Abstract
Nitrogen (N) is one of the most crucial elements for plant growth. However, a deficiency of N affects plant growth and development. Wedelia trilobata is a notorious invasive plant species that exhibits superior tolerance to adapt to environmental stresses. Yet, research on the growth and antioxidant defensive system of invasive Wedelia under low N stress, which could contribute to understanding invasion mechanisms, is still limited. Therefore, this study aims to investigate and compare the tolerance capability of invasive and native Wedelia under low and normal N conditions. Native and invasive Wedelia species were grown in normal and low-N conditions using a hydroponic nutrient solution for 8 weeks to assess the photosynthetic parameters, antioxidant activity, and localization of reactive oxygen species (ROS). The growth and biomass of W. trilobata were significantly (p < 0.05) higher than W. chinensis under low N. The leaves of W. trilobata resulted in a significant increase in chlorophyll a, chlorophyll b, and total chlorophyll content by 40.2, 56.2, and 46%, respectively, compared with W. chinensis. W. trilobata significantly enhanced antioxidant defense systems through catalase, peroxidase, and superoxide dismutase by 18.6%, 20%, and 36.3%, respectively, providing a positive response to oxidative stress caused by low N. The PCA analysis showed that W. trilobata was 95.3% correlated with physiological traits by Dim1 (79.1%) and Dim2 (16.3%). This study provides positive feedback on W. trilobata with respect to its comprehensive invasion mechanism to improve agricultural systems via eco-friendly approaches in N deficit conditions, thereby contributing to the reclamation of barren land.
1. Introduction
Macronutrients are essential for the growth and survival of many living organisms, including plants, animals, and microorganisms. Nitrogen (N) is one of the crucial elements for plant growth and development [1]. It is a primary component of chlorophyll, proteins, and nucleic acids, and is thus involved in photosynthesis, enzyme activity, and growth [2]. Nitrogen optimization and remobilization play a vital role in promoting agricultural sustainability [3]. According to Meng et al. [4], approximately one billion hectares of agricultural land has been protected by the enhancement of nitrogen use efficiency in poor nutrient conditions. The N requirement for plants varies based on the type of plants and their growth stage; therefore, it is recommended to test the soil N level and fertilize [5]. Plants’ roots uptake N from the soil in the form of nitrate (NO3−) and ammonium ion (NH4+) [6]. The absorption of these two types of ions occurs via a membrane transporter located inside the roots, and it is then translocated to the other parts of the plants [7]. Basically, the ammonium transporter (AMT) and nitrate transporter (NRT) are responsible for adapting the N levels in plants. Under normal N conditions, plants exhibited better growth and physiological activities [8]. Excessive or low-N applications can lead to negative consequences, such as nutrient runoff, soil degradation, and altered ecosystem dynamics in agriculture [9,10].
Insufficient N levels in soil inhibit plant growth and secondary metabolites [11]. Generally, a lack of nitrogen obstructs enzymatic activities and increases reactive oxygen species (ROSs), leading to disturbed nutrient uptake activities in plants [12]. Low N reduces photosynthetic pigments, i.e., net fluorescence, stomatal conductance, chlorophyll content, and the assimilation of CO2 in plants [13]. The negative effects of low N were observed in rice, corn, pecan, and barley [8,14]. Physiological and biochemical processes are the main processes that respond to low-N environments via the roots and leaf tissues of plants [15]. The physiological exploration of plants involves the study of how plants acquire, transport, and use water, minerals, and other nutrients. It also includes the study of plant growth, development, photosynthesis, respiration, and senescence [16]. By understanding these processes, plant physiologists contribute to improving crop yields, developing disease-resistant varieties, and identifying ways to mitigate the effects of climate change on plant growth and development. Similarly, the biochemical exploration of plants focuses on the various biochemical pathways that occur within plants [17]. It involves the study of plant metabolism, which includes the synthesis and breakdown of carbohydrates, lipids, and proteins. It also includes the study of plant secondary metabolites, which are chemicals produced by plants that are not involved in primary metabolism under low-N conditions [18].
Different plant species have different responses to N deficiency, encompassing morphological, physiological, or biochemical ones [19]. Plants will undergo these changes to uptake more N in the soil to promote growth under low-N conditions [20,21]. Plant roots have the capability to adapt to different environmental stress conditions, especially under low-N conditions [22]. Besides, root morphology, leaf characteristics, and leaf area are also used as indicators against low-N conditions because chlorophyll content is directly connected with N availability [23]. Qiu et al. [24] reported that N content is directly proportional to photosynthesis, which is associated with chlorophyll content. Moso bombo is one of the woody plants that improved its morphological and physiological response to stress conditions under ammonium ion (NH4) [25]. Chen et al. [26] reported that Populus deltoides significantly promoted root growth that uptakes more N from the soil to improve nitrogen use efficiency under low-N conditions. Invasive plant species also have the capability to live in stressful conditions without any damaging effects because they have strong ecological adaptability [27]. Sitaria italica (L.) is one of the grassy plants that increased its root elongation, physiological response, and antioxidant defense system under low-N conditions [9]. Solidago canadensis enhanced its stem length, root length, and photosynthesis rate under different N conditions [28].
Wedelia trilobata (synonymous with Sphagneticola trilobata) is one of the native plant species of southwest America belonging to the family Asteraceae and was introduced to China as an ornamental base in the 20th century. However, this species has been accepted as an invasive plant in China, because it occupies more humid and subtropical areas [29]. W. trilobata is distributed throughout China, including the Guangxi, Hunan, Fujian, and Guangdong provinces [30]. W. trilobata has a well-developed root system, rapid growth, high nutrient uptake capability, and a strong antioxidant defense system in low-nutrient-availability conditions [31]. Invasive plant species were reported to have strong N adaptability to regulate the toxicity of heavy metals [32]. The responses of W. trilobata have proved to be positive in different environmental stress conditions. For example, its antioxidant activities and gene expression levels were higher compared with native Wedelia under drought stress [33]. The photosynthetic and physiological response of W. trilobata was significantly higher under low-temperature and -light conditions [30]. Previous studies observed that W. trilobata has a strong response to N enrichment and flooding conditions and better antioxidant enzyme activities under different forms of nitrogen compared with native Wedelia [34,35]. Until now, there has been no specific information on the physiological and biochemical application of W. trilobata under N deficit conditions. So, it is important to understand how W. trilobata responds to low N and how this response might be positive for agricultural systems. For this purpose, the aim of the current study was to investigate the comparison between W. trilobata and W. chinensis under N deficient condition and to evaluate its physiological, biochemical, and ecological adaptive invasion mechanism under normal and low-N conditions. This study will provide an in-depth and reliable approach for assessing invasive W. trilobata low-N adaptation, as well as the theoretical underpinnings for improving N application on various low-N-tolerant crops in barren lands. Future studies will be required to evaluate the molecular mechanism involved under low-N conditions.
2. Results
2.1. Comparison of Plants by Phenotypical and Growth Traits
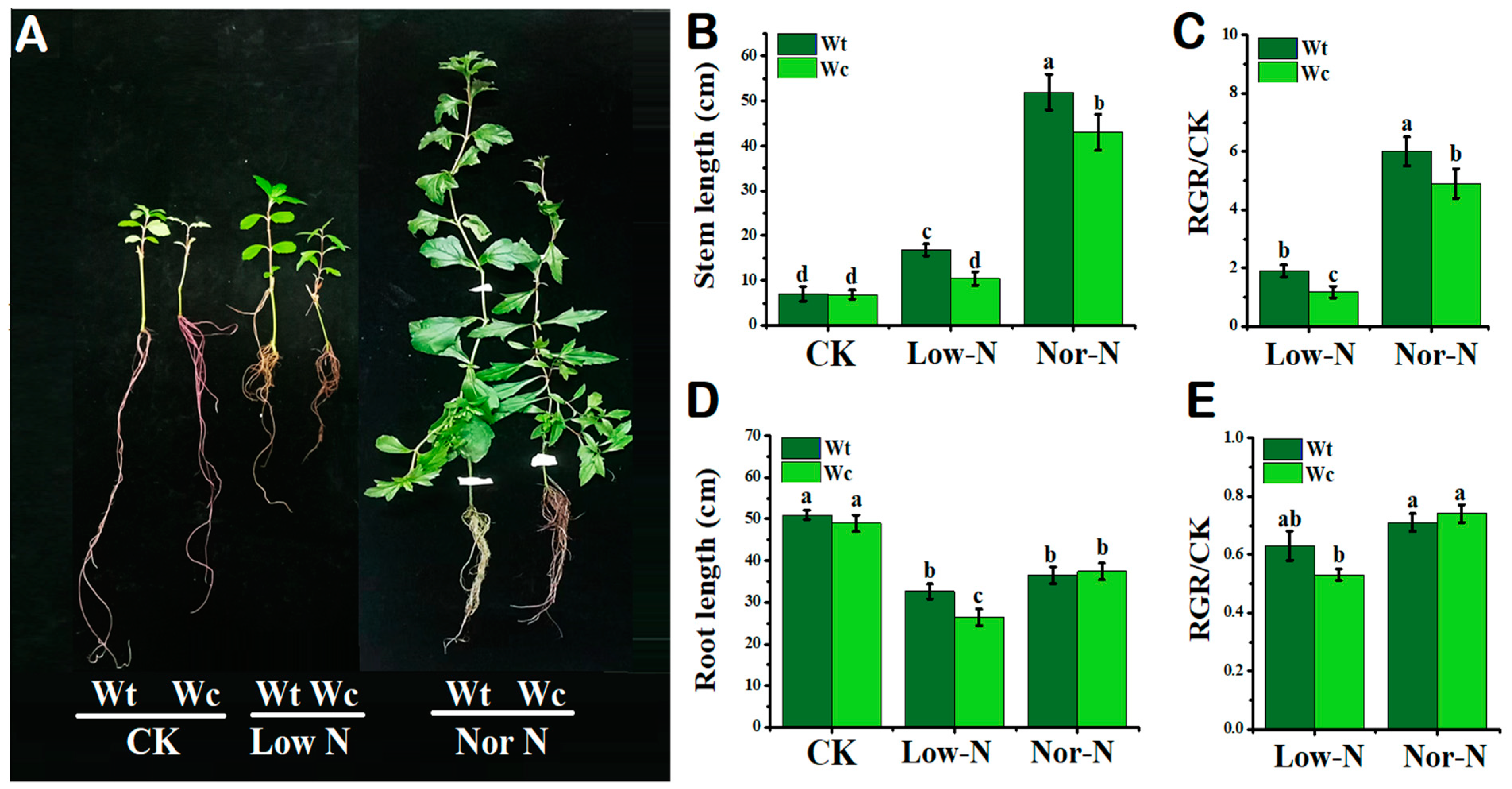
The growth phenotypes of native and invasive Wedelia were not significantly different under control conditions. Under the normal (Nor-N) and low-N (Low-N) Hoagland nutrient conditions, the growth phenotypes of W. trilobata were better compared with W. chinensis (Figure 1A). The stem length of the invasive Wedelia was significantly (p < 0.05) higher than native Wedelia by 17–38%, respectively, under Nor-N and Low-N conditions (Figure 1B). The relative growth rate (RGR) of the W. trilobata was higher than the control and W. chinensis under both levels of N conditions (Figure 1C). The root elongation was not significant from each other under the Nor-N condition; however, the root elongation of W. trilobata was higher than W. chinensis under Low-N conditions (Figure 1D). The number of roots, roots biomass, and root surface area of W. trilobata were significantly (p < 0.05) increased under Low-N conditions compare with W. chinensis (Table 1). This result suggests that W. trilobata has the capability to adjust growths in low-nutrient environments.
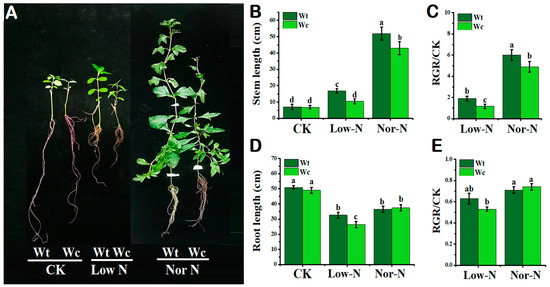
Figure 1.
Growth response of W. trilobata (Wt) and W. chinensis (Wc). Sixteen-day-old plants with or without Hoagland nutrient solution supplied with low concentration of nitrogen (Low-N) and normal concentration of nitrogen (Nor-N) for 8 weeks: (A) Phenotypes of both species. (B,C) Stem length and RGR rate. (D,E) Root length and RGR rate with control, Low-N and Nor-N, respectively. Vertical bars indicate standard deviation in 6 replicates. The different letters represent significant differences in W. trilobata and W. chinensis with ANOVA (Tukey’s test, p < 0.05) analysis.

Table 1.
Growth parameter of W. chinensis and W. trilobata under Low-N and Nor-N conditions.
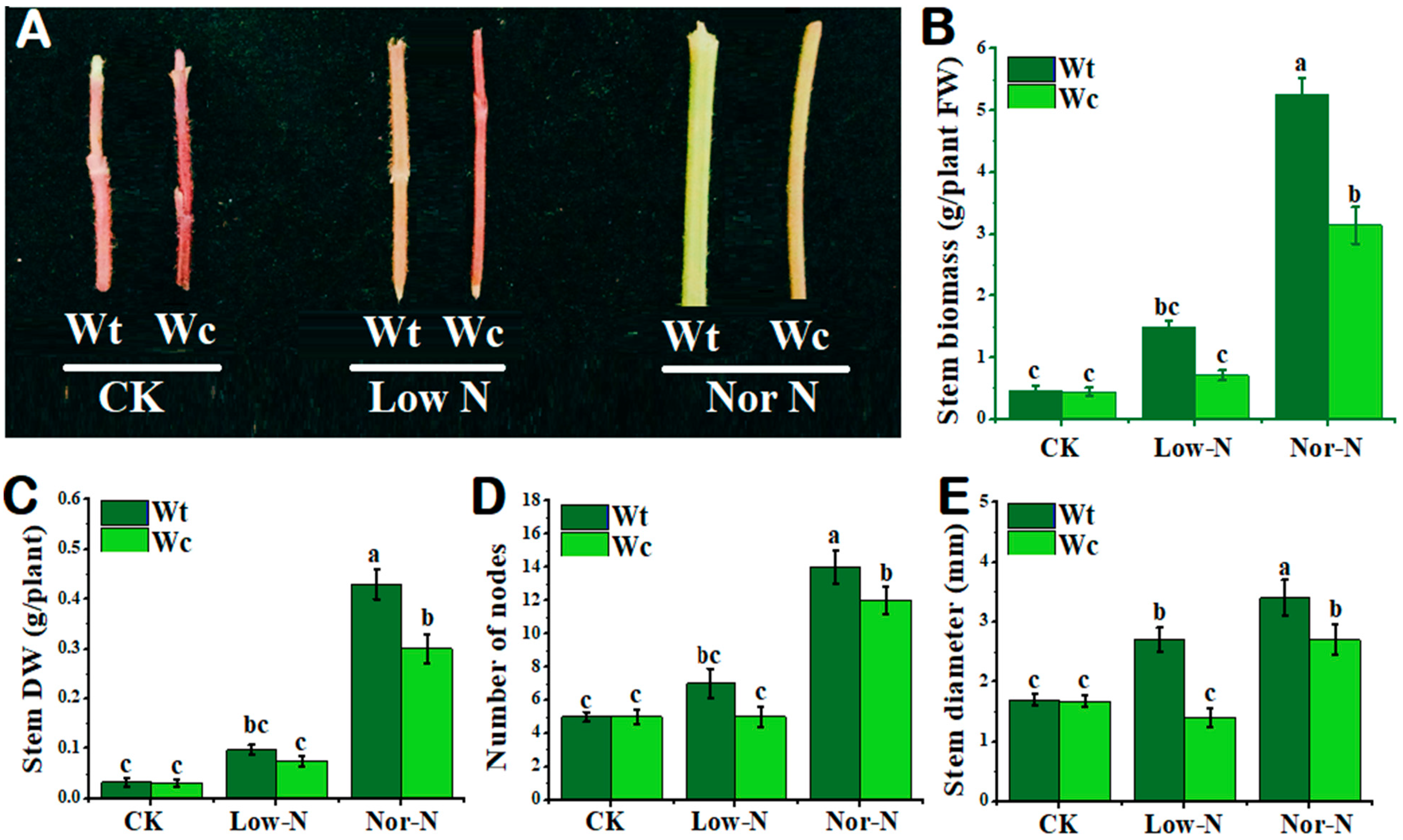
As shown in Figure 2A, the tolerance capability of W. trilobata was higher compared with W. chinensis under both levels of N conditions. The phenotypes of the invasive plant were stouter than native plants under Nor-N and Low-N conditions. The stem biomass (fresh and dry) of invasive Wedelia was significantly (p < 0.05) increased compared with native Wedelia under Nor-N conditions (Figure 2B,C). Similarly, the number of nodes in invasive Wedelia increased significantly compared with the native Wedelia, especially under Nor-N conditions (Figure 2D). The stem diameter of W. trilobata was greater by 48.4–20.5% than W. chinensis under Nor-N and Low-N conditions (Figure 2E).
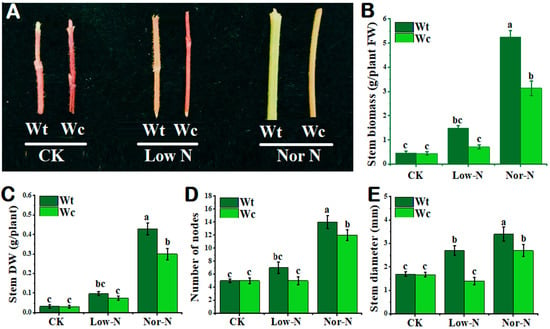
Figure 2.
Growth response of W. trilobata (Wt) and W. chinensis (Wc). Sixteen-day-old plants with or without Hoagland nutrient solution supplied with Low-N and Nor-N for 8 weeks: (A) Stem phenotypes of both species. (B) Stem biomass. (C) Stem dry weight. (D) Number of nodes and (E) stem diameter with control, Low-N, and Nor-N, respectively. Vertical bars indicate standard deviation in 6 replicates. The different letters represent significant differences in W. trilobata and W. chinensis with ANOVA (Tukey’s test, p < 0.05) analysis.
2.2. Tolerance of Leaves under Different N Conditions
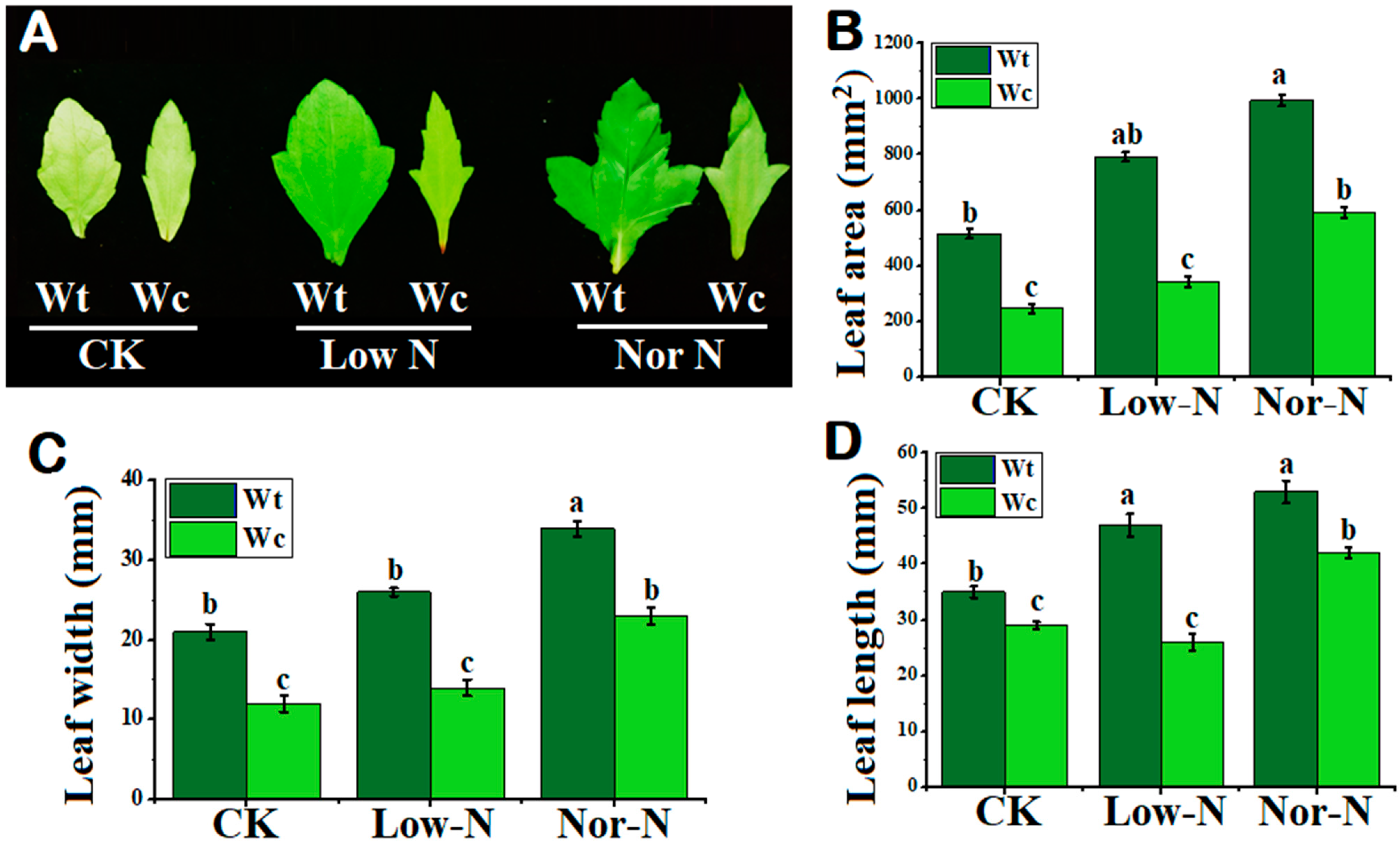
The leaves of W. trilobata were observed to be broader compared with native Wedelia under both levels of N conditions. The leaves of W. trilobata were greener compared with W. chinensis, especially under Low-N conditions (Figure 3A). The leaf area of W. trilobata was greater under normal and Low-N levels compared with native Wedelia (Figure 3B). Leaf width and leaf length were also smaller in the native species. Compared with native Wedelia, invasive Wedelia has a greater response to deficit N by increasing leaf width and length by 44.6% and 46.15%, respectively (Figure 3C,D). The number of leaves of W. trilobata was significantly enhanced under the N conditions compared with W. chinensis (Table 1). This suggested that the invasive Wedelia had a strong tolerance capability to adjust its own characteristics under Low-N conditions.
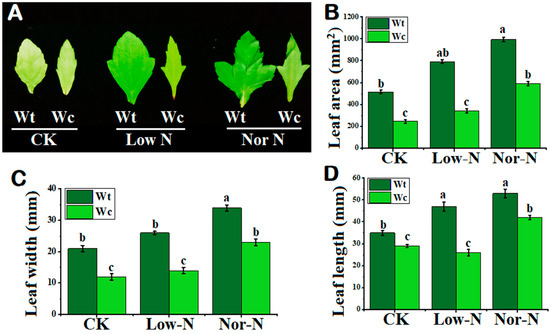
Figure 3.
Leaf morphology of (Wt) W. trilobata and (Wc) W. chinensis. Sixteen-day-old plants with or without Hoagland nutrient solution supplied with Low-N and Nor-N for 8 weeks: (A) Leaf phenotypes of both species. (B) Leaf area. (C) Leaf width. (D) Leaf length with control, Low-N, and Nor-N, respectively. Vertical bars indicate standard deviation in 6 replicates. The different letters represent significant differences in W. trilobata and W. chinensis with ANOVA (Tukey’s test, p < 0.05) analysis.
2.3. Leaf Photosynthetic Response to N Conditions
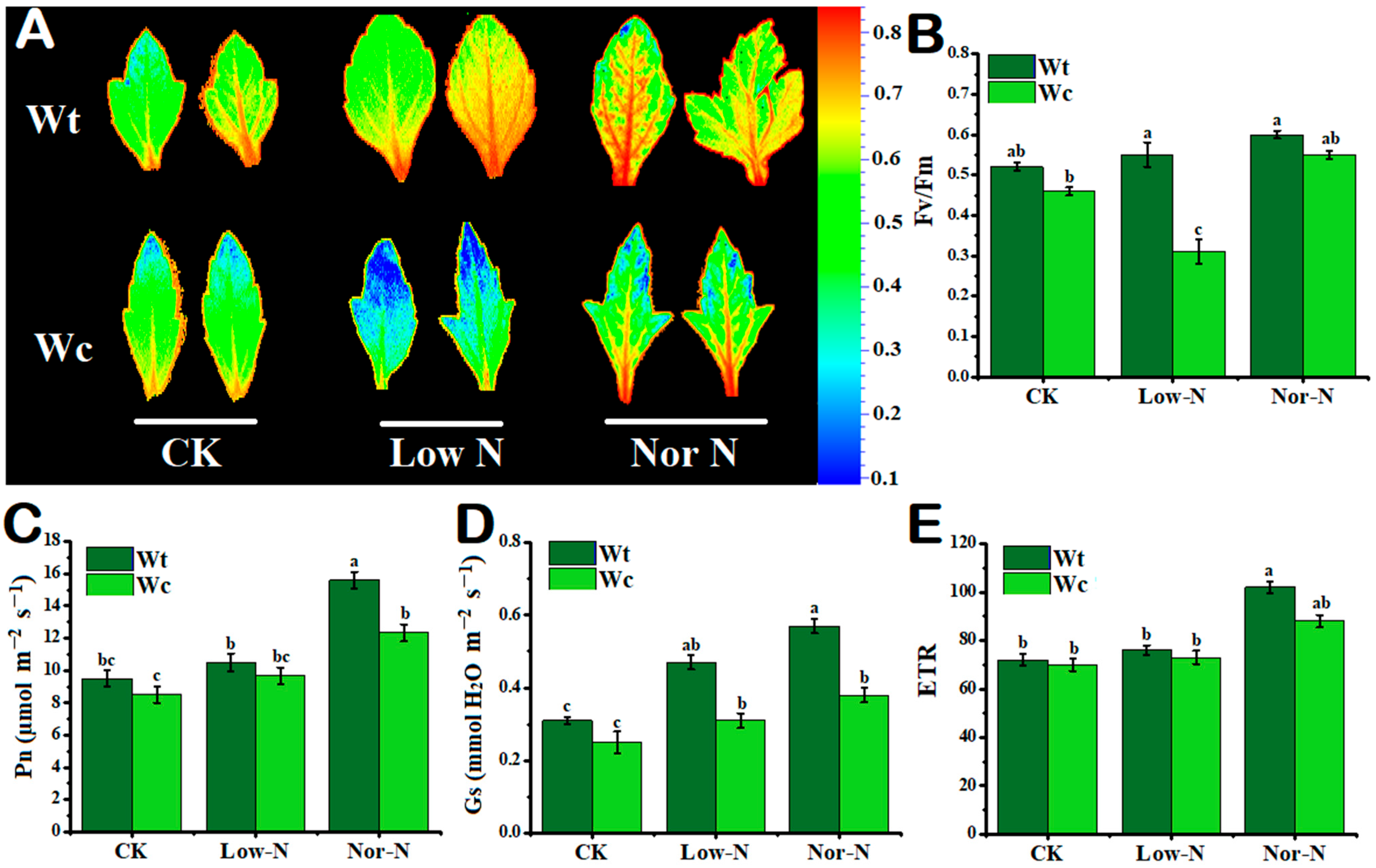
The photosynthetic traits responses of W. trilobata were observed to be stronger than native W. chinensis under Low-N conditions. The chlorophyll fluorescence image clearly indicated that W. chinensis leaves changed from green color to blue, indicating toxicity under Low-N conditions; however, the invasive Wedelia has a strong tolerance to Low-N conditions (Figure 4A). There was no obvious effect observed in Fv/Fm (net fluorescence) under control and Nor-N conditions in both plant species; whereas, under Low-N conditions, the Fv/Fm value was significantly reduced in W. chinensis by 54.4% compared with the control, but W. trilobata does not affect net fluorescence levels (Figure 4B). The net photosynthetic rate was slightly increased in W. trilobata compared with W. chinensis with Nor-N (Figure 4C). The stomatal conductance (Gs) was significantly enhanced by 33.9% under Nor-N conditions in W. trilobata compared with W. chinensis (Figure 4D). The electron transport rate (ETR) did not differ significantly between the two species under both N conditions (Figure 4E).

Figure 4.
Growth response of (Wt) W. trilobata and (Wc) W. chinensis. Sixteen-day-old plants with or without Hoagland nutrient solution supplied with Low-N and Nor-N for 8 weeks: (A) Morphology of chlorophyll fluorescence of both species. (B) Fv/Fm. (C) net photosynthetic rate. (D) Stomatal conductance and (E) electron transport rate with control, Low-N, and Nor-N, respectively. Vertical bars indicate standard deviation in 6 replicates. The different letters represent significant differences in W. trilobata and W. chinensis with ANOVA (Tukey’s test, p < 0.05) analysis.
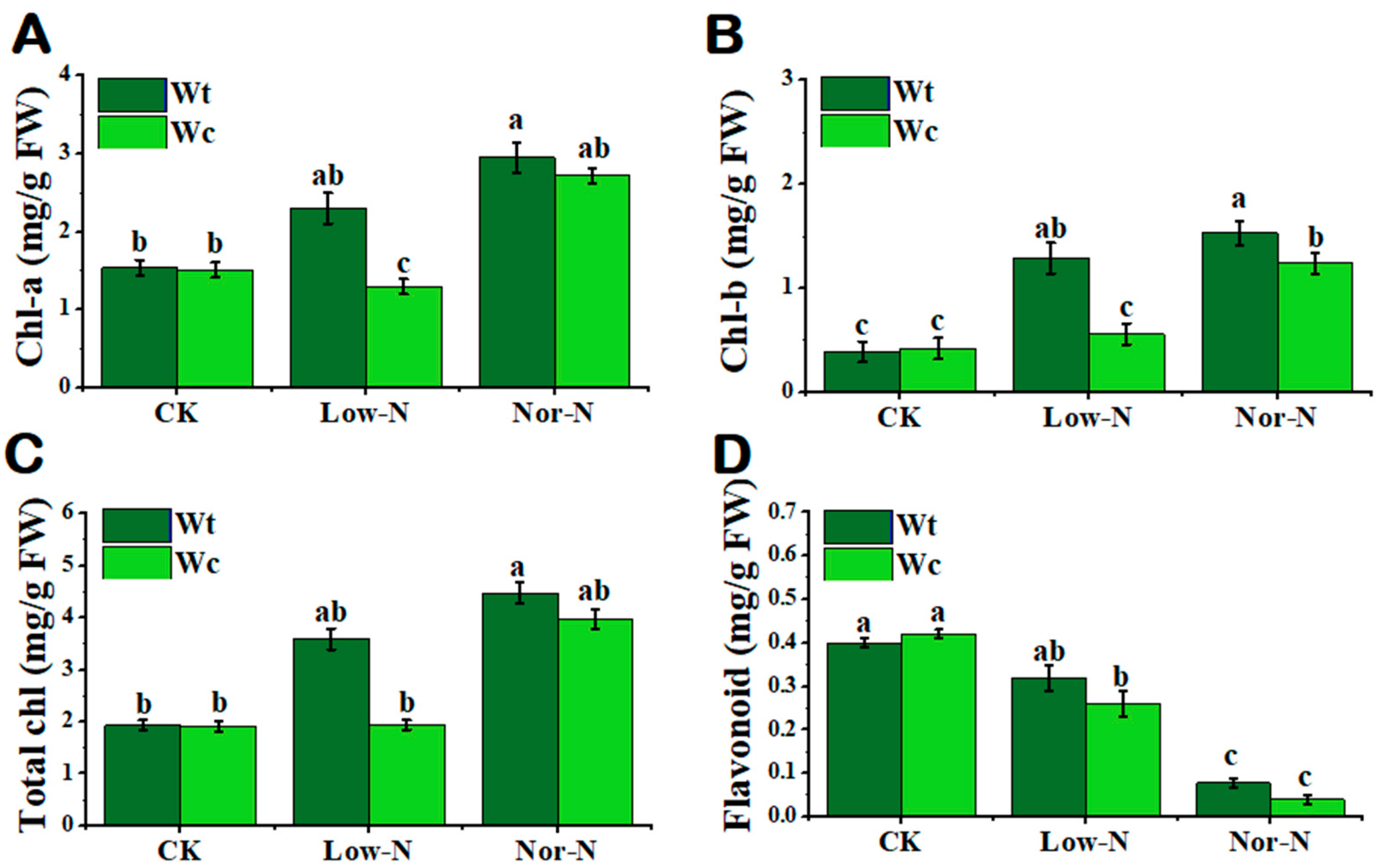
Chlorophyll is another physiological indicator that plants respond to abiotic stress. In this study, the chlorophyll content was significantly inhibited in W. chinensis under Low-N conditions compared with W. trilobata. The chlorophyll-a and b content of W. trilobata was significantly increased by 40.2 and 56.2%, respectively, compared with W. chinensis under Low-N conditions (Figure 5A–C). The nonenzymatic antioxidant flavonoid content of W. trilobata was not significantly enhanced compared with W. chinensis under Low-N conditions (Figure 5D). There was no significant variation in total nitrogen content between both Wedelia species in the leaves; however, the nitrogen content of W trilobata was higher under Low-N and Nor-N conditions in the roots (Table 1).
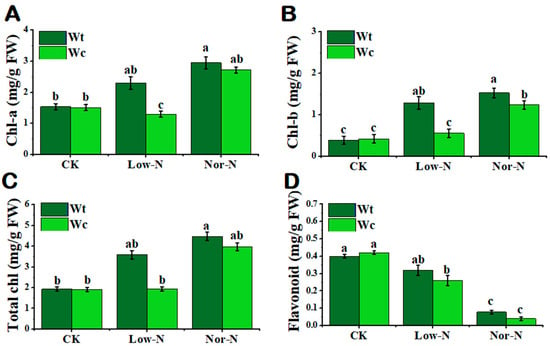
Figure 5.
Physiological response of (Wt) W. trilobata and (Wc) W. chinensis. Sixteen-day-old plants with or without Hoagland nutrient solution supplied with Low-N and Nor-N for 8 weeks: (A) chlorophyll-a. (B) chlorophyll-b and (C) total chlorophyll content of both plant species. (D) Flavonoid content with control, Low-N, and Nor-N, respectively. Vertical bars indicate standard deviation in 6 replicates. The different letters represent significant differences (p < 0.05) in W. trilobata and W. chinensis with ANOVA (Tukey’s test, p < 0.05) analysis.
2.4. Detection of Reactive Oxygen Species and Antioxidant Enzyme Activities of Plants in N Conditions
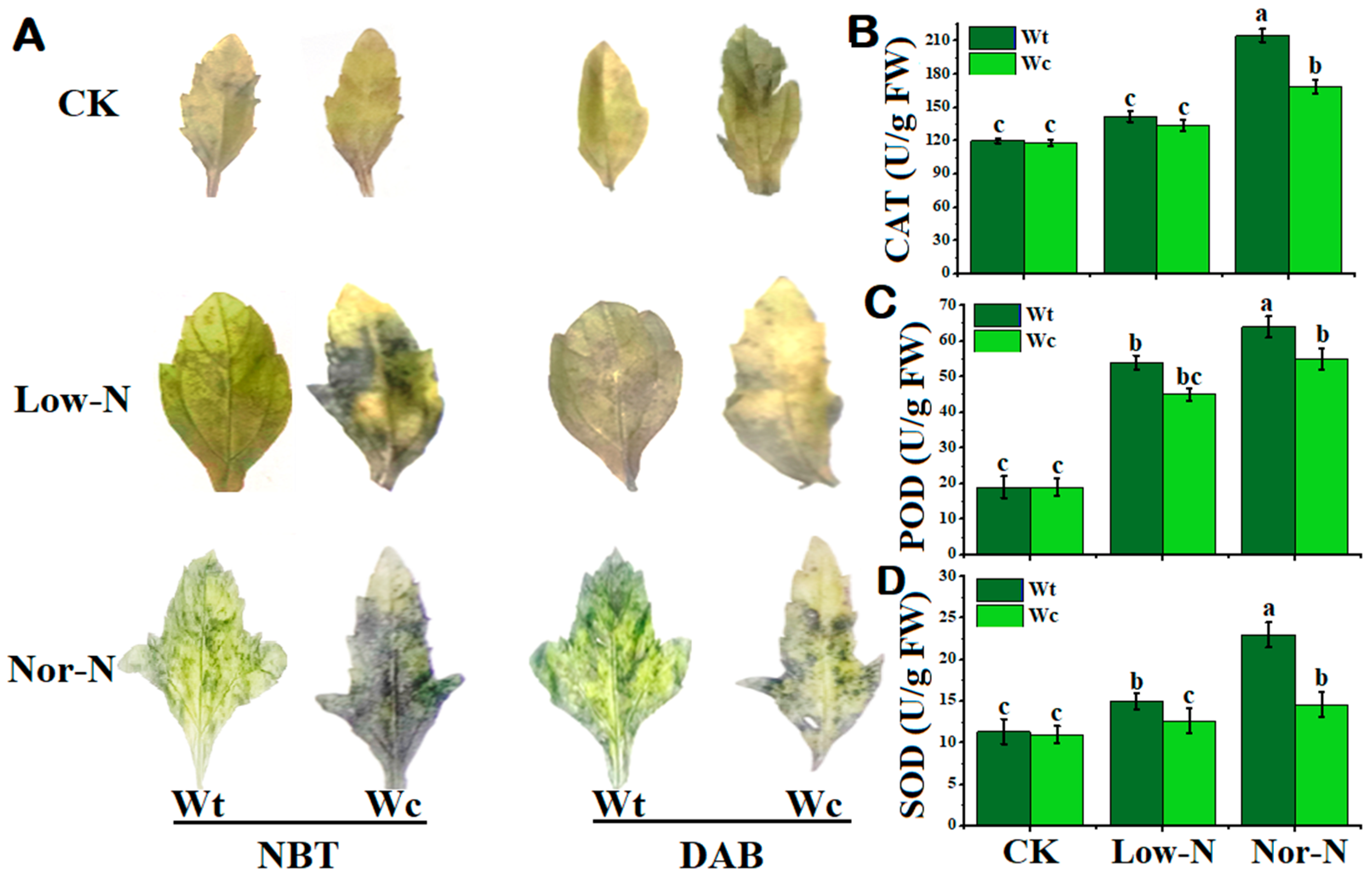
To check the toxicity of W. trilobata and W. chinensis under deficit N conditions, this study used DAB and NBT staining to check for spots in the leaves. The stained spots were observed in both Wedelia plant species under N conditions. The leaves of W. chinensis exhibited a considerable number of deep-colored indigo spots (NBT) and brown spots (DAB) under Low- and Nor-N conditions, showing that more O2− and H2O2 accumulated. W. trilobata’s stained spots were noticeably fewer than W. chinensis, indicating decreased accumulation of ROS and hydrogen peroxide (Figure 6A). Different trends of antioxidants were observed at different levels of N conditions in both plant species. The catalase (CAT) activity of both Wedelia plant species was not more significant than the control under Low-N conditions, while the CAT activity of W. trilobata was significantly increased by 66.9% compared with the control and 21.3% compared with W. chinensis under Nor-N conditions (Figure 6B). The peroxidase (POD) activity of both Wedelia species was increased compared with the control. Interestingly, the POD levels of W. trilobata were significantly higher by 16.3% under Nor-N conditions compared with W. chinensis (Figure 6C). The superoxide dismutase (SOD) trend of W. trilobata was significantly higher than the control, while the activity of W. chinensis was comparable to the control. The SOD activities of W. trilobata increased by 36.3% and 109%, respectively, compared with control under both levels of N conditions (Figure 6D). The result suggests that W. trilobata has a positive response to Low-N conditions, including scavenging ROS by antioxidant enzymes.
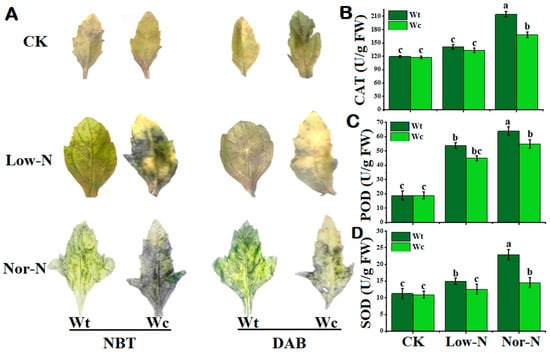
Figure 6.
The accumulation of reactive oxygen species (ROSs) in the leaves and stems of W. trilobata and W. chinensis species under low and normal nitrogen conditions: (A) The changes in superoxide anion (O2−) and changes in hydrogen peroxide (H2O2) in the leaves of the two species after NBT and DAB staining. (B) Catalase activity. (C) Peroxidase activity and (D) superoxide dismutase activity of W. trilobata and W. chinensis. Vertical bars indicate standard deviation in 6 replicates. The different letters represent significant differences (p < 0.05) in W. trilobata and W. chinensis with ANOVA (Tukey’s test, p < 0.05) analysis.
2.5. Correlation of Growth Traits and Physiological Activity
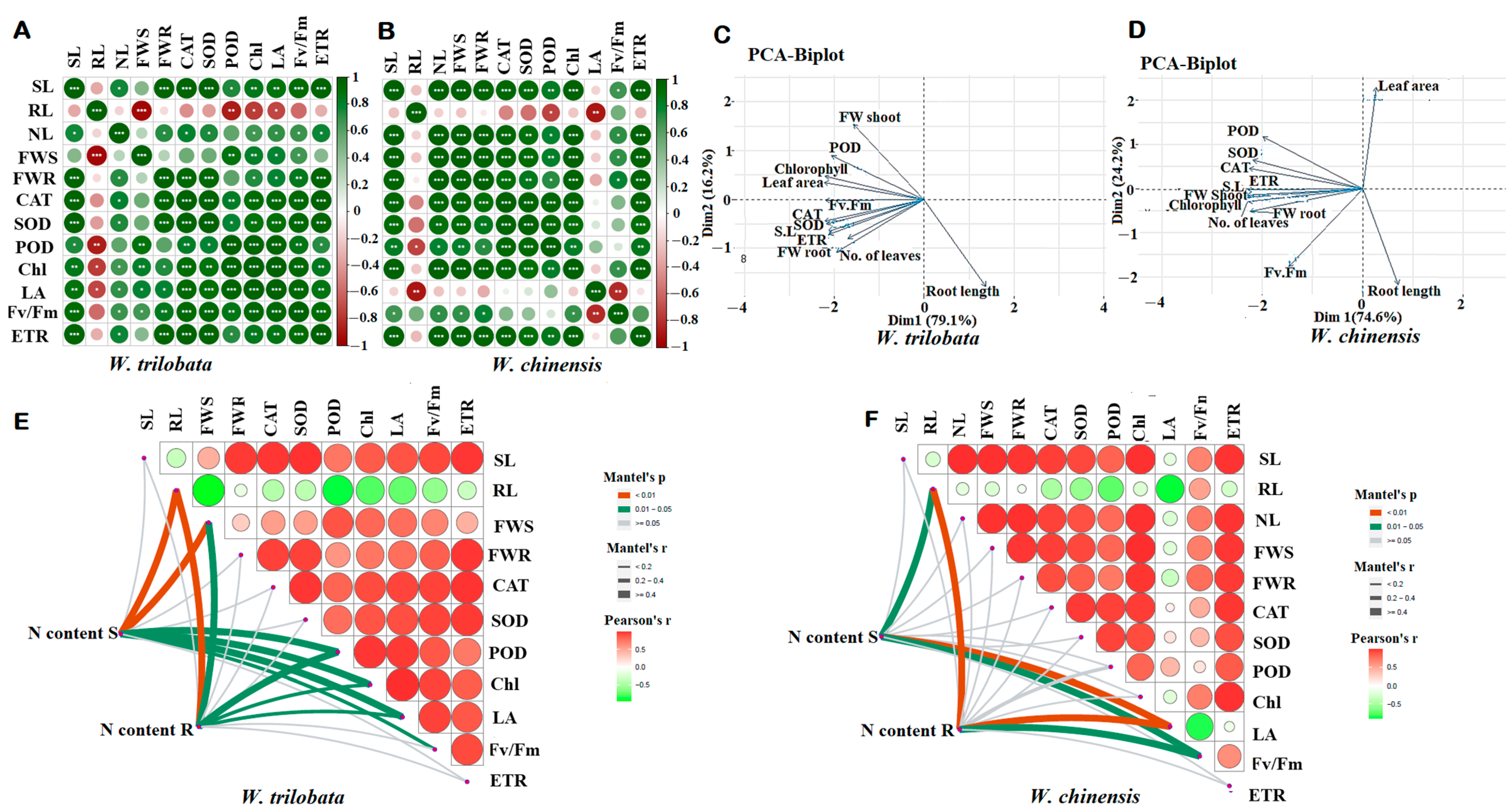
A pairwise correlation was used to identify the positive and negative interaction between the growth traits, i.e., shoot length (SL), root length (RL), number of leaves (NL), fresh weight shoot biomass (FWS), fresh weight root biomass (FWR) and physiological activities such as CAT, POD, SOD, chlorophyll (Chl), leaf area (LA), photosystem (Fv/Fm), and the electron transport rate (ETR) of W. trilobata and W. chinensis (Figure 7A,B). Shoot length, fresh biomass, and number of leaves were strongly positively correlated with physiological indicators in both plant species; however, the root lengths of both plant species were negatively correlated with other traits under different nitrogen conditions. Biplot principal component analysis indicated different variations between the growth traits and physiological traits among the W. trilobata and W. chinensis. The biplot PCA analysis showed that the W. trilobata 95.3% correlated with Dim1 (79.1%) and Dim2 (16.3%), respectively (Figure 7C). Similarly, The PCA biplot correlation of W. chinensis also showed significant a correlation between the growth and physiological traits under different N conditions. PCA biplot analysis showed that both plant species were correlated positively and negatively for different traits (Figure 7C,D). Furthermore, the Pearson correlation was verified by the Mantel test for both Wedelia species for N content with other physiological and biochemical traits. The Mantel test analysis of W. trilobata showed that the N content in shoots and roots was highly significant (p < 0.01) with plant growth traits and other physio-bio traits; however, W. chinensis showed less significant correlation of growth traits and other traits compared with W. trilobata (Figure 7E,F).
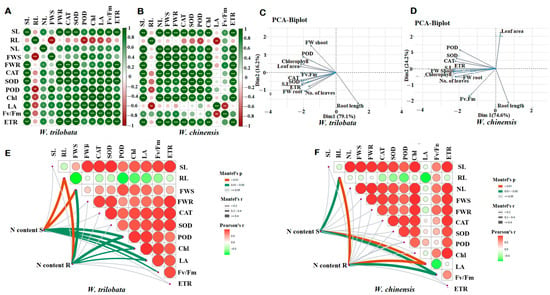
Figure 7.
Pairwise correlation was used to identify the positive and negative interaction between the growth traits, i.e., shoot length (SL), root length (RL), number of leaves (NL), fresh weight shoot biomass (FWS), fresh weight root biomass (FWR), and CAT, POD, SOD, chlorophyll (Chl), leaf area (LA), photosystem (Fv/Fm), and the electron transport rate (ETR) of W. trilobata (Wt) and W. chinensis (Wc). Different asterisks show different correlations with each trait: (A) W. trilobata. (B) W. chinensis. Single, double, and triple asterisks show significant correlation levels. Similarly, principal component and Mantel test analysis revealed that different physiological parameters correlated with different growth traits: (C,E) W. trilobata and (D,F) W. chinensis.
3. Discussion
This study showed that the invasive plant species adapted its growth to environmental stressors, with green leaves being the principal photosynthetic part that responded to stressors. Similarly, the stem is a nonphotosynthetic organ that plays an important role in the invasion process. The current study used invasive plant species (W. trilobata) and native plant species (W. chinensis) cultured in hydroponic nutrient solution to investigate responses to low-N conditions. This study investigated the comparison between native and invasive plant species under low and normal N conditions. Moreover, we also revealed the invasion mechanism of W. trilobata and its antioxidant defense system under low-N conditions. The current study helps us to truly understand the contribution of plants’ own physiological and ecological advantages to successful invasion.
3.1. The Growth Response of W. trilobata Is Better Than W. chinensis under Low-N Conditions
Generally, plant growth and development require all the essential micro- and macronutrients in normal conditions [36]. Nitrogen is one of the major macronutrients that plays an important role in plants, such as the formation of proteins, DNA preparation, chlorophyll, and plant hormones [22]. When the concentration of N is insufficient, the plant will decrease its growth, development, and other life activities [23]. A deficiency of N affects plant height, root length, leaf traits, stem width, biomass, and other growth factors [37]. Different plant species have different effects and responses to low-nutrient conditions, such as modifying root architecture, enhancing N assimilation, leaf photosynthetic responses, lignin, chloroplast, and carbohydrate metallization [38,39,40]. Similarly, native and invasive plant species are also affected under low-N environment conditions [27]. This study observed a positive response in W. trilobata to low-N conditions, namely via root elongation, an increase in the number of roots, leaf morphology, and increased leaf numbers and stem width. However, the root length growth of native Wedelia was inhibited, as well as stem length and leaf structure, under low-N conditions. This result suggests that the invasive Wedelia has a positive response to low N because it absorbs and stores more nutrients and organic compounds through roots to adjust its life activities, including changing the resource allocation strategies under poor nutrient conditions to cope with adverse habitats. In N deficit conditions, this essential nutrient is transported to the upper part of the plant via the xylem transporter, which provides a normal situation [41]. Foxtail millet developed specific root lengths and diameters to adapt to the growth under low-N conditions that are similar to the findings of the current study [9]. It has already been proved that invasive plant species have great responses and stronger competitive capabilities under low-nutrient environments by improving their regulatory mechanisms, as well as better growth adjustment compared with native plant species [40,42,43].
The leaf is the main plant organ that is directly linked to nitrogen content and provides positive responses to low-nutrient environments [44,45]. The leaf morphology of W. trilobata was larger and thinner to increase the specific leaf area that enhanced its tolerance under low-N conditions. In contrast, in native Wedelia, the size of its leaf is thicker and softer due to decreased leaf-specific area under low-N conditions compared with invasive plants and the control. Likewise, the leaf width, leaf area, leaf length, and number of leaves increased compared with native W. chinensis under both levels of N conditions because t has more absorption and transport activities of N from the soils and adapted quickly to the environments [46]. Cai et al. [47] revealed that the invasive W. trilobata leaves are denser, larger, and wider compared with native S. trilobata due to the capability of the accumulated essential hormones (auxin and cytokinin) to increase the specific leaf area under poor nutrient conditions. The leaves of W. trilobata were much improved compared with native W. chinensis after the amendments of different forms of N such as nitrate ion and ammonium ion (NO3−, NH4−), both alone and in mixed forms [27]. This study concluded that the invasive Wedelia has a strong capability to adjust its life characteristics under different stressful conditions, such as low-N conditions, through invasion mechanisms compared with native plant species.
3.2. Physiological Response of W. trilobata to Low-N Conditions
Photosynthesis and chlorophyll are the main physiological indicators of plant responses to abiotic stress [36]. Invasive plant species have strong photosynthetic capacity compared with native plant species. For example, the invasive Wedelia had greater net photosynthetic pigment, stomatal conductance, transpiration rate, and gas exchange capacity compared with native Wedelia under different environmental conditions [30]. According to the current study, the photosynthetic trait responses of W. trilobata were observed to be stronger than native W. chinensis under low-N conditions. The chlorophyll fluorescence image clearly indicated that W. chinensis leaves change from green color to blue, showing toxicity under low-N conditions; however, the invasive Wedelia has a strong tolerance to low-N conditions because the leaf size and area are wide, storing more essential nutrients by chlorophyll. Similarly, the Fv/Fm value was greater than W. chinensis under low-N conditions. Similar results were also reported by Ke et al. [35]. W. trilobata have stronger net fluorescence Fv/Fm than W. chinensis under low-light conditions, demonstrating that low-light conditions accelerate the photosystem II reaction that converts light energy to heat energy. Net photochemical efficiency (Fv/Fm) is commonly used for the detection of fluorescence traits, with the normal range being from 0.7 to 0.8 and significantly decreased under stressful conditions [48]. The present study also observed that W. trilobata increased its stomatal conductance and net transpiration rate under the Nor-N conditions (Figure 4). Consistent with the current study, Cai et al. [47] indicated that S. trilobata decreases its trend of Fv/Fm and stomatal conductance under low-temperature conditions compared with native plant species. This result suggests that when contrasted with extant native plants, W. trilobata exhibits greater invasion, allelopathy, and phenotypic adaptability attributes that allow it to quickly adapt and colonize under low-N conditions.
Nitrogen content directly influences the net photosynthesis of plants through chlorophyll formation, which regulates a particular photosynthetic enzyme in plant tissues, as well as chloroplast quantity, size, and composition [49]. Under low-N conditions, the plant’s chloroplast size, quantity, and composition decreased due to the decreased expression levels of photosynthetic genes [39]. This study also observed a decreasing trend in chlorophyll a, b, and total chlorophyll content under low-N conditions compared with the normal provision of N content, which may be due to the destruction of the chloroplast and photosynthetic organ. However, the chlorophyll a and b content of invasive Wedelia was significantly enhanced compared with native Wedelia, which means W. trilobata has strong photosynthetic pigments and chloroplast activities under N deficit conditions. Similar findings have been reported under low-temperature conditions [30,47]. The second reason that might cause W. trilobata’s response to low-N conditions is the decreased synthesis of specific organic compounds, such as amino acids, nucleic acids, proteins, and other essential compound that are required for good photosynthetic properties [50]. Switch grass (low-land-type) enhanced the chlorophyll content under low-N conditions because it has a strong capability to survive in poor nutrient conditions, which is consistent with the current study [51]. Overall, the results suggest that the invasive W. trilobata has a strong tolerance compared with native W. chinensis under different environmental stressors.
3.3. Alleviation of ROS by Defensive Antioxidant Enzyme Activities
Abiotic stressors lead to the generation of numerous reactive oxygen species (ROSs). Among these, O2•− plays a pivotal role in plants, serving as the primary catalyst for the formation of other ROS [52]. Few reports indicated that the scavenging systems may be lost by the production of high amounts of ROS, and antioxidant enzyme activity can efficiently maintain balanced levels of ROS in plants [53,54,55]. The present study clearly indicated the accumulation of ROS with the help of DAB and NBT staining by observing the different spots in the leaves of W. trilobata and W. chinensis under low-N conditions. The current observation indicated that the invasive Wedelia had fewer spots than native Wedelia, which means W. trilobata activated antioxidant activities to maintain its levels of ROS under low-N conditions. Reactive oxygen molecules (ROMs), such as H2O2 and OH, accumulate in plants under stressful conditions and are harmful to crops because they interfere with their regular biological processes [31]. The H2O2 and O2•− species activate various antioxidant enzyme defense systems in plants [39].
In order to absorb radicals and minimize ROS-mediated injury in plants, the concentrations of CAT, POD, and SOD are elevated. This study also checked the tolerance capability of invasive and native Wedelia to activate the antioxidant defensive systems under low-nutrient conditions. This study showed that the invasive Wedelia has a strong antioxidant system, including CAT, POD, and SOD, in poor nutrient conditions compared with native Wedelia. The CAT, POD, and SOD levels of W. trilobata were significantly higher compared with the control and native plant species. SOD is a catalyst for decreasing the cytotoxic conversion of O2•− to H2O2, which may be crucial for plant cells to increase their antioxidant defense systems for tolerance [56]. When the antioxidant enzyme defensive system was activated, the production of H2O2 and ROS was catalyzed to help W. trilobata species resist environmental stress better than native plant species [47]. Farid et al. [57] reported that increasing the concentration of CAT and POD occurred when the plants were struggling to maintain their normal growth under environmental stress conditions. Similar to the current study, Cai et al. [30] reported that S. trilobata increased its flavonoid content, phenol content, and antioxidant defense systems in the stem/leaf compared with native plant species. Electrolyte leakage and membrane damage occur due to ROS accumulation, either directly or indirectly, to initiate membrane lipid peroxidation in plants [42]. Huang et al. [27] also reported W. trilobata to have activated strong antioxidant defense systems under the provision of excess nitrogen compared with W. chinensis to adapt itself by various chemical reactions. Plants produce a vast number of antioxidants, including antioxidant enzymes and nonenzymatic compounds, to eliminate too many ROS from their cells, for example, under drought stress conditions. The concentration of flavonoids and total phenols, as well as the activity of antioxidant enzymes (SOD, CAT, and POD), significantly increased in the leaves of the native and invasive plant species [22,42,58,59].
In summary, both plant species (W. trilobata and W. chinensis) were correlated positively and negatively with each other under low and normal N conditions in terms of shoot length, root length, biomass, and physiological parameters. The PCA biplot and Pearson correlation essay indicated that W. trilobata has strong physiological parameters to adjust its normal growth under low-N conditions compared with W. chinensis, which agrees with the idea of Iqbal et al. [60], who described the Pearson correlation and PCA analysis under different N conditions with different physiological parameters in different crops.
4. Materials and Methods
For this study, the invasive W. trilobata and indigenous W. chinensis plant species were obtained from Guangxi Province, Nanning City (22°38′ N and 108°13′ E) and the greenhouse of the Institute of Environment and Safety Engineering (32°12′2″ N, 119°30′50″ E) at Jiangsu University, respectively. The ramets of these two plant species were cut in two nodes and grown in the growth chamber till to two leaves were opened. For roots and germination, the stem segments were placed into an open, transparent glass jar in a chamber with an ambient light level of 95 mol m−2 s−1, a light cycle of 14/10 h, and an ambient temperature of 28 °C. After 16 d, the seedlings were transplanted in different experimental 500 mL plastic pots.
4.1. Experimental Design
Sixteen days later, nitrogen-free Hoagland nutrient solution was provided to each pot and a normal concentration of nitrogen (Nor-N) 91.05 mg/L and a low concentration of nitrogen (Low-N) 0.9105 mg/L were added in the form of eques calcium nitrate tetrahydrate (Ca(NO3)2·4(H2O)). The detailed research design included control CK (only water), low N (0.9105 mg/L with Hoagland nutrient), and normal N (Nor-N, 91.05 with Hoagland nutrient). The plants were grown in the treatments for 8 weeks with six replicates. Totally, 3 treatments × 2 species × 6 replicates equaled 36 pots, respectively (Table 2). Hoagland nutrient solution was renewed three times a week, and the pH levels were maintained at 5.8. The plastic pots’ openings were sealed with a hygienic air-filtering sheet.

Table 2.
The table indicates different concentrations of N used in the present study.
4.2. Phenotypes and Growth Measurement
We used a DSLR digital camera (D7000 Camera Price-030I, Jiaxing factory, Jiaxing, city, Zhejiang, China.) for the phenotypical analysis of W. trilobata and W. chinensis at the end of the experiment. All parameters of each replicate of both plant species were taken and measured. We measured both plant species’ stem length, root height (cm), number of nodes, number of leaves, and number of branches. Roots, stems, and leaves were separated by seizer, and the fresh biomass was recorded in an experimental notebook [61]. Some fresh samples of both plant species were stored in liquid nitrogen at −80 °C for further analysis, and the remaining samples were kept in an oven for drying at 80 °C. WinRHIZO root analyzer system was used to analyze the root length, root volume, and number of root forks of W. trilobata and W. chinensis [38].
4.3. Analysis of Leaf Morphology
At the end of the experiment, new leaves of both plant species were cut in each treatment with 6 replicates. The leaf area (mm2) was estimated using a portable hand leaf area meter (Yaxin-1241, Shanghai, China). We used leaf image area software (ImageJ software, Version10.12.6), and leaf width was determined by Vernier caliper. After that, all leaf samples were placed in an oven for drying for 2–3 d to obtain biomass [46].
4.4. Analysis of Photosynthetic Parameters
Photosynthetic pigmentation was determined using a Fluor-Pen handheld chlorophyll fluorescence meter (Li Cor-USA Biosciences, Lincoln, NE, USA). This study used chlorophyll fluorescence image systems (CF Imager, Technological Ltd., Colchester, UK) to check Fv/Fm values in dark and light conditions. For 20 min, the leaves were left in the dark. The dark-adapted leaves’ minimum fluorescence (Fo) and maximum fluorescence (Fm) were measured. The minimum fluorescence (F0) after 15 m in a light environment and maximum fluorescence (Fm) were measured after dark with the formula Fv/Fm = (Fm − Fo/Fm) in control and treatments of the W. trilobata and W. chinensis [34]. A CIRAS-3 portable photosynthesis system (PP Systems, Amesbury, MA, USA) was used to measure the net photosynthetic rate (Pn), transpiration rate (ETR), and stomatal conductance (gs) of W. trilobata and W. chinensis [62]. Nitrogen content was analyzed with the protocol of Khan et al. [62].
For the analysis of chlorophyll content, fresh leaves of both W. trilobata and W. chinensis were crushed into small pieces, kept in 20 mL tubes, and stored in a dark place with the addition of 85% acetone to remove all the green color. The chlorophyll-a, chlorophyll-b, and total chlorophyll (mg/g−1 FW) content was checked with a spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan) at 645 and 663 nm after 2 to 3 days according to Khan et al. [36].
Chlorophyll content (mg/g FW): Chl-a = 12.71 (OD663) − 2.59 (OD645); Chl-b = 22.88 (OD645) − 4.67 (OD663); Total Chl = Chl-a + Chl-b.
4.5. Measurement of Nonenzymatic Flavonoid Content
Flavonoid concentration was assessed using a colorimetric method on frozen plant tissues that were earlier preserved at −80 °C [47]. With the addition of 0.3 mL of 5% sodium nitrite, an aliquot of 1 mL of the extract was diluted with 4 mL of distilled water. Then, 10% AlCl3 was added to the solution after 5 min. After 6 min, 2 mL of 1 M NaOH was added, and 10 mL of distilled water was added to make the sample volume. An EON microplate spectrophotometer (Bio Tek, Vermont, VT, USA) was used to measure the absorbance at 510 nm after the solution was thoroughly mixed.
4.6. Localization of ROS with NBT and DAB Solution
The full leaves of W. trilobata and W. chinensis with control and treatment were immersed in diaminobenzidine (DAB) solution containing 0.5 mg/mL−1 with pH 8.0. Phosphate buffer was used as the solvent for 15 min before being covered with gauze and kept in the dark for 7 h. The accumulation of ROS could be seen as brown dots after DAB staining [35]. Similarly, both plant species were immersed in a solution using 0.1% nitro-blue tetrazolium (NBT) and 10 mM sodium azide using pH 6.4 phosphate buffer as the solvent. After being vacuum-covered for 20 min, the gauze was left in complete darkness for 2–3 h. The greenery in the leaves was removed by boiling 90% ethanol solution. Blue specks were observed after staining.
4.7. Analysis of Antioxidant Enzyme Activity
The antioxidant enzyme activity was assessed following the protocol outlined by Khattak et al. [63] with slight modification. Three (3) mg fresh leaf samples of W. trilobata and W. chinensis were collected and homogenized thoroughly in ice and crushed in liquid nitrogen. After crushing, a 4 mL phosphate buffer (PBS) (0.05 M Na2HPO4 + 0.05 M NaH2PO4) with pH 6 to 7 was added. The obtained samples were centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatants were placed in ice for analysis of antioxidant enzyme activity.
The 3 mL enzymatic reaction mixture contains 1.9 mL of PBS (50 mM, pH 7), 0.1 mL of enzyme extract, and 1 mL of hydrogen peroxide (0.3%), and 0.2% of guaiacol with PBS was used as blank control. The peroxidase (POD) activity was determined by visible spectrophotometer at 470 nm [64]. The enzymatic reaction mixture for catalyzing (CAT) activity consists of 1 mL of 0.3% H2O2, 1.9 mL of H2O, and 0.1 mL of enzyme extract solution, with distilled water used as blank control. The CAT activity was determined by spectrophotometrically at 240 nm, and the enzyme was recorded every 30 s. At least 6 points were measured. The CAT was measured with the following formula, CAT = (△OD240 × V)/(0.01 × VS × m), where m is the fresh biomass of the sample, vs. is the enzyme extract, and V is the total volume of the samples. The SOD and CAT values are expressed in U/g FW.
4.8. Statistical Analysis of the Study
All value parameters of W. trilobata and W. chinensis were put in Microsoft Excel software 2010. This study exemplifies experimental design, with six replicates for each treatment and species, allowing for a comprehensive representation of standard error. ANOVA, along with the Tukey t-test (p < 0.05), was conducted to derive meaningful statistical analysis from the data. The different letters indicate significant differences in all physicochemical and biochemical analyses. Correlation arrays were made by the heatmap to examine relationships between physicochemical and plant growth parameters. For the purpose of calculating PCA bilateral correlation factors between the variables, we used the “Performance Analytics” package, which uses the Pearson method. The Mantel test analysis was analyzed between the two matrices. The larger the correlation coefficient of the Mantel test, the smaller the p-value of both Wedelia species by online correlation tool (https://www.cloudtutu.com/#/index, accessed on 2 December 2023).
5. Conclusions
In conclusion, this study clearly indicated that the invasive Wedelia trilobata has a strong response to low-N conditions by improving plant height, stem length, leaf morphology, and root length. The physiological parameters, such as net photosynthesis, stomatal conductance, chlorophyll content, and flavonoid content, were significantly increased, showing a tolerance capability against low-N conditions. Under low-N conditions, the W. trilobata activated its self-enzymatic defensive mechanism to adjust its growth characteristic by catalase, peroxidase, and superoxide dismutase to resist the poor nutrient conditions. The NBT and DAB staining clearly show that W. trilobata has a strong response to low-N conditions compared with native W. chinensis. From the result of this study, it is concluded that W. trilobata could be a valuable option for ecosystem restoration and land management efforts. Additionally, it has the potential to contribute to environmentally friendly approaches, promoting ecological balance and reducing the negative impacts of low nitrogen. Further study will be required to explore the full potential of W. trilobata in environmental management and interactions with other plants, as well as its role in ecosystem restoration.
Author Contributions
Conceptualization, Z.-C.D.; methodology, F.-L.K.; formal analysis, Y.-F.L.; data curation, Y.-F.Z.; writing—original draft preparation and supervision, I.U.K.; review and editing, F.G.; validation, H.J.; visualization, N.U.; supervision, S.-S.Q. and I.U.K.; project administration, D.-L.D.; funding acquisition, R.U. and E.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
Data Availability Statement
The data presented in this study are available on request from the corresponding author (e-mail: irfanullahkhan195@yahoo.com). The data are not publicly available due to some part of ongoing research to keep it confidential until further publications or patents are completed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Asma Bano, S. Biological Nitrogen Fixation to Improve Plant Growth and Productivity Vaccine Designing View Project Antibiotic Producing Bacteria View Project. Int. J. Agric. Innov. Res. 2016, 4, 597–599. [Google Scholar]
- Bhuvaneswari, G.; Sivaranjani, R.; Reetha, S.; Ramakrishnan, K. Application of Nitrogen Fertilizer on Plant Density, Growth, Yield and Fruit of Bell Peppers (Capsicum annuum L.). Int. Lett. Nat. Sci. 2014, 13, 81–90. [Google Scholar] [CrossRef]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen Nutrition of Fruit Trees to Reconcile Productivity and Environmental Concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yue, S.; Hou, P.; Cui, Z.; Chen, X. Improving Yield and Nitrogen Use Efficiency Simultaneously for Maize and Wheat in China: A Review. Pedosphere 2016, 26, 137–147. [Google Scholar] [CrossRef]
- Yotsova, E.; Dobrikova, A.; Stefanov, M.; Misheva, S.; Bardáčová, M.; Matušíková, I.; Žideková, L.; Blehová, A.; Apostolova, E. Effects of Cadmium on Two Wheat Cultivars Depending on Different Nitrogen Supply. Plant Physiol. Biochem. 2020, 155, 789–799. [Google Scholar] [CrossRef]
- Xuan, W.; Beeckman, T.; Xu, G. Plant Nitrogen Nutrition: Sensing and Signaling. Curr. Opin. Plant Biol. 2017, 39, 57–65. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Dai, S.; Meng, L.; He, M.; Chen, S.; Zhao, C.; Dan, X.; Cai, Z.; Zhang, J.; et al. Effects of Solidago Canadensis L. on Mineralization-Immobilization Turnover Enhance Its Nitrogen Competitiveness and Invasiveness. Sci. Total Environ. 2023, 882, 163641. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.; Gao, J.; Zhang, W.; Yi, J.; Zhen, X.; Bi, C.; He, D.; Liu, S.; Zhao, X. Adaptation Mechanism of Roots to Low and High Nitrogen Revealed by Proteomic Analysis. Rice 2021, 14, 5. [Google Scholar] [CrossRef]
- Nadeem, F.; Mahmood, R.; Sabir, M.; Khan, W.u.D.; Haider, M.S.; Wang, R.; Zhong, Y.; Ishfaq, M.; Li, X. Foxtail Millet [Setaria italica (L.) Beauv.] over-Accumulates Ammonium under Low Nitrogen Supply. Plant Physiol. Biochem. 2022, 185, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jia, X.; Huo, L.; Che, R.; Gong, X.; Wang, P.; Ma, F. MdATG18a Overexpression Improves Tolerance to Nitrogen Deficiency and Regulates Anthocyanin Accumulation through Increased Autophagy in Transgenic Apple. Plant Cell Environ. 2018, 41, 469–480. [Google Scholar] [CrossRef]
- Sousa, H.C.; Sousa, G.G.d.; Lessa, C.I.N.; Lima, A.F.d.S.; Ribeiro, R.M.R.; Rodrigues, F.H.d.C. Growth and Gas Exchange of Corn under Salt Stress and Nitrogen Doses TT—Crescimento e Trocas Gasosas Do Milho Sob Estresse Salino e Doses de Nitrogênio. Rev. Bras. Eng. Agrícola Ambient. 2021, 25, 174–181. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.; Ou, Y.; Zheng, X.; Feng, Q.; Zhang, H.; Fei, Y.; Luo, J.; Resco de Dios, V.; Yao, Y. Pretreating Poplar Cuttings with Low Nitrogen Ameliorates Salt Stress Responses by Increasing Stored Carbohydrates and Priming Stress Signaling Pathways. Ecotoxicol. Environ. Saf. 2021, 225, 112801. [Google Scholar] [CrossRef]
- Ullah, I.; Mao, H.; Rasool, G.; Gao, H.; Javed, Q.; Sarwar, A.; Khan, M.I. Effect of Deficit Irrigation and Reduced n Fertilization on Plant Growth, Root Morphology and Water Use Efficiency of Tomato Grown in Soilless Culture. Agronomy 2021, 11, 228. [Google Scholar] [CrossRef]
- Cruz-Alvarez, O.; Hernández-Rodríguez, O.A.; Jacobo-Cuellar, J.L.; Ávila-Quezada, G.; Morales-Maldonado, E.; Parra-Quezada, R.Á.; Robles-Hernandez, L.; Ojeda-Barrios, D.L. Nitrogen fertilization in pecan and its effect on leaf nutrient concentration, yield and nut quality. Rev. Chapingo Ser. Hort. 2020, 26, 163–173. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and Biochemical Responses of Soybean Plants Inoculated with Arbuscular Mycorrhizal Fungi and Bradyrhizobium under Drought Stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P. Symbiotic Nitrogen Fixation and Phosphorus Acquisition. Plant Nutrition in a World of Declining Renewable Resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of Phosphorus and Nitrogen in the Rhizosphere and Plant Growth Promotion by Microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Kabbadj, A.; Makoudi, B.; Mouradi, M.; Pauly, N.; Frendo, P.; Ghoulam, C. Physiological and Biochemical Responses Involved in Water Deficit Tolerance of Nitrogen-Fixing Vicia Faba. PLoS ONE 2017, 12, e0190284. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, Y.; Ma, J.Y.; Wang, H.; Wang, K.L.; Wang, T.; Jiang, S.Y.; Jiao, J.B.; Sun, Y.K.; Jiang, X.L.; et al. Nitrogen Deposition Effects on Invasive and Native Plant Competition: Implications for Future Invasions. Ecotoxicol. Environ. Saf. 2023, 259, 115029. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J.; Masclaux-Daubresse, C.; Wang, N.; Wang, H.; Zheng, B. Morphological and Physiological Responses to Contrasting Nitrogen Regimes in Populus Cathayana Is Linked to Resources Allocation and Carbon/Nitrogen Partition. Environ. Exp. Bot. 2019, 162, 247–255. [Google Scholar] [CrossRef]
- Guo, Q.; Song, H.; Kang, J.; Korpelainen, H.; Li, C. Different Responses in Leaf-Level Physiology to Competition and Facilitation under Different Soil Types and N Fertilization. Environ. Exp. Bot. 2018, 150, 69–78. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Shi, D.; Li, Y.; Zhang, J.; Liu, P.; Zhao, B.; Dong, S. The Mechanisms of Low Nitrogen Induced Weakened Photosynthesis in Summer Maize (Zea mays L.) under Field Conditions. Plant Physiol. Biochem. 2016, 105, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qin, S.; Tang, J.; Chen, G.; Xie, J.; Chen, L.; Han, S.; Wang, X.; Zhu, T.; Liu, Y.; et al. Exogenous Nitrogen Enhances Poplar Resistance to Leaf Herbivory and Pathogen Infection after Exposure to Soil Cadmium Stress. Ecotoxicol. Environ. Saf. 2021, 208, 111688. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Xie, X.Y.; Qiu, J.; Fang, W.H.; Liang, R.; Ren, X.; Ji, X.; Cui, G.; Asiri, A.M.; Cui, G.; et al. High-Performance Artificial Nitrogen Fixation at Ambient Conditions Using a Metal-Free Electrocatalyst. Nat. Commun. 2018, 9, 3485. [Google Scholar] [CrossRef] [PubMed]
- Zou, N.; Huang, L.; Chen, H.; Huang, X.; Song, Q.; Yang, Q.; Wang, T. Nitrogen Form Plays an Important Role in the Growth of Moso Bamboo (Phyllostachys edulis) Seedlings. PeerJ 2020, 8, e9938. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chu, Y.; Huang, Q.; Ding, C.; Zhang, W.; Li, B.; Zhang, J.; Su, X. Morphological and Physiological Plasticity Response to Low Nitrogen Stress in Black Cottonwood (Populus deltoides Marsh.). J. For. Res. 2022, 33, 51–62. [Google Scholar] [CrossRef]
- Huang, P.; Shen, F.; Abbas, A.; Wang, H.; Du, Y.; Du, D.; Hussain, S.; Javed, T.; Alamri, S. Effects of Different Nitrogen Forms and Competitive Treatments on the Growth and Antioxidant System of Wedelia Trilobata and Wedelia Chinensis Under High Nitrogen Concentrations. Front. Plant Sci. 2022, 13, 851099. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, J.X.; Zhou, X.H.; Peng, P.H.; Li, J.J.; Zhang, S.M.; He, W.M. An Invasive Population of Solidago Canadensis Is Less Sensitive to Warming and Nitrogen-Addition than Its Native Population in an Invaded Range. Biol. Invasions 2019, 21, 151–162. [Google Scholar] [CrossRef]
- Qi, S.S.; Dai, Z.C.; Miao, S.L.; Zhai, D.L.; Si, C.C.; Huang, P.; Wang, R.P.; Du, D.L. Light Limitation and Litter of an Invasive Clonal Plant, Wedelia Trilobata, Inhibit Its Seedling Recruitment. Ann. Bot. 2014, 114, 425–433. [Google Scholar] [CrossRef]
- Cai, M.L.; Zhang, Q.L.; Zhang, J.J.; Ding, W.Q.; Huang, H.Y. Comparative Physiological and Transcriptomic Analyses of Photosynthesis in Sphagneticola calendulacea (L.) Pruski and Sphagneticola trilobata (L.) Pruski. Sci. Rep. 2020, 10, 17810. [Google Scholar] [CrossRef]
- Sun, J.; Rutherford, S.; Saif Ullah, M.; Ullah, I.; Javed, Q.; Rasool, G.; Ajmal, M.; Azeem, A.; Nazir, M.J.; Du, D. Plant-Soil Feedback during Biological Invasions: Effect of Litter Decomposition from an Invasive Plant (Sphagneticola trilobata) on Its Native Congener (S. calendulacea). J. Plant Ecol. 2022, 15, 610–624. [Google Scholar] [CrossRef]
- Zhang, L.D.; Song, L.Y.; Dai, M.J.; Guo, Z.J.; Wei, M.Y.; Li, J.; Xu, C.Q.; Zhu, X.Y.; Zheng, H.L. Cadmium Promotes the Absorption of Ammonium in Hyperaccumulator Solanum nigrum L. Mediated by Ammonium Transporters and Aquaporins. Chemosphere 2022, 307, 136031. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Ke, W.; Cai, M.; Chen, G.; Peng, C. Responses of Sphagneticola trilobata, Sphagneticola calendulacea and Their Hybrid to Drought Stress. Int. J. Mol. Sci. 2021, 22, 1288. [Google Scholar] [CrossRef]
- Azeem, A.; Wenxuan, M.; Changyan, T.; Javed, Q.; Abbas, A. Competition and plant trait plasticity of invasive (Wedelia trilobata) and native species (Wedelia chinensis, WC) under nitrogen enrichment and flooding condition. Water 2021, 13, 3472. [Google Scholar] [CrossRef]
- Ke, W.Q.; Pan, Y.R.; Chen, L.H.; Huang, J.D.; Zhang, J.J.; Long, X.Y.; Cai, M.L.; Peng, C.L. Adaptive Photosynthetic Strategies of the Invasive Plant Sphagneticola Trilobata and Its Hybrid to a Low-Light Environment. Photosynthetica 2022, 60, 549–561. [Google Scholar] [CrossRef]
- Khan, I.U.; Rono, J.K.; Liu, X.S.; Feng, S.J.; Li, H.; Chen, X.; Yang, Z.M. Functional Characterization of a New Metallochaperone for Reducing Cadmium Concentration in Rice Crop. J. Clean. Prod. 2020, 272, 123152. [Google Scholar] [CrossRef]
- Lin, Z.H.; Chen, C.S.; Zhao, S.Q.; Liu, Y.; Zhong, Q.S.; Ruan, Q.C.; Chen, Z.H.; You, X.M.; Shan, R.Y.; Li, X.L.; et al. Molecular and Physiological Mechanisms of Tea (Camellia sinensis (L.) O. Kuntze) Leaf and Root in Response to Nitrogen Deficiency. BMC Genom. 2023, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, D.; Shi, X.; Luo, J.; Ren, G.; Dai, Z.; Qi, S.; Du, D. Different Responses of Invasive Weed Alternanthera Philoxeroides and Oryza Sativa to Plant Growth Regulators. Life 2022, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, X.; Wang, H.; Dang, K.; Deng, X.; Feng, B. Low-Nitrogen Tolerance Comprehensive Evaluation and Physiological Response to Nitrogen Stress in Broomcorn Millet (Panicum miliaceum L.) Seedling. Plant Physiol. Biochem. 2020, 151, 233–242. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Wang, P.; Chen, F.; Yuan, L.; Mi, G. Low Nitrogen Induces Root Elongation via Auxin-Induced Acid Growth and Auxin-Regulated Target of Rapamycin (TOR) Pathway in Maize. J. Plant Physiol. 2020, 254, 153281. [Google Scholar] [CrossRef]
- Luo, J.S.; Zhang, Z. Proteomic Changes in the Xylem Sap of Brassica Napus under Cadmium Stress and Functional Validation. BMC Plant Biol. 2019, 19, 280. [Google Scholar] [CrossRef]
- Gul, F.; Khan, I.U.; Rutherford, S.; Dai, Z.C.; Li, G.; Du, D.L. Plant growth promoting rhizobacteria and biochar production from Parthenium hysterophorus enhance seed germination and productivity in barley under drought stress. Front. Plant Sci. 2023, 14, 1175097. [Google Scholar] [CrossRef]
- Khan, I.U.; Qi, S.-S.; Gul, F.; Manan, S.; Rono, J.K.; Naz, M.; Shi, X.-N.; Zhang, H.; Dai, Z.-C.; Du, D.-L. A Green Approach Used for Heavy Metals ‘Phytoremediation’ Via Invasive Plant Species to Mitigate Environmental Pollution: A Review. Plants 2023, 12, 725. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xiong, Y.; Wang, Y.; Li, Q. Combination Effects of Heavy Metal and Inter-Specific Competition on the Invasiveness of Alternanthera Philoxeroides. Environ. Exp. Bot. 2021, 189, 104532. [Google Scholar] [CrossRef]
- Tahir, M.; Ahmad, I.; Shahid, M.; Shah, G.M.; Farooq, A.B.U.; Akram, M.; Tabassum, S.A.; Naeem, M.A.; Khalid, U.; Ahmad, S.; et al. Regulation of Antioxidant Production, Ion Uptake and Productivity in Potato (Solanum tuberosum L.) Plant Inoculated with Growth Promoting Salt Tolerant Bacillus Strains. Ecotoxicol. Environ. Saf. 2019, 178, 33–42. [Google Scholar] [CrossRef]
- Javed, Q.; Sun, J.; Rutherford, S.; Li, J.; Iqbal, B.; Xiang, Y.; Ren, G.; He, F.; Pan, L.; Bo, Y.; et al. Soil Pollution and the Invasion of Congener Sphagneticola in Crop Lands. J. Environ. Manag. 2023, 340, 118013. [Google Scholar] [CrossRef]
- Cai, M.L.; Ding, W.Q.; Zhai, J.J.; Zheng, X.T.; Yu, Z.C.; Zhang, Q.L.; Lin, X.H.; Chow, W.S.; Peng, C.L. Photosynthetic Compensation of Non-Leaf Organ Stems of the Invasive Species Sphagneticola trilobata (L.) Pruski at Low Temperature. Photosynth. Res. 2021, 149, 121–134. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen Supply Influences Photosynthesis Establishment along the Sugarcane Leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; ISBN 9780123849052. [Google Scholar]
- Zhu, Y.; Fan, X.; Hou, X.; Wu, J.; Wang, T. Effect of Different Levels of Nitrogen Deficiency on Switchgrass Seedling Growth. Crop J. 2014, 2, 223–234. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant Growth under Water/Salt Stress: ROS Production; Antioxidants and Significance of Added Potassium under Such Conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Modulation of Phytoremediation and Plant Growth by the Treatment with PGPR, Ag Nanoparticle and Untreated Municipal Wastewater. Int. J. Phytoremediation 2016, 18, 1258–1269. [Google Scholar] [CrossRef]
- Fu, W.; Huang, K.; Cai, H.H.; Li, J.; Zhai, D.L.; Dai, Z.C.; Du, D.L. Exploring the Potential of Naturalized Plants for Phytoremediation of Heavy Metal Contamination. Int. J. Environ. Res. 2017, 11, 515–521. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of Antioxidant Defense System Is Associated with Combined Drought and Heat Stress Tolerance in Citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Sajjad, A.; Asam, Z.U.Z.; Zubair, M.; Rizwan, M.; Abbas, M.; Farid, S.; Ali, S.; Alharby, H.F.; Alzahrani, Y.M.; et al. Phytoremediation of Contaminated Industrial Wastewater by Duckweed (Lemna minor L.): Growth and Physiological Response under Acetic Acid Application. Chemosphere 2022, 304, 135262. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Wang, J.G.; Li, M.; Zhang, S.; Gao, Y.; Fan, M.; Han, C.; Xiang, F.; Li, G.; Wang, Y.; et al. HBI Transcription Factor-Mediated ROS Homeostasis Regulates Nitrate Signal Transduction. Plant Cell 2021, 33, 3004–3021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.D.; Liu, X.; Wei, M.Y.; Guo, Z.J.; Zhao, Z.Z.; Gao, C.H.; Li, J.; Xu, J.X.; Shen, Z.J.; Zheng, H.L. Ammonium Has Stronger Cd Detoxification Ability than Nitrate by Reducing Cd Influx and Increasing Cd Fixation in Solanum nigrum L. J. Hazard. Mater. 2022, 425, 127947. [Google Scholar] [CrossRef]
- Iqbal, B.; Khan, I.; Javed, Q.; Alabbosh, K.; Inamullah, I.; Zhou, Z.; Rehman, A. The High Phosphorus Incorporation Promotes the Soil Enzymatic Activity, Nutritional Status, and Biomass of the Crop. Polish J. Environ. Stud. 2023, 32, 2125–2139. [Google Scholar] [CrossRef]
- Qi, S.; Wang, J.; Wan, L.; Dai, Z.; da Silva Matos, D.M.; Du, D.; Egan, S.; Bonser, S.P.; Thomas, T.; Moles, A.T. Arbuscular Mycorrhizal Fungi Contribute to Phosphorous Uptake and Allocation Strategies of Solidago Canadensis in a Phosphorous-Deficient Environment. Front. Plant Sci. 2022, 13, 831654. [Google Scholar] [CrossRef]
- Khan, I.U.; Zhang, Y.F.; Shi, X.N.; Qi, S.S.; Zhang, H.Y.; Du, D.L.; Gul, F.; Wang, J.H.; Naz, M.; Shah, S.W.A.; et al. Dose dependent effect of nitrogen on the phyto extractability of Cd in metal contaminated soil using Wedelia trilobata. Ecotoxicol. Environ. Saf. 2023, 264, 115419. [Google Scholar] [CrossRef] [PubMed]
- Khattak, W.A.; He, J.; Abdalmegeed, D.; Hu, W.; Wang, Y.; Zhou, Z. Foliar Melatonin Stimulates Cotton Boll Distribution Characteristics by Modifying Leaf Sugar Metabolism and Antioxidant Activities during Drought Conditions. Physiol. Plant. 2022, 174, e13526. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Chen, J.; Zhang, S.; Xu, J.; Han, X.; Feng, Y.; Chen, Y.; Zhang, X.; Dong, G.; et al. Xylem Development, Cadmium Bioconcentration, and Antioxidant Defense in Populus × Euramericana Stems under Combined Conditions of Nitrogen and Cadmium. Environ. Exp. Bot. 2019, 164, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).