Ethnopharmacological Study of Garrya laurifolia and Its Antidiabetic Effect in Rats
Abstract
1. Introduction
2. Results
2.1. Descriptive Ethnobotanical Study
- Ethnomedicinal information of Gl
2.2. In Vivo Studies of Gl
2.2.1. Acute Toxicity Study
2.2.2. Pharmacologic Study
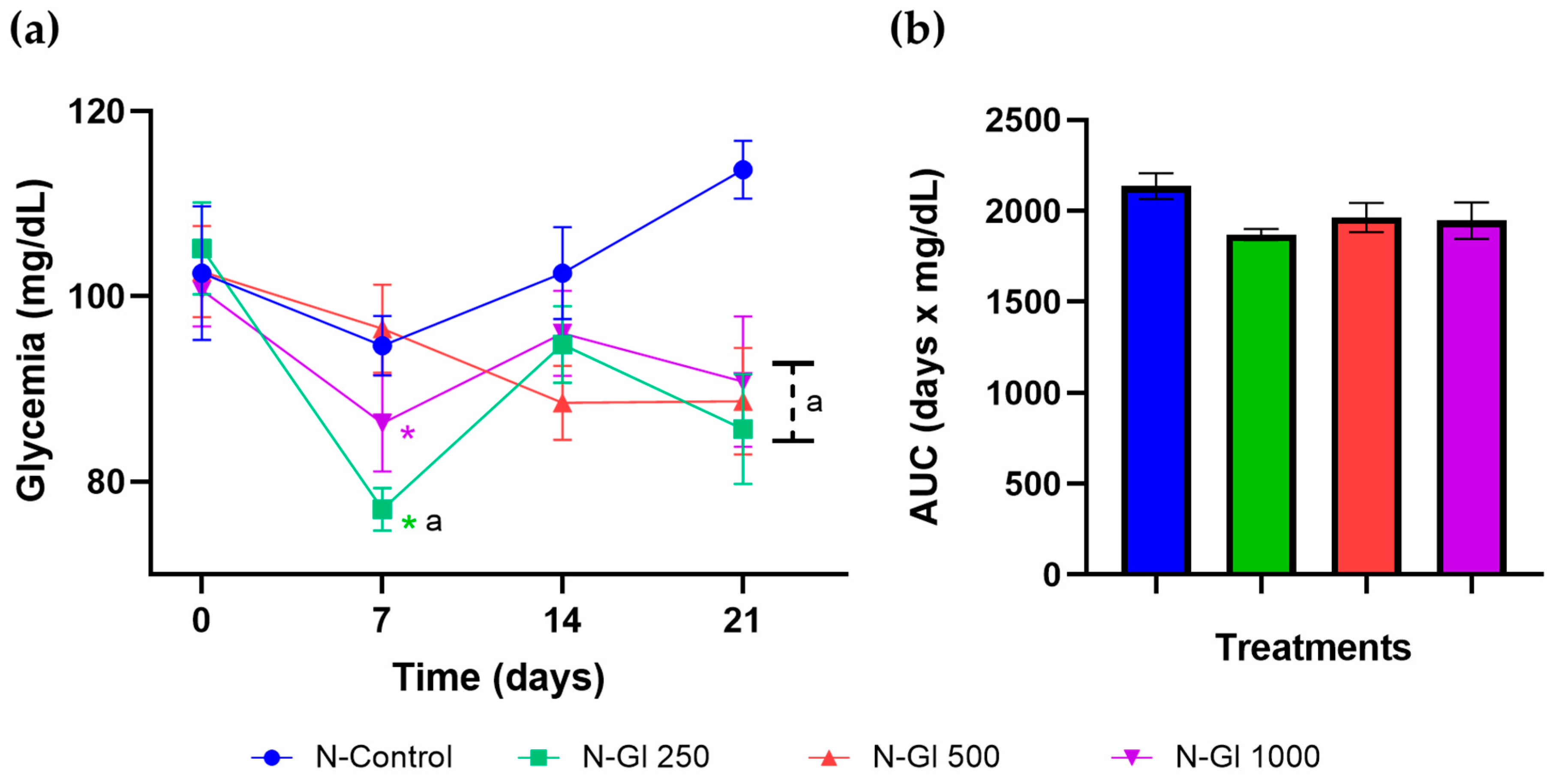
- Effect on glycemia in the experimental DM model
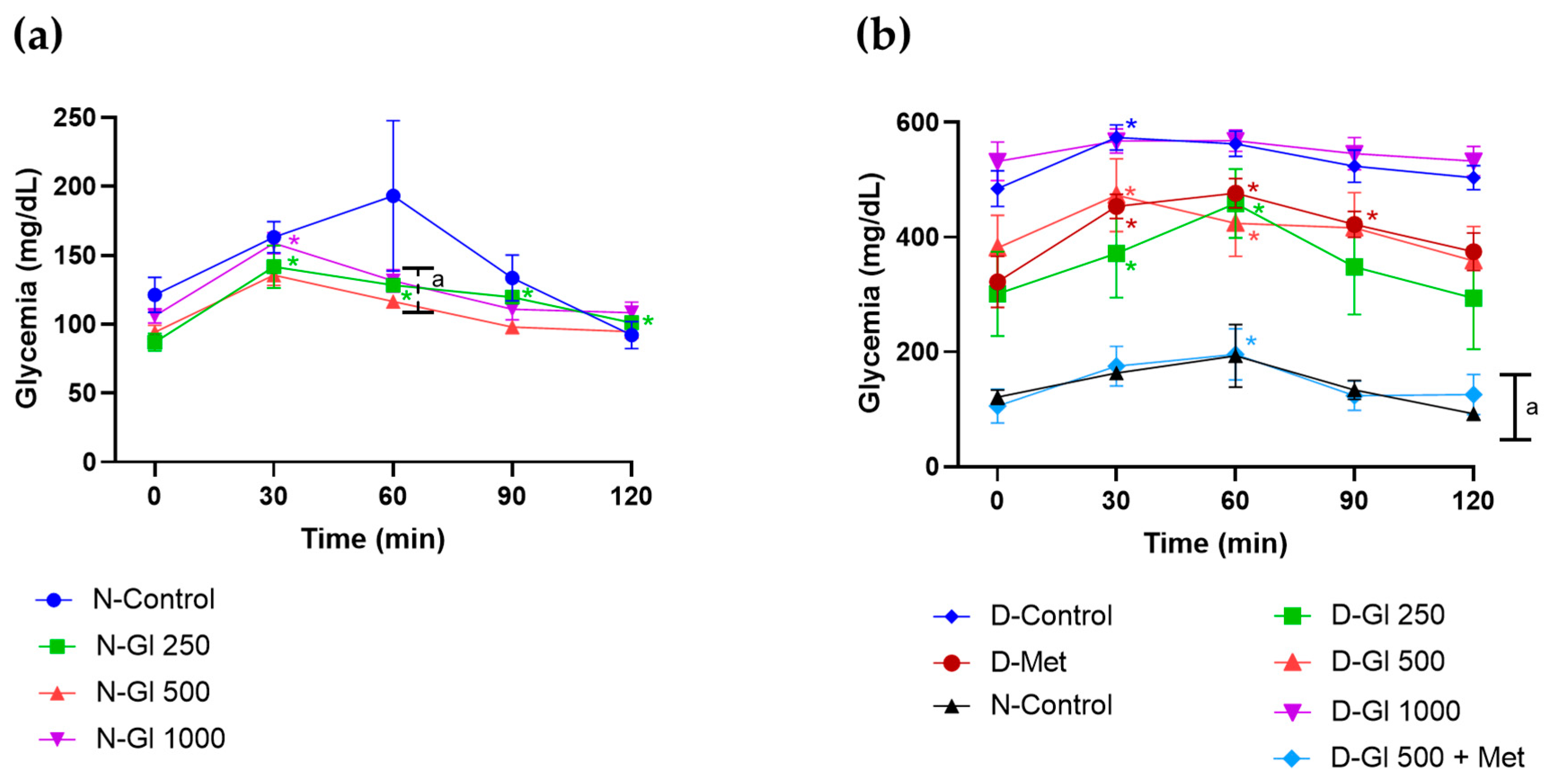
- Effect on Glucose Tolerance Test (GTT)
2.3. Antioxidant Potential In Vitro
2.4. Phytochemical Studies of the Infusion of Gl Leaves
- Preliminary phytochemical screening
- Total phenolic and flavonoid
- Ultra-High-Performance Liquid Chromatography Mass Spectrometry (UHPLC-MS/MS) analysis
3. Discussion
4. Materials and Methods
4.1. Descriptive Ethnobotanical Study
- Description of the study area
- Field work
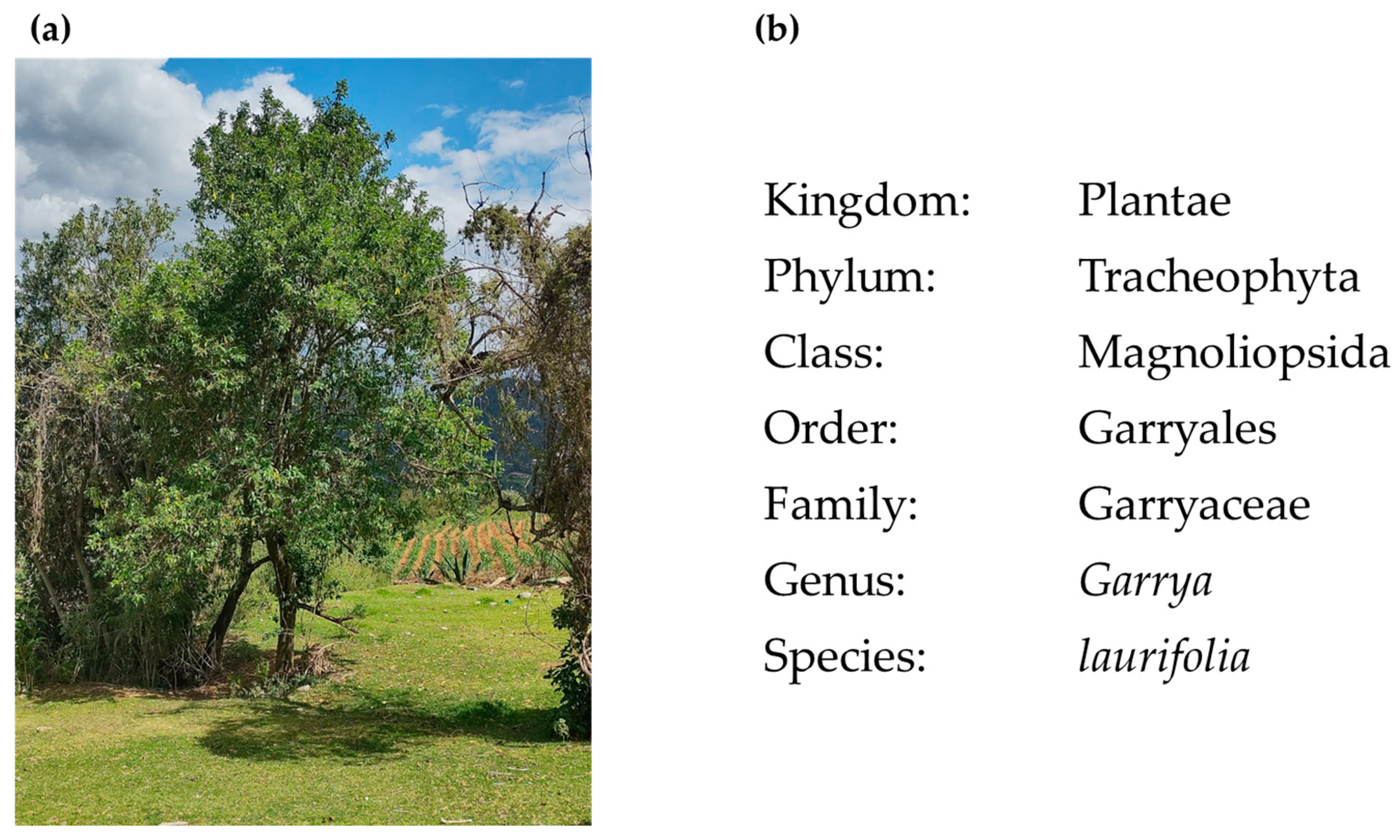
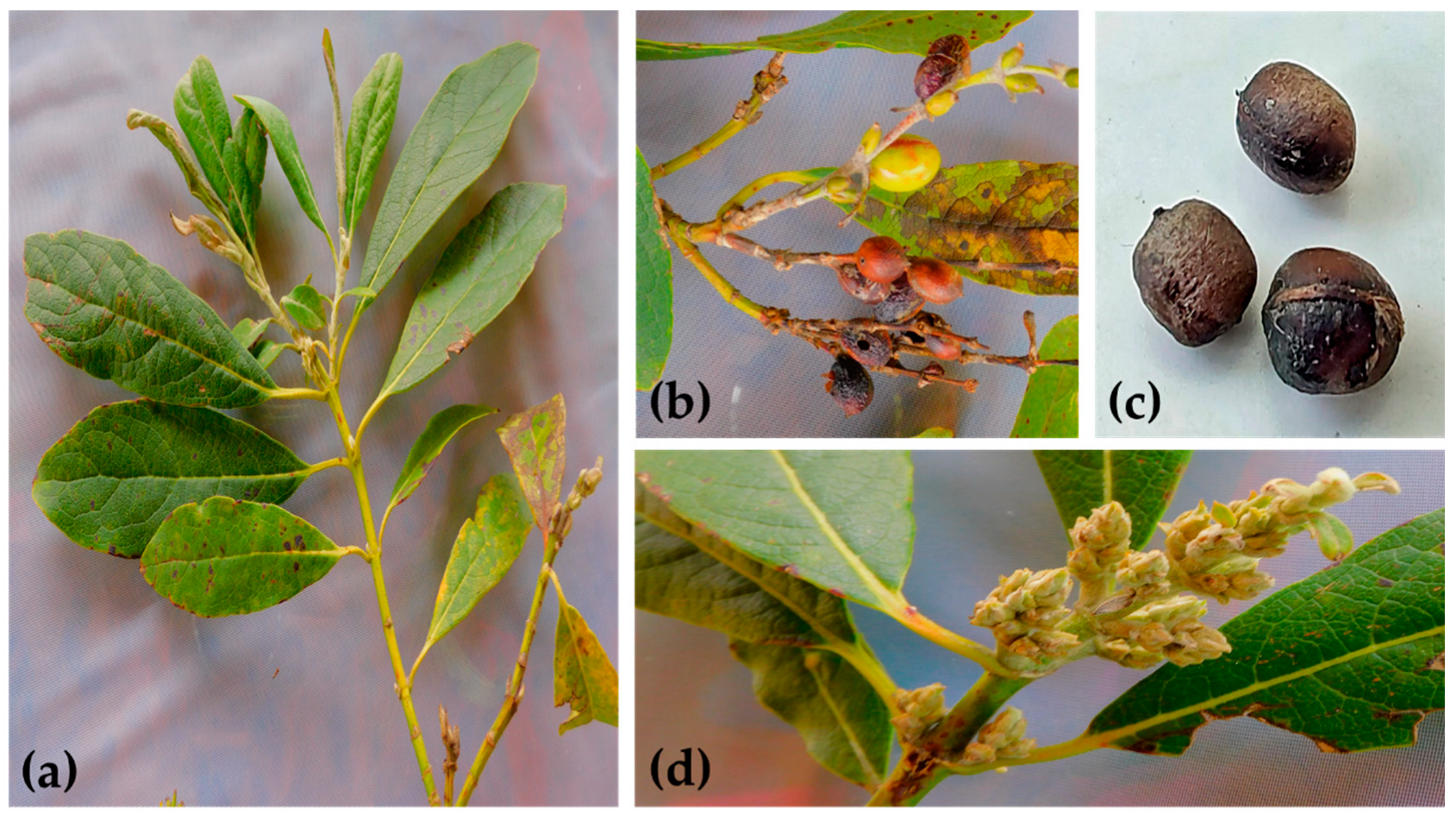
4.2. Collection and Identification of Botanical Material
4.3. Reagents and Drugs
4.4. Preparation of Infusion of G. laurifolia Leaves
4.5. In Vivo Studies of Gl
- Animal care and housing
4.5.1. Acute Toxicity Study
4.5.2. Pharmacologic Study
- Induction of experimental DM in rats
- Effect of Gl on glycemia
- Effect of Gl on Glucose Tolerance Test (GTT)
4.6. Antioxidant In Vitro Activity of Gl
4.7. Phytochemical Studies of Gl
- Preliminary phytochemical screening
- Total phenolic content
- Total flavonoid content
- Ultra-High-Performance Liquid Chromatography Mass Spectrometry (UHPLC-MS) analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation (IDF). IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; p. 6. [Google Scholar]
- Diabetes. World Health Organization (WHO). 2023. Available online: https://www.who.int/es/news-room/fact-sheets/detail/diabetes (accessed on 7 October 2024).
- Pang, M.; Li, Y.; Gu, W.; Sun, Z.; Wang, Z.; Li, L. Recent advances in epigenetics of macrovascular complications in diabetes mellitus. Heart Lung Circ. 2021, 30, 186–196. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Global Report on Traditional and Complementary Medicine; World Health Organization: Geneva, Switzerland, 2019; p. 45. [Google Scholar]
- Esquivel-Gutiérrez, E.; Noriega-Cisneros, R.; Bello-González, M.; Saavedra-Molina, A.; Salgado-Garciglia, R. Plantas utilizadas en la medicina tradicional mexicana con propiedades antidiabéticas y antihipertensivas. Biológicas 2012, 14, 45–52. [Google Scholar]
- Bello-Chavolla, O.Y.; Rojas-Martinez, R.; Aguilar-Salinas, C.A.; Hernández-Avila, M. Epidemiology of diabetes mellitus in Mexico. Nutr. Rev. 2017, 75, 4–12. [Google Scholar] [CrossRef]
- Carranza, G.E. Familia Garryaceae. In Flora del Bajío y de Regiones Adyacentes. Fascículo 49; Instituto de Ecología, A.C. (INECOL): Michoacán, Mexico, 1996; pp. 1–16. [Google Scholar]
- Powell, C.E.; Herrmann, R.G.; Chen, K.K. Pharmacological action of four Garrya alkaloids. J. Am. Pharm. Assoc. (Sci. Ed.) 1956, 45, 733–734. [Google Scholar] [CrossRef]
- Enciclovida. CONABIO. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Available online: https://enciclovida.mx/especies/164188-garrya-laurifolia (accessed on 29 October 2024).
- Ahmed-Sagin, S. Ethnobotanical survey of medicinal plants in Bozyazt, district or Mersin, Turkey. J. Ethnopharmacol. 2015, 173, 105–126. [Google Scholar] [CrossRef]
- OECD. Test No. 423: Acute oral toxicity—Acute toxic class method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002; pp. 1–14. [Google Scholar] [CrossRef]
- Oliver-Bever, B. Drug plants in ancient and modern Mexico. Q. J. Crude Drug Res. 1972, 12, 1957–1972. [Google Scholar] [CrossRef]
- Djerassi, C.; Smith, C.R.; Lippman, A.E.; Figdor, S.K.; Herran, J. Alkaloid studies. VIII. The structures of the diterpenoid alkaloids laurifoline and cuauchichicine. J. Am. Chem. Soc. 1955, 77, 4801–4807. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.A.; Rad, D.L. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. EXCLI J. 2023, 22, 274–294. [Google Scholar] [CrossRef]
- Ouassou, H.; Zahidi, T.; Bouknana, S.; Bouhrim, M.; Mekhfi, H.; Ziyyat, A.; Legssyer, A.; Aziz, M.; Bnouham, M. Inhibition of α-glucosidase, intestinal glucose absorption, and antidiabetic properties by Caralluma europaea. eCAM 2018, 2018, 9589472. [Google Scholar] [CrossRef]
- Meddah, B.; Ducroc, R.; El Abbes Faouzi, M.; Eto, B.; Mahraoui, L.; Benhaddou-Andaloussi, A.; Martineau, L.C.; Cherrah, Y.; Haddad, P.S. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J. Ethnopharmacol. 2009, 121, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Liang, H.; Ye, J.; Weng, J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 2011, 6, e16556. [Google Scholar] [CrossRef]
- Panfoli, I.; Puddu, A.; Bertola, N.; Ravera, S.; Maggi, D. The hormetic effect of metformin: “less is more”? Int. J. Mol. Sci. 2021, 22, 6297. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis and Ginseng: Ginseng mixtures and individual constituents commonly display hormesis dose responses, especially for neuroprotective effects. Molecules 2020, 25, 2719. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, D. Interactions between antidiabetic drugs and herbs: An overview of mechanisms of action and clinical implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 4, e13394. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Li, Z.; Niu, L.; Chen, Y.; Qiu, X.; Du, T.; Zhu, M.; Wang, M.; Mo, H.; Xiao, S. Recent advance in the biological activity of chlorogenic acid and its application in food industry. Int. J. Food Sci. Technol. 2023, 58, 4931–4947. [Google Scholar] [CrossRef]
- Nicasio, P.; Aguilar-Santamaría, L.; Aranda, E.; Ortiz, S.; González, M. Hypoglycemic effect and chlorogenic acid content in two Cecropia species. Phytother. Res. 2005, 19, 661–664. [Google Scholar] [CrossRef]
- Hunyadi, A.; Martins, A.; Hsieh, T.-J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Kartini, K.; Irawan, M.A.; Setiawan, F.; Jayani, N.I.E. Characteristics, isolation methods, and biological properties of Aucubin. Molecules 2023, 28, 4154. [Google Scholar] [CrossRef] [PubMed]
- Kengo, H.; Devkota, H.P. Phenolic Compounds from the aerial parts of Adenophora triphylla (Thunb.) A. DC. var. triphylla and their free radical scavenging activity. Nepal J. Biotechnol. 2020, 8, 12–16. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Djerassi, C.; Smith, C.R.; Lippman, A.E.; Figdor, S.K.; Herran, J. The structures of the diterpenoid alkaloids laurifoline and cuachichicine. Nomenclature alteration. Am. Chem. Soc. 1955, 77, 6633. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Geografía (INEGI). Available online: https://www.inegi.org.mx/app/geo2/ahl/ (accessed on 7 October 2024).
- Martinez, E.M.; Bieski, I.G.C.; de Oliveira, M.D.T. Probability sampling design in the ethnobotanical surveys of medicinal plants. Rev. Bras. Farmacogn. 2012, 22, 1362–1367. [Google Scholar] [CrossRef]
- Martin, G.J. Ethnobotany. A Methods Manual; Chapman and Hall: London, UK, 1995; pp. 95–135. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-de-Santayana, M. Cultural importance indices: A comparative analysis based on the useful wild plants of southern Cantabria (northern Spain). Econ. Bot. 2008, 62, 24–39. [Google Scholar] [CrossRef]
- Calderon, R.G.; Rzedowski, J. Flora Fanerogámica del Valle de México, 2nd ed.; Instituto de Ecología, A.C. y Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Michoacán, Mexico, 2005; pp. 78–80. [Google Scholar]
- Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. NOM-062-ZOO-1999, Especificaciones Técnicas para la Producción, Cuidado y Uso de Animales de Laboratorio; Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación: Mexico City, Mexico, 1999. [Google Scholar]
- National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Palm, F.; Ortsäter, H.; Hansell, P.; Liss, P.; Carlsson, P. Differentiating between effects of streptozotocin per se and subsequent hyperglycemia on renal function and metabolism in the streptozotocin-diabetic rat model. Diabetes Metab. Res. Rev. 2004, 20, 452–459. [Google Scholar] [CrossRef]
- Ghamarian, A.; Abdollahi, M.; Su, X.; Amiri, A.; Ahadi, A.; Nowrozi, A. Effect of chicory seed extraction on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU J. Pharm. Sci. 2012, 20, 56. [Google Scholar] [CrossRef]
- Brand-Williams, W.W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Domínguez, X.A. Métodos de Investigación Fitoquímica; Limusa: Monterrey, Mexico, 1988; pp. 41, 81–219. [Google Scholar]
- Waterhouse, A.L. Supplement 6: Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, E.R.E., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002; pp. l1.1.1–l1.1.8. [Google Scholar] [CrossRef]
- Lamaison, J.L.C.; Carnet, A. Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la vegetation. Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Bruker Daltonics Technical Note 008, Bremen, Germany. 2004.

| # Peak | Rt (min) | Name | [M-H]−obs | [M-H]−exact | Formula | Error | %RA |
|---|---|---|---|---|---|---|---|
| 1 | 0.9 | Unknown | 533.1740 | ------ | ------ | ---- | 8.0 |
| 2 | 3.6 | Aucubin | 345.1176 | 345.1191 | C15H22O9 | −4.4 | 2.1 |
| 2 | 3.6 | Benzyl 4-[2-(2-methoxyphenoxy)acetoxy]benzoate | 391.1211 | 391.1187 | C23H20O6 | 6.2 | 2.1 |
| 3 | 5.7 | Ixoside | 387.0895 | 387.0933 | C16H20O11 | 5.4 | 9.5 |
| 4 | 5.8 | [4-Methyl-7-(1-naphthylmethoxy)-2-oxo-2H-chromen-3-yl]acetic acid | 373.1100 | 373.1081 | C23H18O5 | 4.9 | 30.0 |
| 5 | 6.0 | Chlorogenic acid | 353.0837 | 353.0819 | C16H18O9 | −4.9 | 1.8 |
| 6–7 | 7.0–7.1 | Chlorogenic acid isomer | 353.0839 | 353.0819 | C16H18O9 | -4.6 | 25.0 |
| 8 | 7.7 | Scopolin | 353.0841 | 353.0878 | C16H18O9 | −6.3 | 0.8 |
| 9 | 7.8 | 7-[(4-Methoxybenzyl)oxy]-3-(4-methoxyphenyl)-2-methyl-4H-chromen-4-one | 401.1411 | 401.1394 | C25H22O5 | −4.1 | 0.7 |
| 10 | 8.2 | Clovin | 755.2013 | 755.2040 | C33H40O20 | −3.6 | 1.3 |
| 11 | 8.8 | Rutin | 609.1448 | 609.1461 | C27H30O16 | −2.1 | 13.7 |
| 12 | 9.0 | Myricitrin | 463.0861 | 463.0882 | C21H20O12 | 4.6 | 0.4 |
| 13 | 9.3 | Luteolin 7-O-neohesperidoside | 593.1487 | 593.1512 | C27H30O15 | 4.2 | 2.0 |
| 14 | 10.0 | Unknown | 1127.3501 | ------ | ------ | 0.6 | |
| 15 | 10.3 | Unknown | 1483.4842 | ------ | ------ | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estévez-Carmona, M.M.; Pablo-Pérez, S.S.; Almanza-Cruz, J.E.; Meléndez-Camargo, M.E.; Arrieta-Baez, D.; Cristóbal-Luna, J.M.; Franco-Colín, M. Ethnopharmacological Study of Garrya laurifolia and Its Antidiabetic Effect in Rats. Plants 2024, 13, 3235. https://doi.org/10.3390/plants13223235
Estévez-Carmona MM, Pablo-Pérez SS, Almanza-Cruz JE, Meléndez-Camargo ME, Arrieta-Baez D, Cristóbal-Luna JM, Franco-Colín M. Ethnopharmacological Study of Garrya laurifolia and Its Antidiabetic Effect in Rats. Plants. 2024; 13(22):3235. https://doi.org/10.3390/plants13223235
Chicago/Turabian StyleEstévez-Carmona, María Mirian, Saudy Saret Pablo-Pérez, Jesús Eduardo Almanza-Cruz, María Estela Meléndez-Camargo, Daniel Arrieta-Baez, José Melesio Cristóbal-Luna, and Margarita Franco-Colín. 2024. "Ethnopharmacological Study of Garrya laurifolia and Its Antidiabetic Effect in Rats" Plants 13, no. 22: 3235. https://doi.org/10.3390/plants13223235
APA StyleEstévez-Carmona, M. M., Pablo-Pérez, S. S., Almanza-Cruz, J. E., Meléndez-Camargo, M. E., Arrieta-Baez, D., Cristóbal-Luna, J. M., & Franco-Colín, M. (2024). Ethnopharmacological Study of Garrya laurifolia and Its Antidiabetic Effect in Rats. Plants, 13(22), 3235. https://doi.org/10.3390/plants13223235








