New Insight into the Related Candidate Genes and Molecular Regulatory Mechanisms of Waterlogging Tolerance in Tree Peony Paeonia ostii
Abstract
1. Introduction
2. Results
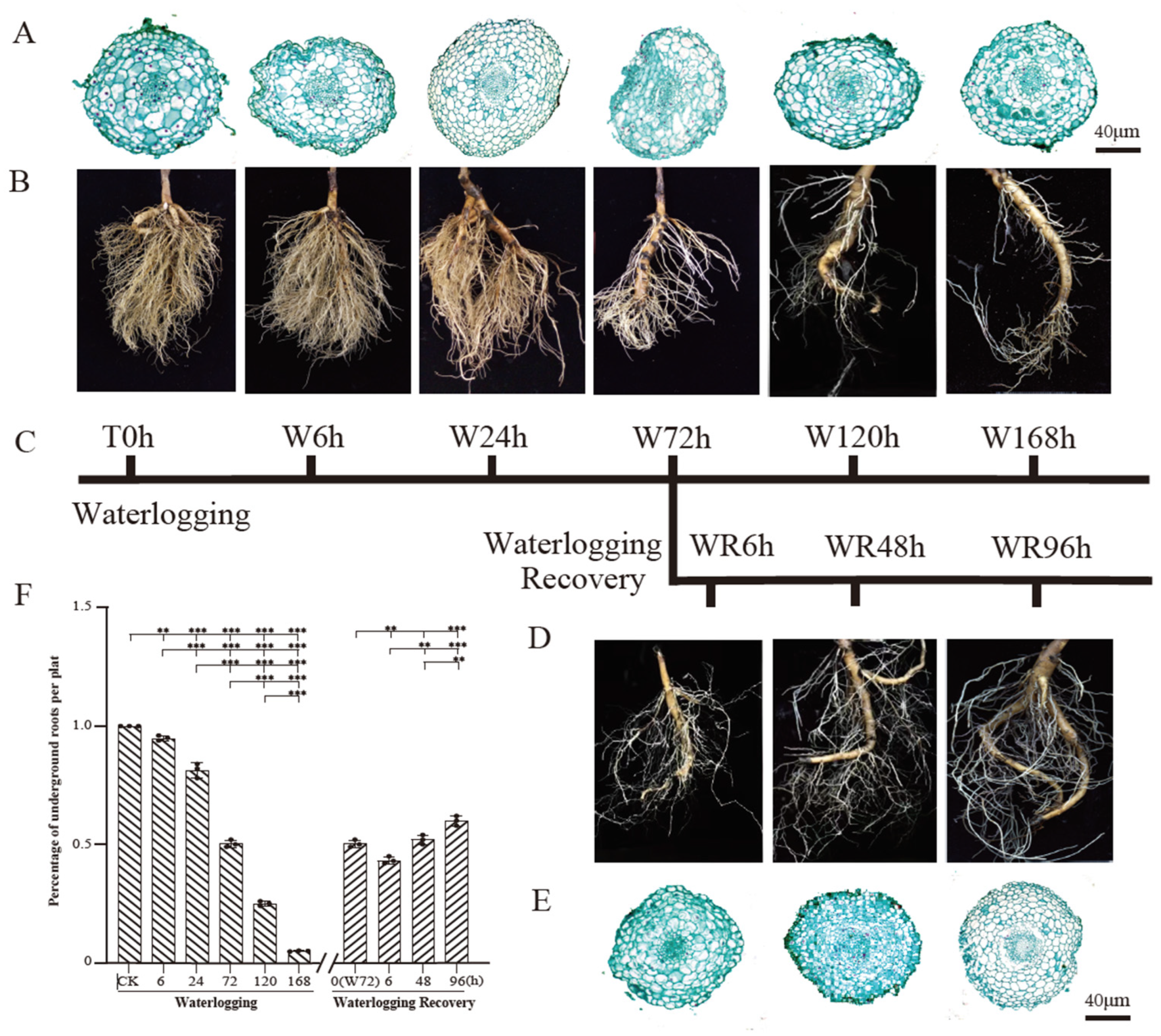
2.1. The Root Number and Root Tip Cell Morphology Underwent Significant Changes over the Course of the Waterlogging and Waterlogging Recovery Treatment
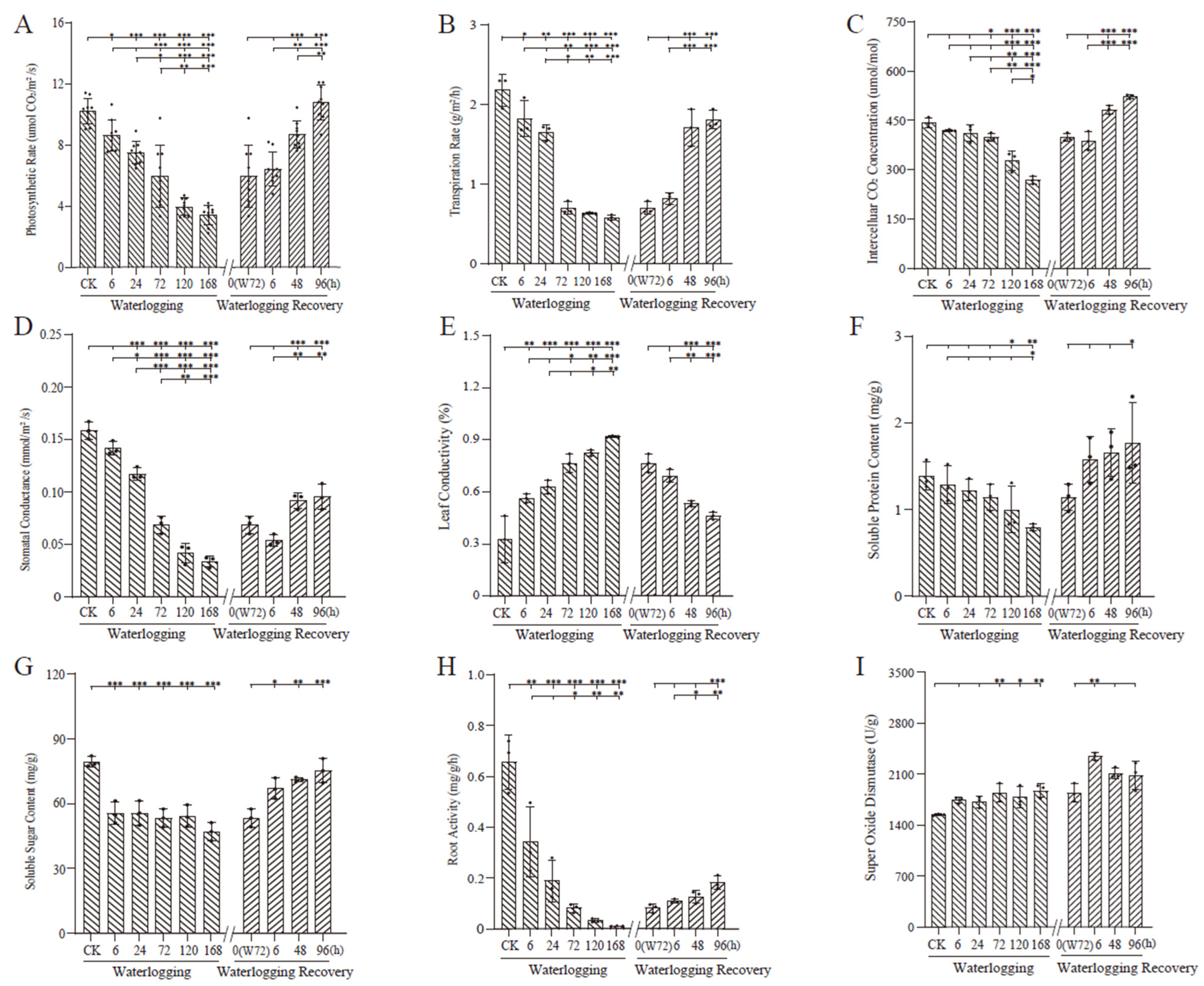
2.2. Nine Physiological and Biochemical Indicators Under Waterlogging Treatment and Recovery Conditions
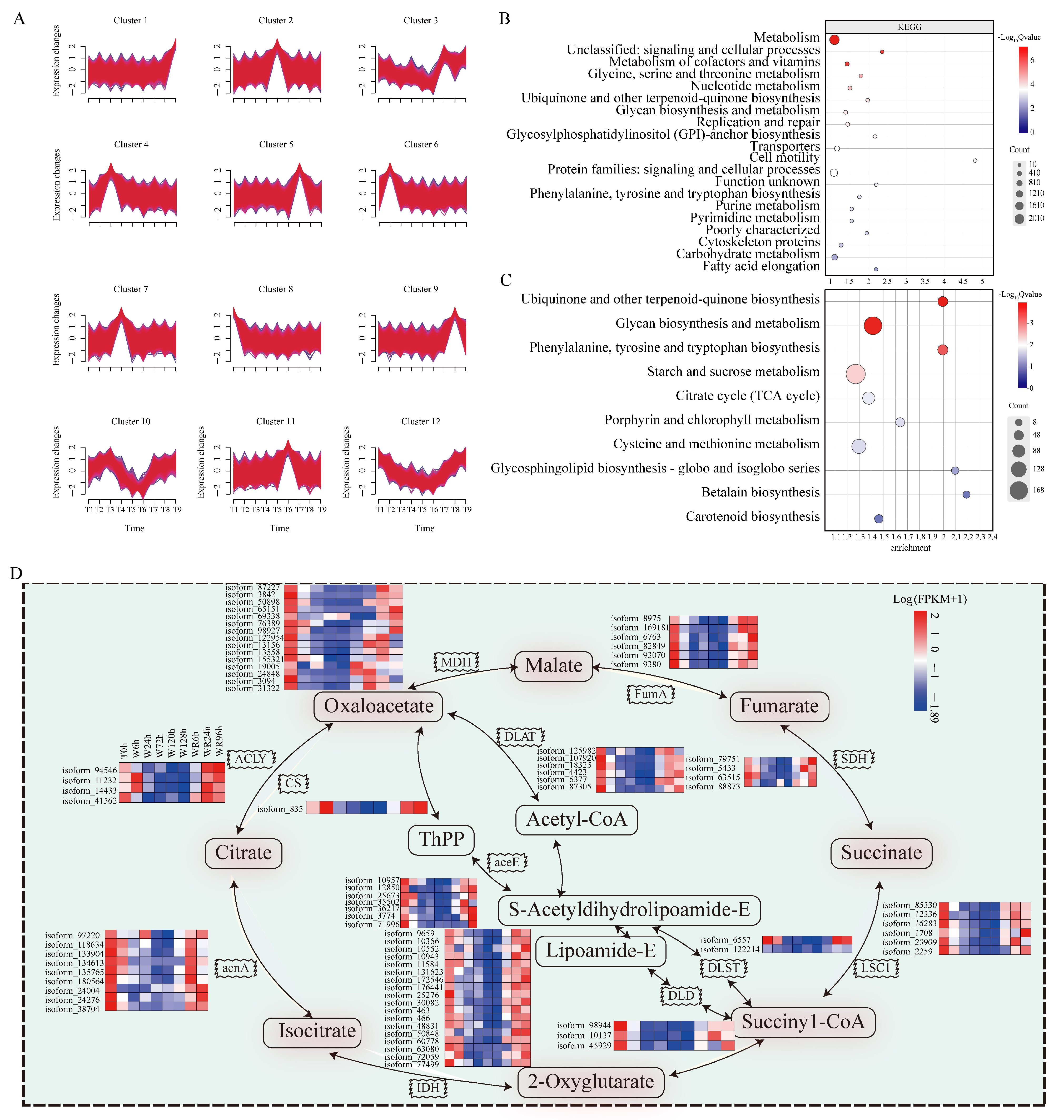
2.3. A Time Sequence Analysis Unveiled Hundreds of Candidate Genes Related Waterlogging Stress Across Different Temporal Stages
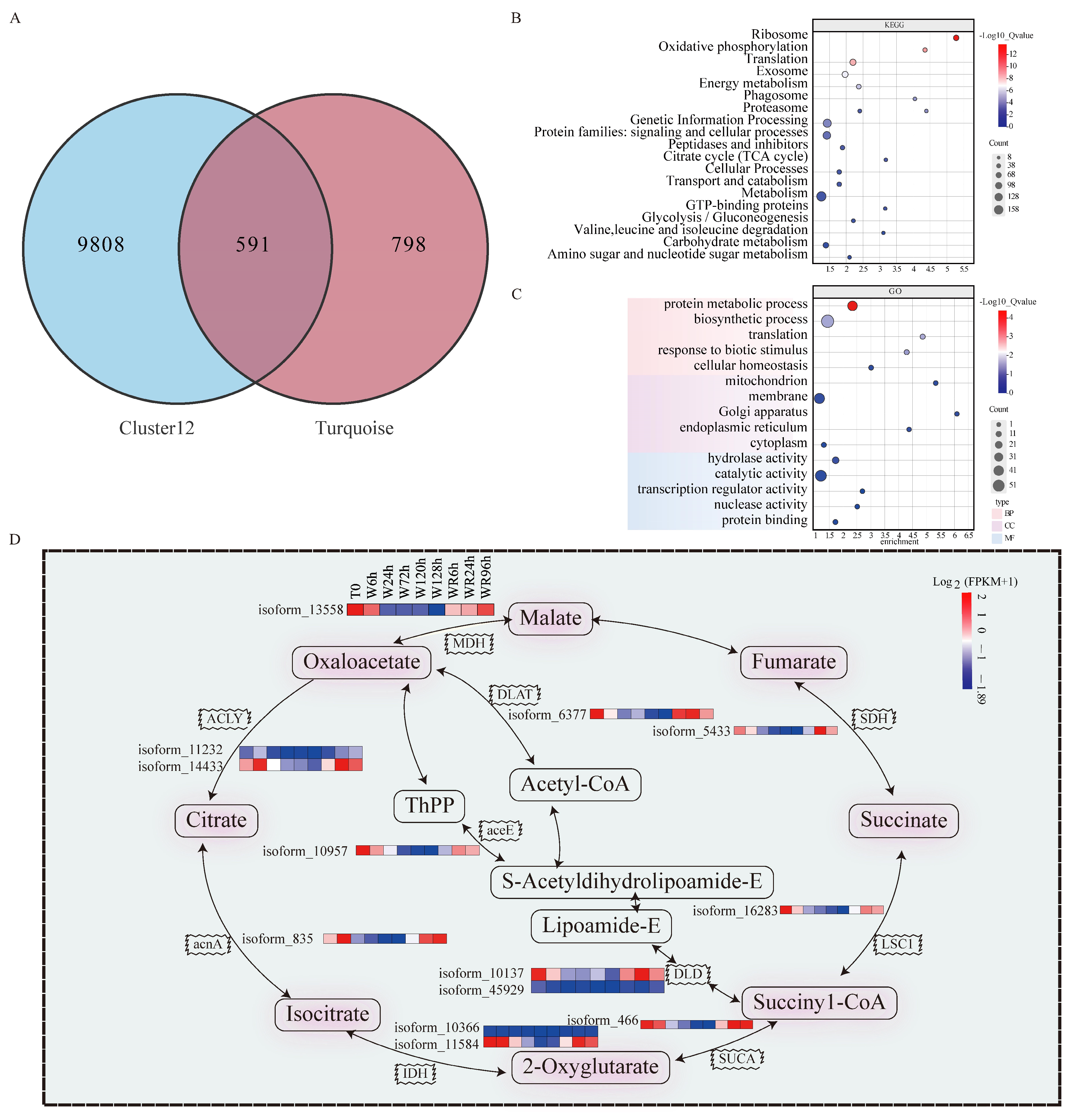
2.4. Weighted Gene Co-Expression Network Analysis (WGCNA) of the Co-Expression Network Reveals the Association of the Turquoise Module with Physiological Traits
2.5. Screening for Candidate Genes Under Waterlogging Stress
3. Discussion
3.1. P. ostii Root Growth Is Inhibited Under Waterlogging Stress
3.2. P. ostii Physiological and Biochemical Indices Were Significantly Affected Under Waterlogging Stress
3.3. The P. ostii Tricarboxylic Acid (TCA) Cycle Is Inhibited Under Waterlogging Stress
4. Materials and Methods
4.1. Plant Material
4.2. Indicator Measurement
4.2.1. Scanning Morphology of the Root System
4.2.2. Root Tip Slices
4.2.3. Physical Indices of Leaf Gas Exchange Parameters
4.2.4. Determination of the Relative Leaf Conductivity
4.2.5. Measurement of Soluble Protein and Sugar
4.2.6. Determination of the Root Superoxide Dismutase (SOD) Activity
4.2.7. Determination of Root Activity
4.2.8. Statistical Analysis
4.3. RNA Extraction, Sequencing and Analysis
4.4. Time Sequence Analysis of the Transcriptome of the P. ostii Root System
4.5. WGCNA of the Transcriptome of the P. ostii Root System
4.6. qRT-PCR Validation of Gene Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Cheng, F.; Zhong, Y. In vitro immature embryo culture of Paeonia ostii ‘Feng Dan’. HortScience 2022, 57, 599–605. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zuo, J.Q.; Wang, Y.T.; Duan, H.Y.; Zhou, M.H.; Li, H.J.; Hu, Y.H.; Yuan, J.H. PoDPBT, a BAHD acyltransferase, catalyses the benzoylation in paeoniflorin biosynthesis in Paeonia ostii. Plant Biotechnol. J. 2023, 21, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, B.; Liu, T.; Zhu, Y.; Wang, L.; Wang, X.; Niu, X.; Xiao, Y.; Sun, Q. Evaluation of paeonol-loaded transethosomes as transdermal delivery carriers. Eur. J. Pharm. Sci. 2017, 99, 240–245. [Google Scholar] [CrossRef]
- Eastmond, P.J.; Germain, V.; Lange, P.R.; Bryce, J.H.; Smith, S.M.; Graham, I.A. Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc. Natl. Acad. Sci. USA 2000, 97, 5669–5674. [Google Scholar] [CrossRef]
- Jürgen, K.; Heinz, R. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for coping with submergence and waterlogging in rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef]
- Jackson, M.; Colmer, T.D. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Hou, Y.B.; Jiang, F.; Zheng, X.L.; Wu, Z. Identification and analysis of oxygen responsive microRNAs in the root of wild tomato (S. habrochaites). BMC Plant Biol. 2019, 19, 1471–2229. [Google Scholar] [CrossRef]
- Irene, M.; Ann, C.; Rose, M. Flooding effects on soil microbial communities. Appl. Soil Ecol. 2009, 42, 1–8. [Google Scholar]
- Ponnamperuma, F. The Chemistry of Submerged Soils; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Lucassen, E.C.; Smolders AJ, P.; Roelofs JG, M. Increased groundwater levels cause iron toxicity in Glyceria fluitans (L.). Aquat. Bot. 2000, 66, 321–327. [Google Scholar] [CrossRef]
- Fukuju, Y.; Tsutomu, S.; Kazuhiko, T. Physiological, morphological and anatomical responses of Fraxinus mandshurica seedlings to flooding. Tree Physiol. 1995, 15, 713–719. [Google Scholar]
- Parelle, J.; Brendel, O.; Bodénès, C.; Berveiller, D.; Dizengremel, P.; Jolivet, Y.; Dreyer, E. Differences in morphological and physiological responses to water-logging between two sympatric oak species. Ann. For. Sc. 2006, 63, 849–859. [Google Scholar] [CrossRef]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Schortemeyer, M. Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Funct. Plant Biol. 2001, 28, 1121–1131. [Google Scholar] [CrossRef]
- Voesenek, L.; Julia, B. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Mendiondo, G.M.; Gibbs, D.J.; Szurman-Zubrzycka, M.; Korn, A.; Marquez, J.; Szarejko, I.; Maluszynski, M.; King, J.; Axcell, B.; Smart, K.; et al. Enhanced waterlogging tolerance in barley by manipulation of expression of the N-end rule pathway E3 ligase PROTEOLYSIS6. Plant Biotechnol. J. 2016, 14, 40–50. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef]
- Gibbs, D.J.; Lee, S.C.; Md Isa, N.; Gramuglia, S.; Fukao, T.; Bassel, G.W.; Correia, C.S.; Corbineau, F.; Theodoulou, F.L.; Bailey-Serres, J.; et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011, 479, 415–418. [Google Scholar] [CrossRef]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.C.J.; Perata, P.; van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, S.; Angelika, M. Plant oxygen sensing is mediated by the N-end rule pathway: A milestone in plant anaerobiosis. Plant Cell 2011, 23, 4173–4183. [Google Scholar]
- Sasidharan, R.; Mustroph, A.; Boonman, A.; Akman, M.; Ammerlaan, A.M.; Breit, T.; Schranz, M.E.; Voesenek, L.A.; van Tienderen, P.H. Root transcript profiling of two Rorippa species reveals gene clusters associated with extreme submergence tolerance. Plant Physiol. 2013, 163, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- van Veen, H.; Mustroph, A.; Barding, G.A.; Vergeer-van Eijk, M.; Welschen-Evertman, R.A.; Pedersen, O.; Visser, E.J.; Larive, C.K.; Pierik, R.; Bailey-Serres, J.; et al. Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell 2013, 25, 4691–4707. [Google Scholar] [CrossRef]
- Yuan, J.; Jiang, S.; Jian, J.; Liu, M.; Yue, Z.; Xu, J.; Li, J.; Xu, C.; Lin, L.; Jing, Y.; et al. Genomic basis of the giga-chromosomes and giga-genome of tree peony Paeonia ostii. Nat. Commun. 2022, 13, 7328. [Google Scholar] [CrossRef]
- Liu, C.Y.; Jiao, S.Q.; Zhang, Q.; Yao, P.Q.; Xie, L.H.; Wang, Z.; Cheng, S.P. Transcriptome profiling provides preliminary molecular insights into adventitious bud formation in herbaceous peony (Paeonia x ‘Coral Charm’). J. Hortic. Sci. Biotechnol. 2022, 4, 97. [Google Scholar] [CrossRef]
- Shao, D.; Abubakar, A.S.; Chen, J.; Zhao, H.; Chen, P.; Chen, K.; Wang, X.; Shawai, R.S.; Chen, Y.; Zhu, A.; et al. Physiological, molecular, and morphological adjustment to waterlogging stress in ramie and selection of waterlogging-tolerant varieties. Plant Physiol. Biochem. 2024, 216, 109101. [Google Scholar] [CrossRef]
- Walne, C.; Reddy, K. Developing Functional Relationships between Soil Waterlogging and Corn Shoot and Root Growth and Development. Plants 2021, 10, 2095. [Google Scholar] [CrossRef]
- Qian, L.; Huang, T.; Zeng, W.; Chen, X.; Wang, X. Determining the dynamic responses of cotton root morphological characteristics under waterlogging stress using the minirhizotron technique. Irrig. Drain. 2023, 72, 60–74. [Google Scholar] [CrossRef]
- Rajhi, I.; Yamauchi, T.; Takahashi, H.; Nishiuchi, S.; Shiono, K.; Watanabe, R.; Mliki, A.; Nagamura, Y.; Tsutsumi, N.; Nishizawa, N.K.; et al. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol. 2011, 190, 351–368. [Google Scholar] [CrossRef]
- Muhammad, A.; Nudrat, A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar]
- Kumutha, D.; Ezhilmathi, K.; Sairam, R.K.; Srivastava, G.C.; Deshmukh, P.S.; Meena, R.C. Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biol. Plant. 2009, 53, 75–84. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Li, Y.; Shi, L.-C.; Yang, J.; Qian, Z.-H.; He, Y.-X.; Li, M.-W. Physiological and transcriptional changes provide insights into the effect of root waterlogging on the aboveground part of Pterocarya stenoptera. Genomics 2021, 113, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Bashar, K.; Tareq, M.; Islam, M. Unlocking the mystery of plants’ survival capability under waterlogging stress. Plant Sci. Today 2020, 7, 142. [Google Scholar] [CrossRef]
- Li, H.S. Experimental Principle and Technology of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Kumar, L.; Futschik, M. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Liu, Y.; Shen, X.; Guo, Y.; Ma, X.; Zhang, X.; Li, X.; Cheng, T.; Wen, H.; et al. RNA-Seq-based WGCNA and association analysis reveal the key regulatory module and genes responding to salt stress in wheat roots. Plants 2024, 13, 274. [Google Scholar] [CrossRef]
- Lu, L.; Tang, Y.; Xu, H.; Qian, Y.; Tao, J.; Zhao, D. Selection and verification of reliable internal reference genes instem development of herbaceous peony(Paeonia lactiflora Pall.). Physiol. Mol. Biol. Plants 2023, 29, 773–782. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhou, M.; Hu, Y.; Yuan, J. PacBio full length sequencing integrated with RNA-seq reveals the molecular mechanism of waterlogging and its recovery in Paeonia ostii. Front. Plant Sci. 2022, 13, 1030584. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Liu, X.; Zhao, J.; Jiang, F.; Li, W.; Yan, X.; Hu, Y.; Yuan, J. New Insight into the Related Candidate Genes and Molecular Regulatory Mechanisms of Waterlogging Tolerance in Tree Peony Paeonia ostii. Plants 2024, 13, 3324. https://doi.org/10.3390/plants13233324
Zhou M, Liu X, Zhao J, Jiang F, Li W, Yan X, Hu Y, Yuan J. New Insight into the Related Candidate Genes and Molecular Regulatory Mechanisms of Waterlogging Tolerance in Tree Peony Paeonia ostii. Plants. 2024; 13(23):3324. https://doi.org/10.3390/plants13233324
Chicago/Turabian StyleZhou, Minghui, Xiang Liu, Jiayan Zhao, Feng Jiang, Weitao Li, Xu Yan, Yonghong Hu, and Junhui Yuan. 2024. "New Insight into the Related Candidate Genes and Molecular Regulatory Mechanisms of Waterlogging Tolerance in Tree Peony Paeonia ostii" Plants 13, no. 23: 3324. https://doi.org/10.3390/plants13233324
APA StyleZhou, M., Liu, X., Zhao, J., Jiang, F., Li, W., Yan, X., Hu, Y., & Yuan, J. (2024). New Insight into the Related Candidate Genes and Molecular Regulatory Mechanisms of Waterlogging Tolerance in Tree Peony Paeonia ostii. Plants, 13(23), 3324. https://doi.org/10.3390/plants13233324




