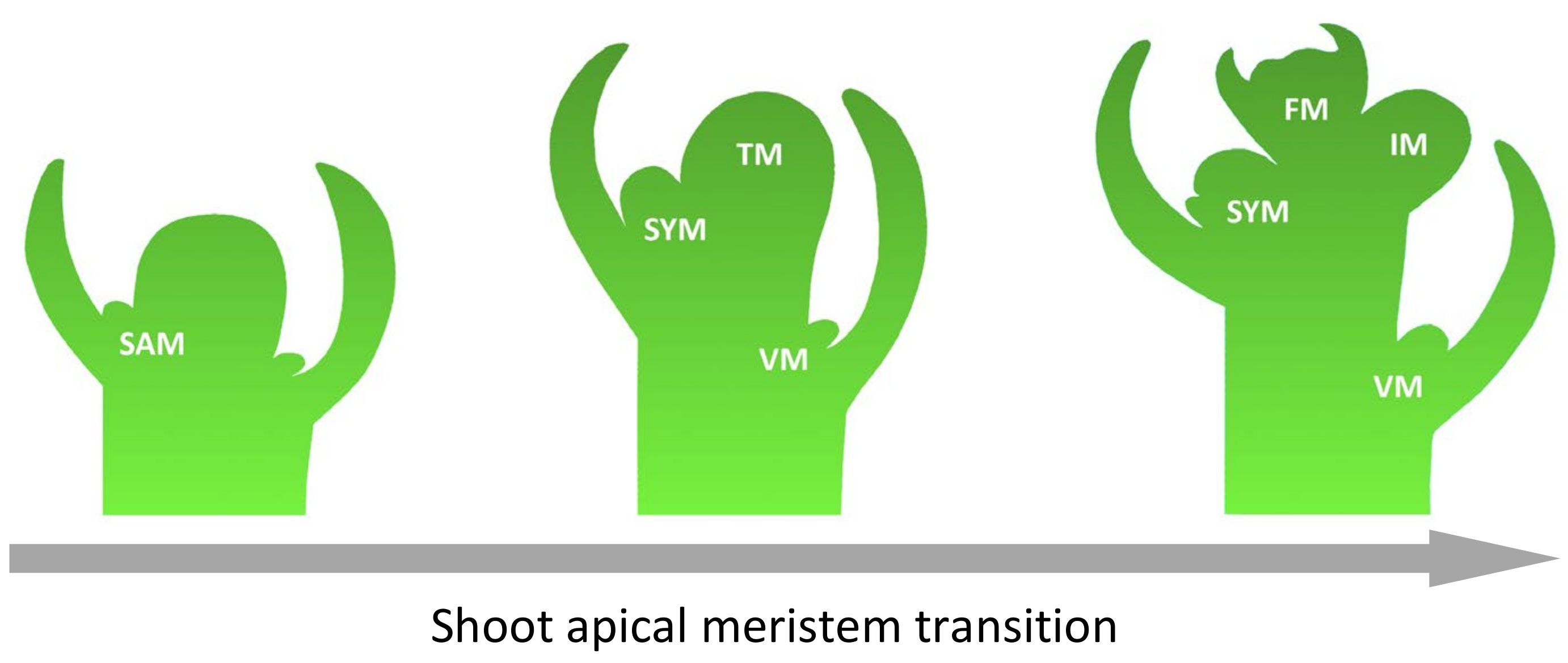
The flowering time, as well as the architecture of the inflorescence, determines the fruit yield. Due to the tomato’s sympodial shoot architecture, its shoot apical meristem (SAM) forms a transition meristem (TM), which results in a floral meristem (FM). At the same time, the flank retains the ability to form a new inflorescence meristem (IM). In addition, tomatoes can form new shoots from the axillary meristem (Figure 3) [23]. The genetic fundamentals of such complex processes are studied on characterized mutants with flowering disorder phenotypes. However, the mechanism of regulation and cross-interaction of genes underlying such phenotypes have yet to be well studied.
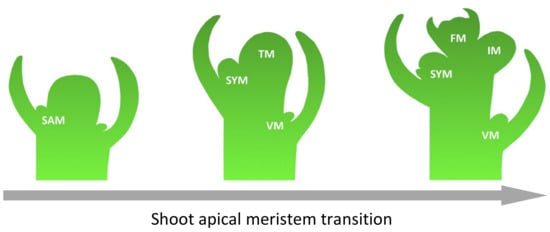
Figure 3.
Steps of inflorescence formation in tomato. Abbreviations: shoot apical meristem (SAM), transitional meristem (TM), flower meristem (FM), sympodial shoot meristem (SYM), vegetative meristem (VM), and inflorescence meristem (IM).
Under suitable conditions, plants transit from the vegetative phase to the reproductive phase. Plants produce florigens in response to endogenous and environmental stimuli. In tomatoes, the main florigen is considered to be SINGLE FLOWER TRUSS (SFT). The sft mutation produces a late-flowering phenotype and causes the replacement of flowers by vegetative shoots [24]. SFT is a member of the CETS gene family [25]. Their proteins bind to adapter proteins in the cytoplasm of SAM cells and translocate into the nucleus, forming florigen activation complexes through binding to the bZIP transcription factor [26]. SFT induces the expression of SAM-to-TM genes, particularly FRUITFUL (FUL) [27]. SFT is regulated by CONSTANS (CO) transcription factors and their homologs due to direct binding to its promoter region, and their suppression stimulates the development of flowers and fruits, which, as a consequence, leads to an increase in the tomato yield [28,29]. Evidence shows that signaling cystine-knot miniproteins (CMPs) interact with CO [30]. CO is regulated by the blue light receptor FKF1 (Flavin-binding Kelch repeat F-box 1) [31]. SQUAMOSA promoter-binding protein (SBP) is another factor that interacts with the SFT promoter region [32]. In addition, SBP-like proteins are directed by miR156 [33]. At the SAM, SBPs activate the expression of FALSIFLORA (FA) while DELLA and miR156-targeted SBPs activate MACROCALYX (MC) to promote inflorescence development [34]. In addition to floral meristem determinacy, miR156-SBP also regulates the locule number [35]. SBP13 inhibits the synthesis of cytokinins (CTKs), thereby suppressing the growth of lateral buds [36]. SBP15 regulates axillary bud development and growth by inhibiting auxin (AUX) transport and GOBLET (GOB) activity and interacts with BRANCHED 1b (BRC1b) to control abscisic acid (ABA) levels in axillary buds [37]. In addition, the miR171-GRAS module restrains the flowering time and trichome distribution by suppressing the activity of miR156-targeted SBP-like proteins [38]. FT-interacting proteins 1 (FTIP1) are involved in transporting the florigen signal [39]. SP acts mainly as an antiflorigen [40]. There is evidence that SP5G, SP5G2, SP5G3 [41,42], and SP3C [43] act as repressors of flowering, and SP3D/SFT as an activator [41]. It was also demonstrated that the night break and red-to-far-red light ratio are the reasons for the accumulation of SFT-like gene transcripts during the late-flowering stages of tomato [41]. Epigenetic modifications could regulate CETS because the ectopic expression of DNA demethylase in tomato had a similar phenotype to CEN1.1-overexpressing plants, manifested by the non-stable transition of meristems to IM, delayed growth, and increased number of leaves between inflorescences [44]. The balance between SFT and SP signaling is the main switch between the determinate and indeterminate development of meristem [45,46,47], and its possible regulatory mechanisms have been discussed previously [22].
ANANTHA (AN) and FALSIFLORA (FA) are considered inflorescence modifiers. The an mutant produces cauliflower-like inflorescences [48]. AN is an F-box protein involved in transcriptional co-activation with the transcription factor FA. AN activation occurs in the late stage of FM development [23]. The tomato fa mutation alters the development of the inflorescence, resulting in the replacement of flowers by secondary shoots, but also produces a late-flowering phenotype with an increased number of leaves below the first and successive inflorescences [49]. GAMYB could be involved in gibberellin-regulated flowering by activating FA gene transcription [50].
TERMINATING FLOWER (TMF), a transcription factor containing a conserved DNA-binding ALOG domain, maintains the meristem in a vegetative state. Single and multiplex knockout mutations of TMF and its paralogs, called TMF FAMILY MEMBERs (TFAMs), demonstrated the dominant role of TMF in the formation of condensates with other members of the ALOG family of genes [51]. These condensates bind to the AN promoter and suppress its expression at the vegetative meristem (VM) stage [52]. An exciting model for regulating the transition of SAM to FM has been proposed [53]. According to the source, naturally produced reactive oxygen species (ROS) contribute to the formation of transcriptional condensates of the TMF transcription factor proteins in tomato meristems due to the oxidation of their cysteine domains with the subsequent formation of disulfide bonds. TMF condensates sequester the AN locus, preventing its premature activation during meristem maturation, thereby regulating the transition to flowering. There is also evidence that TMF acts with cofactor BLADE-ON-PETIOLE (BOP) by forming a transcriptional complex [54] controlling the pleiotropic functions [55].
The absence of pedicel abscission phenotypes characterizes the natural mutants jointless (j) and jointless2 (j2). In addition, j has IMs that develop into vegetative growth, and j2 has bifurcated inflorescences and sepals in the form of leaf-like structures. The j2 phenotype is due to two independent mutations in the MADS-box gene MBP21, caused by the insertion of the Rider transposon and a single-nucleotide substitution that led to the appearance of a premature stop codon [56].
As suggested before, a MADS-box protein complex comprising at least J, MACROCALYX (MC), and MBP21 regulates pedicel abscission in tomatoes [57]. It was shown that MBP21 is involved in the ethylene and auxin regulatory pathway of sepal development [58]. It is also noted that one of the FANTASTIC FOUR family members, FAF1/2c reduces the stability of the COP9 signalosome, thereby regulating the expression of SFT and J [59].
At the TM and IM stages, three MADS-box genes of the SEPALLATA 4 (SEP4) family are expressed—J2, ENHANCER OF JOINTLESS 2 (EJ2, homolog of MADS1), and LONG INFLORESCENCE (LIN) [60]. Cross-interactions were detected between them, and their functional redundancy was confirmed in a collection of knockout mutants. Recently, the RNAi of tomato SEP4-like CMB1 led to longer, branched, and indeterminate inflorescences that exhibited a transition from reproductive to vegetative growth and enlarged and abnormally fused sepals [61] and a yeast two-hybrid assay showed that CMB1 could interact with MC, J, and MBP21.
The J2/EJ2 phenotype of excessive flowering and low fertility can be compensated by introducing a copy of the SB (SUPPRESSOR OF BRANCHING) locus, resulting in unbranched inflorescences [62]. It contains the TM3 (tomato MADS 3) and STM3 (SISTER OF TM 3) genes, which are antagonists of J2 and EJ2 [63]. Therefore, the regulation of flowering can be carried out by changing the dose of the antagonist factors, i.e., by increasing the copy number of their genes. There is cross-regulation between STM3 and J2 through direct binding, and their common target is FUL1 [64,65]. Recently, TARGET OF EAT 1 (TOE1) from the APETALA 2 (AP2) gene family was found to be a regulator of STM3 [66].
FULs are other MADS-box genes that play an important role in flowering. The FUL genes fall into two paralogous clades. Tomato has two genes in each clade: FUL1 and FUL2 in the former and MBP10 and MBP20 in the latter [67]. The analysis of CRISPR-Cas9 knockout plants showed that FUL2 and MBP20 promote the transition from vegetative to reproductive development and control inflorescence architecture [68]. At the same time, FUL1 most likely exhibits secondary functions during flowering, which the authors could not clarify. TM3/STM3 presumably controls floral transition by interacting with FUL2/MBP20 in a protein complex and repressing cytokinin inhibitors but subsequently exhibits antagonistic functions in determining FM and IM identity [69]. MBP10 is expressed at weak to moderate levels, and its atypical short first intron lacks putative transcription factor binding sites, indicating possible pseudogenization [67].
The MACROCALYX (MC) gene belongs to the APETALA 1/FRUITFULL (AP1/FUL) subfamily of the MADS-box gene family and is closely linked to the RIPENING INHIBITOR (RIN) gene that regulates fruit ripening, see review [70]. The MC regulates sepal development, and the transcription factors J and EJ2 were recently shown to interact with MC directly [71,72]. It was demonstrated that MBP22 can form condensates with MC and the SEP proteins TM5 and TM29 [73]. MC is probably downregulated by AGL (AGAMOUS-like) genes [74,75]. TM5 is the only representative of the SEPALLATA3 clade, and it is involved in determining the identity of petals, stamens, and carpels in tomatoes [76]. TM5, together with other transcription factors like RIN, is involved in the regulation of fruit development [77]. TM5 may act altogether with TM29 [78]. It has also been shown that the expression of AGL and TMs is controlled by histone deacetylases [79] and WRKY factors [80].
Transcription factors containing a DNA-binding domain with one zinc finger, called DOF (DNA-binding with one zinc finger), regulate photoperiodic flowering. DOF10 is involved in the control of cell proliferation during the development of vascular tissue in flowers [81]. DOF9 is involved in inflorescence meristem control and floral meristem differentiation by regulating cell division genes and regulating the inflorescence architecture LIN [82]. Additionally, DDF1 has been shown to mediate circadian regulation via protein–protein interaction with the floral inducer SFT [83]. CDFs delay the flowering time by regulating various FT-like genes [84].
A reversal of flower development is an interesting event consisting of a change from floral to vegetative development. It has been shown that the silencing of the GT11 gene, which belongs to the family of photosensitive transcription factors with a trihelix structure, leads to the formation of sepal-like petals in the second radial arrangement, carpel-like stamens in the third radial arrangement, and abnormal stem-like, leaf-like, and flower-like structures in the fourth radial arrangement in tomato [85]. These phenotypic manifestations, as well as the suppression of MADS-box gene expression, suggest the participation of GT11 in forming the pattern of floral organs and maintaining floral determinacy in tomatoes.
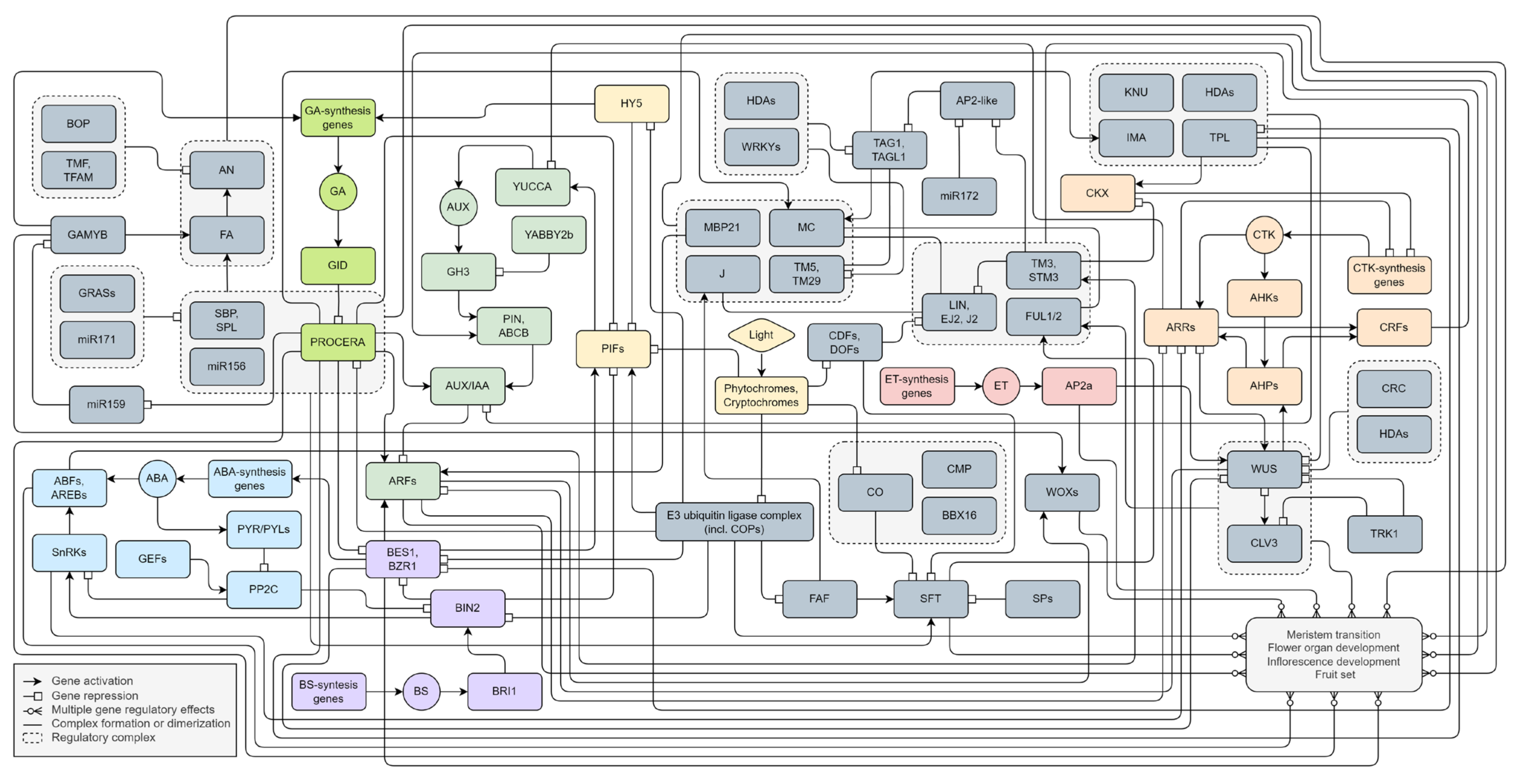
We have presented a putative model of flowering factor regulatory pathways (Figure 4). The primary external stimulus to flowering is considered to be light signaling. It includes both the spectrum and the photoperiod. Plants perceive and respond to light signals via multiple sensory photoreceptors, including phytochromes and cryptochromes. The shared signaling molecules of these photoreceptors are the COPs E3 ubiquitin ligase complex and bHLH transcription factors PIFs. They trigger cascades of hormonal regulatory pathways, mainly AUX, GA, and CTK signaling, that activate meristem transition, inflorescence formation, and flower organ development. The most abundant transcription factors in such processes are MADS-box genes. It appears they form homo- or/and heterologous multimers and provide both the positive and negative regulation of tomato flowering. Due to overlapping targets, they may contribute to regulating flowering-related genes through dosage compensation. The disruption of such balance is possible due to other factors and cofactors by hormonal signaling. We assume three possible relationships of transcription factors: redundancy, additivity, and dependency. Redundancy is manifested in the functional identity of transcription factors. Additivity is associated with the provision of function through the joint contribution of each element. Direct dependence involves the activation or repression of the role of one factor only after interaction with another. Moreover, the autocatalytic regulation of the participants of regulatory cascades is possible.
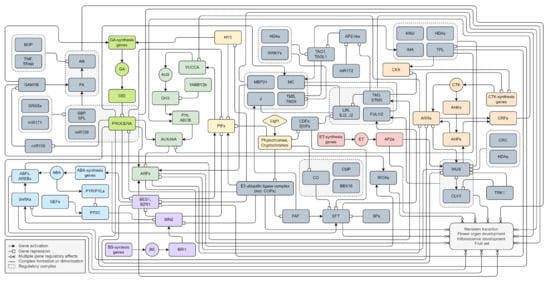
Figure 4.
The flowering gene regulatory pathways in S. lycopersicum. All interactions are based on experimental data reported in scientific publications. A molecular interaction network model was created using the free online web application draw.io (www.drawio.com (accessed on 24 January 2024)).
3. Flower Development
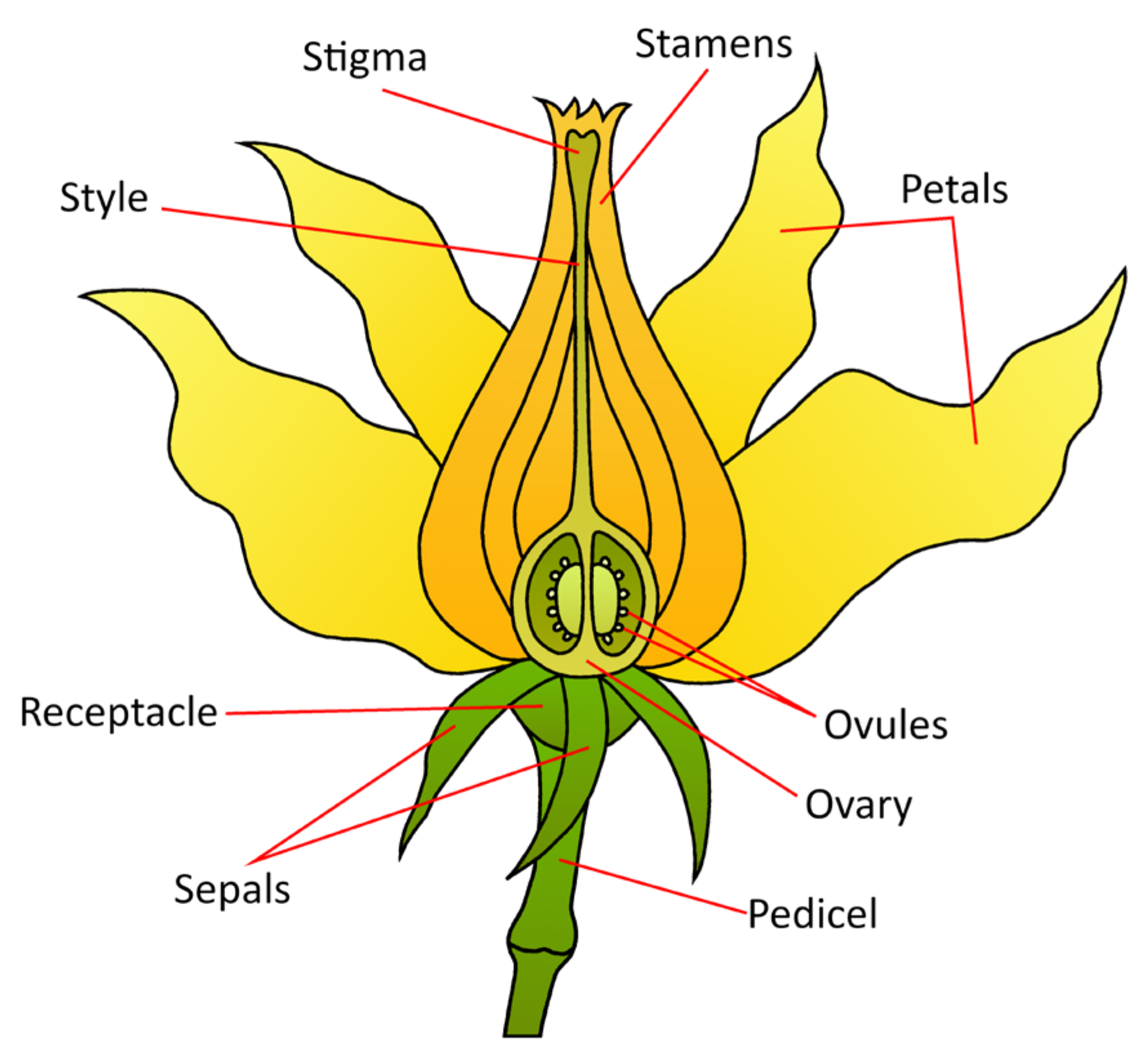
The bisexual tomato flower, like most angiosperm flowers, consists of four distinctive whorls of floral organs: sepals in the outmost whorl, petals in the second whorl, and stamens and carpels in the respective third and fourth whorls. The number and position of floral organs in each whorl are governed by floral homeotic genes and cadastral genes controlling the floral organ boundary (Figure 5).
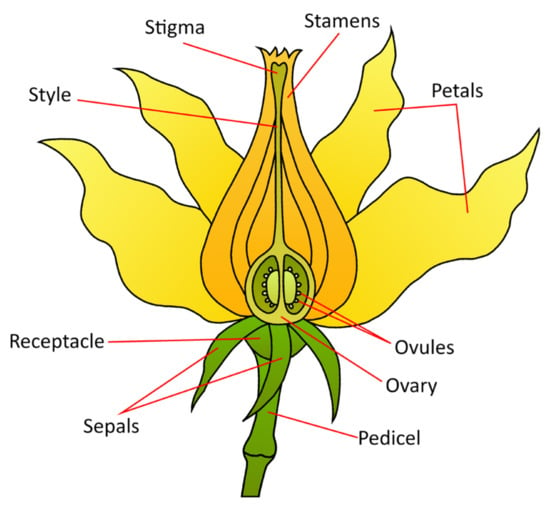
Figure 5.
A stylized diagram of the tomato floral anatomy. This general morphology is consistent across angiosperms.
The tomato fruit develops from the fertilized ovary, which is the broad base of the pistil. It is a berry consisting of a pericarp formed from the ovary wall, placenta, and pulp, containing seeds [86]. Therefore, many traits of fruit morphology are determined during flower development.
3.1. Flower and Fruit Abscission
Plant reproductive organ rejection occurs in the abscission zone (Figure 6) and is one of the causes of low crop yields. It is triggered by developmental and environmental signals. An alteration in the auxin and ethylene contents in the abscission zone to a threshold value is known to result in abscission [87]. Abscission zone cells may remain in a non-dividing state without external stimuli and behave as meristematic cells [88]. Suppression of fruit abscission is also a valuable trait, leading to the convenience of tomato harvesting and processing. For example, in abscission mutants, pedicels and calyxes remain attached to the inflorescence axis, which reduces the mechanical damage to the fruit during transportation. Here, we present some recent studies of tomato genes conducting flower and fruit abscission.
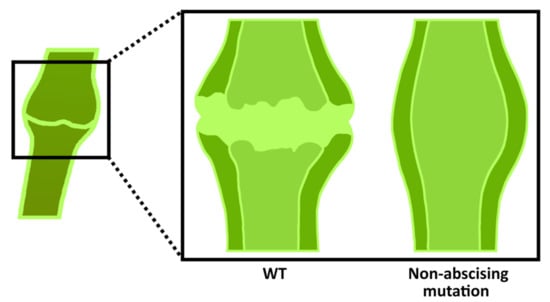
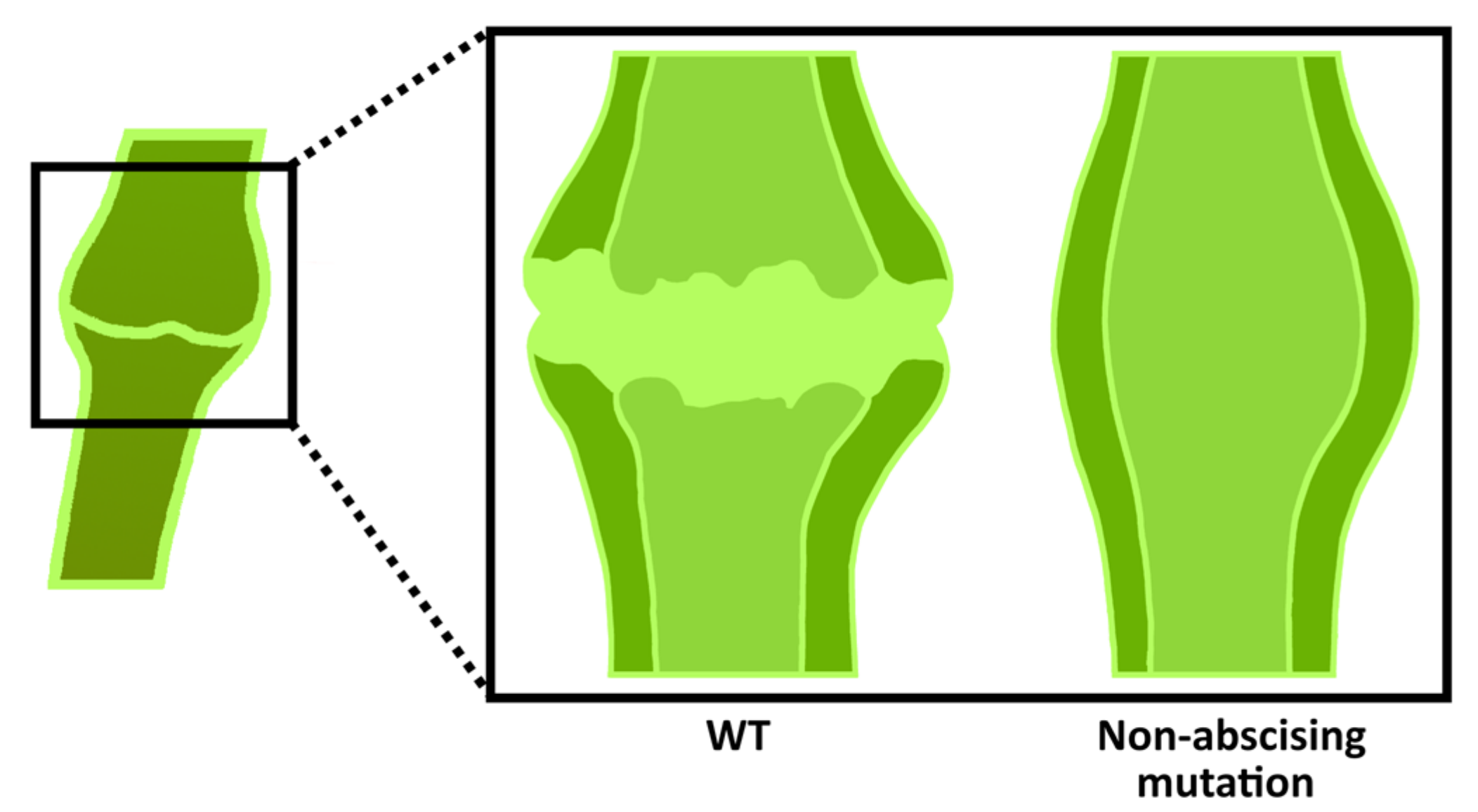
Figure 6.
Representation of tomato abscission zone. It forms in the pedicels and has a knuckle-like structure in which a groove forms for abscission.
LSD (LESION SIMULATING DISEASE) genes encode a family of zinc finger proteins essential in hypersensitive responses and programmed cell death induced by biotic and abiotic stresses. LSD and a bHLH-type transcription factor are involved in cytokinin-induced petal abscission via the regulation of Aux/IAA gene expression [89]. Evidence shows that the IDA (INFLORESCENCE DEFICIENT IN ABSCISSION) peptide and its IDA-like (IDL) homologs play a conserved and central role in this process. For example, the knockout of IDL6 [90] suppressed TAPG1 (polygalacturonase), TAPG4, and CEL2 (cellulase), which is quite similar to the function of the phytosulfokine peptide. However, their regulation of abscission is different. The authors hypothesize that ethylene triggers IDA signaling, which promotes the expression of cell wall hydrolases that cleave polysaccharides in the cell wall and middle lamina, leading to abscission. Here, WRKY17 acts as a positive regulator of IDL6 by directly binding to the W-box elements of its promoter. IDA-regulated genes are KNOX, which also control flower abscission. KNOX has been shown to inhibit the expression of the ethylene synthesis genes by directly binding to their promoters, and its ectopic expression in tomatoes suppresses flower abscission [91].
A lack of auxin induces the production of ethylene, which in turn is an abscission activator. Morphologically, this is characterized by the destruction of undifferentiated cells in the meristem to initiate their differentiation into the abscission zone. ILR and ILL hydrolyze amino acid-type IAA conjugates. Auxin is readily degraded in its free form, so the inactivation of the hydrolases of IAA conjugates may mediate delayed abscission [92].
Many transcription factors are involved in these processes. For example, it has been previously shown that the loss of MBP21 function denied abscission zone formation and the consequent separation of tomato fruit from the parent plant [56]. A BELL gene family member, BL4, is also involved in abscission zone formation [93]. It was confirmed by the anatomical analysis of the peduncle, after which it was evident that the zone was not formed in the BL4-RNAi lines, and more epidermal cell layers were observed at this location compared with WT. Also, the silencing of the intercellular auxin transporter PIN1 accelerates flower abscission by increasing auxin accumulation in the ovule and decreasing the auxin content in the abscission zone [94]. In turn, its negative regulator is the transcription factor MBP9 [95].
ROS are involved in the biosynthesis and signaling of ethylene-dependent regulation. TIP (TONOPLAST INTRINSIC PROTEIN) aquaporins mediate the transport of ROS and water through the cell wall. Therefore, the suppression or overexpression of their corresponding genes shows delayed or accelerated abscission, respectively [96].
At the late stages of abscission, several key enzymes play an essential role in organ abscission. Cellulase and polygalacturonase are involved in cell wall degradation, and pectin methylesterase changes the chemical structure of the abscission zone through hydrolysis and causes cell wall and membrane degradation. However, the signaling proteins at this stage are hybrid products of the PRP (proline-rich protein) gene, which are involved in activating cell wall hydrolysis. In [97], as expected, the silencing of HyPRP (HYBRID PRP) resulted in delayed peduncle abscission.
P4H (prolyl 4-hydroxylase) catalyzes the posttranslational modification proline hydroxylation of cell wall hydrolases (TAPG and CEL) and expansins (EXPs). The RNAi of the P4H3 gene showed decreased TAPG, CEL, and EXP transcript levels [98]. Phenotypically, this was manifested as a delayed abscission of tomato fruits in the latter stages of senescence.
Ultimately, we summarized the abscission-related studies on tomatoes from 2017 to 2023 in Table 1.

Table 1.
Recent studies of tomato genes that facilitate flower abscission.
Despite the abundance of factors in the table, the main regulatory complex of abscission processes appears to be CLV-WUS (CLAVATA-WUSCHEL), which controls auxin and ethylene homeostasis in the abscission zone in response to external stimuli [108].
3.2. Ovary Development and Fruit Size
Fruit organogenesis begins in the early stages of flower development with the differentiation of carpels into locules, the number of which determines the final size of the fruit. Two loci, fasciated (fas) and locule number (lc), are responsible for their formation, and they affect the expression of CLV3, WUS, YABBY2b, and TAG1 (tomato AGAMOUS 1) [112]. Previously, YABBY2b was reported as the candidate gene for the fas allele [113]. However, it was shown that CLV3 is associated with fas [112,114], while YABBY2b regulates auxin synthesis by suppressing GH3.8, which encodes indole-3-acetic acid-amido synthetase [115]. Meanwhile, WUS appears to mediate lc. The RNAi lines of the WUS gene confirmed the involvement of WUS in controlling the formation of floral organs and the number of locules in tomato fruits [116]. The expressions of TAG1 and CLV3 were changed in such lines.
WUS encodes a homeodomain transcription factor of the WOX (WUSCHEL-like homeobox) family. WOX controls the growth and development of plants by regulating the formation and maturation of stem cells in meristems. Null mutations in WOX9 (related with natural mutation in COMPOUND INFLORESCENCES) and WOX8 result in embryonic lethality, and mutations in the promoter region cause various defects in meristem development [117]. The knockout of the WOX1 resulted in a phenotype with defects in the fusion of petals, carpels, and stamens, suggesting the involvement of the gene in the regulation of tomato flowering [118]. The knockout of the WOX1 homolog LAM1 (LAMINA DELETION MUTANT 1) also showed impairments in floral organ development, fruit size, secondary leaflet initiation, and leaf complexity [119,120].
There is evidence that WUS expression is regulated redundantly. For instance, BRI1-EMS-SUPPRESSOR 1 (BES1) repressed the regulation ability of WUS via their heterodimerization, thus inhibiting WUS binding to the CLV3 promoter [121]. WUS can also be repressed by KNUCKLES (KNU) through histone deacetylation [122]. The adapter proteins in this process are most likely INHIBITOR OF MERISTEM ACTIVITY (IMA). IMA was proposed to recruit KNU to form a transcriptional repressor complex with TOPLESS (TPL) and histone deacetylase [123]. In turn, IMA is induced by the MADS-box transcription factor AGAMOUS (AG) [124]. Moreover, CRABS CLAW (CRC) can interact with members of the chromatin remodeling complex that epigenetically represses WUS expression through histone deacetylation [125].
It was shown that the expression of CLV3 and WUS appears to be restricted by the LITTLE ZIPPER protein (ZPR), encoded by the DTM (DEFECTIVE TOMATO MERISTEM) gene [126]. As suggested by the authors, DTM has negative feedback with HD-ZIP III homeodomain transcription factors. It has been established that the receptor-like cytoplasmic kinase TRK1 is necessary for maintaining the proper growth of meristems in tomato [127]. It turned out that TRK1 silencing induced the formation of fasciated branches, altered inflorescences, and fruits of tomatoes with a significantly increased number of locules compared to wild-type plants. This phenotype, supported by further studies, is due to the interaction of TRK1 with CLV1, the receptor for the CLV3 peptide. In addition, TRK1 also interacts with and phosphorylates WUS. However, such cross-molecular interactions require further study.
Regulation of the formation of multiple locules in tomato fruits is achieved by modulating the levels of auxin and gibberellin (GA) in apical meristems, for which the transcriptional repressor TPL3 and WUS are responsible [128]. A protein interaction was found between them, ensuring the regulation of auxin transporter genes and enzymes for gibberellin biosynthesis. However, CLV3 and WUS are not the only genes determining fruit size. It was previously shown that a representative of the AP2/ERF (ethylene responsive factor) superfamily of transcription factors, the ENO (EXCESSIVE NUMBER OF FLORAL ORGANS), regulates the activity of the floral meristem [129]. The authors demonstrated that ENO exhibits synergistic effects with mutations at the fas and ls loci. This is explained by the interaction of ENO with the cis-regulatory element of the WUS promoter. The clarified role of the AP2a factor was revealed in [130]. Loss-of-function mutants had a much higher ethylene production, leading the authors to infer a role as a negative regulator of ripening initiation, with deficient lycopene production and defects in chlorophyll degradation suggesting positive regulation during ripening. A CLV3-WUS module was shown to regulate auxin and ethylene homeostasis in low light-induced tomato flower abscission via the induction of the WUS target genes KD1 (KNOTTED1-LIKE HOMEOBOX PROTEIN) and FUL2 [108].
WUS is a bifunctional protein that can repress and activate gene transcription in SAM [131]. It induces CLV3 expression dose-dependently [132]. CLV3-mediated signaling through CLV1, CLV2-CRN, and BAM-RLK2 complexes restricts WUS expression [133,134]. In addition to the CLV-WUS complex, which determines the tomato fruit size, new regulatory elements are identified. For instance, a new tomato fruit size control module was recently disclosed [135]. Here, UV-damaged DNA-binding protein 1 (DDB1), which is a significant component of the Cullin4-RING E3 (CRL4) ubiquitin ligase complex, promotes the degradation of casein kinase (CK2). In turn, CK2 stabilizes cell division protein kinase (CDK2), acting as a positive regulator of fruit size.
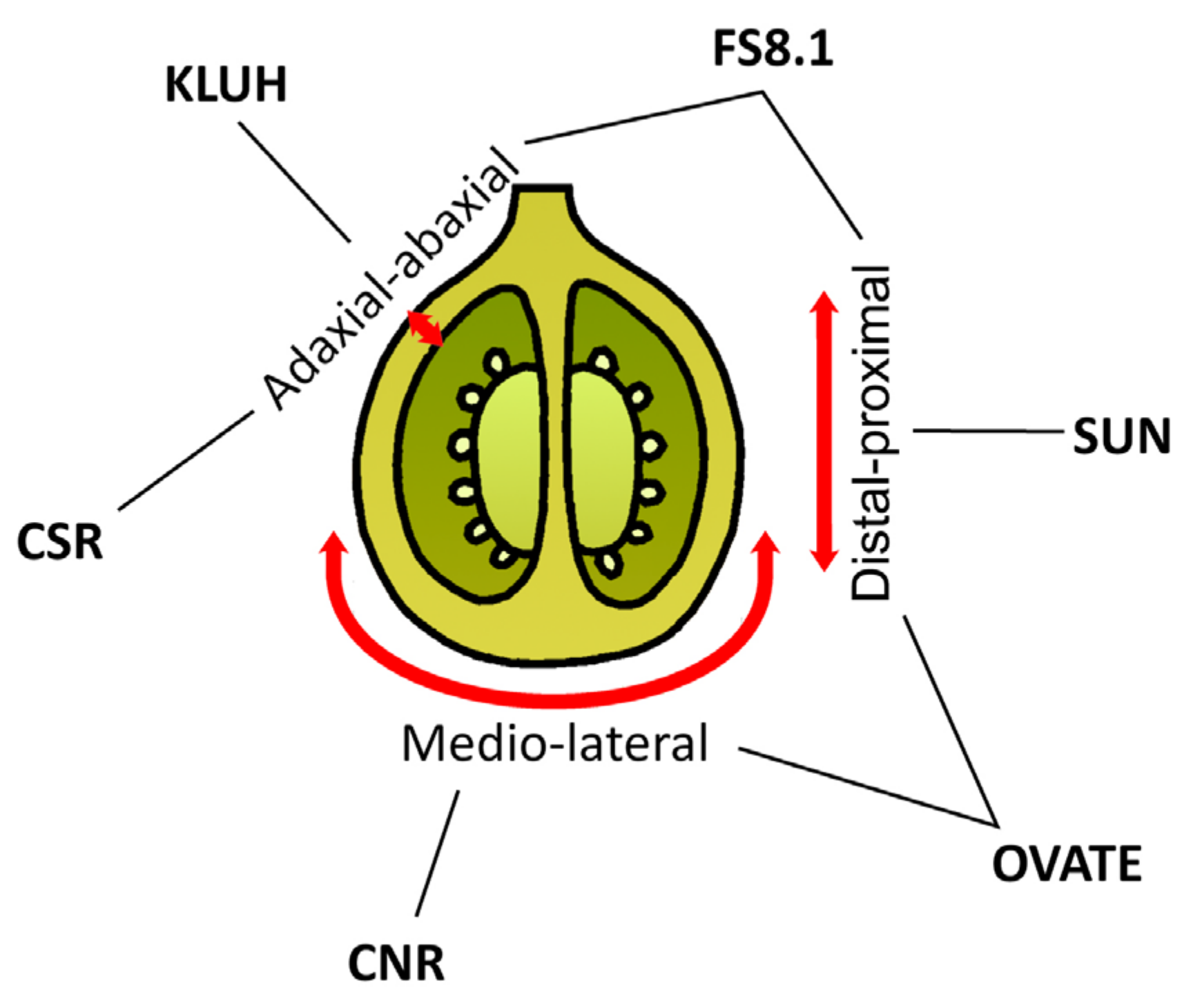
In addition to fruit size, the diversity of fruit shapes of tomato varieties is also attractive to consumers. Tomato loci responsible for fruit size are identified (Figure 7). Fas and lc control fruit locule numbers and their flat shapes, whereas sun and ovate control elongated shapes [136].
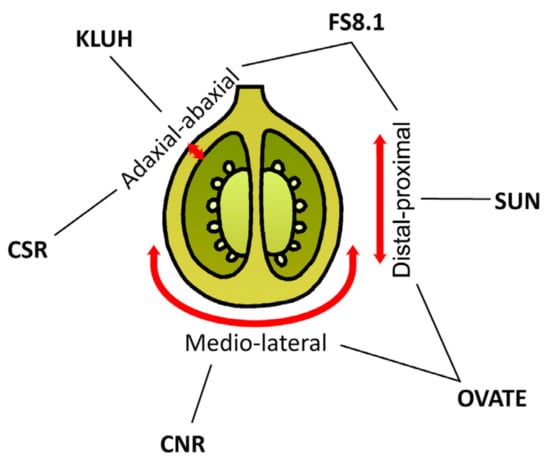
Figure 7.
Genes influencing tomato fruit shape and weight. The red arrows indicate axes of growth: from proximal to distal, medio-lateral, and from adaxial to abaxial.
SUN and OVATE determine the shape of the ovary. Compared with WT, ovate primarily increases the fruit proximal end by increasing cell numbers in the proximal–distal direction and decreasing cell numbers in the medio-lateral direction, leading to a pear-shaped fruit [137]. OFPs (OVATE family proteins ) are a class of proteins with a conserved OVATE domain. OFPs interact with various transcription factors, including KNOX and BELL [93,138], and also directly regulate the expression of auxins, gibberellins [139,140], and ethylene-related genes [141]. The heterologous expression of OFP1 and MADS1 in the ovate mutant compensated for the elongated fruit phenotype [142]. The heterologous overexpression of citrus OFP19 in tomatoes resulted in the pear-shaped ovary and fruit shape [143]. Transgenic tomato plants that overexpressed bottle gourd OVATE1 had cone-shaped fruit, calyx hypertrophy, petal degeneration, and petal retention after flowering [144]. The overexpression of OFP20 has been shown to regulate abscisic acid accumulation [145]. In addition, OFP20 may play an essential role in the crosstalk between brassinosteroids (BSs) and gibberellins [146]. On the other hand, the sun results in long fruit by increasing cell numbers along the entire proximal–distal direction in the pericarp and columella while decreasing cell numbers in the medio-lateral direction in the columella and septum [147].
TONNEAU1-recruiting motif proteins (TRMs) have been implicated in the control of organ shapes in tomatoes, which is contributed to via interaction with OFPs [148]. The TRM1-5-like genes promote fruit elongation, whereas OFP1-like genes play an antagonistic role [149]. The IQ67-domain (IQD) proteins regulate Ca2+ signaling and plant development through interactions with calmodulins, and SUN is one of them. SUN affects the expression of auxin-related genes [150,151] and brassinosteroids [140]. SUN24 negatively regulates the expression of crucial abscisic acid signaling genes [152]. The ectopic overexpression of watermelon IQD24 promoted tomato fruit elongation [153]. MAP70 (microtubule-associated protein) interacts with IQD21a to regulate tomato fruit shape [154]. It becomes clear that the interaction of OFP-TRM and MAP-IQD modules with microtubules lies in the physiology of fetal shape changes, as discussed in [155].
KNOTTED-like (KNOX, KN) proteins and BELL1-like (BLH) proteins belong to the same TALE homeodomain family. KN2 from CLASS-II KNOX genes regulate fruit anatomy via gibberellin [156] and ethylene-dependent pathways [157]. The ectopic expression of litchi KNOX represses tomato flower abscission [91]. The heterologous overexpression of grape KNOX63 in tomato induced smaller fruits and seeds than in wild-type or KN1-deficient plants [158]. There is also KN4, which affects pollen development via the regulation of GA and auxin genes [159]. BLHs have been recently shown to regulate chloroplast development and chlorophyll synthesis in tomato fruit [160,161]. KNOX and BLH proteins act together by forming heterodimer modules [162,163,164].
KLUH is a positive regulator of fruit size, and it was recently demonstrated that introducing point mutations into its promoter region can “improve” the attractive appearance of large fruits [165]. The duplication of KLUH and STM3 and the corresponding increase in gene expression are decisive for fw3.2, one of the primary loci responsible for tomato fruit mass [63].
The fw2.2 (fruit weight 2.2) gene was found to control a significant QTL for tomato fruit size by negatively affecting cell numbers [166]. FW2.2 is a transmembrane protein containing a PLAC8 (PLACENTA-SPECIFIC 8) domain. Unfortunately, no functional analysis is yet available for FW2.2 [167]. However, like in the sun, the presence of a PLAC8 domain can be related to a putative function in regulating Ca2+ signaling [168].
In addition to CNR (COLORLESS NON-RIPENING)/FW2.2 and KLUH/FW3.2, a new tomato cell size regulator gene was recently discovered—CSR (CELL SIZE REGULATOR)/FW11.3 [169]. Its CSR-like 1/2/3 paralogs were also discovered. Co-expression analysis of these genes revealed a role for the CSR gene in cell differentiation during later stages of fruit development, including vascular development. The antagonistic roles of auxin and cytokinin could be related to CSR function, as several genes associated with these pathways were found in co-expression clusters. The authors also associate cell enlargement with increased endoreduplication.
Genes underlying the fs8.1 locus that contribute to rectangular elongated tomato fruit have yet to be identified. Compared with sun and ovate, fs8.1 showed a cellular patterning that was different from the effect of the other two genes: fs8.1 led to increased fruit shape by increased cell number in the proximal–distal direction without a change in the medio-lateral direction [170]. Recently, the candidate genes of fs8.1 have been proposed [171]. Its list includes genes encoding ERECTA (LRR receptor-like kinase), cytokinin oxidase, F-box protein SLOMO (SLOW MOTION), aminopeptidase protein, pentatricopeptide repeat protein, LRR domain-containing protein, and trihelix transcription factor. Thus, the fs8.1 locule requires further investigation.
The sun, ovate, fs8.1, kluh, csr, and cnr loci display individual molecular mechanisms of ovary development in tomato. Although the ovary shape changes they exhibit differ, they can contribute synergistically by strengthening the effects of other mutations, allowing for the combination of these traits in breeding. In general, the effects of fruit shape loci are likely due to changes in the expression of genes related to phytohormones, cytoskeleton, and sugar transport and degradation genes.
4. Pollen Development and Fertilization
Developing functional pollen grains is a crucial aspect of plant sexual reproduction. The process involves three stages: microsporogenesis, postmeiotic microspore development, and microspore mitosis [172]. Pollen grains are developed from microspores in the microsporangium of an anther. The cell wall of a mature pollen grain is a multilayered structure consisting of sporopollenin-based exine [173] and cellulose-based intine [174,175]. Pollen development and maturation involve multiple cellular changes mediated via the precisely organized regulation of gene expression. They could be influenced by many factors, such as tapetum irregularity, cytoskeleton alteration, auxin metabolism aberration, altered sugar utilization, and ROS accumulation [176].
Fruit set depends on successful pollination and fertilization. Pollination involves the transfer of pollen grains from the anther to the pistil’s stigma. Fertilization requires the growth of the pollen tube in the pistil tissue to the ovary [177]. Fertilization of the ovary initiates the development of the ovary into a fruit [178].
4.1. Pollen Maturation and Sterility
During the growth and development of plant pollen, the autophagy of the tapetum provides the necessary nutrients for the development of microspores. Transcription factors induced by various signaling pathways act as molecular regulators in these processes. For instance, the MYB72 inhibits the autophagy in tomato anthers [179] while HB8, which is an HD-Zip III transcription factor, accelerates tapetum degradation [180]. Also, the knockout of genes encoding the bHLH transcription factors leads to dysfunctional meiosis and the development of an abnormal tapetum during flower development [181,182]. Moreover, SINA (SEVEN IN ABSENTIA) proteins with E3 ubiquitin ligase activity impact tomato floral structure [183].
Delayed or early tapetum degradation can affect pollen development, leading to male sterility. Genetic male sterility is attractive when obtaining hybrid seeds, ensuring high varietal purity. The genes that mediate male sterility are being actively studied. For example, the knockout of the ABORTED MICROSPORES (AMS) gene, encoding a bHLH transcription factor, led to critical changes in the morphology of tomato pollen grains and, as a result, their nonviability [184]. It has also been shown that the knockout of the strictosidine synthase gene STR1 results in abnormally small pollen grains with a structurally weakened exine, and the plants themselves do not set fruit after selfing [185]. In [186], it was demonstrated that the mitogen-activated protein kinase MPK20 positively regulates the development of mononuclear microspores during mid-to-late life. Silencing or null mutations of MPK20 led to male sterility. In addition, the abortive nature of MPK4-silenced tomato pollen was confirmed in [187]. A dramatic increase in ACO expression occurs during pollen and seed maturation. The knockout of ACO2 results in male sterility but reduces free proline levels [188].
ROS have been reported to act as regulators of anther development. RBOH (respiratory burst oxidase homolog) plays a key role in regulating ROS accumulation in anthers and mediates tapetum development [189]. Therefore, RBOH/RBOHE double knockout mutants exhibited complete male sterility, showing abnormal programmed cell death in the anthers. There is also evidence that the cytoplasmic invertase CIN7 is involved in pollen viability, which the authors also associate with increased ROS accumulation in CIN7-silenced tomatoes [190]. Meanwhile, the knockout of IDA (inflorescence deficient in abscission), which acts as the RLKs ligand, destabilizes ROS homeostasis that leads to a programmed cell death defect in the tapetum and septum and a failure of anther dehiscence [104]. The accumulation of ROS in anthers is related to heat shock transcription factors, which confers pollen thermotolerance [191].
Hormone-mediated regulation pathways of pollen development and maturation are actively studied. For instance, the study of the mediator complex is required to control the transcription of RNA polymerase II. The function of the MED18 (mediator complex subunit) subunit in pollen ontogeny has been reported [192]. The expression profiles of tapetum degradation genes and pollen maturation genes are in RNAi-silenced lines. There is also evidence that silencing MED18 suppresses the expression of gibberellin biosynthesis genes, auxin transport genes, and regulators of leaf morphogenesis [193]. The importance of auxins in developing tomato anthers was demonstrated using the PIN8 transporter gene as an example [194]. Tomato lines with PIN8 (PIN-FORMED 8) silencing had shortened anthers. They observed the abortion of microspores in anthers, low pollen fertility, and parthenocarpic fruits, which the authors attribute to a greater extent to the increased content of IAA conjugates in transgenes. There is also a clue that abscisic acid plays a role in the primary formation of pollen grains [195]. Ascorbic acid impairs tomato pollen fertility [196].
Meanwhile, salicylic acid has also been shown to impact pollen development [197]. At the same time, ethylene signaling is shown to modulate tomato pollen tube growth through modifications of cell wall remodeling and calcium gradient [198]. Moreover, tapetum degradation and pollen fertility are affected by brassinosteroid-mediated regulation [199]. Jasmonic acid (JA) facilitates flower opening and pollen maturation through the expression of MYB21 [200].
Because sepals and petals only support limited photoassimilates, pollen growth largely depends on the import of carbon resources. SWEET (SUGAR WILL EVENTUALLY BE EXPORTED TRANSPORTER) members are critical players in sugar allocation between source and sink organs [201], and it was shown that SWEET5b is required for pollen maturation in tomato [202]. Meanwhile, the overexpression of Vitis vinifera sucrose transporter SUC27 in tomatoes resulted in longer petals and pistils, an abnormal stigma, and much less and shrunken pollen, while the SUC11- and SUC12-overexpressing lines had similar flower phenotypes compared with those of the wild type [203]. The silencing of hexokinase HXK1 resulted in a decrease in flower numbers, increased rate of flower abscission, abnormal thickening of the anther wall, and reduced pollen and seed viability [111]. Here, it was shown that phytochrome-interacting factor 4 (PIF4) inhibited the transcriptional expression of HXK1.
The growth of a pollen tube requires the coordination of membrane receptor signaling, GTPase activity, and actin cytoskeleton assembly. Kinase partner protein (KPP) is a guanine nucleotide exchange factor, and it plays a crucial role in pollen tube growth by recruiting the actin-related protein complex to the membrane-localized receptors [204].
Tapetum cell formation requires a leucine-rich repeat (LRR) receptor kinase and its ligand TAPETUM DETERMINANT 1 (TPD1). Tomato TPD1 loss-of-function mutants showed an alteration in redox homeostasis during male gametogenesis and are expected to regulate BES1, DYSFUNCTIONAL TAPETUM 1 (DYT1), DEFECTIVE IN TAPETAL DEVELOPMENT AND FUNCTION 1 (TDF1), and MYB33 [205]. In addition to LRR receptor kinases, there are lectin receptor kinases, which are also shown to have an essential role in the correct development and maturation of tomato pollen grains [206]. Here, it was shown that carboxypeptidase, cytochrome P450, and DNA mismatch repair proteins are associated with lectin receptor kinase activity.
PIFs act as central regulators in integrating light and temperature signals to optimize plant growth and development. It was shown that PIF4 regulates the anther’s adaptation to low temperature by directly activating DYT1 expression [207]. Moreover, low temperature promotes the transcriptional activation of TDF1 by the PIF4-DYT1 complex, thereby postponing tapetal autophagy [207]. There is also evidence that PIF4 could be a key transcription factor regulating YUCCA (YUC) genes, which are the main auxin synthesis genes, in tomato stamens [110]. For another PIF, it was shown that both glutamate synthase 1 (GLT1) and cell wall invertase 9 (CWIN9) involved in auxin and sugar homeostasis in anthers are directly regulated by PIF3 [208].
As for MYB33, its knockdown has been shown to restrict the expression of genes controlling flowering (AN, FA, WOX9, SP) and sugar metabolism genes (CWIN, sucrose-phosphate synthase, sucrose synthase, trehalose-6-phosphate synthase) [50]. The authors proposed that MYB33 contributes to brassinosteroid–gibberellin crosstalk in flowering. MYBs are commonly regulated by microRNAs. Therefore, they are also involved in the regulation of sporogenesis. The knockdown of miR171 was reported to result in male sterility in tomatoes due to the production of small amounts of deformed and nonviable pollen due to delayed tapetum ontogeny and reduced callose deposition around tetrads [209]. Due to GRAS24 being one of the miR171 target genes, it is a participant in gibberellin and auxin homeostasis regulation in pollen development [210]. MiR172, miR156, and miR160 are also shown to be involved in transcriptome remodeling during pollen development [211]. Epigenetic regulation is integral to transcript accumulation. DNA methyltransferase CMT4, which is actively expressed during the flowering and early fruit development stages of tomato, has been shown to activate the genes of pollen wall development and pollen tube elongation [212].
The diversity of regulatory genes responsible for anther cell differentiation and pollen formation illustrated here allows us to characterize the gene crosstalk occurring in tomato and other crops. Although a complex system of gene expression and interactions, the molecular network of anther and pollen development is highly conserved. It involves various hormones and transcriptional and epigenetic factors. They regulate gene expression involved in tapetum degradation, cytoskeleton rearrangement, sugar metabolism, transport, and ROS accumulation.
4.2. Self- and Cross-Incompatibility
Modern domesticated tomatoes have been derived from many consecutive selections, resulting in a partial loss of genetic diversity. One way to expand the pool of available genes in tomato to achieve new desirable agronomic traits or for fundamental purposes is to obtain hybrids with its wild relatives. In this case, the problem of interspecific incompatibility arises. The physiological basis of interspecific incompatibility is still emerging. The nature of tomatoes’ reproductive barriers includes prezygotic and postzygotic isolating mechanisms. Tomato wild relatives are characterized by various reproductive biology systems [213], including self-compatible and self-incompatible taxons and facultative and unilateral incompatibility.
Most wild tomato plants cannot self-pollinate due to the protruding stigmas on the flowers. The elongated style prevents pollen grains from becoming trapped on the stigma, resulting in male sterility. In this case, high temperature is a prerequisite for lengthening the column, activating a shift in the hormonal balance. LST (LONG STYLES) is identified as a candidate gene mediating the elongation phenotype [214]. It encodes the ethylene receptor protein and is homologous to the Arabidopsis EIN4 (ETHYLENE-INSENSITIVE 4) and tomato ETR5 (ETHYLENE RECEPTOR 5) genes. Its overexpression reduced the severity of the style elongation phenotype in male-sterile tomatoes. The genes controlling the conversion from flush stigmas to inserted stigmas were identified in [215]. They appear to be C2H2-type zinc finger transcription factors—one controls the conversion of exserted to flush stigmas, while the other regulates the conversion of flush to inserted stigmas.
The callose wall surrounds the sporocytes while meiosis occurs. The temporary isolation of the sporocyte may be connected with the sporocyte’s differentiation process. Callose accumulates in the walls of incompatible pollen grains and tubes [216,217]. It has been shown that the β-1,3-glucanase encoding gene (BG10) regulates pollen development and seed production by modulating callose deposition [218].
MSH2 (MutatorS-Homolog 2) is involved in recognizing and repairing DNA errors. The suppression of MSH2 gene expression in [219] using RNAi resulted in significant phenotypic abnormalities. This suppression contributed to the disruption of the light-dependent repair of thymine dimers. MSH2 silencing also affected the progression of male meiosis, either arresting at the zygotene stage or forming diploid tetrads. Thus, MSH2 may be a ploidy regulator in addition to its reparative function. Indeed, using MSH2 silencing, it was possible to establish that changing the lighting period may regulate the frequency of meiotic recombination within certain limits [220].
An example of overcoming interspecific Solanum incompatibility is given in [221]. The overexpression of the farnesyl pyrophosphate synthase gene FPS2 from Solanum pennellii in S. lycopersicum has been shown to compensate for the pollen incompatibility phenotype of the latter. The authors propose that FPS activity is required to prenylation a specific pollen rejection factor that interacts with the corresponding pistil barrier factor in S. pennellii. The S. pennellii barrier factor gene was also identified [222]. It has been shown that CRISPR/Cas9 mutations in the DIR1-like (DEFECTIVE IN INDUCED RESISTANCE 1) gene of S. pennellii allow S. lycopersicum pollen tubes to grow to the lower third of the style.
In summary, there are several reasons for incompatibility. Flower size and the distance the stigma extends beyond the anther determine the chance of self-fertilization. The size of the pollen grains is also important, as larger grains contain more nutrients, allowing the pollen tubes to grow to longer lengths. The molecular mechanisms of pollen rejection are also affected. At the postzygotic stage, incompatibility is caused by the disruption of the conjugation and segregation of homologous chromosomes due to their structural differences and epigenetic modifications. The genetic basis of these processes is poorly known and needs further study.
4.3. Parthenocarpy
Seed formation is the most crucial stage in fruit development; despite this, seedless fruits and fruits with underdeveloped or few seeds are found in both wild and cultivated tomatoes. This is possible due to parthenocarpy, in which the fruits develop without fertilization, and asthenospermia, in which pollination and fertilization are necessary. However, the embryos cannot form or are aborted before the seeds are formed. The complete penetration of pollen tubes can initiate fruit set independently of fertilization by activating genes regulating cell division and expansion [223]. Exposure to extreme conditions results in the suppression of fruit set due to low pollen viability. Seedless fruits are not only interesting from a developmental point of view. However, they are also a desirable agronomic trait due to their high soluble solids content and the lack of need to separate the seeds for cooking. Parthenocarpy has several advantages for growers, including avoiding emasculation in F1 hybrids and reducing the threat of heat stress during fruit set [224]. Parthenocarpy can increase winter and early yields, allowing for tomato harvests all year round [225]. In addition, the postharvest storage time of seedless fruits is longer than that of seeded fruits because seeds produce senescence hormones [226]. Parthenocarpy can be induced forcibly by external stimulation with plant hormones [227,228]. However, this may lead to undesirable pleiotropic effects, so studying the molecular basis of parthenocarpy is a priority.
The regulation of parthenocarpy is presumably carried out by auxin and gibberellin signaling cascades. The auxin and gibberellin regulatory pathways interact hierarchically and are the primary hormones that promote fruit set [229]. Auxin and gibberellin signaling cascades are negatively regulated by the DELLA component (GRAS gene encodes protein-containing “D-E-L-L-A” amino acid sequences) and the ARF7/IAA9 complex (auxin response factor/IAA), and cross-signaling between them controls the initiation of maturation fruits. As was shown, the inhibition of any of the components in these interactions leads to the appearance of seedless tomato fruits [230]. IAA9 is involved in the control of ARFs, and its suppression in tomato induces parthenocarpy [231].
Based on the assumption that genes of the TOPLESS (TPL) family are involved in auxin-mediated signals in the ovary, the authors silenced TPL1 in tomatoes [232]. These plants did not exhibit pleiotropic effects under normal conditions and produced seedless fruits upon flower emasculation and heat shock, which was associated with changes in cytokinin levels. TPL1 interacts with IAA9, and a mutation of the IAA9 gene leads to parthenocarpic fruit formation [233,234].
DELLA proteins localized in the nucleus are negative growth regulators. DELLA is encoded by the PROCERA gene, and its loss of function in the homozygous state results in dwarfism [235] and parthenocarpy [236]. DELLA proteolysis is mediated by the gibberellin-activated GID receptor [237]. The binding of gibberellin to the GID receptor increases the affinity of the latter for DELLA [238].
MADS-box factors are another participant in signaling regulation mediating parthenocarpy [1]. Indeed, seven MADS-box genes were previously reported to be expressed during flower development and the early stages of fruit and seed development [239]. Here, it was hypothesized that ovary and fruit development are a continuation of the flower development program. The MADS-box factors TAG1 and TAGL1 (tomato AGAMOUS-like 1) are involved in tomato fruit set [240,241] and have redundant and divergent functions [75,242]. Some other members of the MADS-box factor family are also involved in regulating tomato reproduction. For example, in [243], the authors found that the mutated MADS-box alleles of the AGL6 gene ensure the tomato yield under heat stress conditions. CRISPR/Cas9-mediated mutations in AGL6 produce facultative parthenocarpy, manifested by the development of seedless fruits comparable in weight and shape to wild-type fruits. One of the genes with increased expression induced by fertilization is the cell proliferation regulator cytochrome P450 KLUH gene. The ectopic overexpression of KLUH in tomato stimulated both integument growth in unfertilized ovules and parthenocarpy, indicating that its suppression by AGL6 is of primary importance for preventing fertilization-independent fruit set [244]. Also, silencing AGL6 resulted in abnormally fused sepals and smaller, light green petals [74]. In such lines, MC, which is involved in the development of sepals, and GOB (GOBLET), which affects the initiation of the formation and division of leaf blades, were suppressed. AGL11 gene expression correlates with early fruit development. Thus, the phenotypes associated with AGL11 resemble those of other representatives of the MADS-box—TAG1 and TAGL1 [245]. In addition, metabolic reprogramming was observed to occur in sepals and fruits, with strong effects on cell wall-related genes.
Parthenocarpy is often accompanied by sterility due to either defects in the pollen or changes in the ovary. For instance, the formation of parthenocarpic fruits in the hydra mutant is associated with the lack of development of both male and female sporocytes. It was established that the HYDRA gene encodes the tomato ortholog of SPOROCYTELESS (SPL). The connection with sporogenesis in tomatoes was confirmed in RNAi lines and hydra lines overexpressing SPL/HYD [246]. In this case, parthenocarpy is explained by an increase in the level of expression of auxin and gibberellin genes in the ovary. SES (SEXUAL STERILITY) is another SPL homolog exhibiting male and female sterility [247].
Other actors in regulatory pathways are miRNAs. The authors of [248] showed that miRNAs’ modulation of hormone-dependent transcription factors affects the development of ovules and fruit set. Indeed, they found changes in the expression dynamics of the miR159/GAMYB system during the early stages of fruit development. Thus, the overexpression of the gene encoding the miR159 precursor in tomato suppressed GAMYB genes in developing ovaries, which led to earlier fruit initiation and parthenocarpy. Altered responses to auxins and gibberellins explained this. The above conclusions were confirmed later [249]. The overexpression of GAMYB2, a significant target of miR159, resulted in a flat fruit phenotype, while the loss of GAMYB2 function had the opposite effect, resulting in smaller and elongated fruits. This regulation was mediated mainly via the direct repression of the GA3ox2 gene. Confirmation of the connection between the parthenocarpic phenotype and disturbances in the expression of auxins and cytokinins in flowers is given in another publication [250]. Here, parthenocarpy was caused by a loss-of-function mutation in the gene encoding a receptor-like protein kinase expressed in vascular bundles of young buds. The mutation resulted in the increased expression of the gibberellin metabolism gene GA20ox1. Thus, MYB21 is a negative regulator of parthenocarpic fruit development, exerting regulation directly or through the JA signaling pathway [251].
The spectrum of genes responsible for parthenocarpy is broad. Thus, we summarize related studies on it from 2017 to 2023 in Table 2.

Table 2.
Recent studies of tomato genes that facilitate parthenocarpy trait.
Thus, auxins, gibberellins, and homologous MADS-box transcription factors are the most critical players in regulating fruit set. Unfortunately, a clear picture of the regulation has not yet been formed due to the redundancy of MADS-box factors showing both positive and negative regulation in tomato fruit development, as well as the possible existence of other actors involved in this process, such as brassinosteroids, abscisic acid, jasmonates, and salicylates (SAs). For example, cytokinins induce parthenocarpy by modulating GA and AUX metabolism [266]. In turn, ABA acts as an antagonist of GA and AUX by inhibiting ovary development [267,268]. Ethylene suppresses tomato fruit set by stabilizing DELLA repressors [269]. BSs can increase ethylene production in tomato fruit, so they should stimulate parthenocarpy. However, their exogenous application on tomato flowers does not produce a seedless phenotype [270]. Regarding JA, there is evidence that a loss-of-function mutation of the lipoxygenase gene leads to the formation of parthenocarpic fruits in Cucurbita pepo [271]. Presumably, the increased expression of SA genes should favor the formation of seedless fruits due to their antagonistic nature toward JA. These findings provide fertile ground for further studies of the regulatory systems controlling fruit development.
5. Conclusions and Future Prospects
The number of studies on regulating plant life-cycle processes conducted on model objects such as tomatoes continue to increase. Recently, significant progress has been made in understanding the systems and processes of signal transmission. The discovery of gene functions and the regulation systems of tomato reproduction systems allows us to better understand the biology of the processes under consideration of their fundamentals. This knowledge also has a high potential for use in the applied aspect of developing agricultural plant varieties with improved properties.
In the future, the search for new candidate genes involved in tomato reproductive signaling cascades will predominantly be carried out using genetic and bioinformatic methods, possibly leading to the discovery of other groups of regulatory signaling pathways. There is still no clear understanding of their signaling regulation systems. Only now is the functional redundancy of transcription factors involved in flowering becoming apparent. Understanding the “dark horses” of regulatory cascades, such as ABA [195,268] and BSs [199,272,273], is also expanding. The ideas about the influence of epigenetic modifications [125,274,275], ncRNAs [248,262,276,277,278], and external factors [3,279,280] on signaling regulation have been greatly expanded recently. Modern genetic engineering approaches, including targeted genome editing using CRISPR/Cas9 technology, the use of base [281,282] and prime editing [283,284] for precision gene correction, application of omics technologies, and the collection and processing of bioinformatic data by new powerful algorithms are helping researchers to solve problems in this area.
Some limitations should be taken into account when interpreting new data. For example, orthologous genes are unreliable predictors of expression in different species because they often have different regulatory mechanisms. Simplified laboratory experimental conditions cannot establish all possible gene regulation and expression subtleties because reproductive processes are discrete and occur in different tissues at certain life-cycle stages. The studied gene regulators are often represented by large families of gene paralogs, which impose objective difficulties in understanding the function of each of them; confusion in gene numbering is a common phenomenon for such studies.
As a complementary approach in agriculture, growing crops under urban conditions is an option. So-called “urban farms” require crop varieties that are both compact and fast-growing. Although the yield of such plants may be lower, this can be compensated for by growing plants at higher densities, thus maintaining productivity in limited space. This direction has also been taken for tomatoes, e.g., in [42]. CRISPR/Cas9 mutations in SP5G and SP induced rapid flowering and enhanced the compact determinant growth in tomato. Consequently, this resulted in early harvest. Similar results were obtained in [285], where, in addition to creating double SP5G-SP knockouts, triple deficient mutants SP5G-SP-ER (where ER is the gene controlling internode length) were obtained, showing even greater compactness. Increased fruit size combined with determinant growth is a promising approach for tomato cultivation under urban conditions [286,287]. Thus, in addition to SP5G-SP null mutants, mutations can be made in the regulatory regions of CLV3 and WUS genes, thereby increasing the number of locules in fruits. In [288], the tomato was edited for six loci: general plant morphology (sp), fruit shape (ovate) and size (fas and fw2.2), number of fruits (multiflora), and nutrient composition (lycopene beta cyclase gene). The authors obtained 15 combinations of independent alleles with a loss of function of the target genes. In the future, the discovery of new genes associated with improved agronomic traits is potentially compatible with existing genes.
Producers tend to avoid the exogenous application of hormones to stimulate specific physiological processes in plants in favor of controlling the endogenous regulatory systems, which is explained by the economic benefits, lower labor costs, and the possibility of promoting their products as eco-friendly. In addition, physiological changes in such plants have little or no undesirable pleiotropic effects. As for the practical application of the study of the considered reproduction genes, these are “golden opportunities” in overcoming interspecific incompatibility [62,221,222], pollen fertility [186,196], changing the size of the fruit due to changes in the number of locules and pericarp growth [129,165,289], changing the number of fruits due to an increasing number of inflorescences [119,290], and much more. All this is possible by inserting expression cassettes into the genome, i.e., developing genetically modified (GM) plants. Currently, society has not formed a clear opinion on the safety of GM plants. Thus, there are several risks associated with them. Cross-pollination ensures the flow of genes between populations and related species. In particular, the spread of transgenes through pollen can lead to the introgression of marker genes, whether antibiotic or herbicide-resistance genes, into the weed genome, leading to the emergence of herbicide-resistant weeds [291]. A possible solution to these problems lies in studying flowering genes, particularly those associated with the sterility trait of genetically modified crops. In addition, available modern genetic engineering methods for crop improvement allow for the selection of plants that do not contain foreign genes [292]. Obtaining plants with edited genomes makes it possible to bypass legal restrictions on the production and distribution of GM organisms in some countries [293].
This review has considered recent advances in studying tomato reproductive factors using different genetic approaches. Current research on tomato reproductive factors continues to expand the understanding of the molecular basis and physiological mechanisms of these processes, which opens up new opportunities for the practical application of this knowledge. We hope the new data presented here will add to the existing mechanisms describing the regulation of tomato reproductive systems.
Author Contributions
Conceptualization, D.B. and V.T.; writing—original draft preparation, D.B.; writing—review and editing, V.T.; funding acquisition, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant No. 22-14-00118.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ABA, abscisic acid; ABC, ATP-binding cassette transporter; ACO, acotinase; AFB2, auxin signaling F-box 2; AG/AGL, AGAMOUS/AGAMOUS-like; AHK, Arabidopsis histidine kinase receptor; AHP, Arabidopsis histidine phosphotransfer protein; ALOG, Arabidopsis LSH1 and Oryza G1; AMS, ABORTED MICROSPORES; AN, ANANTHA; AP, APETALA; ARF, auxin response factor; ARR, Arabidopsis response regulator; AUX, auxin; BAM1, BARELY ANY MERISTEM1; BBX, B-box domain protein; BES1, BRI1-EMS-SUPPRESSOR 1; BG, β-1,3-glucanase; bHLH, basic helix–loop–helix domain; BIN, BRASSINOSTEROID-INSENSITIVE; BL/BLH, BELL/BELL-homolog; BOP, BLADE-ON-PETIOLE; BRC, BRANCHED; BRI1, BRASSINOSTEROID INSENSITIVE 1; BS, brassinosteroid; bZIP, basic leucine zipper domain; BZR1, BRASSINAZOLE-RESISTANT 1; C2H2 zinc fingers, Cys2-His2 domain; CDF, cycling DOF; CDK, cell division protein kinase; CEL, cellulase; CEN, CENTRORADIALIS; CETS, CEN/TFL1/SP; CIN, cytoplasmic invertase; CK, casein kinase; CLE, CLV3/EMBRYO SURROUNDING REGION; CLV, CLAVATA; CMB1, carnation MADS-box 1; CMP, cystine-knot miniprotein; CMT, chromomethylase; CNR, COLORLESS NON-RIPENING; CO, CONSTANS; COP, CONSTITUTIVE PHOTOMORPHOGENIC; CRC, CRABS CLAW; CRF, cytokinin response factor; CRISPR, clustered regularly interspaced short palindromic repeats; CRN, CORYNE; CSR, CELL SIZE REGULATOR; CTK, cytokinin; CWIN, cell wall invertase; CYP, cytochrome P; DDB, DNA damage-binding protein; DDF1, DOF DAILY FLUCTUATIONS 1; DIR1, DEFECTIVE IN INDUCED RESISTANCE 1; DOF, DNA-binding with one zinc finger; DTM, DEFECTIVE TOMATO MERISTEM; DYT1, DYSFUNCTIONAL TAPETUM 1; EBF3, EIN3 binding F-box; EIN, ETHYLENE INSENSITIVE; EJ2, ENHANCER OF JOINTLESS 2; ENO, EXCESSIVE NUMBER OF FLORAL ORGANS; ER, ERECTA; ERF, ethylene responsive factor; ET, ethylene; ETR, ethylene receptor; EXP, expansin; FA, FALSIFLORA; FAF, FANTASTIC FOUR; FAS, FASCIATED; FKF1, Flavin-binding Kelch repeat F-box 1; FM, floral meristem; FPS, farnesyl pyrophosphate synthase; FRK, fructokinase; FS, FRUIT SHAPE; FTIP, FT-interacting protein; FUL, FRUITFUL; FW, FRUIT WEIGHT; FZY, FLOOZY; GA, gibberellin; GA_ox, gibberellin oxidase; GAMYB, gibberellin-associated MYB; GEF, guanine exchange factor; GH3, GRETCHEN HAGEN 3; GID1, GA INSENSITIVE DWARF 1; GLT, glutamate synthase; GM, genetically modified; GNT, N-acetyl-glucosaminyltransferase; GOB, GOBLET; GRAS, GIBBERELLIC-ACID INSENSITIVE/REPRESSOR OF GAI/SCARECROW; GT, galactosyltransferase; GTPase, guanosine triphosphate hydrolase; HB, homeobox; HD-ZIP III, class III homeodomain-leucine zipper protein; HWS, HAWAIIAN SKIRT; HXK, hexokinase; HY, ELONGATED HYPOCOTYL; HYD, HYDRA; HyPRP, hybrid PRP; IAA, indole-3-acetic acid; IAA9, IAA INDUCIBLE 9; IDA, INFLORESCENCE DEFICIENT IN ABSCISSION/INFLORESCENCE DEFICIENT IN ABSCISSION-LIKE; ILR/ILL, IAA-LEUCINE RESISTANT/IAA-LEUCINE RESISTANT-LIKE; IM, inflorescence meristem; IMA, INHIBITOR OF MERISTEM ACTIVITY; IQD, IQ67-domain protein; J, JOINTLESS; JA, jasmonate; KD, KN-like domain protein; KLUH, HULK backward; KNOX/KN, KNOTTED-LIKE; KNU, KNUCKLES; KPP, kinase partner protein; LAM, LAMINA; LC, LOCULE NUMBER; LIN, LONG INFLORESCENCE; LRR, leucine-rich repeat; LSD/LOL, LESION SIMULATING DISEASE/LESION SIMULATING DISEASE-LIKE; LST, LONG STYLES; MADS1, MADS-box transcription factor 1; MAP, microtubule-associated protein; MBP, MADS-box protein; MC, MACROCALYX; MED, mediator complex subunit; MET, methyltransferase; miR, microRNA; MPK, mitogen-activated protein kinase; MSH2, MutatorS-Homolog 2; MYB, myeloblastosis; NCED, 9-cis-epoxycarotenoid dioxygenase; ncRNA, non-coding RNA; OE, overexpression; OFP, OVATE family protein; P4H, proline 4-hydroxylase; PAD, PARENTAL ADVICE; PIF, phytochrome-interacting factor; PIN, PIN-FORMED; PLAC, PLACENTA ASSOCIATED; POD, POLLEN DEFICIENT; PP, protein phosphatase; PRP, proline-rich protein; PYR/PYL, PYRABACTIN RESISTANCE/PYRABACTIN RESISTANCE-LIKE; QTL, quantitative trait locus; RBOH, respiratory burst oxidase homolog; RIN, RIPENING INHIBITOR; RLK, receptor-like kinase; ROS, reactive oxygen species; SA, salicylate; SAM, shoot apical meristem; SB, SUPPRESSOR OF BRANCHING; SBP, SQUAMOSA promoter-binding protein; SEP, SEPALLATA; SES, SEXUAL STERILITY; SFT, SINGLE FLOWER TRUSS; SINA, SEVEN IN ABSENTIA; SLOMO, SLOW MOTION; SP, SELF-PRUNING; SPL, SPOROCYTELESS; SPMS, spermidine synthase; STM3, SISTER OF TM 3; STR, strictosidine synthase; SUC, sucrose; SWEET, SUGAR WILL EVENTUALLY BE EXPORTED TRANSPORTER; TAG/TAGL, tomato AGAMOUS/tomato AGAMOUS-like; TALE, three amino acid loop extension; TAPG, tomato abscission polygalacturonase; TDF, DEFECTIVE IN TAPETAL DEVELOPMENT AND FUNCTION; TFAM, TMF FAMILY MEMBER; TFL, TERMINAL FLOWER; TIP, tonoplast intrinsic protein; TIR, TRANSPORT INHIBITOR RESPONSE; TM, transition meristem; TM_, tomato MADS; TMF, TERMINATING FLOWER; TOE, TARGET OF EAT; TPD, TAPETUM DETERMINANT; TPL, TOPLESS; TRK, tomato receptor-like cytoplasmic kinase; TRM, TONNEAU1-recruiting motif; VM, vegetative meristem; WOX, WUS-related homeobox; WT, wild type; WUS, WUSCHEL; YUC, YUCCA; ZPR, LITTLE ZIPPER.
References
- Molesini, B.; Dusi, V.; Pennisi, F.; Pandolfini, T. How Hormones and MADS-Box Transcription Factors Are Involved in Controlling Fruit Set and Parthenocarpy in Tomato. Genes 2020, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Ezura, K.; Nomura, Y.; Ariizumi, T. Molecular, Hormonal, and Metabolic Mechanisms of Fruit Set, the Ovary-to-Fruit Transition, in Horticultural Crops. J. Exp. Bot. 2023, 74, 6254–6268. [Google Scholar] [CrossRef]
- Hoshikawa, K.; Pham, D.; Ezura, H.; Schafleitner, R.; Nakashima, K. Genetic and Molecular Mechanisms Conferring Heat Stress Tolerance in Tomato Plants. Front. Plant Sci. 2021, 12, 786688. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in Fruit Development and Maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Z.; Huang, X.; Xu, C. Molecular Regulation of Tomato Male Reproductive Development. Abiotech 2023, 4, 72–82. [Google Scholar] [CrossRef]
- Pandolfini, T.; Molesini, B.; Spena, A. Molecular Dissection of the Role of Auxin in Fruit Initiation. Trends Plant Sci. 2007, 12, 327–329. [Google Scholar] [CrossRef]
- Serrani, J.C.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Auxin-induced Fruit-set in Tomato Is Mediated in Part by Gibberellins. Plant J. 2008, 56, 922–934. [Google Scholar] [CrossRef]
- Llop-Tous, I.; Barry, C.S.; Grierson, D. Regulation of Ethylene Biosynthesis in Response to Pollination in Tomato Flowers. Plant Physiol. 2000, 123, 971–978. [Google Scholar] [CrossRef]
- de Jong, M.; Wolters-Arts, M.; García-Martínez, J.L.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) Mediates Cross-Talk between Auxin and Gibberellin Signalling during Tomato Fruit Set and Development. J. Exp. Bot. 2011, 62, 617–626. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T.; Kononowicz, A.K. Tomato (Solanum lycopersicum L.) in the Service of Biotechnology. Plant Cell Tissue Organ Cult. 2015, 120, 881–902. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Zhou, L.; You, S.; Deng, H.; Chen, Y.; Alseekh, S.; Yuan, Y.; Fu, R.; Zhang, Z.; et al. MicroTom Metabolic Network: Rewiring Tomato Metabolic Regulatory Network throughout the Growth Cycle. Mol. Plant 2020, 13, 1203–1218. [Google Scholar] [CrossRef]
- Bastías, A.; López-Climent, M.; Valcárcel, M.; Rosello, S.; Gómez-Cadenas, A.; Casaretto, J.A. Modulation of Organic Acids and Sugar Content in Tomato Fruits by an Abscisic Acid-regulated Transcription Factor. Physiol. Plant. 2011, 141, 215–226. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene Is Enriched in Tomato Fruit by CRISPR/Cas9-Mediated Multiplex Genome Editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, M.; Zhang, M.; Yang, M.; Dai, S.; Meng, Q.; Lv, W.; Zhuang, K. ETHYLENE-INSENSITIVE 3-LIKE 2 Regulates β-Carotene and Ascorbic Acid Accumulation in Tomatoes during Ripening. Plant Physiol. 2023, 192, 2067–2080. [Google Scholar] [CrossRef]
- Ratto, F.; Franchini, F.; Musicco, M.; Caruso, G.; Di Santo, S.G. A Narrative Review on the Potential of Tomato and Lycopene for the Prevention of Alzheimer’s Disease and Other Dementias. Crit. Rev. Food Sci. Nutr. 2022, 62, 4970–4981. [Google Scholar] [CrossRef]
- Landrier, J.-F.; Breniere, T.; Sani, L.; Desmarchelier, C.; Mounien, L.; Borel, P. Effect of Tomato, Tomato-Derived Products and Lycopene on Metabolic Inflammation: From Epidemiological Data to Molecular Mechanisms. Nutr. Res. Rev. 2023, 1–17. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Rothan, C.; Diouf, I.; Causse, M. Trait Discovery and Editing in Tomato. Plant J. 2019, 97, 73–90. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Singh, A.K.; Behera, T.K. CRISPR/Cas Genome Editing in Tomato Improvement: Advances and Applications. Front. Plant Sci. 2023, 14, 1121209. [Google Scholar] [CrossRef]
- Sussex, I.M.; Kerk, N.M. The Evolution of Plant Architecture. Curr. Opin. Plant Biol. 2001, 4, 33–37. [Google Scholar] [CrossRef] [PubMed]
- McGarry, R.C.; Ayre, B.G. Manipulating Plant Architecture with Members of the CETS Gene Family. Plant Sci. 2012, 188–189, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lippman, Z.B.; Cohen, O.; Alvarez, J.P.; Abu-Abied, M.; Pekker, I.; Paran, I.; Eshed, Y.; Zamir, D. The Making of a Compound Inflorescence in Tomato and Related Nightshades. PLoS Biol. 2008, 6, e288. [Google Scholar] [CrossRef]
- Molinero-Rosales, N.; Latorre, A.; Jamilena, M.; Lozano, R. SINGLE FLOWER TRUSS Regulates the Transition and Maintenance of Flowering in Tomato. Planta 2004, 218, 427–434. [Google Scholar] [CrossRef]
- Robledo, J.M.; Medeiros, D.; Vicente, M.H.; Azevedo, A.A.; Thompson, A.J.; Peres, L.E.P.; Ribeiro, D.M.; Araújo, W.L.; Zsögön, A. Control of Water-use Efficiency by Florigen. Plant Cell Environ. 2020, 43, 76–86. [Google Scholar] [CrossRef]
- Park, S.J.; Jiang, K.; Tal, L.; Yichie, Y.; Gar, O.; Zamir, D.; Eshed, Y.; Lippman, Z.B. Optimization of Crop Productivity in Tomato Using Induced Mutations in the Florigen Pathway. Nat. Genet. 2014, 46, 1337–1342. [Google Scholar] [CrossRef]
- Shalit-Kaneh, A.; Eviatar-Ribak, T.; Horev, G.; Suss, N.; Aloni, R.; Eshed, Y.; Lifschitz, E. The Flowering Hormone Florigen Accelerates Secondary Cell Wall Biogenesis to Harmonize Vascular Maturation with Reproductive Development. Proc. Natl. Acad. Sci. USA 2019, 116, 16127–16136. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, F.; Wang, J.; Zhang, C.; Zhang, D.; Gao, S.; Zhang, C.; Ye, J.; Zhang, Y.; Ouyang, B.; et al. The Tomato CONSTANS-LIKE Protein SlCOL1 Regulates Fruit Yield by Repressing SFT Gene Expression. BMC Plant Biol. 2022, 22, 429. [Google Scholar] [CrossRef]
- Yang, T.; He, Y.; Niu, S.; Yan, S.; Zhang, Y. Identification and Characterization of the CONSTANS (CO)/CONSTANS-like (COL) Genes Related to Photoperiodic Signaling and Flowering in Tomato. Plant Sci. 2020, 301, 110653. [Google Scholar] [CrossRef]
- Molesini, B.; Dusi, V.; Pennisi, F.; Di Sansebastiano, G.P.; Zanzoni, S.; Manara, A.; Furini, A.; Martini, F.; Rotino, G.L.; Pandolfini, T. TCMP-2 Affects Tomato Flowering and Interacts with BBX16, a Homolog of the Arabidopsis B-box MiP1b. Plant Direct 2020, 4, e00283. [Google Scholar] [CrossRef]
- Shibuya, T.; Nishiyama, M.; Kato, K.; Kanayama, Y. Characterization of the FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 Homolog SlFKF1 in Tomato as a Model for Plants with Fleshy Fruit. Int. J. Mol. Sci. 2021, 22, 1735. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, F.; Wang, J.; Zhang, C.; Xiao, F.; Ye, J.; Li, C.; Ye, Z.; Zhang, J. MiR156a-targeted SBP-Box Transcription Factor SlSPL13 Regulates Inflorescence Morphogenesis by Directly Activating SFT in Tomato. Plant Biotechnol. J. 2020, 18, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.F.F.E.; Silva, E.M.; da Silva Azevedo, M.; Guivin, M.A.C.; Ramiro, D.A.; Figueiredo, C.R.; Carrer, H.; Peres, L.E.P.; Nogueira, F.T.S. MicroRNA156-targeted SPL/SBP Box Transcription Factors Regulate Tomato Ovary and Fruit Development. Plant J. 2014, 78, 604–618. [Google Scholar] [CrossRef]
- Silva, G.F.F.; Silva, E.M.; Correa, J.P.O.; Vicente, M.H.; Jiang, N.; Notini, M.M.; Junior, A.C.; De Jesus, F.A.; Castilho, P.; Carrera, E.; et al. Tomato Floral Induction and Flower Development Are Orchestrated by the Interplay between Gibberellin and Two Unrelated MicroRNA-controlled Modules. New Phytol. 2019, 221, 1328–1344. [Google Scholar] [CrossRef]
- Ferigolo, L.F.; Vicente, M.H.; Correa, J.P.O.; Barrera-Rojas, C.H.; Silva, E.M.; Silva, G.F.F.; Carvalho, A., Jr.; Peres, L.E.P.; Ambrosano, G.B.; Margarido, G.R.A.; et al. Gibberellin and MiRNA156-Targeted SlSBP Genes Synergistically Regulate Tomato Floral Meristem Determinacy and Ovary Patterning. Development 2023, 150, dev201961. [Google Scholar] [CrossRef]
- Chen, S.; Song, X.; Zheng, Q.; Liu, Y.; Yu, J.; Zhou, Y.; Xia, X. The Transcription Factor SPL13 Mediates Strigolactone Suppression of Shoot Branching by Inhibiting Cytokinin Synthesis in Solanum lycopersicum. J. Exp. Bot. 2023, 74, 5722–5735. [Google Scholar] [CrossRef]
- Barrera-Rojas, C.H.; Vicente, M.H.; Pinheiro Brito, D.A.; Silva, E.M.; Lopez, A.M.; Ferigolo, L.F.; do Carmo, R.M.; Silva, C.M.S.; Silva, G.F.F.; Correa, J.P.O.; et al. Tomato MiR156-Targeted SlSBP15 Represses Shoot Branching by Modulating Hormone Dynamics and Interacting with GOBLET and BRANCHED1b. J. Exp. Bot. 2023, 74, 5124–5139. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, Z.; Li, F.; Yu, X.; Naeem, M.; Zhang, Y.; Chen, G. Manipulation of Plant Architecture and Flowering Time by Down-Regulation of the GRAS Transcription Factor SlGRAS26 in Solanum lycopersicum. Plant Sci. 2018, 271, 81–93. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Ma, Y.-J.; Wang, M.; Zhou, H.; Gan, Z.-M.; Zeng, R.-F.; Ye, L.-X.; Zhou, J.-J.; Zhang, J.-Z.; Hu, C.-G. Mobility of FLOWERING LOCUS T Protein as a Systemic Signal in Trifoliate Orange and Its Low Accumulation in Grafted Juvenile Scions. Hortic. Res. 2022, 9, uhac056. [Google Scholar] [CrossRef]
- Kang, M.-S.; Kim, Y.J.; Heo, J.; Rajendran, S.; Wang, X.; Bae, J.H.; Lippman, Z.; Park, S.J. Newly Discovered Alleles of the Tomato Antiflorigen Gene SELF PRUNING Provide a Range of Plant Compactness and Yield. Int. J. Mol. Sci. 2022, 23, 7149. [Google Scholar] [CrossRef]
- Cao, K.; Yan, F.; Xu, D.; Ai, K.; Yu, J.; Bao, E.; Zou, Z. Phytochrome B1-Dependent Control of SP5G Transcription Is the Basis of the Night Break and Red to Far-Red Light Ratio Effects in Tomato Flowering. BMC Plant Biol. 2018, 18, 158. [Google Scholar] [CrossRef]
- Soyk, S.; Müller, N.A.; Park, S.J.; Schmalenbach, I.; Jiang, K.; Hayama, R.; Zhang, L.; Van Eck, J.; Jiménez-Gómez, J.M.; Lippman, Z.B. Variation in the Flowering Gene SELF PRUNING 5G Promotes Day-Neutrality and Early Yield in Tomato. Nat. Genet. 2017, 49, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.D.R.; Quiñones, A.; Lira, B.S.; Robledo, J.M.; Curtin, S.J.; Vicente, M.H.; Ribeiro, D.M.; Ryngajllo, M.; Jiménez-Gómez, J.M.; Peres, L.E.P.; et al. SELF PRUNING 3C Is a Flowering Repressor That Modulates Seed Germination, Root Architecture, and Drought Responses. J. Exp. Bot. 2022, 73, 6226–6240. [Google Scholar] [CrossRef] [PubMed]
- Hollwey, E.; Out, S.; Watson, M.R.; Heidmann, I.; Meyer, P. TET3-Mediated Demethylation in Tomato Activates Expression of a CETS Gene That Stimulates Vegetative Growth. Plant Direct 2017, 1, e00022. [Google Scholar] [CrossRef]
- Lifschitz, E.; Eviatar, T.; Rozman, A.; Shalit, A.; Goldshmidt, A.; Amsellem, Z.; Alvarez, J.P.; Eshed, Y. The Tomato FT Ortholog Triggers Systemic Signals That Regulate Growth and Flowering and Substitute for Diverse Environmental Stimuli. Proc. Natl. Acad. Sci. USA 2006, 103, 6398–6403. [Google Scholar] [CrossRef]
- Shalit, A.; Rozman, A.; Goldshmidt, A.; Alvarez, J.P.; Bowman, J.L.; Eshed, Y.; Lifschitz, E. The Flowering Hormone Florigen Functions as a General Systemic Regulator of Growth and Termination. Proc. Natl. Acad. Sci. USA 2009, 106, 8392–8397. [Google Scholar] [CrossRef]
- Pnueli, L.; Carmel-Goren, L.; Hareven, D.; Gutfinger, T.; Alvarez, J.; Ganal, M.; Zamir, D.; Lifschitz, E. The SELF-PRUNING Gene of Tomato Regulates Vegetative to Reproductive Switching of Sympodial Meristems and Is the Ortholog of CEN and TFL1. Development 1998, 125, 1979–1989. [Google Scholar] [CrossRef]
- Allen, K.; Sussex, I. Falsiflora and Anantha Control Early Stages of Floral Meristem Development in Tomato (Lycopersicon esculentum Mill.). Planta 1996, 200, 254–264. [Google Scholar] [CrossRef]
- Molinero-Rosales, N.; Jamilena, M.; Zurita, S.; Gómez, P.; Capel, J.; Lozano, R. FALSIFLORA, the Tomato Orthologue of FLORICAULA and LEAFY, Controls Flowering Time and Floral Meristem Identity. Plant J. 1999, 20, 685–693. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Yang, T.; Zhang, J.; Liu, B.; Zhan, X.; Liang, Y. The GAMYB-like Gene SlMYB33 Mediates Flowering and Pollen Development in Tomato. Hortic. Res. 2020, 7, 133. [Google Scholar] [CrossRef]
- Huang, X.; Tang, L.; Yu, Y.; Dalrymple, J.; Lippman, Z.B.; Xu, C. Control of Flowering and Inflorescence Architecture in Tomato by Synergistic Interactions between ALOG Transcription Factors. J. Genet. Genomics 2018, 45, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiao, N.; Zou, Y.; Xie, Y.; Tang, L.; Zhang, Y.; Yu, Y.; Li, Y.; Xu, C. Heterotypic Transcriptional Condensates Formed by Prion-like Paralogous Proteins Canalize Flowering Transition in Tomato. Genome Biol. 2022, 23, 78. [Google Scholar] [CrossRef]
- Huang, X.; Chen, S.; Li, W.; Tang, L.; Zhang, Y.; Yang, N.; Zou, Y.; Zhai, X.; Xiao, N.; Liu, W.; et al. ROS Regulated Reversible Protein Phase Separation Synchronizes Plant Flowering. Nat. Chem. Biol. 2021, 17, 549–557. [Google Scholar] [CrossRef]
- Izhaki, A.; Alvarez, J.P.; Cinnamon, Y.; Genin, O.; Liberman-Aloni, R.; Eyal, Y. The Tomato BLADE ON PETIOLE and TERMINATING FLOWER Regulate Leaf Axil Patterning along the Proximal-Distal Axes. Front. Plant Sci. 2018, 9, 1126. [Google Scholar] [CrossRef]
- Xu, C.; Park, S.J.; Van Eck, J.; Lippman, Z.B. Control of Inflorescence Architecture in Tomato by BTB/POZ Transcriptional Regulators. Genes Dev. 2016, 30, 2048–2061. [Google Scholar] [CrossRef]
- Roldan, M.V.G.; Périlleux, C.; Morin, H.; Huerga-Fernandez, S.; Latrasse, D.; Benhamed, M.; Bendahmane, A. Natural and Induced Loss of Function Mutations in SlMBP21 MADS-Box Gene Led to Jointless-2 Phenotype in Tomato. Sci. Rep. 2017, 7, 4402. [Google Scholar] [CrossRef]
- Liu, D.; Wang, D.; Qin, Z.; Zhang, D.; Yin, L.; Wu, L.; Colasanti, J.; Li, A.; Mao, L. The SEPALLATA MADS-box Protein SLMBP21 Forms Protein Complexes with JOINTLESS and MACROCALYX as a Transcription Activator for Development of the Tomato Flower Abscission Zone. Plant J. 2014, 77, 284–296. [Google Scholar] [CrossRef]
- Li, N.; Huang, B.; Tang, N.; Jian, W.; Zou, J.; Chen, J.; Cao, H.; Habib, S.; Dong, X.; Wei, W.; et al. The MADS-Box Gene SlMBP21 Regulates Sepal Size Mediated by Ethylene and Auxin in Tomato. Plant Cell Physiol. 2017, 58, 2241–2256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ai, G.; Ji, K.; Huang, R.; Chen, C.; Yang, Z.; Wang, J.; Cui, L.; Li, G.; Tahira, M.; et al. EARLY FLOWERING Is a Dominant Gain-of-function Allele of FANTASTIC FOUR 1/2c That Promotes Early Flowering in Tomato. Plant Biotechnol. J. 2023. ahead of print. [Google Scholar] [CrossRef]
- Soyk, S.; Lemmon, Z.H.; Oved, M.; Fisher, J.; Liberatore, K.L.; Park, S.J.; Goren, A.; Jiang, K.; Ramos, A.; van der Knaap, E.; et al. Bypassing Negative Epistasis on Yield in Tomato Imposed by a Domestication Gene. Cell 2017, 169, 1142–1155.e12. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Wang, Y.; Yu, X.; Liao, C.; Zhu, M.; Chen, G. Suppression of a Tomato SEPALLATA MADS-Box Gene, SlCMB1, Generates Altered Inflorescence Architecture and Enlarged Sepals. Plant Sci. 2018, 272, 75–87. [Google Scholar] [CrossRef]
- Soyk, S.; Lemmon, Z.H.; Sedlazeck, F.J.; Jiménez-Gómez, J.M.; Alonge, M.; Hutton, S.F.; Van Eck, J.; Schatz, M.C.; Lippman, Z.B. Duplication of a Domestication Locus Neutralized a Cryptic Variant That Caused a Breeding Barrier in Tomato. Nat. Plants 2019, 5, 471–479. [Google Scholar] [CrossRef]
- Alonge, M.; Wang, X.; Benoit, M.; Soyk, S.; Pereira, L.; Zhang, L.; Suresh, H.; Ramakrishnan, S.; Maumus, F.; Ciren, D.; et al. Major Impacts of Widespread Structural Variation on Gene Expression and Crop Improvement in Tomato. Cell 2020, 182, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Bai, J.; Sun, S.; Song, J.; Li, R.; Cui, X. Antagonistic Regulation of Target Genes by the SISTER OF TM3–JOINTLESS2 Complex in Tomato Inflorescence Branching. Plant Cell 2023, 35, 2062–2078. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Sun, S.; Wu, J.; Li, R.; Wang, H.; Cui, X. SISTER OF TM3 Activates FRUITFULL1 to Regulate Inflorescence Branching in Tomato. Hortic. Res. 2021, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, X.; Liu, Z.; Bai, J.; Song, J.; Li, R.; Cui, X. Tomato APETALA2 Family Member SlTOE1 Regulates Inflorescence Branching by Repressing SISTER OF TM3. Plant Physiol. 2023, 192, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Maheepala, D.C.; Emerling, C.A.; Rajewski, A.; Macon, J.; Strahl, M.; Pabón-Mora, N.; Litt, A. Evolution and Diversification of FRUITFULL Genes in Solanaceae. Front. Plant Sci. 2019, 10, 43. [Google Scholar] [CrossRef]
- Jiang, X.; Lubini, G.; Hernandes-Lopes, J.; Rijnsburger, K.; Veltkamp, V.; de Maagd, R.A.; Angenent, G.C.; Bemer, M. FRUITFULL-like Genes Regulate Flowering Time and Inflorescence Architecture in Tomato. Plant Cell 2022, 34, 1002–1019. [Google Scholar] [CrossRef]
- Zahn, I.E.; Roelofsen, C.; Angenent, G.C.; Bemer, M. TM3 and STM3 Promote Flowering Together with FUL2 and MBP20, but Act Antagonistically in Inflorescence Branching in Tomato. Plants 2023, 12, 2754. [Google Scholar] [CrossRef]
- Baranov, D.; Timerbaev, V. Recent Advances in Studying the Regulation of Fruit Ripening in Tomato Using Genetic Engineering Approaches. Int. J. Mol. Sci. 2024, 25, 760. [Google Scholar] [CrossRef]
- Yuste-Lisbona, F.J.; Quinet, M.; Fernández-Lozano, A.; Pineda, B.; Moreno, V.; Angosto, T.; Lozano, R. Characterization of Vegetative Inflorescence (Mc-Vin) Mutant Provides New Insight into the Role of MACROCALYX in Regulating Inflorescence Development of Tomato. Sci. Rep. 2016, 6, 18796. [Google Scholar] [CrossRef]
- Xing, M.; Li, H.; Liu, G.; Zhu, B.; Zhu, H.; Grierson, D.; Luo, Y.; Fu, D. A MADS-Box Transcription Factor, SlMADS1, Interacts with SlMACROCALYX to Regulate Tomato Sepal Growth. Plant Sci. 2022, 322, 111366. [Google Scholar] [CrossRef]
- Li, F.; Jia, Y.; Zhou, S.; Chen, X.; Xie, Q.; Hu, Z.; Chen, G. SlMBP22 Overexpression in Tomato Affects Flower Morphology and Fruit Development. J. Plant Physiol. 2022, 272, 153687. [Google Scholar] [CrossRef]
- Yu, X.; Chen, G.; Guo, X.; Lu, Y.; Zhang, J.; Hu, J.; Tian, S.; Hu, Z. Silencing SlAGL6, a Tomato AGAMOUS-LIKE6 Lineage Gene, Generates Fused Sepal and Green Petal. Plant Cell Rep. 2017, 36, 959–969. [Google Scholar] [CrossRef]
- Gimenez, E.; Castañeda, L.; Pineda, B.; Pan, I.L.; Moreno, V.; Angosto, T.; Lozano, R. TOMATO AGAMOUS1 and ARLEQUIN/TOMATO AGAMOUS-LIKE1 MADS-Box Genes Have Redundant and Divergent Functions Required for Tomato Reproductive Development. Plant Mol. Biol. 2016, 91, 513–531. [Google Scholar] [CrossRef]
- Nezhdanova, A.V.; Slugina, M.A.; Dyachenko, E.A.; Kamionskaya, A.M.; Kochieva, E.Z.; Shchennikova, A.V. Analysis of the Structure and Function of the Tomato Solanum Lycopersicum L. MADS-Box Gene SlMADS5. Vavilovskii Zh. Genet. Selektsii 2021, 25, 492–501. [Google Scholar] [CrossRef]
- Slugina, M.A.; Dyachenko, E.A.; Kochieva, E.Z.; Shchennikova, A.V. Structural and Functional Diversification of SEPALLATA Genes TM5 and RIN in Tomato Species (Section lycopersicon). Dokl. Biochem. Biophys. 2020, 492, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ampomah-Dwamena, C.; Morris, B.A.; Sutherland, P.; Veit, B.; Yao, J.-L. Down-Regulation of TM29, a Tomato SEPALLATA Homolog, Causes Parthenocarpic Fruit Development and Floral Reversion. Plant Physiol. 2002, 130, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, J.; Zhang, J.; Wu, P.-Y.; Yang, S.; Wu, K. Identification and Characterization of Histone Deacetylases in Tomato (Solanum lycopersicum). Front. Plant Sci. 2015, 5, 760. [Google Scholar] [CrossRef]
- Arhondakis, S.; Bita, C.E.; Perrakis, A.; Manioudaki, M.E.; Krokida, A.; Kaloudas, D.; Kalaitzis, P. In Silico Transcriptional Regulatory Networks Involved in Tomato Fruit Ripening. Front. Plant Sci. 2016, 7, 1234. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Gracia, P.; Roque, E.; Medina, M.; López-Martín, M.J.; Cañas, L.A.; Beltrán, J.P.; Gómez-Mena, C. The DOF Transcription Factor SlDOF10 Regulates Vascular Tissue Formation during Ovary Development in Tomato. Front. Plant Sci. 2019, 10, 216. [Google Scholar] [CrossRef]
- Hu, G.; Wang, K.; Huang, B.; Mila, I.; Frasse, P.; Maza, E.; Djari, A.; Hernould, M.; Zouine, M.; Li, Z.; et al. The Auxin-Responsive Transcription Factor SlDOF9 Regulates Inflorescence and Flower Development in Tomato. Nat. Plants 2022, 8, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Ewas, M.; Khames, E.; Ziaf, K.; Shahzad, R.; Nishawy, E.; Ali, F.; Subthain, H.; Amar, M.H.; Ayaad, M.; Ghaly, O.; et al. The Tomato DOF Daily Fluctuations 1, TDDF1 Acts as Flowering Accelerator and Protector against Various Stresses. Sci. Rep. 2017, 7, 10299. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, X.; Wu, X.; Meng, L.; Zou, Z.; Bao, E.; Bian, Z.; Cao, K. Tomato SlCDF3 Delays Flowering Time by Regulating Different FT-like Genes under Long-Day and Short-Day Conditions. Front. Plant Sci. 2021, 12, 650068. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qi, S.; Touqeer, A.; Li, H.; Zhang, X.; Liu, X.; Wu, S. SlGT11 Controls Floral Organ Patterning and Floral Determinacy in Tomato. BMC Plant Biol. 2020, 20, 562. [Google Scholar] [CrossRef]
- Bertin, N. Analysis of the Tomato Fruit Growth Response to Temperature and Plant Fruit Load in Relation to Cell Division, Cell Expansion and DNA Endoreduplication. Ann. Bot. 2004, 95, 439–447. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in Abscission. New Phytol. 2001, 151, 323–340. [Google Scholar] [CrossRef]
- Butenko, M.A.; Simon, R. Beyond the Meristems: Similarities in the CLAVATA3 and INFLORESCENCE DEFICIENT IN ABSCISSION Peptide Mediated Signalling Pathways. J. Exp. Bot. 2015, 66, 5195–5203. [Google Scholar] [CrossRef]
- Jiang, C.; Liang, Y.; Deng, S.; Liu, Y.; Zhao, H.; Li, S.; Jiang, C.-Z.; Gao, J.; Ma, C. The RhLOL1–RhILR3 Module Mediates Cytokinin-induced Petal Abscission in Rose. New Phytol. 2023, 237, 483–496. [Google Scholar] [CrossRef]
- Li, R.; Shi, C.-L.; Wang, X.; Meng, Y.; Cheng, L.; Jiang, C.-Z.; Qi, M.; Xu, T.; Li, T. Inflorescence Abscission Protein SlIDL6 Promotes Low Light Intensity-Induced Tomato Flower Abscission. Plant Physiol. 2021, 186, 1288–1301. [Google Scholar] [CrossRef]
- Zhao, M.; Li, C.; Ma, X.; Xia, R.; Chen, J.; Liu, X.; Ying, P.; Peng, M.; Wang, J.; Shi, C.-L.; et al. KNOX Protein KNAT1 Regulates Fruitlet Abscission in Litchi by Repressing Ethylene Biosynthetic Genes. J. Exp. Bot. 2020, 71, 4069–4082. [Google Scholar] [CrossRef]
- Fu, X.; Shi, Z.; Jiang, Y.; Jiang, L.; Qi, M.; Xu, T.; Li, T. A Family of Auxin Conjugate Hydrolases from Solanum lycopersicum and Analysis of Their Roles in Flower Pedicel Abscission. BMC Plant Biol. 2019, 19, 233. [Google Scholar] [CrossRef]
- Yan, F.; Gong, Z.; Hu, G.; Ma, X.; Bai, R.; Yu, R.; Zhang, Q.; Deng, W.; Li, Z.; Wuriyanghan, H. Tomato SlBL4 Plays an Important Role in Fruit Pedicel Organogenesis and Abscission. Hortic. Res. 2021, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Jiang, Y.; Han, X.; Liu, X.; Cao, R.; Qi, M.; Xu, T.; Li, T. SlPIN1 Regulates Auxin Efflux to Affect Flower Abscission Process. Sci. Rep. 2017, 7, 14919. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, G.; Yu, X.; Zhu, Z.; Zhang, L.; Zhou, S.; Hu, Z. The Tomato MADS-Box Gene SlMBP9 Negatively Regulates Lateral Root Formation and Apical Dominance by Reducing Auxin Biosynthesis and Transport. Plant Cell Rep. 2019, 38, 951–963. [Google Scholar] [CrossRef]
- Wang, R.; Li, R.; Cheng, L.; Wang, X.; Fu, X.; Dong, X.; Qi, M.; Jiang, C.; Xu, T.; Li, T. SlERF52 Regulates SlTIP1;1 Expression to Accelerate Tomato Pedicel Abscission. Plant Physiol. 2021, 185, 1829–1846. [Google Scholar] [CrossRef]
- Sundaresan, S.; Philosoph-Hadas, S.; Ma, C.; Jiang, C.-Z.; Riov, J.; Mugasimangalam, R.; Kochanek, B.; Salim, S.; Reid, M.S.; Meir, S. The Tomato Hybrid Proline-Rich Protein Regulates the Abscission Zone Competence to Respond to Ethylene Signals. Hortic. Res. 2018, 5, 28. [Google Scholar] [CrossRef]
- Perrakis, A.; Bita, C.E.; Arhondakis, S.; Krokida, A.; Mekkaoui, K.; Denic, D.; Blazakis, K.N.; Kaloudas, D.; Kalaitzis, P. Suppression of a Prolyl 4 Hydroxylase Results in Delayed Abscission of Overripe Tomato Fruits. Front. Plant Sci. 2019, 10, 348. [Google Scholar] [CrossRef]
- Qi, X.; Hu, S.; Zhou, H.; Liu, X.; Wang, L.; Zhao, B.; Huang, X.; Zhang, S. A MADS-Box Transcription Factor of ‘Kuerlexiangli’(Pyrus Sinkiangensis Yu) PsJOINTLESS Gene Functions in Floral Organ Abscission. Gene 2018, 642, 163–171. [Google Scholar] [CrossRef]
- Deng, H.; Pirrello, J.; Chen, Y.; Li, N.; Zhu, S.; Chirinos, X.; Bouzayen, M.; Liu, Y.; Liu, M. A Novel Tomato F-box Protein, SlEBF3, Is Involved in Tuning Ethylene Signaling during Plant Development and Climacteric Fruit Ripening. Plant J. 2018, 95, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Jiang, L.; Kai, W.; Liang, B.; Wang, J.; Du, Y.; Zhai, X.; Wang, J.; Zhang, Y.; et al. Suppressing Type 2C Protein Phosphatases Alters Fruit Ripening and the Stress Response in Tomato. Plant Cell Physiol. 2018, 59, 142–154. [Google Scholar] [CrossRef]
- Kai, W.; Wang, J.; Liang, B.; Fu, Y.; Zheng, Y.; Zhang, W.; Li, Q.; Leng, P. PYL9 Is Involved in the Regulation of ABA Signaling during Tomato Fruit Ripening. J. Exp. Bot. 2019, 70, 6305–6319. [Google Scholar] [CrossRef]
- Kai, W.; Fu, Y.; Wang, J.; Liang, B.; Li, Q.; Leng, P. Functional Analysis of SlNCED1 in Pistil Development and Fruit Set in Tomato (Solanum lycopersicum L.). Sci. Rep. 2019, 9, 16943. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shi, C.; Wang, X.; Li, R.; Meng, Y.; Cheng, L.; Qi, M.; Xu, T.; Li, T. Tomato SlIDA Has a Critical Role in Tomato Fertilization by Modifying Reactive Oxygen Species Homeostasis. Plant J. 2020, 103, 2100–2118. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, S.; Philosoph-Hadas, S.; Ma, C.; Jiang, C.-Z.; Riov, J.; Kochanek, B.; Salim, S.; Reid, M.S.; Meir, S. Role of the KNOTTED1-LIKE HOMEOBOX Protein (KD1) in Regulating Abscission of Tomato Flower Pedicels at Early and Late Stages of the Process. Physiol. Plant. 2021, 173, 2103–2118. [Google Scholar] [CrossRef]
- Kaulfürst-Soboll, H.; Mertens-Beer, M.; Brehler, R.; Albert, M.; von Schaewen, A. Complex N-Glycans Are Important for Normal Fruit Ripening and Seed Development in Tomato. Front. Plant Sci. 2021, 12, 16943. [Google Scholar] [CrossRef]
- Wang, X.; Meng, J.; Deng, L.; Wang, Y.; Liu, H.; Yao, J.-L.; Nieuwenhuizen, N.J.; Wang, Z.; Zeng, W. Diverse Functions of IAA-Leucine Resistant PpILR1 Provide a Genic Basis for Auxin-Ethylene Crosstalk during Peach Fruit Ripening. Front. Plant Sci. 2021, 12, 655758. [Google Scholar] [CrossRef]
- Cheng, L.; Li, R.; Wang, X.; Ge, S.; Wang, S.; Liu, X.; He, J.; Jiang, C.-Z.; Qi, M.; Xu, T.; et al. A SlCLV3-SlWUS Module Regulates Auxin and Ethylene Homeostasis in Low Light-Induced Tomato Flower Abscission. Plant Cell 2022, 34, 4388–4408. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, L.; Li, R.; Cai, Y.; Wang, X.; Fu, X.; Dong, X.; Qi, M.; Jiang, C.-Z.; Xu, T.; et al. The HD-Zip Transcription Factor SlHB15A Regulates Abscission by Modulating Jasmonoyl-Isoleucine Biosynthesis. Plant Physiol. 2022, 189, 2396–2412. [Google Scholar] [CrossRef]
- Meng, S.; Xiang, H.; Yang, X.; Ye, Y.; Han, L.; Xu, T.; Liu, Y.; Wang, F.; Tan, C.; Qi, M.; et al. Effects of Low Temperature on Pedicel Abscission and Auxin Synthesis Key Genes of Tomato. Int. J. Mol. Sci. 2023, 24, 9186. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, J.; Cao, L.; Xie, Q.; Chen, G.; Chen, X.; Hu, Z. Suppression of a Hexokinase Gene SlHXK1 in Tomato Affects Fruit Setting and Seed Quality. Plant Physiol. Biochem. 2023, 205, 108160. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-H.; Jang, J.-C.; Huang, Z.; van der Knaap, E. Tomato Locule Number and Fruit Size Controlled by Natural Alleles of Lc and Fas. Plant Direct 2019, 3, e00142. [Google Scholar] [CrossRef]
- Cong, B.; Barrero, L.S.; Tanksley, S.D. Regulatory Change in YABBY-like Transcription Factor Led to Evolution of Extreme Fruit Size during Tomato Domestication. Nat. Genet. 2008, 40, 800–804. [Google Scholar] [CrossRef]
- Xu, C.; Liberatore, K.L.; MacAlister, C.A.; Huang, Z.; Chu, Y.-H.; Jiang, K.; Brooks, C.; Ogawa-Ohnishi, M.; Xiong, G.; Pauly, M.; et al. A Cascade of Arabinosyltransferases Controls Shoot Meristem Size in Tomato. Nat. Genet. 2015, 47, 784–792. [Google Scholar] [CrossRef]
- Sun, M.; Li, H.; Li, Y.; Xiang, H.; Liu, Y.; He, Y.; Qi, M.; Li, T. Tomato YABBY2b Controls Plant Height through Regulating Indole-3-Acetic Acid-Amido Synthetase (GH3.8) Expression. Plant Sci. 2020, 297, 110530. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, M.; Sun, M.; Liu, Y.; Liu, Y.; Xu, T.; Li, Y.; Li, T. Tomato Transcription Factor SlWUS Plays an Important Role in Tomato Flower and Locule Development. Front. Plant Sci. 2017, 8, 457. [Google Scholar] [CrossRef]
- Hendelman, A.; Zebell, S.; Rodriguez-Leal, D.; Dukler, N.; Robitaille, G.; Wu, X.; Kostyun, J.; Tal, L.; Wang, P.; Bartlett, M.E.; et al. Conserved Pleiotropy of an Ancient Plant Homeobox Gene Uncovered by Cis-Regulatory Dissection. Cell 2021, 184, 1724–1739.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Wang, X.; Li, C.; Ye, Z.; Zhang, J. UF, a WOX Gene, Regulates a Novel Phenotype of Un-Fused Flower in Tomato. Plant Sci. 2020, 297, 110523. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, B.; He, L.; Zhou, S.; Liu, Y.; Zhao, W.; Guo, S.; Wang, R.; Bai, Q.; Li, Y.; et al. The WOX Family Transcriptional Regulator SlLAM1 Controls Compound Leaf and Floral Organ Development in Solanum lycopersicum. J. Exp. Bot. 2021, 72, 1822–1835. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Mo, Y.; Israeli, A.; Wang, Q.; Yifhar, T.; Ori, N.; Jiao, Y. Leaflet Initiation and Blade Expansion Are Separable in Compound Leaf Development. Plant J. 2020, 104, 1073–1087. [Google Scholar] [CrossRef]
- Su, D.; Wen, L.; Xiang, W.; Shi, Y.; Lu, W.; Liu, Y.; Xian, Z.; Li, Z. Tomato Transcriptional Repressor SlBES1.8 Influences Shoot Apical Meristem Development by Inhibiting the DNA Binding Ability of SlWUS. Plant J. 2022, 110, 482–498. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, Y.; Cai, J.; Shang, E.; Yamaguchi, N.; Xiao, J.; Looi, L.-S.; Wee, W.-Y.; Gao, X.; Wagner, D.; et al. Integration of Transcriptional Repression and Polycomb-Mediated Silencing of WUSCHEL in Floral Meristems. Plant Cell 2019, 31, 1488–1505. [Google Scholar] [CrossRef]
- Bollier, N.; Sicard, A.; Leblond, J.; Latrasse, D.; Gonzalez, N.; Gévaudant, F.; Benhamed, M.; Raynaud, C.; Lenhard, M.; Chevalier, C.; et al. At-MINI ZINC FINGER2 and Sl-INHIBITOR OF MERISTEM ACTIVITY, a Conserved Missing Link in the Regulation of Floral Meristem Termination in Arabidopsis and Tomato. Plant Cell 2018, 30, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Bollier, N.; Sicard, A.; Gonzalez, N.; Chevalier, C.; Hernould, M.; Delmas, F. Induced Ovule-to-Flower Switch by Interfering with SlIMA Activity in Tomato. Plant Signal. Behav. 2018, 13, e1473687. [Google Scholar] [CrossRef]
- Castañeda, L.; Giménez, E.; Pineda, B.; García-Sogo, B.; Ortiz-Atienza, A.; Micol-Ponce, R.; Angosto, T.; Capel, J.; Moreno, V.; Yuste-Lisbona, F.J.; et al. Tomato CRABS CLAW Paralogues Interact with Chromatin Remodelling Factors to Mediate Carpel Development and Floral Determinacy. New Phytol. 2022, 234, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, R.; Weng, L.; Sun, Y.; Li, M.; Xiao, H. Domain-Specific Expression of Meristematic Genes Is Defined by the LITTLE ZIPPER Protein DTM in Tomato. Commun. Biol. 2019, 2, 134. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Liao, C.-J.; Mengesha, B.; Han, H.; Lee, S.; Sharon, A.; Zhou, Y.; Mengiste, T. Regulation of Plant Immunity and Growth by Tomato Receptor-like Cytoplasmic Kinase TRK1. New Phytol. 2022, 233, 458–478. [Google Scholar] [CrossRef]
- Song, S.; Huang, B.; Pan, Z.; Zhong, Q.; Yang, Y.; Chen, D.; Zhu, L.; Hu, G.; He, M.; Wu, C.; et al. The SlTPL3–SlWUS Module Regulates Multi-locule Formation in Tomato by Modulating Auxin and Gibberellin Levels in the Shoot Apical Meristem. J. Integr. Plant Biol. 2022, 64, 2150–2167. [Google Scholar] [CrossRef] [PubMed]
- Yuste-Lisbona, F.J.; Fernández-Lozano, A.; Pineda, B.; Bretones, S.; Ortíz-Atienza, A.; García-Sogo, B.; Müller, N.A.; Angosto, T.; Capel, J.; Moreno, V.; et al. ENO Regulates Tomato Fruit Size through the Floral Meristem Development Network. Proc. Natl. Acad. Sci. USA 2020, 117, 8187–8195. [Google Scholar] [CrossRef]
- Wang, R.; Tavano, E.C.D.R.; Lammers, M.; Martinelli, A.P.; Angenent, G.C.; de Maagd, R.A. Re-Evaluation of Transcription Factor Function in Tomato Fruit Development and Ripening with CRISPR/Cas9-Mutagenesis. Sci. Rep. 2019, 9, 1696. [Google Scholar] [CrossRef]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis WUSCHEL Is a Bifunctional Transcription Factor That Acts as a Repressor in Stem Cell Regulation and as an Activator in Floral Patterning. Plant Cell 2009, 21, 3493–3505. [Google Scholar] [CrossRef]
- Perales, M.; Rodriguez, K.; Snipes, S.; Yadav, R.K.; Diaz-Mendoza, M.; Reddy, G.V. Threshold-Dependent Transcriptional Discrimination Underlies Stem Cell Homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, E6298–E6306. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhu, Y.; Cui, Y.; Cheng, K.; Liang, W.; Wei, Z.; Zhu, M.; Yin, H.; Zeng, L.; Xiao, Y.; et al. A Group of Receptor Kinases Are Essential for CLAVATA Signalling to Maintain Stem Cell Homeostasis. Nat. Plants 2018, 4, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.X.; Jürgens, G.; Laux, T. The Stem Cell Population of Arabidopsis Shoot Meristems Is Maintained by a Regulatory Loop between the CLAVATA and WUSCHEL Genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Liu, Y. SlCK2α as a Novel Substrate for CRL4 E3 Ligase Regulates Fruit Size through Maintenance of Cell Division Homeostasis in Tomato. Planta 2023, 257, 38. [Google Scholar] [CrossRef]
- Rodríguez, G.R.; Muños, S.; Anderson, C.; Sim, S.-C.; Michel, A.; Causse, M.; Gardener, B.B.M.; Francis, D.; van der Knaap, E. Distribution of SUN, OVATE, LC, and FAS in the Tomato Germplasm and the Relationship to Fruit Shape Diversity. Plant Physiol. 2011, 156, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Van Eck, J.; Cong, B.; Tanksley, S.D. A New Class of Regulatory Genes Underlying the Cause of Pear-Shaped Tomato Fruit. Proc. Natl. Acad. Sci. USA 2002, 99, 13302–13306. [Google Scholar] [CrossRef]
- Rodríguez, G.R.; Kim, H.J.; van der Knaap, E. Mapping of Two Suppressors of OVATE (Sov) Loci in Tomato. Heredity 2013, 111, 256–264. [Google Scholar] [CrossRef]
- Wang, S.; Chang, Y.; Ellis, B. Overview of OVATE FAMILY PROTEINS, A Novel Class of Plant-Specific Growth Regulators. Front. Plant Sci. 2016, 7, 417. [Google Scholar] [CrossRef]
- Chen, J.; Pan, B.; Li, Z.; Xu, Y.; Cao, X.; Jia, J.; Shen, H.; Sun, L. Fruit Shape Loci Sun, Ovate, Fs8.1 and Their Interactions Affect Seed Size and Shape in Tomato. Front. Plant Sci. 2023, 13, 1091639. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Miao, H.; Jia, C.; Wang, J.; Xu, B.; Jin, Z. Elucidating the Mechanisms of the Tomato Ovate Mutation in Regulating Fruit Quality Using Proteomics Analysis. J. Agric. Food Chem. 2017, 65, 10048–10057. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Wang, J.; Zhang, J.; Miao, H.; Jia, C.; Wang, Z.; Xu, B.; Jin, Z. MuMADS1 and MaOFP1 Regulate Fruit Quality in a Tomato Ovate Mutant. Plant Biotechnol. J. 2018, 16, 989–1001. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, J.; Fu, J.; Yu, H.; Wang, X.; Wang, S.; Adhikari, P.B.; Deng, X.; Xu, Q. Genome-Wide Identification of Ovate Family in Citrus and Functional Characterization of CitOFP19. Plant Sci. 2022, 321, 111328. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, X.; Wang, J.; Wu, X.; Wang, B.; Lu, Z.; Ye, Z.; Li, G.; Wang, Y. Identification of Bottle Gourd (Lagenaria siceraria) OVATE Family Genes and Functional Characterization of LsOVATE1. Biomolecules 2022, 13, 85. [Google Scholar] [CrossRef]
- Zhou, S.; Cheng, X.; Li, F.; Feng, P.; Hu, G.; Chen, G.; Xie, Q.; Hu, Z. Overexpression of SlOFP20 in Tomato Affects Plant Growth, Chlorophyll Accumulation, and Leaf Senescence. Front. Plant Sci. 2019, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, Z.; Li, F.; Tian, S.; Zhu, Z.; Li, A.; Chen, G. Overexpression of SlOFP20 Affects Floral Organ and Pollen Development. Hortic. Res. 2019, 6, 125. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, H.; Cabrera, A.; Meulia, T.; van der Knaap, E. SUN Regulates Vegetative and Reproductive Organ Shape by Changing Cell Division Patterns. Plant Physiol. 2011, 157, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Q.; Keyhaninejad, N.; Taitano, N.; Sapkota, M.; Snouffer, A.; van der Knaap, E. A Combinatorial TRM-OFP Module Bilaterally Fine-tunes Tomato Fruit Shape. New Phytol. 2023, 238, 2393–2409. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, B.; Keyhaninejad, N.; Rodríguez, G.R.; Kim, H.J.; Chakrabarti, M.; Illa-Berenguer, E.; Taitano, N.K.; Gonzalo, M.J.; Díaz, A.; et al. A Common Genetic Mechanism Underlies Morphological Diversity in Fruits and Other Plant Organs. Nat. Commun. 2018, 9, 4734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Clevenger, J.P.; Illa-Berenguer, E.; Meulia, T.; van der Knaap, E.; Sun, L. A Comparison of Sun, Ovate, Fs8.1 and Auxin Application on Tomato Fruit Shape and Gene Expression. Plant Cell Physiol. 2019, 60, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Dong, Y.; Nong, H.; Huang, L.; Liu, J.; Yu, X.; Zhang, Y.; Yang, L.; Hong, B.; Wang, W.; et al. VvSUN May Act in the Auxin Pathway to Regulate Fruit Shape in Grape. Hortic. Res. 2022, 9, uhac200. [Google Scholar] [CrossRef]
- Bi, L.; Weng, L.; Jiang, Z.; Xiao, H. The Tomato IQD Gene SUN24 Regulates Seed Germination through ABA Signaling Pathway. Planta 2018, 248, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Duan, S.; Umer, M.J.; Xie, K.; Wang, Y.; Kang, Q.; Yang, S.; Yang, L.; Liu, D.; Liu, L.; et al. Genome-Wide Analysis of IQD Proteins and Ectopic Expression of Watermelon ClIQD24 in Tomato Suggests Its Important Role in Regulating Fruit Shape. Front. Genet. 2022, 13, 993218. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Guo, Y.; Deng, Y.; Zang, J.; Zhang, J.; Deng, Y.; Ouyang, B.; Qu, X.; Bürstenbinder, K.; Wang, P. Microtubule-Associated Protein SlMAP70 Interacts with IQ67-Domain Protein SlIQD21a to Regulate Fruit Shape in Tomato. Plant Cell 2023, 35, 4266–4283. [Google Scholar] [CrossRef]
- Lazzaro, M.D.; Wu, S.; Snouffer, A.; Wang, Y.; van der Knaap, E. Plant Organ Shapes Are Regulated by Protein Interactions and Associations with Microtubules. Front. Plant Sci. 2018, 9, 1766. [Google Scholar] [CrossRef] [PubMed]
- Shtern, A.; Keren-Keiserman, A.; Mauxion, J.-P.; Furumizu, C.; Alvarez, J.P.; Amsellem, Z.; Gil, N.; Motenko, E.; Alkalai-Tuvia, S.; Fallik, E.; et al. Solanum lycopersicum CLASS-II KNOX Genes Regulate Fruit Anatomy via Gibberellin-Dependent and Independent Pathways. J. Exp. Bot. 2023, 74, 848–863. [Google Scholar] [CrossRef]
- Keren-Keiserman, A.; Shtern, A.; Levy, M.; Chalupowicz, D.; Furumizu, C.; Alvarez, J.P.; Amsalem, Z.; Arazi, T.; Alkalai-Tuvia, S.; Efroni, I.; et al. CLASS-II KNOX Genes Coordinate Spatial and Temporal Ripening in Tomato. Plant Physiol. 2022, 190, 657–668. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, S.; Wu, N.; Li, X.; Ahmad, B.; Wu, J.; Guo, R.; Wang, X. KNOX Transcription Factor VvHB63 Affects Grape Seed Development by Interacting with Protein VvHB06. Plant Sci. 2023, 330, 111665. [Google Scholar] [CrossRef]
- Yan, F.; Deng, W.; Pang, X.; Gao, Y.; Chan, H.; Zhang, Q.; Hu, N.; Chen, J.; Li, Z. Overexpression of the KNOX Gene Tkn4 Affects Pollen Development and Confers Sensitivity to Gibberellin and Auxin in Tomato. Plant Sci. 2019, 281, 61–71. [Google Scholar] [CrossRef]
- Yan, F.; Gao, Y.; Pang, X.; Xu, X.; Zhu, N.; Chan, H.; Hu, G.; Wu, M.; Yuan, Y.; Li, H.; et al. BEL1-LIKE HOMEODOMAIN4 Regulates Chlorophyll Accumulation, Chloroplast Development, and Cell Wall Metabolism in Tomato Fruit. J. Exp. Bot. 2020, 71, 5549–5561. [Google Scholar] [CrossRef]
- Meng, L.; Fan, Z.; Zhang, Q.; Wang, C.; Gao, Y.; Deng, Y.; Zhu, B.; Zhu, H.; Chen, J.; Shan, W.; et al. BEL1-LIKE HOMEODOMAIN 11 Regulates Chloroplast Development and Chlorophyll Synthesis in Tomato Fruit. Plant J. 2018, 94, 1126–1140. [Google Scholar] [CrossRef]
- Ezura, K.; Nakamura, A.; Mitsuda, N. Genome-Wide Characterization of the TALE Homeodomain Family and the KNOX-BLH Interaction Network in Tomato. Plant Mol. Biol. 2022, 109, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, P.; Cheng, B.; Zhang, Y.; Shen, Y.; Wang, X.; Zhang, Q.; Lou, Q.; Zhang, S.; Wang, B.; et al. Identification of TALE Transcription Factor Family and Expression Patterns Related to Fruit Chloroplast Development in Tomato (Solanum lycopersicum L.). Int. J. Mol. Sci. 2022, 23, 4507. [Google Scholar] [CrossRef]
- He, Y.; Yang, T.; Yan, S.; Niu, S.; Zhang, Y. Identification and Characterization of the BEL1-like Genes Reveal Their Potential Roles in Plant Growth and Abiotic Stress Response in Tomato. Int. J. Biol. Macromol. 2022, 200, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feng, Q.; Snouffer, A.; Zhang, B.; Rodríguez, G.R.; van der Knaap, E. Increasing Fruit Weight by Editing a Cis-Regulatory Element in Tomato KLUH Promoter Using CRISPR/Cas9. Front. Plant Sci. 2022, 13, 879642. [Google Scholar] [CrossRef] [PubMed]
- Grandillo, S.; Ku, H.M.; Tanksley, S.D. Identifying the Loci Responsible for Natural Variation in Fruit Size and Shape in Tomato. Züchter Genet. Breed. Res. 1999, 99, 978–987. [Google Scholar] [CrossRef]
- Thibivilliers, S.; Farmer, A.; Libault, M. Biological and Cellular Functions of the Microdomain-Associated FWL/CNR Protein Family in Plants. Plants 2020, 9, 377. [Google Scholar] [CrossRef]
- Beauchet, A.; Gévaudant, F.; Gonzalez, N.; Chevalier, C. In Search of the Still Unknown Function of FW2.2/CELL NUMBER REGULATOR, a Major Regulator of Fruit Size in Tomato. J. Exp. Bot. 2021, 72, 5300–5311. [Google Scholar] [CrossRef]
- Mu, Q.; Huang, Z.; Chakrabarti, M.; Illa-Berenguer, E.; Liu, X.; Wang, Y.; Ramos, A.; van der Knaap, E. Fruit Weight Is Controlled by Cell Size Regulator Encoding a Novel Protein That Is Expressed in Maturing Tomato Fruits. PLoS Genet. 2017, 13, e1006930. [Google Scholar] [CrossRef]
- Wu, S.; Clevenger, J.P.; Sun, L.; Visa, S.; Kamiya, Y.; Jikumaru, Y.; Blakeslee, J.; van der Knaap, E. The Control of Tomato Fruit Elongation Orchestrated by Sun, Ovate and Fs8.1 in a Wild Relative of Tomato. Plant Sci. 2015, 238, 95–104. [Google Scholar] [CrossRef]
- Sun, L.; Rodriguez, G.R.; Clevenger, J.P.; Illa-Berenguer, E.; Lin, J.; Blakeslee, J.J.; Liu, W.; Fei, Z.; Wijeratne, A.; Meulia, T.; et al. Candidate Gene Selection and Detailed Morphological Evaluations of Fs8.1, a Quantitative Trait Locus Controlling Tomato Fruit Shape. J. Exp. Bot. 2015, 66, 6471–6482. [Google Scholar] [CrossRef]
- Chaudhury, A.M. Nuclear Genes Controlling Male Fertility. Plant Cell 1993, 5, 1277–1283. [Google Scholar] [CrossRef]
- McNeil, K.J.; Smith, A.G. A Glycine-Rich Protein That Facilitates Exine Formation during Tomato Pollen Development. Planta 2010, 231, 793–808. [Google Scholar] [CrossRef]
- Jaffri, S.R.F.; Scheer, H.; MacAlister, C.A. The Hydroxyproline O-Arabinosyltransferase FIN4 Is Required for Tomato Pollen Intine Development. Plant Reprod. 2023, 36, 173–191. [Google Scholar] [CrossRef]
- Ochoa-Jiménez, V.-A.; Berumen-Varela, G.; Burgara-Estrella, A.; Orozco-Avitia, J.-A.; Ojeda-Contreras, Á.-J.; Trillo-Hernández, E.-A.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Datsenka, T.; et al. Functional Analysis of Tomato Rhamnogalacturonan Lyase Gene Solyc11g011300 during Fruit Development and Ripening. J. Plant Physiol. 2018, 231, 31–40. [Google Scholar] [CrossRef]
- Jaffri, S.R.F.; MacAlister, C.A. Sequential Deposition and Remodeling of Cell Wall Polymers during Tomato Pollen Development. Front. Plant Sci. 2021, 12, 703713. [Google Scholar] [CrossRef]
- Mascarenhas, J.P. Molecular Mechanisms of Pollen Tube Growth and Differentiation. Plant Cell 1993, 5, 1303–1314. [Google Scholar] [CrossRef]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A Developmental Perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Q.; Wu, G.; Zhang, L.; Xu, X.; Hu, X.; Gong, Z.; Chen, Y.; Li, Z.; Li, H.; et al. SlMYB72 Affects Pollen Development by Regulating Autophagy in Tomato. Hortic. Res. 2023, 10, uhac286. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, Y.; Su, D.; Yu, C.; Xian, Z.; Pan, Z.; Guan, H.; Hu, G.; Chen, D.; Li, Z.; et al. The SlHB8 Acts as a Negative Regulator in Tapetum Development and Pollen Wall Formation in Tomato. Hortic. Res. 2022, 9, uhac185. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Kim, D.H.; Lee, H.J.; Nam, K.H.; Bae, S.; Nou, I.S.; Cho, Y.-G.; Kim, M.K.; Kang, K.K. Knockout of SlMS10 Gene (Solyc02g079810) Encoding BHLH Transcription Factor Using CRISPR/Cas9 System Confers Male Sterility Phenotype in Tomato. Plants 2020, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, M.; Liu, X.; Wei, K.; Cao, X.; Wang, X.; Wang, X.; Guo, Y.; Du, Y.; Li, J.; et al. A Putative BHLH Transcription Factor Is a Candidate Gene for Male Sterile 32, a Locus Affecting Pollen and Tapetum Development in Tomato. Hortic. Res. 2019, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, Y.; Niu, X.; Miao, M.; Kud, J.; Zhou, B.; Zeng, L.; Liu, Y.; Xiao, F. Functional Analysis of the Seven in Absentia Ubiquitin Ligase Family in Tomato. Plant Cell Environ. 2018, 41, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Ding, Y.; Yang, F.; Zhang, J.; Xie, J.; Zhao, C.; Du, K.; Zeng, Y.; Zhao, K.; Li, Z.; et al. Gene Silencing, Knockout and over-Expression of a Transcription Factor ABORTED MICROSPORES (SlAMS) Strongly Affects Pollen Viability in Tomato (Solanum lycopersicum). BMC Genom. 2022, 23, 346. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhou, K.; Liu, Y.; Deng, L.; Zhang, X.; Lin, L.; Zhou, M.; Zhao, W.; Wen, C.; Xing, J.; et al. A Biotechnology-based Male-sterility System for Hybrid Seed Production in Tomato. Plant J. 2020, 102, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, D.; Zhang, Y.; Wu, L.; Zhang, Y.; Ye, L.; Pan, C.; He, Y.; Huang, L.; Ruan, Y.-L.; et al. Evidence for a Specific and Critical Role of Mitogen-activated Protein Kinase 20 in Uni-to-binucleate Transition of Microgametogenesis in Tomato. New Phytol. 2018, 219, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Zhuo, S.; Liu, Y.; Yu, X.; Mukhtar, S.; Ali, M.; Lu, G. Mitogen-Activated Protein Kinase 4 Is Obligatory for Late Pollen and Early Fruit Development in Tomato. Hortic. Res. 2022, 9, uhac048. [Google Scholar] [CrossRef] [PubMed]
- Secgin, Z.; Uluisik, S.; Yıldırım, K.; Abdulla, M.F.; Mostafa, K.; Kavas, M. Genome-Wide Identification of the Aconitase Gene Family in Tomato (Solanum lycopersicum) and CRISPR-Based Functional Characterization of SlACO2 on Male-Sterility. Int. J. Mol. Sci. 2022, 23, 13963. [Google Scholar] [CrossRef]
- Dai, X.; Han, H.; Huang, W.; Zhao, L.; Song, M.; Cao, X.; Liu, C.; Niu, X.; Lang, Z.; Ma, C.; et al. Generating Novel Male Sterile Tomatoes by Editing Respiratory Burst Oxidase Homolog Genes. Front. Plant Sci. 2022, 12, 817101. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, S.; Zhang, Q.; Jiang, J. RNA Interference Silencing of the Cytoplasmic Invertases SlCIN7 Leads to Reduction in Pollen Viability and Parthenocarpic Fruit in Tomato. Gene 2021, 771, 145367. [Google Scholar] [CrossRef]
- Xie, D.-L.; Huang, H.-M.; Zhou, C.-Y.; Liu, C.-X.; Kanwar, M.K.; Qi, Z.-Y.; Zhou, J. HsfA1a Confers Pollen Thermotolerance through Upregulating Antioxidant Capacity, Protein Repair, and Degradation in Solanum lycopersicum L. Hortic. Res. 2022, 9, uhac163. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martín, F.; Yuste-Lisbona, F.J.; Pineda, B.; García-Sogo, B.; del Olmo, I.; de Dios Alché, J.; Egea, I.; Flores, F.B.; Piñeiro, M.; Jarillo, J.A.; et al. Developmental Role of the Tomato Mediator Complex Subunit MED18 in Pollen Ontogeny. Plant J. 2018, 96, 300–315. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Zhang, J.; Yu, X.; Guo, J.-E.; Liang, H.; Liao, C.; Chen, G. Silencing SlMED18, Tomato Mediator Subunit 18 Gene, Restricts Internode Elongation and Leaf Expansion. Sci. Rep. 2018, 8, 3285. [Google Scholar] [CrossRef]
- Gan, Z.; Feng, Y.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. Downregulation of the Auxin Transporter Gene SlPIN8 Results in Pollen Abortion in Tomato. Plant Mol. Biol. 2019, 99, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Kai, W.; Liang, B.; Wang, J.; Jiang, L.; Du, Y.; Sun, Y.; Leng, P. The Functional Analysis of SlNCED1 in Tomato Pollen Development. Cell. Mol. Life Sci. 2018, 75, 3457–3472. [Google Scholar] [CrossRef]
- Deslous, P.; Bournonville, C.; Decros, G.; Okabe, Y.; Mauxion, J.-P.; Jorly, J.; Gadin, S.; Brès, C.; Mori, K.; Ferrand, C.; et al. Overproduction of Ascorbic Acid Impairs Pollen Fertility in Tomato. J. Exp. Bot. 2021, 72, 3091–3107. [Google Scholar] [CrossRef]
- Jansma, S.Y.; Sergeeva, L.I.; Tikunov, Y.M.; Kohlen, W.; Ligterink, W.; Rieu, I. Low Salicylic Acid Level Improves Pollen Development under Long-Term Mild Heat Conditions in Tomato. Front. Plant Sci. 2022, 13, 828743. [Google Scholar] [CrossRef]
- Althiab-Almasaud, R.; Chen, Y.; Maza, E.; Djari, A.; Frasse, P.; Mollet, J.-C.; Mazars, C.; Jamet, E.; Chervin, C. Ethylene Signaling Modulates Tomato Pollen Tube Growth through Modifications of Cell Wall Remodeling and Calcium Gradient. Plant J. 2021, 107, 893–908. [Google Scholar] [CrossRef]
- Yan, M.-Y.; Xie, D.-L.; Cao, J.-J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Brassinosteroid-mediated Reactive Oxygen Species Are Essential for Tapetum Degradation and Pollen Fertility in Tomato. Plant J. 2020, 102, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Suzuki, T.; Takebayashi, Y.; Ishiguro, R.; Higashiyama, T.; Sakakibara, H.; Ishiguro, S. Jasmonic Acid Facilitates Flower Opening and Floral Organ Development through the Upregulated Expression of SlMYB21 Transcription Factor in Tomato. Biosci. Biotechnol. Biochem. 2018, 82, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef]
- Ko, H.-Y.; Tseng, H.-W.; Ho, L.-H.; Wang, L.; Chang, T.-F.; Lin, A.; Ruan, Y.-L.; Neuhaus, H.E.; Guo, W.-J. Hexose Translocation Mediated by SlSWEET5b Is Required for Pollen Maturation in Solanum lycopersicum. Plant Physiol. 2022, 189, 344–359. [Google Scholar] [CrossRef]
- Cai, Y.; Yin, L.; Tu, W.; Deng, Z.; Yan, J.; Dong, W.; Gao, H.; Xu, J.; Zhang, N.; Wang, J.; et al. Ectopic Expression of VvSUC27 Induces Stenospermocarpy and Sugar Accumulation in Tomato Fruits. Front. Plant Sci. 2021, 12, 759047. [Google Scholar] [CrossRef]
- Liu, H.-K.; Li, Y.-J.; Wang, S.-J.; Yuan, T.-L.; Huang, W.-J.; Dong, X.; Pei, J.-Q.; Zhang, D.; McCormick, S.; Tang, W.-H. Kinase Partner Protein Plays a Key Role in Controlling the Speed and Shape of Pollen Tube Growth in Tomato. Plant Physiol. 2020, 184, 1853–1869. [Google Scholar] [CrossRef]
- Salazar-Sarasua, B.; López-Martín, M.J.; Roque, E.; Hamza, R.; Cañas, L.A.; Beltrán, J.P.; Gómez-Mena, C. The Tapetal Tissue Is Essential for the Maintenance of Redox Homeostasis during Microgametogenesis in Tomato. Plant J. 2022, 112, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- Micol-Ponce, R.; García-Alcázar, M.; Lebrón, R.; Capel, C.; Pineda, B.; García-Sogo, B.; Alché, J.D.D.; Ortiz-Atienza, A.; Bretones, S.; Yuste-Lisbona, F.J.; et al. Tomato POLLEN DEFICIENT 2 Encodes a G-Type Lectin Receptor Kinase Required for Viable Pollen Grain Formation. J. Exp. Bot. 2023, 74, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Yang, D.; Zhao, X.; Liu, Y.; Li, M.; Ye, L.; Ali, M.; Yu, F.; Lamin-Samu, A.T.; Fei, Z.; et al. PIF4 Negatively Modulates Cold Tolerance in Tomato Anthers via Temperature-Dependent Regulation of Tapetal Cell Death. Plant Cell 2021, 33, 2320–2339. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Y.; Ali, M.; Ye, L.; Pan, C.; Li, M.; Zhao, X.; Yu, F.; Zhao, X.; Lu, G. Phytochrome Interacting Factor 3 Regulates Pollen Mitotic Division through Auxin Signalling and Sugar Metabolism Pathways in Tomato. New Phytol. 2022, 234, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Kravchik, M.; Stav, R.; Belausov, E.; Arazi, T. Functional Characterization of MicroRNA171 Family in Tomato. Plants 2019, 8, 10. [Google Scholar] [CrossRef]
- Huang, W.; Peng, S.; Xian, Z.; Lin, D.; Hu, G.; Yang, L.; Ren, M.; Li, Z. Overexpression of a Tomato MiR171 Target Gene SlGRAS24 Impacts Multiple Agronomical Traits via Regulating Gibberellin and Auxin Homeostasis. Plant Biotechnol. J. 2017, 15, 472–488. [Google Scholar] [CrossRef]
- Keller, M.; Schleiff, E.; Simm, S. MiRNAs Involved in Transcriptome Remodeling during Pollen Development and Heat Stress Response in Solanum lycopersicum. Sci. Rep. 2020, 10, 10694. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, J.; Chen, Z.; Qiao, J.; Zhang, Y.; Shen, H.; Hu, Z. CRISPR/Cas9-Targeted Mutagenesis of SlCMT4 Causes Changes in Plant Architecture and Reproductive Organs in Tomato. Hortic. Res. 2022, 9, uhac081. [Google Scholar] [CrossRef]
- Rick, C.M. Tomato-like Nightshades: Affinities, Autoecology, and Breeders’ Opportunities. Econ. Bot. 1988, 42, 145–154. [Google Scholar] [CrossRef]
- Cheng, M.-Z.; Gong, C.; Zhang, B.; Qu, W.; Qi, H.-N.; Chen, X.-L.; Wang, X.-Y.; Zhang, Y.; Liu, J.-Y.; Ding, X.-D.; et al. Morphological and Anatomical Characteristics of Exserted Stigma Sterility and the Location and Function of SlLst (Solanum lycopersicum Long Styles) Gene in Tomato. Züchter Genet. Breed. Res. 2021, 134, 505–518. [Google Scholar] [CrossRef]
- Shang, L.; Song, J.; Yu, H.; Wang, X.; Yu, C.; Wang, Y.; Li, F.; Lu, Y.; Wang, T.; Ouyang, B.; et al. A Mutation in a C2H2-Type Zinc Finger Transcription Factor Contributed to the Transition toward Self-Pollination in Cultivated Tomato. Plant Cell 2021, 33, 3293–3308. [Google Scholar] [CrossRef] [PubMed]
- Dumas, C.; Knox, R.B. Callose and Determination of Pistil Viability and Incompatibility. Züchter Genet. Breed. Res. 1983, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nal, M.; Vardar, F.; Ayturk, Z. Callose in Plant Sexual Reproduction. In Current Progress in Biological Research; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Pei, Y.; Xue, Q.; Zhang, Z.; Shu, P.; Deng, H.; Bouzayen, M.; Hong, Y.; Liu, M. β-1,3-GLUCANASE10 Regulates Tomato Development and Disease Resistance by Modulating Callose Deposition. Plant Physiol. 2023, 192, 2785–2802. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Pandey, A.K.; Sharma, K.; Ravi, M.; Sreelakshmi, Y.; Sharma, R. MutS-Homolog2 Silencing Generates Tetraploid Meiocytes in Tomato (Solanum lycopersicum). Plant Direct 2018, 2, e00017. [Google Scholar] [CrossRef] [PubMed]
- Strelnikova, S.R.; Krinitsina, A.A.; Komakhin, R.A. Effective RNAi-Mediated Silencing of the Mismatch Repair MSH2 Gene Induces Sterility of Tomato Plants but Not an Increase in Meiotic Recombination. Genes 2021, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, W.; Liu, Y.; Tan, M.; Ganal, M.; Chetelat, R.T. A Farnesyl Pyrophosphate Synthase Gene Expressed in Pollen Functions in S-RNase-independent Unilateral Incompatibility. Plant J. 2018, 93, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sanz, J.V.; Tovar-Méndez, A.; Lu, L.; Dai, R.; McClure, B. A Cysteine-Rich Protein, SpDIR1L, Implicated in S-RNase-Independent Pollen Rejection in the Tomato (Solanum Section Lycopersicon) Clade. Int. J. Mol. Sci. 2021, 22, 13067. [Google Scholar] [CrossRef]
- Tran, L.T.; Sugimoto, K.; Kasozi, M.; Mitalo, O.W.; Ezura, H. Pollination, Pollen Tube Growth, and Fertilization Independently Contribute to Fruit Set and Development in Tomato. Front. Plant Sci. 2023, 14, 1205816. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.; Gramazio, P.; Ezura, H. Increase in Phloem Area in the Tomato Hawaiian Skirt Mutant Is Associated with Enhanced Sugar Transport. Genes 2021, 12, 932. [Google Scholar] [CrossRef]
- Kusano, M.; Worarad, K.; Fukushima, A.; Kamiya, K.; Mitani, Y.; Okazaki, Y.; Higashi, Y.; Nakabayashi, R.; Kobayashi, M.; Mori, T.; et al. Transcriptomic, Hormonomic and Metabolomic Analyses Highlighted the Common Modules Related to Photosynthesis, Sugar Metabolism and Cell Division in Parthenocarpic Tomato Fruits during Early Fruit Set. Cells 2022, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Gorguet, B.; van Heusden, A.W.; Lindhout, P. Parthenocarpic Fruit Development in Tomato. Plant Biol. 2005, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Mariani, C.; Vriezen, W.H. The Role of Auxin and Gibberellin in Tomato Fruit Set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef]
- Serrani, J.C.; Fos, M.; Atarés, A.; García-Martínez, J.L. Effect of Gibberellin and Auxin on Parthenocarpic Fruit Growth Induction in the Cv Micro-Tom of Tomato. J. Plant Growth Regul. 2007, 26, 211–221. [Google Scholar] [CrossRef]
- Srivastava, A.; Handa, A.K. Hormonal Regulation of Tomato Fruit Development: A Molecular Perspective. J. Plant Growth Regul. 2005, 24, 67–82. [Google Scholar] [CrossRef]
- Hu, J.; Israeli, A.; Ori, N.; Sun, T.-P. The Interaction between DELLA and ARF/IAA Mediates Crosstalk between Gibberellin and Auxin Signaling to Control Fruit Initiation in Tomato. Plant Cell 2018, 30, 1710–1728. [Google Scholar] [CrossRef]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Delalande, C.; Regad, F.; Chaabouni, S.; Latché, A.; Pech, J.-C.; Bouzayen, M. The Tomato Aux/IAA Transcription Factor IAA9 Is Involved in Fruit Development and Leaf Morphogenesis. Plant Cell 2005, 17, 2676–2692. [Google Scholar] [CrossRef]
- He, M.; Song, S.; Zhu, X.; Lin, Y.; Pan, Z.; Chen, L.; Chen, D.; Hu, G.; Huang, B.; Chen, M.; et al. SlTPL1 Silencing Induces Facultative Parthenocarpy in Tomato. Front. Plant Sci. 2021, 12, 860. [Google Scholar] [CrossRef]
- Kim, J.-S.; Ezura, K.; Lee, J.; Ariizumi, T.; Ezura, H. Genetic Engineering of Parthenocarpic Tomato Plants Using Transient SlIAA9 Knockdown by Novel Tissue-Specific Promoters. Sci. Rep. 2019, 9, 18871. [Google Scholar] [CrossRef] [PubMed]
- Ueta, R.; Abe, C.; Watanabe, T.; Sugano, S.S.; Ishihara, R.; Ezura, H.; Osakabe, Y.; Osakabe, K. Rapid Breeding of Parthenocarpic Tomato Plants Using CRISPR/Cas9. Sci. Rep. 2017, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, L.; Yang, Y.; Emenecker, R.; Smoker, M.; Taylor, J.; Perkins, S.; Smith, J.; MacLean, D.; Olszewski, N.E.; Jones, J.D.G. Using CRISPR/Cas9 Genome Editing in Tomato to Create a Gibberellin-responsive Dominant Dwarf DELLA Allele. Plant Biotechnol. J. 2019, 17, 132–140. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Ezura, K.; Hu, J.; Okabe, Y.; Bénard, C.; Prodhomme, D.; Gibon, Y.; Sun, T.-P.; Ezura, H.; Ariizumi, T. Identification and Functional Study of a Mild Allele of SlDELLA Gene Conferring the Potential for Improved Yield in Tomato. Sci. Rep. 2018, 8, 12043. [Google Scholar] [CrossRef] [PubMed]
- Illouz-Eliaz, N.; Ramon, U.; Shohat, H.; Blum, S.; Livne, S.; Mendelson, D.; Weiss, D. Multiple Gibberellin Receptors Contribute to Phenotypic Stability under Changing Environments. Plant Cell 2019, 31, 1506–1519. [Google Scholar] [CrossRef]
- Illouz-Eliaz, N.; Nissan, I.; Nir, I.; Ramon, U.; Shohat, H.; Weiss, D. Mutations in the Tomato Gibberellin Receptors Suppress Xylem Proliferation and Reduce Water Loss under Water-Deficit Conditions. J. Exp. Bot. 2020, 71, 3603–3612. [Google Scholar] [CrossRef]
- María Victoria, B.; Claudia, B.; Cecilia, D.; Mauricio, H.-C.; Silvana, B.B.; Estela, M.V.; Eduardo, Z. MADS-box genes expressed during tomato seed and fruit development. Plant Mol. Biol. 2003, 52, 801–815. [Google Scholar] [CrossRef]
- Pnueli, L.; Hareven, D.; Rounsley, S.D.; Yanofsky, M.F.; Lifschitz, E. Isolation of the Tomato AGAMOUS Gene TAG1 and Analysis of Its Homeotic Role in Transgenic Plants. Plant Cell 1994, 6, 163–173. [Google Scholar] [CrossRef]
- Vrebalov, J.; Pan, I.L.; Arroyo, A.J.M.; McQuinn, R.; Chung, M.; Poole, M.; Rose, J.; Seymour, G.; Grandillo, S.; Giovannoni, J.; et al. Fleshy Fruit Expansion and Ripening Are Regulated by the Tomato SHATTERPROOF Gene TAGL1. Plant Cell 2009, 21, 3041–3062. [Google Scholar] [CrossRef] [PubMed]
- Ribelles, C.; García-Sogo, B.; Yuste-Lisbona, F.J.; Atarés, A.; Castañeda, L.; Capel, C.; Lozano, R.; Moreno, V.; Pineda, B. Alq Mutation Increases Fruit Set Rate and Allows the Maintenance of Fruit Yield under Moderate Saline Conditions. J. Exp. Bot. 2019, 70, 5731–5744. [Google Scholar] [CrossRef] [PubMed]
- Klap, C.; Yeshayahou, E.; Bolger, A.M.; Arazi, T.; Gupta, S.K.; Shabtai, S.; Usadel, B.; Salts, Y.; Barg, R. Tomato Facultative Parthenocarpy Results from SlAGAMOUS-LIKE 6 Loss of Function. Plant Biotechnol. J. 2017, 15, 634–647. [Google Scholar] [CrossRef]
- Gupta, S.K.; Barg, R.; Arazi, T. Tomato Agamous-Like6 Parthenocarpy Is Facilitated by Ovule Integument Reprogramming Involving the Growth Regulator KLUH. Plant Physiol. 2021, 185, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Routaboul, J.-M.; Liu, M.; Deng, W.; Maza, E.; Mila, I.; Hu, G.; Zouine, M.; Frasse, P.; Vrebalov, J.T.; et al. Overexpression of the Class D MADS-Box Gene Sl-AGL11 Impacts Fleshy Tissue Differentiation and Structure in Tomato Fruits. J. Exp. Bot. 2017, 68, 4869–4884. [Google Scholar] [CrossRef]
- Rojas-Gracia, P.; Roque, E.; Medina, M.; Rochina, M.; Hamza, R.; Angarita-Díaz, M.P.; Moreno, V.; Pérez-Martín, F.; Lozano, R.; Cañas, L.; et al. The Parthenocarpic Hydra Mutant Reveals a New Function for a SPOROCYTELESS-like Gene in the Control of Fruit Set in Tomato. New Phytol. 2017, 214, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Ariizumi, T.; Ezura, H. SEXUAL STERILITY Is Essential for Both Male and Female Gametogenesis in Tomato. Plant Cell Physiol. 2017, 58, cw214. [Google Scholar] [CrossRef]
- da Silva, E.M.; Silva, G.F.F.E.; Bidoia, D.B.; da Silva Azevedo, M.; de Jesus, F.A.; Pino, L.E.; Peres, L.E.P.; Carrera, E.; López-Díaz, I.; Nogueira, F.T.S. MicroRNA159-targeted SlGAMYB Transcription Factors Are Required for Fruit Set in Tomato. Plant J. 2017, 92, 95–109. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, F.; Deng, Y.; Zhong, F.; Tian, P.; Lin, D.; Deng, J.; Zhang, Y.; Huang, T. Sly-miR159 Regulates Fruit Morphology by Modulating GA Biosynthesis in Tomato. Plant Biotechnol. J. 2022, 20, 833–845. [Google Scholar] [CrossRef]
- Takei, H.; Shinozaki, Y.; Yano, R.; Kashojiya, S.; Hernould, M.; Chevalier, C.; Ezura, H.; Ariizumi, T. Loss-of-Function of a Tomato Receptor-like Kinase Impairs Male Fertility and Induces Parthenocarpic Fruit Set. Front. Plant Sci. 2019, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Dobritzsch, S.; Gruber, C.; Hause, G.; Athmer, B.; Schreiber, T.; Marillonnet, S.; Okabe, Y.; Ezura, H.; Acosta, I.F.; et al. Tomato MYB21 Acts in Ovules to Mediate Jasmonate-Regulated Fertility. Plant Cell 2019, 31, 1043–1062. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Cui, L.; Zhang, T.; Wu, Z.; Zhu, P.-Y.; Meng, Y.-J.; Zhang, K.-J.; Yu, X.-Q.; Lou, Q.-F.; et al. New Insights into the Roles of Cucumber TIR1 Homologs and miR393 in Regulating Fruit/Seed Set Development and Leaf Morphogenesis. BMC Plant Biol. 2017, 17, 130. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Feng, Q.; Qin, L.; Pan, C.; Lamin-Samu, A.T.; Lu, G. Tomato AUXIN RESPONSE FACTOR 5 Regulates Fruit Set and Development via the Mediation of Auxin and Gibberellin Signaling. Sci. Rep. 2018, 8, 2971. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, K.; Datsenka, T.U.; Liu, W.; Lv, S.; Lang, Z.; Wang, X.; Gao, J.; Wang, W.; Nie, W.; et al. Critical Function of DNA Methyltransferase 1 in Tomato Development and Regulation of the DNA Methylome and Transcriptome. J. Integr. Plant Biol. 2019, 61, 1224–1242. [Google Scholar] [CrossRef] [PubMed]
- Nambeesan, S.U.; Mattoo, A.K.; Handa, A.K. Nexus between Spermidine and Floral Organ Identity and Fruit/Seed Set in Tomato. Front. Plant Sci. 2019, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Yamaoka, T.; Ariizumi, T.; Ushijima, K.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Kusano, M.; Shinozaki, Y.; Pulungan, S.I.; et al. Aberrant Stamen Development Is Associated with Parthenocarpic Fruit Set through Up-Regulation of Gibberellin Biosynthesis in Tomato. Plant Cell Physiol. 2019, 60, 38–51. [Google Scholar] [CrossRef]
- Wang, H.; Wu, T.; Liu, J.; Cong, L.; Zhu, Y.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. PbGA20ox2 Regulates Fruit Set and Induces Parthenocarpy by Enhancing GA4 Content. Front. Plant Sci. 2020, 11, 113. [Google Scholar] [CrossRef]
- Matsuo, S.; Miyatake, K.; Endo, M.; Urashimo, S.; Kawanishi, T.; Negoro, S.; Shimakoshi, S.; Fukuoka, H. Loss of Function of the Pad-1 Aminotransferase Gene, Which Is Involved in Auxin Homeostasis, Induces Parthenocarpy in Solanaceae Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 12784–12790. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Beauvoit, B.P.; Takahara, M.; Hao, S.; Ezura, K.; Andrieu, M.-H.; Nishida, K.; Mori, K.; Suzuki, Y.; Kuhara, S.; et al. Fruit Setting Rewires Central Metabolism via Gibberellin Cascades. Proc. Natl. Acad. Sci. USA 2020, 117, 23970–23981. [Google Scholar] [CrossRef]
- Abe-Hara, C.; Yamada, K.; Wada, N.; Ueta, R.; Hashimoto, R.; Osakabe, K.; Osakabe, Y. Effects of the Sliaa9 Mutation on Shoot Elongation Growth of Tomato Cultivars. Front. Plant Sci. 2021, 12, 627832. [Google Scholar] [CrossRef]
- Nagata, T.; Lombardo, F.; Ezura, H. Complementation of the Tomato HWS Gene with Its Arabidopsis Counterpart Demonstrates Conservation of the Gene Function between Both Species. Plant Biotechnol. 2021, 38, 387–390. [Google Scholar] [CrossRef]
- Clepet, C.; Devani, R.S.; Boumlik, R.; Hao, Y.; Morin, H.; Marcel, F.; Verdenaud, M.; Mania, B.; Brisou, G.; Citerne, S.; et al. The miR166–SlHB15A Regulatory Module Controls Ovule Development and Parthenocarpic Fruit Set under Adverse Temperatures in Tomato. Mol. Plant 2021, 14, 1185–1198. [Google Scholar] [CrossRef]
- Zhang, H.; Han, W.; Wang, H.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Xu, L. Downstream of GA4, PbCYP78A6 Participates in Regulating Cell Cycle-Related Genes and Parthenogenesis in Pear (Pyrus bretshneideri Rehd.). BMC Plant Biol. 2021, 21, 292. [Google Scholar] [CrossRef]
- Kashojiya, S.; Lu, Y.; Takayama, M.; Komatsu, H.; Minh, L.H.T.; Nishida, K.; Shirasawa, K.; Miura, K.; Nonaka, S.; Masuda, J.-I.; et al. Modification of Tomato Breeding Traits and Plant Hormone Signaling by Target-AID, the Genome-Editing System Inducing Efficient Nucleotide Substitution. Hortic. Res. 2022, 9, uhab004. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Sun, T.-P. Four Class A AUXIN RESPONSE FACTORs Promote Tomato Fruit Growth despite Suppressing Fruit Set. Nat. Plants 2023, 9, 706–719. [Google Scholar] [CrossRef]
- Ding, J.; Chen, B.; Xia, X.; Mao, W.; Shi, K.; Zhou, Y.; Yu, J. Cytokinin-Induced Parthenocarpic Fruit Development in Tomato Is Partly Dependent on Enhanced Gibberellin and Auxin Biosynthesis. PLoS ONE 2013, 8, e70080. [Google Scholar] [CrossRef]
- Vriezen, W.H.; Feron, R.; Maretto, F.; Keijman, J.; Mariani, C. Changes in Tomato Ovary Transcriptome Demonstrate Complex Hormonal Regulation of Fruit Set. New Phytol. 2008, 177, 60–76. [Google Scholar] [CrossRef]
- Nitsch, L.M.C.; Oplaat, C.; Feron, R.; Ma, Q.; Wolters-Arts, M.; Hedden, P.; Mariani, C.; Vriezen, W.H. Abscisic Acid Levels in Tomato Ovaries Are Regulated by LeNCED1 and SlCYP707A1. Planta 2009, 229, 1335–1346. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Hao, S.; Kojima, M.; Sakakibara, H.; Ozeki-Iida, Y.; Zheng, Y.; Fei, Z.; Zhong, S.; Giovannoni, J.J.; Rose, J.K.C.; et al. Ethylene Suppresses Tomato (Solanum lycopersicum) Fruit Set through Modification of Gibberellin Metabolism. Plant J. 2015, 83, 237–251. [Google Scholar] [CrossRef]
- Bishop, G.J.; Nomura, T.; Yokota, T.; Harrison, K.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Jones, J.D.G.; Kamiya, Y. The Tomato DWARF Enzyme Catalyses C-6 Oxidation in Brassinosteroid Biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, G.; Segura, M.; Martínez, J.; Iglesias-Moya, J.; Martínez, C.; Garrido, D.; Jamilena, M. Jasmonate-Deficient Mutant Lox3a Reveals Crosstalk between Jasmonate and Ethylene in the Differential Regulation of Male and Female Flower Opening and Early Fruit Development in Cucurbita Pepo. J. Exp. Bot. 2023, 74, 1258–1274. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Luo, B.; Hu, M.; Fu, S.; Liu, J.; Jiang, M.; Zhao, Y.; Huang, S.; Wang, S.; Wang, X. Brassinosteroid Signaling Downstream Suppressor BIN2 Interacts with SLFRIGIDA-LIKE to Induce Early Flowering in Tomato. Int. J. Mol. Sci. 2022, 23, 11264. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lee, H.; Lee, J.; Kim, H.; Ryu, H. ABSCISIC ACID-INSENSITIVE 3 Is Involved in Brassinosteroid-Mediated Regulation of Flowering in Plants. Plant Physiol. Biochem. 2019, 139, 207–214. [Google Scholar] [CrossRef]
- Hu, G.; Huang, B.; Wang, K.; Frasse, P.; Maza, E.; Djari, A.; Benhamed, M.; Gallusci, P.; Li, Z.; Zouine, M.; et al. Histone Posttranslational Modifications Rather than DNA Methylation Underlie Gene Reprogramming in Pollination-dependent and Pollination-independent Fruit Set in Tomato. New Phytol. 2021, 229, 902–919. [Google Scholar] [CrossRef] [PubMed]
- Hawar, A.; Xiong, S.; Yang, Z.; Sun, B. Histone Acetyltransferase SlGCN5 Regulates Shoot Meristem and Flower Development in Solanum lycopersicum. Front. Plant Sci. 2022, 12, 805879. [Google Scholar] [CrossRef] [PubMed]
- Molesini, B.; Pennisi, F.; Vitulo, N.; Pandolfini, T. MicroRNAs Associated with AGL6 and IAA9 Function in Tomato Fruit Set. BMC Res. Notes 2023, 16, 242. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, C.; Wang, Z.; Yang, Z.; Chen, D.; Wu, Y. LncRNA Expression Profile and ceRNA Analysis in Tomato during Flowering. PLoS ONE 2019, 14, e0210650. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, Z.; Yang, C.; Wang, Z.; Chen, D.; Xie, Y.; Wu, Y. Identification and Genetic Analysis of Alternative Splicing of Long Non-Coding RNAs in Tomato Initial Flowering Stage. Genomics 2020, 112, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Mubarok, S.; Jadid, N.; Widiastuti, A.; Derajat Matra, D.; Budiarto, R.; Lestari, F.W.; Nuraini, A.; Suminar, E.; Pradana Nur Rahmat, B.; Ezura, H. Parthenocarpic Tomato Mutants, Iaa9-3 and Iaa9-5, Show Plant Adaptability and Fruiting Ability under Heat-Stress Conditions. Front. Plant Sci. 2023, 14, 1090774. [Google Scholar] [CrossRef]
- Chong, L.; Xu, R.; Huang, P.; Guo, P.; Zhu, M.; Du, H.; Sun, X.; Ku, L.; Zhu, J.-K.; Zhu, Y. The Tomato OST1–VOZ1 Module Regulates Drought-Mediated Flowering. Plant Cell 2022, 34, 2001–2018. [Google Scholar] [CrossRef]
- Yuan, S.; Kawasaki, S.; Abdellatif, I.M.Y.; Nishida, K.; Kondo, A.; Ariizumi, T.; Ezura, H.; Miura, K. Efficient Base Editing in Tomato Using a Highly Expressed Transient System. Plant Cell Rep. 2021, 40, 667–676. [Google Scholar] [CrossRef]
- Hunziker, J.; Nishida, K.; Kondo, A.; Kishimoto, S.; Ariizumi, T.; Ezura, H. Multiple Gene Substitution by Target-AID Base-Editing Technology in Tomato. Sci. Rep. 2020, 10, 20471. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, Y.; Shen, R.; Yao, Q.; Zhong, D.; Zhang, X.; Zhu, J.-K. Precise Genome Modification in Tomato Using an Improved Prime Editing System. Plant Biotechnol. J. 2021, 19, 415–417. [Google Scholar] [CrossRef]
- Van Vu, T.; Nguyen, N.T.; Kim, J.; Das, S.; Lee, J.; Kim, J.-Y. The Obstacles and Potential Solution Clues of Prime Editing Applications in Tomato. Biodes. Res. 2022, 2022, 0001. [Google Scholar] [CrossRef]
- Kwon, C.-T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid Customization of Solanaceae Fruit Crops for Urban Agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480.e8. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of Wild Tomato Is Accelerated by Genome Editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De Novo Domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Sierra-Orozco, E.; Shekasteband, R.; Illa-Berenguer, E.; Snouffer, A.; van der Knaap, E.; Lee, T.G.; Hutton, S.F. Identification and Characterization of GLOBE, a Major Gene Controlling Fruit Shape and Impacting Fruit Size and Marketability in Tomato. Hortic. Res. 2021, 8, e574-3. [Google Scholar] [CrossRef]
- Lin, W.; Gupta, S.K.; Arazi, T.; Spitzer-Rimon, B. MIR172d Is Required for Floral Organ Identity and Number in Tomato. Int. J. Mol. Sci. 2021, 22, 4659. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Nawaz, M.A.; Kouretas, D.; Balias, G.; Savolainen, K.; Tutelyan, V.A.; Golokhvast, K.S.; Lee, J.D.; Yang, S.H.; Chung, G. Environmental Impacts of Genetically Modified Plants: A Review. Environ. Res. 2017, 156, 818–833. [Google Scholar] [CrossRef]
- Timerbaev, V.; Pushin, A.; Dolgov, S. Production of Marker-Free Tomato Plants Expressing the Supersweet Protein Thaumatin II Gene under the Control of Predominantly Fruit-Specific Promoters. Plant Cell Tissue Organ Cult. 2019, 139, 621–634. [Google Scholar] [CrossRef]
- Ahmad, A.; Jamil, A.; Munawar, N. GMOs or Non-GMOs? The CRISPR Conundrum. Front. Plant Sci. 2023, 14, 1232938. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).