Bioactive Properties of Campomanesia lineatifolia: Correlation Between Anti-Helicobacter pylori Activity, Antioxidant Potential and Chemical Composition
Abstract
1. Introduction
2. Results
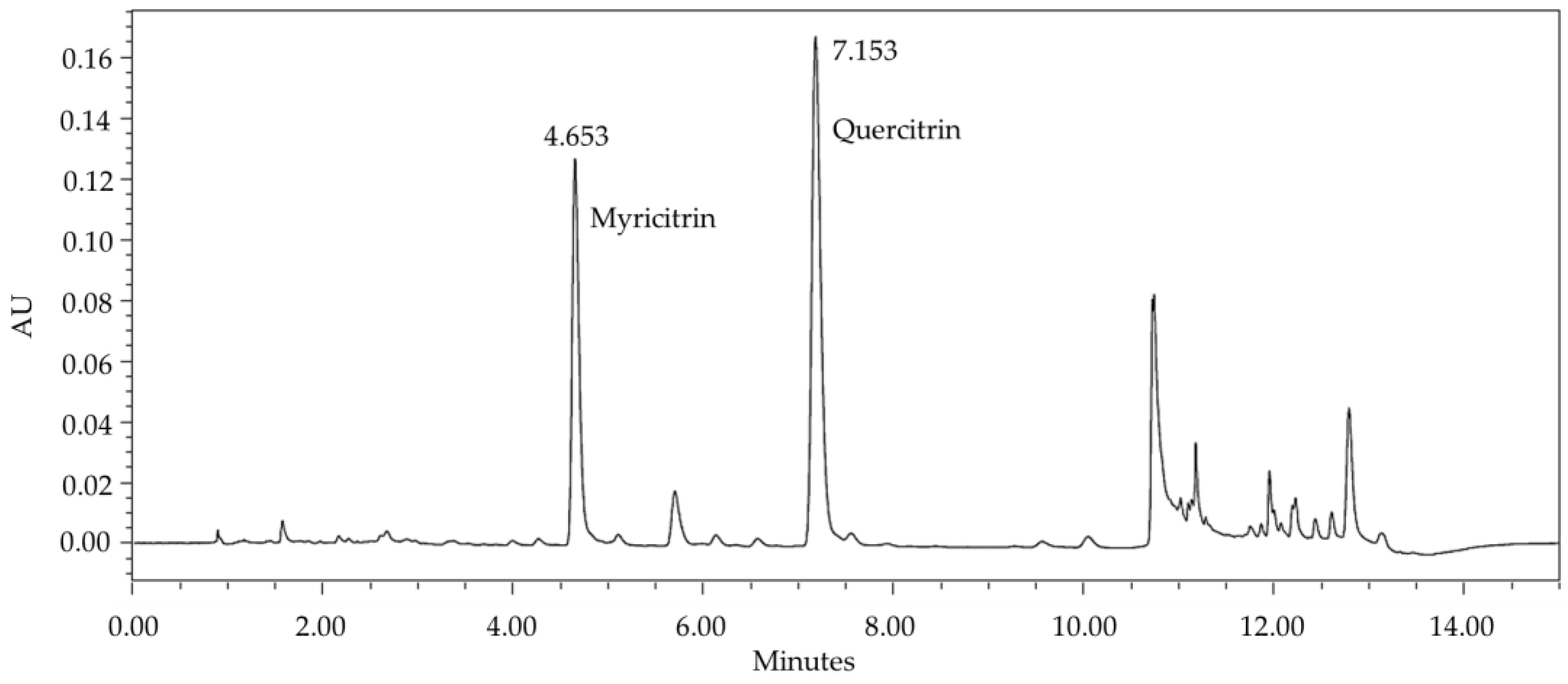
2.1. Extraction and Chemical Profile of PEE, Fractions and Isolated Flavonols
2.2. Tannin and Flavonoid Content
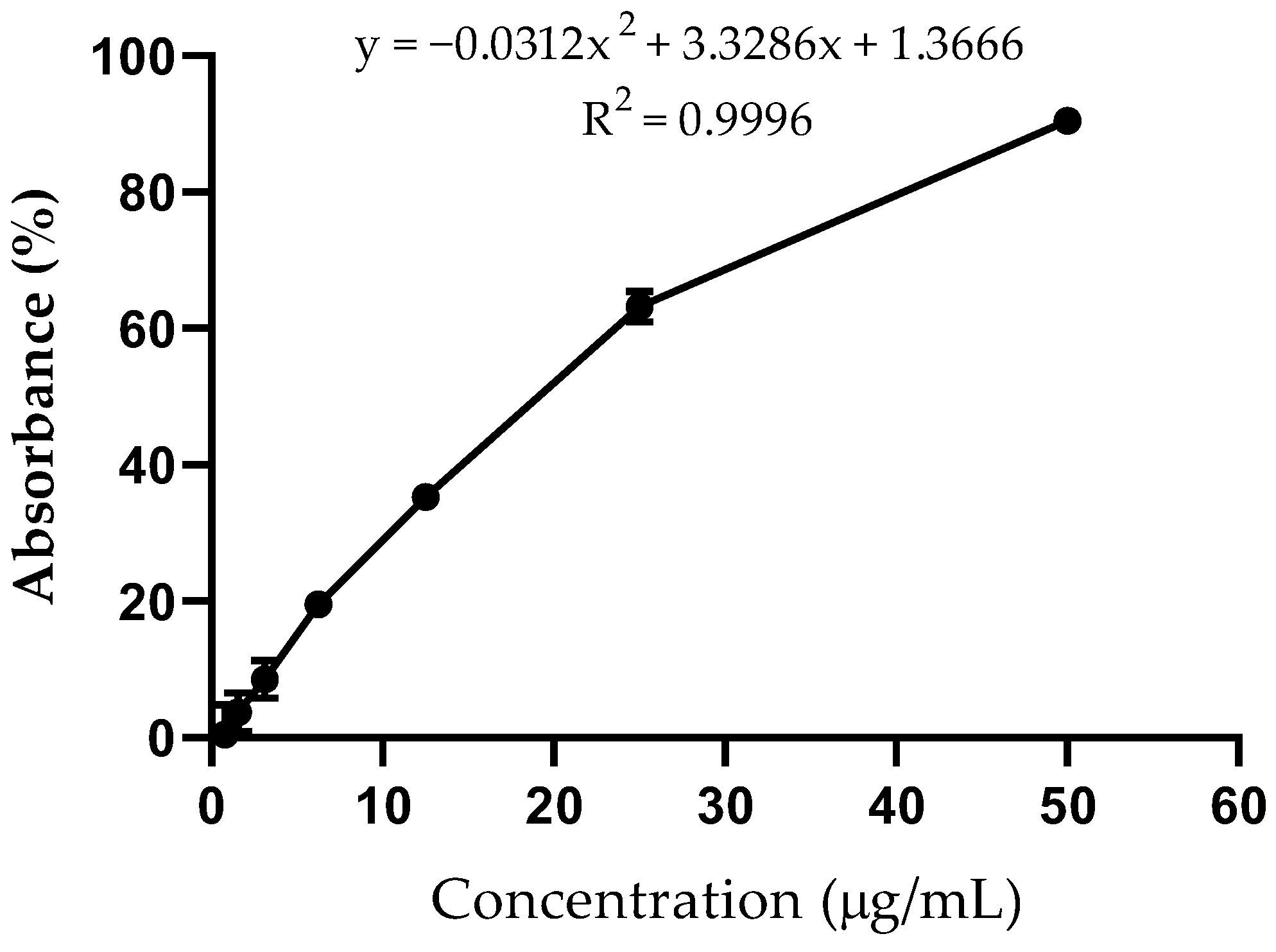
2.3. Antioxidant Activity
2.4. Anti-Helicobacter pylori Activity
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Phenolic-Rich Ethanol Extract
4.4. Chemical Profile of Phenolic-Rich Extract by UHPCL
4.5. Polar and Non-Polar Fractions
4.6. Aqueous Extract
4.7. Myricitrin and Myricitrin+Quercitrin
4.8. Determination of Tannin Content
4.9. Determination of Flavonoid Content
4.10. Evaluation of Antioxidant Activity
= [(A1 − A0)/A1] × 100
4.11. Evaluation of Anti-Helicobacter pylori Activity
4.11.1. Bacterial Strains and Culture Conditions
4.11.2. Broth Microdilution Assays
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Q.; Yuan, C.; Zhou, S.; Lu, J.; Zeng, M.; Cai, X.; Song, H. Helicobacter pylori Infection: A Dynamic Process from Diagnosis to Treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1257817. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Katelaris, P.; Hunt, R.; Bazzoli, F.; Cohen, H.; Fock, K.M.; Gemilyan, M.; Malfertheiner, P.; Mégraud, F.; Piscoya, A.; Quach, D.; et al. Helicobacter pylori World Gastroenterology Organization Global Guideline. J. Clin. Gastroenterol. 2023, 57, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-Analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Bi, W.-P. Efficacy and Safety of Herbal Medicines in Treating Gastric Ulcer: A Review. World J. Gastroenterol. 2014, 20, 17020. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchová, K.; Majerová, M.; Pokorná, M.; Švajdlenka, E.; Vajdlenka, E.Š. In vitro synergistic effect of Hibiscus sabdariffa aqueous extract in combination with standard antibiotics against Helicobacter pylori clinical isolates. Pharm. Biol. 2016, 54, 1736–1740. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium majus and Corydalis cheilanthifolia Extracts against Multidrug-Resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- D’Eeckenbrugge, G.C.; Ferla, D.L. Fruits from America: An Ethonobotanical Inventory; Institute for Plant Genetic Resources (IPGRI)/Centre de Coopéracion Internationale en Recherché Agronomique Pour de Developpement. Département des Production Fruitières et Horticoles (CIRAD-FLHOR): Cali, Colombia, 2000. [Google Scholar]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae in Lista de Espécies Da Flora Do Brasil. Rodriguésia 2015, 66, 1085–1113. [Google Scholar]
- Villachica, H.; Carvalho, J.F.U.; Muller, C.H.; Diaz, C.S.; Almanza, M. Frutales y Hortializas Promisorios de la Amazonia; Tratado de Cooperacion Amazonica, Secretaria Pro-Tempore Publicaciones: Lima, Peru, 1996; Volume 44. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429171482. [Google Scholar]
- Grandtner, M.M.; Chevrette, J. (Eds.) Dictionary of Trees, Volume 2: South America: Nomenclature, Taxonomy and Ecology; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-396490-8. [Google Scholar]
- Rutter, R.A. Catalogo de Plantas Utiles de la Amazonia Peruana, 2nd ed.; Instituto Linguistico de Verano: Lima, Peru, 1990. [Google Scholar]
- Pio Corrêa, M. Dictionary of Useful Brazilian and Exotic Cultivated Plants; National Press: Rio de Janeiro, Brazil, 1952. [Google Scholar]
- de Oliveira Cabral, C.; Campos, A.; da Silva, L.M.; Boeing, T.; de Andrade, S.F.; Filho, V.C.; Nesello, L.Â.N. Gastroprotective Potential of Methanolic Extract and Dimethyl Cardamonin from Campomanesia reitziana Fruits in Mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 661–666. [Google Scholar] [CrossRef]
- Markman, B.E.O.; Bacchi, E.M.; Kato, E.T.M. Antiulcerogenic Effects of Campomanesia xanthocarpa. J. Ethnopharmacol. 2004, 94, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Neves, N.C.V.; de Mello, M.P.; Smith, S.M.; Boylan, F.; Caliari, M.V.; Castilho, R.O. Chemical Composition and In Vitro Anti-Helicobacter pylori Activity of Campomanesia lineatifolia Ruiz & Pavón (Myrtaceae) Essential Oil. Plants 2022, 11, 1945. [Google Scholar] [CrossRef] [PubMed]
- Neves, N.C.V.; de Mello, M.P.; Amorim, J.M.; Faraco, A.A.G.; Castilho, R.O. Optimization of Phenolic Compounds Extraction from Campomanesia lineatifolia Leaves. Rodriguésia 2020, 71, e01072019. [Google Scholar] [CrossRef]
- Neves, N.C.V.; de Mello, M.P.; Zaidan, I.; Sousa, L.P.; Braga, A.V.; Machado, R.R.; Kukula-Koch, W.; Boylan, F.; Caliari, M.V.; Castilho, R.O. Campomanesia lineatifolia Ruiz & Pavón (Myrtaceae): Isolation of Major and Minor Compounds of Phenolic-Rich Extract by High-Speed Countercurrent Chromatography and Anti-Inflammatory Evaluation. J. Ethnopharmacol. 2023, 310, 116417. [Google Scholar] [CrossRef] [PubMed]
- Neves, N.C.V. Campomanesia lineatifolia Ruiz & Pav.: Caracterização Fitoquímica, Estudos Mecanísticos da Atividade Gastroprotetora e Avaliação da Atividade Anti-Helicobacter pylori. Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2021. [Google Scholar]
- Madalosso, R.C.; Oliveira, G.C.; Martins, M.T.; Vieira, A.E.D.; Barbosa, J.; Caliari, M.V.; Castilho, R.O.; Tagliati, C.A. Campomanesia lineatifolia Ruiz & Pav. as a Gastroprotective Agent. J. Ethnopharmacol. 2012, 139, 772–779. [Google Scholar] [CrossRef][Green Version]
- Barbosa, J. Campomanesia lineatifolia Ruiz e Pav.: Estudo Fitoquímico e Avaliação da Atividade Antioxidante. Master’s Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2011. [Google Scholar]
- Davies, G.R.; Simmonds, N.J.; Stevens, T.R.J.; Sheaff, M.T.; Banatvala, N.; Laurenson, I.F.; Blake, D.R.; Rampton, D.S. Helicobacter pylori Stimulates Antral Mucosal Reactive Oxygen Metabolite Production in Vivo. Gut 1994, 35, 179–185. [Google Scholar] [CrossRef]
- Sant’anna, L.S.; Merlugo, L.; Ehle, C.S.; Limberger, J.; Fernandes, M.B.; Santos, M.C.; Mendez, A.S.L.; Paula, F.R.; Moreira, C.M. Chemical Composition and Hypotensive Effect of Campomanesia xanthocarpa. Evid.-Based Complement. Altern. Med. 2017, 2017, 1591762. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Coelho, R.G.; Kataoka, V.M.F.; Honda, N.K.; Silva, J.R.M.; Vilegas, W.; Cardoso, C.A.L. Determination of Phenolic Compounds and Evaluation of Antioxidant Capacity of Campomanesia adamantium Leaves. Eclet. Quim. 2008, 33, 53–60. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Cardoso, C.A.L.; Ré-Poppi, N.; Melo, A.M.; Vieira, M.D.C.; Honda, N.K.; Coelho, R.G. Gas Chromatography-Mass Spectrometry (GC-MS) and Evaluation of Antioxidant and Antimicrobial Activities of Essential Oil of Campomanesia adamantium (Cambess.) O. Berg (Guavira). Braz. J. Pharm. Sci. 2009, 45, 767–776. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Mocan, A.; Sepúlveda, B. High Resolution Metabolite Fingerprinting of the Resin of Baccharis tola Phil. from the Atacama Desert and Its Antioxidant Capacities. Ind. Crops Prod. 2016, 94, 368–375. [Google Scholar] [CrossRef]
- Pascoal, A.C.R.F.; Ehrenfried, C.A.; Eberlin, M.N.; Stefanello, M.É.A.; Salvador, M.J. Free Radical Scavenging Activity, Determination of Phenolic Compounds and HPLC-DAD/ESI-MS Profile of Campomanesia adamantium Leaves. Nat. Prod. Commun. 2011, 6, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Catelan, T.B.S.; Santos Radai, J.A.; Leitão, M.M.; Branquinho, L.S.; Vasconcelos, P.C.d.P.; Heredia-Vieira, S.C.; Kassuya, C.A.L.; Cardoso, C.A.L. Evaluation of the Toxicity and Anti-Inflammatory Activities of the Infusion of Leaves of Campomanesia guazumifolia (Cambess.) O. Berg. J. Ethnopharmacol. 2018, 226, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, V.M.F.; Cardoso, C.A.L. Evaluation of the Chromatographic Profile and the Antioxidant Activity of the Species Campomanesia sessiliflora (O. Berg) Mattos and Campomanesia xanthocarpa O. Berg. Rev. Bras. Plantas Med. 2013, 15, 121–129. [Google Scholar] [CrossRef]
- Klafke, J.Z.; Pereira, R.L.D.; Hirsch, G.E.; Parisi, M.M.; Porto, F.G.; de Almeida, A.S.; Rubin, F.H.; Schmidt, A.; Beutler, H.; Nascimento, S.; et al. Study of Oxidative and Inflammatory Parameters in LDLr-KO Mice Treated with a Hypercholesterolemic Diet: Comparison between the Use of Campomanesia xanthocarpa and Acetylsalicylic Acid. Phytomedicine 2016, 23, 1227–1234. [Google Scholar] [CrossRef]
- Klafke, J.Z.; Arnoldi Da Silva, M.; Fortes Rossato, M.; Trevisan, G.; Banderó Walker, C.I.; Martins Leal, C.A.; Olschowsky Borges, D.; Chitolina Schetinger, M.R.; Noal Moresco, R.; Medeiros Frescura Duarte, M.M.; et al. Antiplatelet, Antithrombotic, and Fibrinolytic Activities of Campomanesia xanthocarpa. Evid.-Based Complement. Altern. Med. 2012, 2012, 954748. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Grabe-Guimarães, A.; De Paula, C.A.; Michel, M.C.P.; Guimarães, R.G.; Rezende, S.A.; De Souza Filho, J.D.; Saúde-Guimarães, D.A. Anti-Inflammatory and Antinociceptive Activities of Campomanesia adamantium. J. Ethnopharmacol. 2013, 145, 100–108. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.l.; Zhang, J.y.; Song, X.n.; Zhang, Z.y.; Li, J.f.; Li, S. Anti-Ulcer and Anti-Helicobacter pylori Potentials of the Ethyl Acetate Fraction of Physalis alkekengi L. Var. Franchetii (Solanaceae) in Rodent. J. Ethnopharmacol. 2018, 211, 197–206. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Cellini, L.; Di Campli, E.; Masulli, M.; Di Bartolomeo, S.; Allocati, N. Inhibition of Helicobacter pylori by Garlic Extract (Allium sativum). FEMS Immunol. Med. Microbiol. 1996, 13, 273–277. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and Curcumin Inhibit the Growth of Helicobacter pylori, a Group 1 Carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar]
- Siddaraju, M.N.; Dharmesh, S.M. Inhibition of Gastric H+,K+-ATPase and Helicobacter pylori Growth by Phenolic Antioxidants of Zingiber officinale. Mol. Nutr. Food Res. 2007, 51, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Shoae Hassani, A.; Ordouzadeh, N.; Ghaemi, A.; Amirmozafari, N.; Hamdi, K.; Nazari, R. In Vitro Inhibition of Helicobacter pylori Urease with Non and Semi Fermented Camellia sinensis. Indian J. Med. Microbiol. 2009, 27, 30–34. [Google Scholar] [CrossRef]
- Voravuthikunchai, S.P.; Limsuwan, S.; Mitchell, H. Effects of Punica granatum Pericarps and Quercus infectoria Nutgalls on Cell Surface Hydrophobicity and Cell Survival of Helicobacter pylori. J. Health Sci. 2006, 52, 154–159. [Google Scholar] [CrossRef][Green Version]
- Mayyas, A.; Abu-Sini, M.; Amr, R.; Akasheh, R.T.; Zalloum, W.; Khdair, A.; Hamad, I.; Aburjai, T.; Darwish, R.M.; Abu-Qatouseh, L. Novel in Vitro and in Vivo Anti-Helicobacter pylori Effects of Pomegranate Peel Ethanol Extract. Vet. World 2021, 14, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, F.; Harada, N.; Yamada, M.; Murohisa, B.; Oguni, I. Inhibitory Effect of Green Tea Catechins in Combination with Sucralfate on Helicobacter pylori Infection in Mongolian Gerbils. J. Gastroenterol. 2004, 39, 61–63. [Google Scholar] [CrossRef]
- Modolo, L.V.; de Souza, A.X.; Horta, L.P.; Araujo, D.P.; de Fátima, Â. An Overview on the Potential of Natural Products as Ureases Inhibitors: A Review. J. Adv. Res. 2015, 6, 35–44. [Google Scholar] [CrossRef]
- Yang, H.; Hu, B. Immunological Perspective: Helicobacter pylori Infection and Gastritis. Mediat. Inflamm. 2022, 2022, 2944156. [Google Scholar] [CrossRef]
- Elloumi, W.; Mahmoudi, A.; Ortiz, S.; Boutefnouchet, S.; Chamkha, M.; Sayadi, S. Wound Healing Potential of Quercetin-3-O-Rhamnoside and Myricetin-3-O-Rhamnoside Isolated from Pistacia lentiscus Distilled Leaves in Rats Model. Biomed. Pharmacother. 2022, 146, 112574. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Ye, W.C.; Che, C.T.; Zhao, S.X. Cycloartane Triterpenes and Glycosides from Cimicifuga acerina. Yaoxue Xuebao 2001, 36, 287–291. [Google Scholar]
- Okba, M.M.; El Gedaily, R.A.; Ashour, R.M. UPLC–PDA–ESI–QTOF-MS Profiling and Potent Anti-HSV-II Activity of Eucalyptus sideroxylon Leaves. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1068–1069, 335–342. [Google Scholar] [CrossRef]
- de Albuquerque, R.L.; Kentopff, M.R.; Machado, M.I.L.; Silva, M.G.V.; Matos, F.J.d.A.; Morais, S.M.; Braz-Filho, R. Diterpenos Tipo Abietano Isolados de Plectranthus barbatus Andrews. Quim. Nova 2007, 30, 1882–1886. [Google Scholar] [CrossRef]
- Costa-Lotufo, L.V.; Araújo, E.C.C.; Lima, M.A.S.; Moraes, M.E.A.; Pessoa, C.; Silviera, E.R.; Moraes, M.O. Antiproliferative Effects of Abietane Diterpenoids Isolated from Hyptis martiusii Benth (Labiatae). Pharmazie 2004, 59, 78–79. [Google Scholar] [PubMed]
- Yoshikawa, M.; Yamaguchi, S.; Kunimi, K.; Matsuda, H.; Okuno, Y.; Yamahara, J.; Murakami, N. Stomachic Principles in Ginger. III. An Anti-Ulcer Principle, 6-Gingesulfonic Acid, and Three Monoacyldigalactosylglycerols, Gingerglycolipids A, B, and C, from Zingiberis Rhizoma Originating in Taiwan. Chem. Pharm. Bull. 1994, 42, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Augusto, A.C.; Miguel, F.; Mendonça, S.; Pedrazzoli, J.; Gurgueira, S.A. Oxidative Stress Expression Status Associated to Helicobacter pylori Virulence in Gastric Diseases. Clin. Biochem. 2007, 40, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus berries for the Control of Gastric Inflammation: In Vitro and In Vivo Studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of Phenolic Compounds and Antioxidant Properties of Glycyrrhiza glabra L. Rhizomes and Roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef]
- Wittschier, N.; Faller, G.; Hensel, A. Aqueous Extracts and Polysaccharides from Liquorice Roots (Glycyrrhiza glabra L.) Inhibit Adhesion of Helicobacter pylori to Human Gastric Mucosa. J. Ethnopharmacol. 2009, 125, 218–223. [Google Scholar] [CrossRef]
- Asha, M.K.; Debraj, D.; Prashanth, D.; Edwin, J.R.; Srikanth, H.S.; Muruganantham, N.; Dethe, S.M.; Anirban, B.; Jaya, B.; Deepak, M.; et al. In Vitro Anti-Helicobacter pylori Activity of a Flavonoid Rich Extract of Glycyrrhiza glabra and Its Probable Mechanisms of Action. J. Ethnopharmacol. 2013, 145, 581–586. [Google Scholar] [CrossRef]
- Rahnama, M.; Mehrabani, D.; Japoni, S.; Edjtehadi, M.; Saberi-Firoozi, M. The Healing Effect of Licorice (Glycyrrhiza glabra) on Helicobacter pylori Infected Peptic Ulcers. J. Res. Med. Sci. 2013, 18, 532–533. [Google Scholar]
- Mukherjee, M.; Bhaskaran, N.; Srinath, R.; Shivaprasad, H.N.; Allan, J.J.; Shekhar, D.; Agarwal, A. Anti-Ulcer and Antioxidant Activity of GutGard™. Indian J. Exp. Biol. 2010, 48, 269–274. [Google Scholar]
- Hajiaghamohammadi, A.A.; Zargar, A.; Oveisi, S.; Samimi, R.; Reisian, S. To Evaluate of the Effect of Adding Licorice to the Standard Treatment Regimen of Helicobacter pylori. Braz. J. Infect. Dis. 2016, 20, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Christoph, F.; Kaulfers, P.M.; Stahl-Biskup, E. A Comparative Study of the in Vitro Antimicrobial Activity of Tea Tree Oils s.I. with Special Reference to the Activity of β-Triketones. Planta Med. 2000, 66, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Christoph, F.; Kaulfers, P.M.; Stahl-Biskup, E. In Vitro Evaluation of the Antibacterial Activity of β-Triketones Admixed to Melaleuca Oils. Planta Med. 2001, 67, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Giordano, O.S.; Guerreiro, E.; Pestchanker, M.J.; Guzman, J.; Pastor, D.; Guardia, T. The Gastric Cytoprotective Effect of Several Sesquiterpene Lactones. J. Nat. Prod. 1990, 53, 803–809. [Google Scholar] [CrossRef]
- Whazin, K.A.; Gatilov, Y.B.; Adekenev, S.M. Gaigranin and Gaigrandin—New Sesquiterpene Lactones from Gaillardia grandiflora. Chem. Nat. Compd. 1995, 31, 63–67. [Google Scholar] [CrossRef]
- Reis, M.G.; De Faria, A.D.; Dos Santos, I.A.; Amaral, M.D.C.E.; Marsaioli, A.J. Byrsonic Acid—The Clue to Floral Mimicry Involving Oil-Producing Flowers and Oil-Collecting Bees. J. Chem. Ecol. 2007, 33, 1421–1429. [Google Scholar] [CrossRef]
- Santos, R.C.; Kushima, H.; Rodrigues, C.M.; Sannomiya, M.; Rocha, L.R.M.; Bauab, T.M.; Tamashiro, J.; Vilegas, W.; Hiruma-Lima, C.A. Byrsonima Intermedia A. Juss.: Gastric and Duodenal Anti-Ulcer, Antimicrobial and Antidiarrheal Effects in Experimental Rodent Models. J. Ethnopharmacol. 2012, 140, 203–212. [Google Scholar] [CrossRef]
- Hu, H.B.; Zheng, X.D.; Hu, H.S. Analysis of Flavonoids from Leaves of Acanthopanax brachypus Harms. J. Chil. Chem. Soc. 2013, 58, 1549–1552. [Google Scholar] [CrossRef]
- Hu, H.s.; Hu, H.b.; Zheng, X.d. Study on Chemical Constituents and Antimicrobial Activity of the Essential Oil from Acanthopanax brachypus. Zhong Yao Cai 2009, 32, 67–70. [Google Scholar]
- Takasaki, M.; Tokuda, H.; Nishino, H.; Konoshima, T. Cancer Chemopreventive Agents (Antitumor-Promoters) from Ajuga decumbens. J. Nat. Prod. 1999, 62, 972–975. [Google Scholar] [CrossRef]
- Israili, Z.H.; Lyoussi, B. Ethnopharmacology of the Plants of Genus Ajuga. Pak. J. Pharm. Sci. 2009, 22, 425–462. [Google Scholar] [PubMed]
- Grkovic, T.; Blees, J.S.; Colburn, N.H.; Schmid, T.; Thomas, C.L.; Henrich, C.J.; McMahon, J.B.; Gustafson, K.R. Cryptocaryols A-H, α-Pyrone-Containing 1,3-Polyols from Cryptocarya sp. Implicated in Stabilizing the Tumor Suppressor Pdcd4. J. Nat. Prod. 2011, 74, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.T.B. Resistance to Clarithromycin and Gastroenterologist’s Persistence Roles in Nomination for Helicobacter pylori as High Priority Pathogen by World Health Organization. World J. Gastroenterol. 2017, 23, 6379–6384. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.G.V.; Marinho, J.R.; Genta, R.; Ribeiro, L.T.; Passos, M.d.C.F.; Zaterka, S.; Assumpção, P.P.; Barbosa, A.J.A.; Barbuti, R.; Braga, L.L.; et al. IVth Brazilian Consensus Conference on Helicobacter pylori Infection. Arq. Gastroenterol. 2018, 55, 97–121. [Google Scholar] [CrossRef]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Ichijima, R.; Ohyauchi, M.; Ito, H.; Kawamura, M.; Ogata, Y.; Ohtaka, M.; et al. Seven-Day Vonoprazan and Low-Dose Amoxicillin Dual Therapy as First-Line Helicobacter pylori Treatment: A Multicentre Randomised Trial in Japan. Gut 2020, 69, 1019–1026. [Google Scholar] [CrossRef]
- Ginovyan, M.; Trchounian, A. Novel Approach to Combat Antibiotic Resistance: Evaluation of Some Armenian Herb Crude Extracts for Their Antibiotic Modulatory and Antiviral Properties. J. Appl. Microbiol. 2019, 127, 472–480. [Google Scholar] [CrossRef]
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef]
- Brazilian Health Regulatory Agency (Ed.) Brazilian Pharmacopoeia, 6th ed.; ANVISA: Brasilia, Brazil, 2019. [Google Scholar]
- Kurechi, T.; Kikugawa, K.; Kato, T. Studies on the Antioxidants. XIII. Hydrogen Donating Capability of Antioxidants to 2,2-Diphenyl-1-Picrylhydrazyl. Chem. Pharm. Bull. 1980, 28, 2089–2093. [Google Scholar] [CrossRef]
- Patel, J.B.; Cockerill, F.R.; Bradford, P.A. (Eds.) M100-S25 Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 35. [Google Scholar]
- Borges, A.S.; Minozzo, B.R.; Santos, H.; Ardisson, J.S.; Rodrigues, R.P.; Romão, W.; Borges, W.d.S.; Gonçalves, R.d.C.R.; Beltrame, F.L.; Kitagawa, R.R. Plectranthus barbatus Andrews as Anti-Helicobacter pylori Agent with Activity against Adenocarcinoma Gastric Cells. Ind. Crops Prod. 2020, 146, 112207. [Google Scholar] [CrossRef]
| Samples | Helicobacter pylori Strains/MIC * | |||
|---|---|---|---|---|
| ATCC 49503 a | NCTC 11638 a | S SR359 b | S SR366 c | |
| PEE | 0.49 μg/mL | 0.49 μg/mL | 125 μg/mL | 250 μg/mL |
| Polar fraction | 500 μg/mL | 0.49 μg/mL | 125 μg/mL | 250 μg/mL |
| Non-polar fraction | 250 μg/mL | 125 μg/mL | 125 μg/mL | 125 μg/mL |
| MYR | 62.5 μg/mL | 250 μg/mL | NI | 250 μg/mL |
| MYR/QUER | NI | NI | 31.25 μg/mL | NI |
| Tea | 1:100 | 1:100 | 1:100 | 1:100 |
| Antimicrobial control | ||||
| Clarithromycin | 0.03 μg/mL | 0.03 μg/mL | 0.03 μg/mL | 8 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, N.C.V.; de Mello, M.P.; Smith, S.M.; Boylan, F.; Caliari, M.V.; Castilho, R.O. Bioactive Properties of Campomanesia lineatifolia: Correlation Between Anti-Helicobacter pylori Activity, Antioxidant Potential and Chemical Composition. Plants 2024, 13, 3117. https://doi.org/10.3390/plants13223117
Neves NCV, de Mello MP, Smith SM, Boylan F, Caliari MV, Castilho RO. Bioactive Properties of Campomanesia lineatifolia: Correlation Between Anti-Helicobacter pylori Activity, Antioxidant Potential and Chemical Composition. Plants. 2024; 13(22):3117. https://doi.org/10.3390/plants13223117
Chicago/Turabian StyleNeves, Nívea Cristina Vieira, Morgana Pinheiro de Mello, Sinéad Marian Smith, Fabio Boylan, Marcelo Vidigal Caliari, and Rachel Oliveira Castilho. 2024. "Bioactive Properties of Campomanesia lineatifolia: Correlation Between Anti-Helicobacter pylori Activity, Antioxidant Potential and Chemical Composition" Plants 13, no. 22: 3117. https://doi.org/10.3390/plants13223117
APA StyleNeves, N. C. V., de Mello, M. P., Smith, S. M., Boylan, F., Caliari, M. V., & Castilho, R. O. (2024). Bioactive Properties of Campomanesia lineatifolia: Correlation Between Anti-Helicobacter pylori Activity, Antioxidant Potential and Chemical Composition. Plants, 13(22), 3117. https://doi.org/10.3390/plants13223117








