Phenolic Fractions from Walnut Milk Residue: Antioxidant Activity and Cytotoxic Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content
2.2. Antioxidant Activity
2.3. Identification and Quantification of Phenolic Compounds from WMR
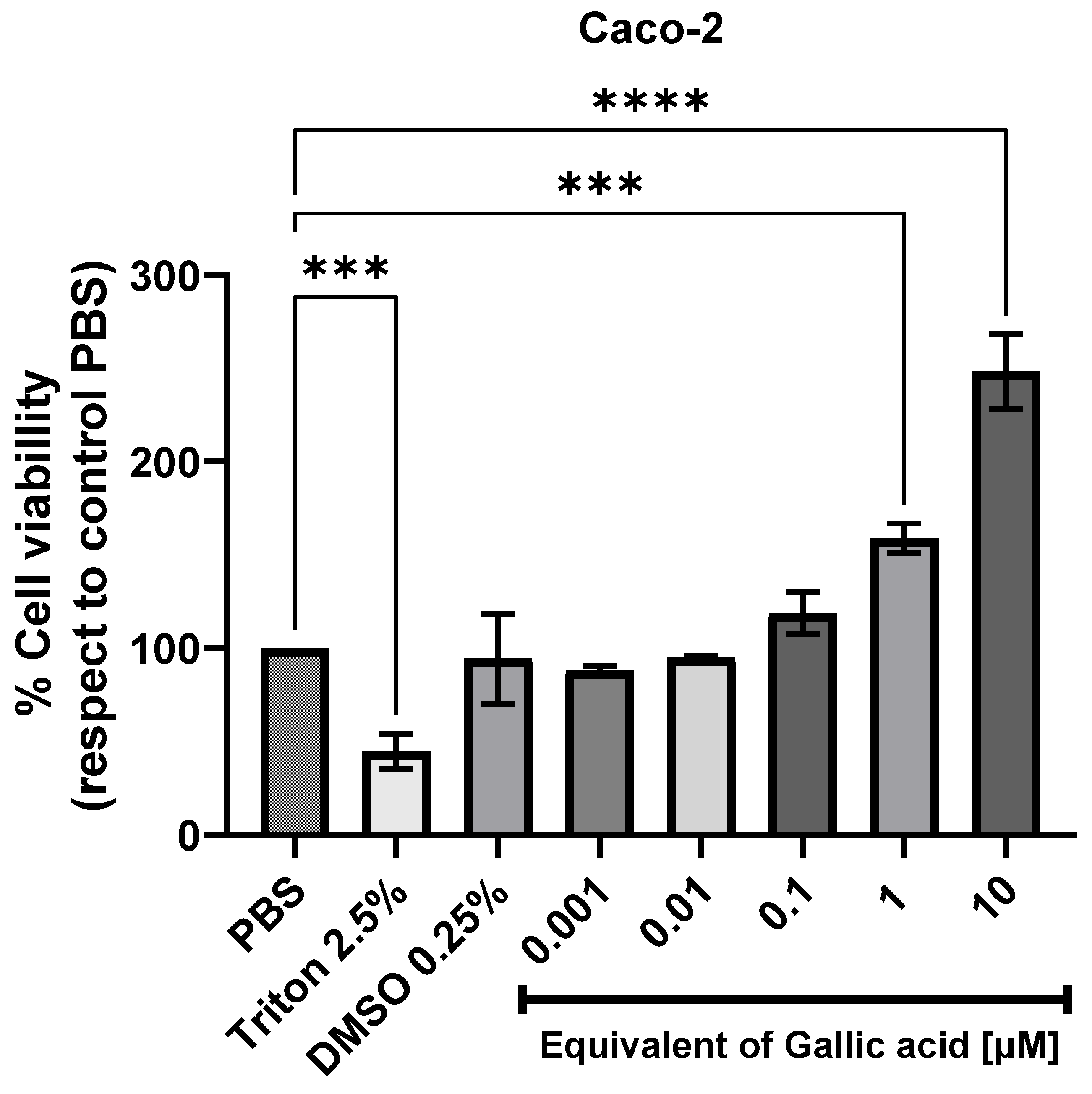
2.4. Cytotoxicity Against the Human Intestinal Cell Line (Caco-2)
3. Materials and Methods
3.1. Raw Material
3.2. Solvents, Chemical Reagents, and Standards
3.3. Walnut Processing Residues Preparation
3.4. Phenolic Fractions Extraction
3.4.1. Soluble Phenolic Compounds Fractions Extraction
3.4.2. Insoluble-Bound Phenolic Hydrolysates
3.5. Total Phenolic Compounds (TPCs)
3.6. Ferric Reducing Antioxidant Power (FRAP)
3.7. Oxygen Radical Absorbance Capacity (ORAC)
3.8. UPLC-ESI-MS/MS Analysis from WMR
3.9. Cell Culture
3.10. 3-[4,5-Dimethylthiazol-2-yl]-2,5-Diphenyltetrazolium Bromide (MTT) Cell Viability Assay
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.P.; Perez-Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, A.; Ficai, D.; Craciun, L.; Ficai, A.; Andronescu, E. Evaluation and exploitation of bioactive compounds of walnut, Juglans regia. Curr. Pharm. Des. 2019, 25, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhong, L.; Yang, H.; Zhu, F.; Hou, X.; Wu, C.; Zhang, R.; Cheng, Y. Comparative analysis of antioxidant activities between dried and fresh walnut kernels by metabolomic approaches. LWT 2022, 155, 112875. [Google Scholar] [CrossRef]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; De Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic profiles and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef]
- Alasalvar, C.; Huang, G.; Bolling, B.W.; Jantip, P.A.; Pegg, R.B.; Wong, X.K.; Chang, S.K.; Pelvan, E.; de Camargo, A.C.; Mandalari, G.; et al. Upcycling commercial nut byproducts for food, nutraceutical, and pharmaceutical applications: A comprehensive review. Food Chem. 2024, 467, 142222. [Google Scholar] [CrossRef]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hernández-Alonso, P.; Drouin-Chartier, J.-P.; Ruiz-Canela, M.; Razquin, C.; Toledo, E.; Li, J.; Dennis, C.; Wittenbecher, C.; Corella, D.; et al. Walnut consumption, plasma metabolomics, and risk of type 2 diabetes and cardiovascular disease. J. Nutr. 2021, 151, 303–311. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. Walnuts decrease risk of cardiovascular disease: A summary of efficacy and biologic mechanisms. J. Nutr. 2014, 144, 547S–554S. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Zhang, Y.-G.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2022, 62, 5113–5129. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-d’Arce, M.A.B.; Shahidi, F. Phenolic profile of peanut by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. J. Am. Oil Chem. Soc. 2017, 94, 959–971. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Kan, H.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Comparison of phenolic compounds extracted from diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020, 321, 126672. [Google Scholar] [CrossRef]
- Kafkas, E.; Attar, S.H.; Gundesli, M.A.; Ozcan, A.; Ergun, M. Phenolic and fatty acid profile, and protein content of different walnut cultivars and genotypes (Juglans regia L.) grown in the USA. Int. J. Fruit Sci. 2020, 20, S1711–S1720. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Arranz, S.; Pérez-Jiménez, J.; Saura-Calixto, F. Antioxidant capacity of walnut (Juglans regia L.): Contribution of oil and defatted matter. Eur. Food Res. Technol. 2008, 227, 425–431. [Google Scholar] [CrossRef]
- Trandafir, I.; Cosmulescu, S.; Botu, M.; Nour, V. Antioxidant activity, and phenolic and mineral contents of the walnut kernel (Juglans regia L.) as a function of the pellicle color. Fruits 2016, 71, 177–184. [Google Scholar] [CrossRef]
- Salem, M.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Fernie, A.R.; Alseekh, S.; Ezzat, S.M. Potential valorization of edible nuts by-products: Exploring the immune-modulatory and antioxidants effects of selected nut shells extracts in relation to their metabolic profiles. Antioxidants 2022, 11, 462. [Google Scholar] [CrossRef]
- Soto-Maldonado, C.; Caballero-Valdés, E.; Santis-Bernal, J.; Jara-Quezada, J.; Fuentes-Viveros, L.; Zúñiga-Hansen, M.E. Potential of solid wastes from the walnut industry: Extraction conditions to evaluate the antioxidant and bioherbicidal activities. Electron. J. Biotechnol. 2022, 58, 25–36. [Google Scholar] [CrossRef]
- Facts and Factors. Research Plant-Based Beverages Market Size, Growth, Global Trends, Forecast to 2030. Available online: https://www.fnfresearch.com/sample/plant-based-beverages-market (accessed on 12 March 2024).
- Statista. Alternativas a la Leche de Vaca: Consumo en América Latina por País. Available online: https://es.statista.com/estadisticas/1421435/consumo-de-alternativas-a-la-leche-en-america-latina-por-pais/ (accessed on 12 March 2024).
- Garcia-Mendoza, M.D.P.; Espinosa-Pardo, F.A.; Savoire, R.; Etchegoyen, C.; Harscoat-Schiavo, C.; Subra-Paternault, P. Recovery and antioxidant activity of phenolic compounds extracted from walnut press-cake using various methods and conditions. Ind. Crops Prod. 2021, 167, 113546. [Google Scholar] [CrossRef]
- Feng, J.-Y.; Wang, R.; Thakur, K.; Ni, Z.-J.; Zhu, Y.-Y.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. Evolution of okara from waste to value added food ingredient: An account of its bio-valorization for improved nutritional and functional effects. Trends Food Sci. Technol. 2021, 116, 669–680. [Google Scholar] [CrossRef]
- Tiurikova, I.S.; Peresichnyi, M.I.; Matsuk, Y.A.; Kainash, A.P.; Budnik, N.V. Technology of dietary supplements from walnuts. J. Chem. Technol. 2020, 28, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Boncler, M.; Golanski, J.; Lukasiak, M.; Redzynia, M.; Dastych, J.; Watala, C. A new approach for the assessment of the toxicity of polyphenol-rich compounds with the use of high content screening analysis. PLoS ONE 2017, 12, e0180022. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–111. ISBN 978-3-319-15791-7. [Google Scholar]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Labuckas, D.O.; Maestri, D.M.; Perelló, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Fuentealba, C.; Hernández, I.; Saa, S.; Toledo, L.; Burdiles, P.; Chirinos, R.; Campos, D.; Brown, P.; Pedreschi, R. Colour and in vitro quality attributes of walnuts from different growing conditions correlate with key precursors of primary and secondary metabolism. Food Chem. 2017, 232, 664–672. [Google Scholar] [CrossRef]
- Vu, D.C.; Vo, P.H.; Coggeshall, M.V.; Lin, C.-H. Identification and characterization of phenolic compounds in black walnut kernels. J. Agric. Food Chem. 2018, 66, 4503–4511. [Google Scholar] [CrossRef]
- Wei, F.; Li, Y.; Sun, D.; Chen, Q.; Fu, M.; Zhao, H.; Chen, X.; Huang, Y.; Xu, H. Odor, tastes, nutritional compounds and antioxidant activity of fresh-eating walnut during ripening. Sci. Hortic. 2022, 293, 110744. [Google Scholar] [CrossRef]
- Canaan, J.M.M.; Brasil, G.S.P.; De Barros, N.R.; Mussagy, C.U.; Guerra, N.B.; Herculano, R.D. Soybean processing wastes and their potential in the generation of high value added products. Food Chem. 2022, 373, 131476. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Croge, C.; Da Silva, D.F.; Araújo, P.J.; Gallina, M.Z.; Matumoto-Pintro, P.T. Okara residue as source of antioxidants against lipid oxidation in milk enriched with omega-3 and bioavailability of bioactive compounds after in vitro gastrointestinal digestion. J. Food Sci. Technol. 2018, 55, 1518–1524. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Low molecular weight phenolics of grape juice and winemaking byproducts: Antioxidant activities and inhibition of oxidation of human low-density lipoprotein cholesterol and DNA strand breakage. J. Agric. Food Chem. 2014, 62, 12159–12171. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, M.V.; Tsantili, E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011, 131, 49–57. [Google Scholar] [CrossRef]
- Ojeda-Amador, R.M.; Salvador, M.D.; Gómez-Alonso, S.; Fregapane, G. Characterization of virgin walnut oils and their residual cakes produced from different varieties. Food Res. Int. 2018, 108, 396–404. [Google Scholar] [CrossRef]
- Wianowska, D.; Olszowy-Tomczyk, M. A concise profile of gallic acid—From its natural sources through biological properties and chemical methods of determination. Molecules 2023, 28, 1186. [Google Scholar] [CrossRef]
- Lu, T.; Shen, Y.; Wu, Z.-X.; Xie, H.-K.; Li, A.; Wang, Y.-F.; Song, L.; Zhou, D.-Y.; Wang, T. Improving the oxidative stability of flaxseed oil with composite antioxidants comprising gallic acid alkyl ester with appropriate chain length. LWT 2021, 138, 110763. [Google Scholar] [CrossRef]
- Roidoung, S.; Dolan, K.D.; Siddiq, M. Gallic acid as a protective antioxidant against anthocyanin degradation and color loss in vitamin-C fortified cranberry juice. Food Chem. 2016, 210, 422–427. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; De Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Ayoub, M.; De Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Enzyme-assisted extraction of phenolics from winemaking byproducts: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.; Adlercreutz, H. Naturally occurring oestrogens in food. Pure Appl. Chem. 1998, 70, 1759–1776. [Google Scholar] [CrossRef]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef]
- Sarfraz, I.; Rasul, A.; Riaz, A.; Ucak, I.; Zahoor, M.K.; Hussain, G.; Nawaz, J.; Sadiqa, A.; Adem, Ş. Biochanin A and biochanin B. In A Centum of Valuable Plant Bioactives; Mushtaq, M., Anwar, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 563–588. ISBN 978-0-12-822923-1. [Google Scholar]
- Rossi, Y.E.; Bohl, L.P.; Vanden Braber, N.L.; Ballatore, M.B.; Escobar, F.M.; Bodoira, R.; Maestri, D.M.; Porporatto, C.; Cavaglieri, L.R.; Montenegro, M.A. Polyphenols of peanut (Arachis hypogaea L.) skin as bioprotectors of normal cells. Studies of cytotoxicity, cytoprotection and interaction with ROS. J. Funct. Foods. 2020, 67, 103862. [Google Scholar] [CrossRef]
- Felix, F.B.; Vago, J.P.; Beltrami, V.A.; Araújo, J.M.D.; Grespan, R.; Teixeira, M.M.; Pinho, V. Biochanin A as a modulator of the inflammatory response: An updated overview and therapeutic potential. Pharmacol. Res. 2022, 180, 106246. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, W.; Yu, H.; Zhang, F.; Zhang, H.-T.; Zhou, Y. Biochanin A alleviates cognitive impairment and hippocampal mitochondrial damage in ovariectomized APP/PS1 mice. Phytomed. 2022, 100, 154056. [Google Scholar] [CrossRef]
- Jaina, V.K.; Eedara, A.; Svs, S.P.; Jadav, S.S.; Chilaka, S.; Sistla, R.; Andugulapati, S.B. Anti-cancer activity of biochanin A against multiple myeloma by targeting the CD38 and cancer stem-like cells. Process Biochem. 2022, 123, 11–26. [Google Scholar] [CrossRef]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Concepción Alvarez, A.; Arias-Santé, M.F.; Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Núñez Pizarro, P.; Bustos, S.M.; Schwember, A.R.; Shahidi, F.; et al. Soluble free, esterified and insoluble-bound phenolic antioxidants from chickpeas prevent cytotoxicity in human hepatoma HuH-7 cells induced by peroxyl radicals. Antioxidants 2022, 11, 1139. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M.C. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Acta. 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Motilva, M.-J.; Serra, A.; Macià, A. Analysis of food polyphenols by ultra high-performance liquid chromatography coupled to mass spectrometry: An overview. J. Chromatog. A. 2013, 1292, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pereira, G.A.; De Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of antioxidant synergisms and antagonisms among phenolic acids in the model matrices using FRAP and ORAC methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid Colorimetric assay for cell growth and survival. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Green, L.M.; Reade, J.L.; Ware, C.F. Rapid colormetric assay for cell viability: Application to the quantitation of cytotoxic and growth inhibitory lymphokines. J. Immunol. Methods 1984, 70, 257–268. [Google Scholar] [CrossRef]
- Marrelli, M.; Argentieri, M.P.; Alexa, E.; Meleleo, D.; Statti, G.; Avato, P.; Conforti, F.; Mallamaci, R. Antioxidant activity and protective effect of the outer scales hydroalcoholic extract of Allium Cepa L. Var. Tropea on toxicity damage induced by cadmium in Caco-2 cells. Food Chem. Toxicol. 2022, 170, 113495. [Google Scholar] [CrossRef]
- Park, J.G.; Kramer, B.S.; Steinberg, S.M.; Carmichael, J.; Collins, J.M.; Minna, J.D.; Gazdar, A.F. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 1987, 47, 5875–5879. [Google Scholar]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Aspevik, T.; Kousoulaki, K.; Barba, F.J.; Ruiz, M.-J. Cytoprotective effects of fish protein hydrolysates against H2O2-induced oxidative stress and mycotoxins in Caco-2/TC7 cells. Antioxidants 2021, 10, 975. [Google Scholar] [CrossRef]
- Bórquez, J.C.; Hidalgo, M.; Rodríguez, J.M.; Montaña, A.; Porras, O.; Troncoso, R.; Bravo-Sagua, R. Sucralose stimulates mitochondrial bioenergetics in Caco-2 cells. Front. Nutr. 2021, 7, 585484. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.-J. Role of quercetin on Caco-2 cells against cytotoxic effects of alternariol and alternariol monomethyl ether. Food Chem. Toxicol. 2016, 89, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Jun, N.; Yi-Ting, C.; Yu-Ting, G.; Cheng-Fa, Z.; Li-Juan, L.; Rong, S.; Xiao-yan, Y.; Wen, X.; Xu, Y. Antioxidant, anti-inflammatory, and anticancer function of Engleromyces goetzei Henn aqueous extract on human intestinal Caco-2 cells treated with t-BHP. Food Sci. Nutr. 2023, 11, 3450–3463. [Google Scholar] [CrossRef] [PubMed]
- Laka, K.; Mapheto, K.B.F.; Mbita, Z. Selective in vitro cytotoxicity effect of drimia calcarata bulb extracts against P53 mutant HT-29 and P53 wild-type Caco-2 colorectal cancer cells through STAT5B regulation. Toxicol. Rep. 2021, 8, 1265–1279. [Google Scholar] [CrossRef]
- Van Eyk, A.D. The effect of five artificial sweeteners on Caco-2, HT-29 and HEK-293 cells. Drug Chem. Toxicol. 2015, 38, 318–327. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, M.; Yang, J.; Pan, H.; Yu, M.; Ji, F. Curcumin improves epithelial barrier integrity of Caco-2 monolayers by inhibiting endoplasmic reticulum stress and subsequent apoptosis. Gastroenterol. Res. Pract. 2021, 2021, 5570796. [Google Scholar] [CrossRef]
- Hung, Y.-K.; Ho, S.-T.; Kuo, C.-Y.; Chen, M.-J. In vitro effects of velvet antler water extracts from formosan sambar deer and red deer on barrier integrity in Caco-2 cell. Int. J. Med. Sci. 2021, 18, 1778–1785. [Google Scholar] [CrossRef]
- Lopez-Escalera, S.; Wellejus, A. Evaluation of Caco-2 and human intestinal epithelial cells as in vitro models of colonic and small intestinal integrity. Biochem. Biophys. Rep. 2022, 31, 101314. [Google Scholar] [CrossRef]
- Zongo, A.W.-S.; Zogona, D.; Youssef, M.; Ye, S.; Zhan, F.; Li, J.; Li, B. Senegalia Macrostachya seed polysaccharides attenuate inflammation-induced intestinal epithelial barrier dysfunction in a Caco-2 and RAW264.7 macrophage co-culture model by inhibiting the NF-κB/MLCK pathway. Food Funct. 2022, 13, 11676–11689. [Google Scholar] [CrossRef]
- Lopes Do Carmo, M.C.; Mateus Martins, I.; Ramos Magalhães, A.E.; Maróstica Júnior, M.R.; Alves Macedo, J. Passion fruit (Passiflora edulis) leaf aqueous extract ameliorates intestinal epithelial barrier dysfunction and reverts inflammatory parameters in Caco-2 cells monolayer. Food Res. Int. 2020, 133, 109162. [Google Scholar] [CrossRef]
- Kaak, J.; Lobo De Sá, F.D.; Turner, J.R.; Schulzke, J.; Bücker, R. Unraveling the intestinal epithelial barrier in cyanotoxin microcystin-treated Caco-2 cell monolayers. Ann. N. Y. Acad. Sci. 2022, 1516, 188–196. [Google Scholar] [CrossRef]
- Gravel, A.; Dubois-Laurin, F.; Doyen, A. Effects of hexane on protein profile and techno-functional properties of pea protein isolates. Food Chem. 2023, 406, 135069. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Gheldiu, A.-M.; Mocan, A.; Moldovan, C.; Popa, D.-S.; Tomuta, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.; Hillis, W.E. The Phenolic constituents of Prunus domestica I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Biasoto, A.C.T.; Schwember, A.R.; Granato, D.; Rasera, G.B.; Franchin, M.; Rosalen, P.L.; Alencar, S.M.; Shahidi, F. Should we ban total phenolics and antioxidant screening methods? The link between antioxidant potential and activation of NF-κB using phenolic compounds from grape by-products. Food Chem. 2019, 290, 229–238. [Google Scholar] [CrossRef]
- Da Silva, M.D.A.S.; Bridi, R.; Arias-Santé, M.F.; Rincón-Cervera, M.A.; Meisel, L.A.; Rhein, S.; Porras, O.; Márquez Calvo, K.; Carrasco, B.; De Camargo, A.C. Soluble and insoluble-bound phenolic bioactive compounds of tortola bean leaf and their antioxidant properties in chemical-based assays and Caco-2 cells. Food Biosci. 2024, 57, 103616. [Google Scholar] [CrossRef]

| Sample | Free | Esterified | Etherified | IBPH | Total |
|---|---|---|---|---|---|
| Total phenolic content (mg GAE 100 g−1 dw) | |||||
| DWR | 384.51 ± 9.8 Aa | 141.50 ± 2.2 Ab | 104.15 ± 2.9 Ac | 373.48 ± 5.0 Aa | 1003.64 |
| WMR | 124.45 ± 3.5 Ba | 78.30 ± 0.9 Bb | 72.55 ± 0.7 Bb | 167.95 ± 3.3 Bc | 443.25 |
| Ferric Reducing Antioxidant Power_FRAP (µmol TE g−1 dw) | |||||
| DWR | 887.56 ± 37.5 Aa | 79.70 ± 5.9 Ab | 79.48 ± 5.3 Ab | 167.07 ± 9.4 Ac | 1213.81 |
| WMR | 109.64 ± 9.5 Ba | 19.52 ± 1.4 Bb | 8.97 ± 0.9 Bb | 57.94 ± 0.9 Bc | 196.07 |
| Oxygen radical absorbance capacity_ORAC (µmol TE g−1 dw) | |||||
| DWR | 36.80 ± 2.0 Ab | 20.24 ± 0.4 Ad | 24.70 ± 0.0 Ac | 50.47 ± 1.8 Aa | 132.21 |
| WMR | 15.52 ± 0.1 Bb | 13.84 ± 0.1 Bc | 16.05 ± 0.8 Bb | 20.84 ± 0.0 Ba | 66.25 |
| Compound | Free | Esterified | Etherified | IBPH | Total |
|---|---|---|---|---|---|
| Phenolic acids | |||||
| Gallic acid | 3061.01 ± 19.71 Aa | 88.87 ± 3.83 Bb | 88.10 ± 0.87 Ab | 530.70 ± 3.89 Bc | 3768.68 |
| p-Coumaric acid | 18.15 ± 1.97 Ba | 25.43 ± 0.08 Ca | tr | 111.73 ± 5.65 Cb | 155.31 |
| Caffeic acid | 54.50 ± 0.63 C | tr | nd | tr | 54.50 |
| Ferulic acid | 5.70 ± 0.44 Ba | 7.38 ± 0.75 Aa | tr | 40.83 ± 3.14 Ab | 53.90 |
| 3,4-Dihydroxybenzoic acid * | 43.32 ± 0.95 C | nd | nd | nd | 43.32 |
| Sinapic acid | tr | tr | 2.11 ± 0.15 B | tr | 2.11 |
| Flavonoids | |||||
| Biochanin A * | 259.17 ± 20.78 Aa | 593.75 ± 1.50 Bb | nd | nd | 852.92 |
| Quercetin | 40.30 ± 1.37 Ba | tr | tr | 34.26 ± 0.64 b | 74.56 |
| Quercitrin * | 37.49 ± 1.18 Ba | 12.53 ± 0.15 Cb | 11.03 ± 0.69 b | tr | 61.05 |
| Chrysin * | tr | 8.67 ± 0.36 A | tr | tr | 8.67 |
| Compound | MRM * Transition 1 | DP | CE | CXP | MRM Transition 2 | DP | CE | CXP |
|---|---|---|---|---|---|---|---|---|
| Gallic acid | 168.9 ** > 124.9 *** | −70 | −18 | −7 | 168.9 ** > 78.9 *** | −70 | −18 | −7 |
| p-Coumaric acid | 162.9 ** > 119.0 *** | −70 | −20 | −5 | 162.9 ** > 119.0 *** | −70 | −38 | −25 |
| Caffeic acid | 178.9 ** > 135.0 *** | −70 | −20 | −5 | 178.9 ** > 133.9 *** | −70 | −32 | −7 |
| Ferulic acid | 193.0 ** > 134.0 *** | −55 | −20 | −7 | 193.0 ** > 177.9 *** | −55 | −16 | −15 |
| Sinapic acid | 223.0 ** > 207.9 *** | −75 | −18 | −7 | 223.0 ** > 148.8 *** | −75 | −26 | −13 |
| 3,4-Dihydroxybenzoic acid | 152.9 ** > 107.9 *** | −50 | −26 | −3 | 152.9 ** > 108.9 *** | −50 | −16 | −3 |
| Biochanin A | 282.9 ** > 267.9 *** | −80 | −32 | −5 | 282.9 ** > 211.1 *** | −80 | −46 | −5 |
| Quercetin | 301.0 ** > 150.9 *** | −15 | −28 | −13 | 301.0 ** > 178.8 *** | −15 | −24 | −11 |
| Quercitrin | 447.0 ** > 299.9 *** | −105 | −32 | −13 | 447.0 ** > 299.9 *** | −105 | −30 | −7 |
| Chrysin | 253.0 ** > 208.9 *** | −130 | −16 | −7 | 253.0 ** > 143.0 *** | −130 | −34 | −3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo-Merma, P.R.; Arias-Santé, M.F.; Rincón-Cervera, M.Á.; Porras, O.; Bridi, R.; Rhein, S.; Sánchez-Contreras, M.; Hernandez-Pino, P.; Tobar, N.; Puente-Díaz, L.; et al. Phenolic Fractions from Walnut Milk Residue: Antioxidant Activity and Cytotoxic Potential. Plants 2024, 13, 3473. https://doi.org/10.3390/plants13243473
Toledo-Merma PR, Arias-Santé MF, Rincón-Cervera MÁ, Porras O, Bridi R, Rhein S, Sánchez-Contreras M, Hernandez-Pino P, Tobar N, Puente-Díaz L, et al. Phenolic Fractions from Walnut Milk Residue: Antioxidant Activity and Cytotoxic Potential. Plants. 2024; 13(24):3473. https://doi.org/10.3390/plants13243473
Chicago/Turabian StyleToledo-Merma, Pamela Ruth, María Fernanda Arias-Santé, Miguel Ángel Rincón-Cervera, Omar Porras, Raquel Bridi, Samantha Rhein, Martina Sánchez-Contreras, Paulina Hernandez-Pino, Nicolás Tobar, Luis Puente-Díaz, and et al. 2024. "Phenolic Fractions from Walnut Milk Residue: Antioxidant Activity and Cytotoxic Potential" Plants 13, no. 24: 3473. https://doi.org/10.3390/plants13243473
APA StyleToledo-Merma, P. R., Arias-Santé, M. F., Rincón-Cervera, M. Á., Porras, O., Bridi, R., Rhein, S., Sánchez-Contreras, M., Hernandez-Pino, P., Tobar, N., Puente-Díaz, L., & de Camargo, A. C. (2024). Phenolic Fractions from Walnut Milk Residue: Antioxidant Activity and Cytotoxic Potential. Plants, 13(24), 3473. https://doi.org/10.3390/plants13243473












