Abstract
Acidic stress is a formidable environmental factor that exerts adverse effects on plant growth and development, ultimately leading to a potential reduction in agricultural productivity. A low pH triggers Ca2+ influx across the plasma membrane (PM), eliciting distinct responses under various acidic pH levels. However, the underlying mechanisms by which Arabidopsis plant cells generate stimulus-specific Ca2+ signals in response to acidic stress remain largely unexplored. The experimentally induced stimulus may elicit spikes in cytosolic free Ca2+ concentration ([Ca2+]i) spikes or complex [Ca2+]i oscillations that persist for 20 min over a long-term of 24 h or even several days within the plant cytosol and chloroplast. This study investigated the increase in [Ca2+]i under a gradient of low pH stress ranging from pH 3.0 to 6.0. Notably, the peak of [Ca2+]i elevation was lower at pH 4.0 than at pH 3.0 during the initial 8 h, while other pH levels did not significantly increase [Ca2+]i compared to low acidic stress conditions. Lanthanum chloride (LaCl3) can effectively suppress the influx of [Ca2+]i from the apoplastic to the cytoplasm in plants under acid stress, with no discernible difference in intracellular calcium levels observed in Arabidopsis. Following 8 h of acid treatment in the darkness, the intracellular baseline Ca2+ levels in Arabidopsis were significantly elevated when exposed to low pH stress. A moderately low pH, specifically 4.0, may function as a spatial-temporal input into the circadian clock system. These findings suggest that acid stimulation can exert a continuous influence on intracellular calcium levels, as well as plant growth and development.
1. Introduction
Acid stress is becoming an increasingly severe environmental issue [1,2]. Both the area affected by acid rain and the acidity of rainwater are increasing, and the situation could worsen soon [3]. While increased fertilizer use has increased crop yields worldwide, improper management has caused severe soil acidification, which has had a negative impact on crop yields, especially in China [4]. Acid rain could damage plant cell membrane systems and could negatively affect respiration, photosynthesis, and the antioxidative enzyme system [5,6,7,8]. The decrease in soil pH value will reduce the utilization of soil nutrients (phosphorus, potassium, calcium, and magnesium) and affect the structure and function of microbial communities while increasing the effects of toxic heavy metals (such as cadmium and lead) and other harmful elements (such as aluminum and manganese), especially at a pH below 4.5 [9]. Plants cannot move and are confronted with changing environmental conditions, such as light, water, biotic and abiotic stress. The vacuole regulates osmotic pressure, cytosolic ion homeostasis, protein degradation, and the storage of nutrients and secondary metabolic compounds to cope with harsh environmental conditions. By using a positive charge surplus on the inside of the concentration and the electrostatic gradient, the vacuole can pump other ions and metabolites against their concentration gradient into the vacuole to maintain osmotic balance and functions [10].
The cytosolic pH in Arabidopsis is at ~pH 7.4, which was maintained by organic acids and phosphates and plasma membrane-resident proton pumps [11]. In the Arabidopsis, two V-ATPases and one vacuolar pyrophosphatase pump assess protons from the cytosol into the vacuole to establish the proton gradient through ATP energy and pyrophosphate [12]. In the Arabidopsis endomembrane system, the pH drops from 7.1 in the endoplasmic reticulum to 5.6 in the trans-Golgi network/early endosome, and vacuoles of Arabidopsis root cells typically have a pH of 5.8 [13,14,15]. Changes in the pH of the medium surrounding Arabidopsis roots have shown a substantial effect on the apoplastic pH but not cytosolic pH [16]. According to the “acid growth hypothesis”, auxin mediates the SCFTIR1/AFB signaling pathway [17,18] and promotes the expression of SMALL Auxin UP RNA (SAUR) [19], which binds to PM-targeted PP2C.D2/PP2C.D5/PP2C.D6 [19,20,21]. The binding to the PM H+ -ATP complex inhibits the dephosphorylation of Thr947, keeping these protons pumping phosphorylation and leading toward proton efflux. The decrease in extracellular pH alters the activity of cell-wall-modifying proteins, including expansins, xyloglucan endoglycosylase/hydrolase (XTH), and PMEs [22,23,24], leading to changes in wall extension. Proton pumping also hyperpolarized PM, activating inward K+ channels and activating H+-coupled anion symporters (X−). This transport increases solute uptake activities and maintains the solute absorption required for water uptake and the pressure that forces the wall to expand.
The effect of auxin on cell expansion depends on both concentration and tissue. In general, auxin concentration can promote stem cell expansion and inhibit root cell expansion in physiology. We found that synthetic auxin inhibits root elongation. In addition, the auxin receptor antagonist auxin can restore the inhibitory effect of exogenous IAA on root growth. In addition, N-1-naphthalophthalic acid, a polar transport inhibitor of auxin, also affects root bending [25]. In studies on the auxin regulation of root cell expansion, apoplastic acidification induced by exogenous auxin can both promote and inhibit [26] root cell expansion and trigger apoplastic alkalization as well as acidification [27]. These paradoxical findings suggest that auxin plays a complex and dynamic role in controlling root cell pH homeostasis and cell expansion. Kinematic studies are needed to evaluate elongation beyond the meristem effect of auxin on root growth. Auxin-induced alkalization occurs within 15s [28,29]. Consistent with this extremely rapid alkalization is increased cytoplasmic Ca2+ ([Ca2+]i) levels. The increase in extracellular pH was inhibited both by pretreatment with the Ca2+ channel blocker La3+ [29,30] and by Ca2+ ionophores [30], suggesting that elevated [Ca2+]i acts as a second messenger mediating auxin-induced alkalization.
Calcium ions (Ca2+) have been adopted as a ubiquitous intracellular second messenger, which plays an essential role in understanding a complicated network of plant growth and responses to abiotic and biotic stimuli, including salinity, cold, and drought [31,32]. Plant cells can rapidly change cytosolic free Ca2+ concentrations in a changing environment in time and space. The spatio-temporal patterning of cellular Ca2+ dynamics has been formulated as a concept of the Ca2+ signature. The Ca2+ signature, namely the alterations in amplitude, duration, frequency, and spatial distribution of the Ca2+ signal, encodes information about the type and the strength of the stimuli [33,34]. Downstream effectors decode such Ca2+-encoded stimulus-specific information. Most downstream effectors are Ca2+-sensing proteins, which bind to Ca2+ to initiate or regulate biochemical processes and ultimately translate information into specific end responses [35,36,37]. However, the mechanisms of how plant cells generate stimulus-specific Ca2+ signals have not yet been explored. There is a need to understand how plant cells initiate stimulus-specific Ca2+ signals and perceive and transduce signals to cope with unfavorable environmental conditions. Low pH stress is representative of such abiotic stress. However, the molecular mechanisms surrounding the initial perception of low pH stress are still unknown. Arabidopsis as a model plant was used to better understand the effects of low pH stress in cotyledons on basal Ca2+ signaling.
There are 24 h [Ca2+]i oscillations of the cytosol and chloroplastic in light and dark cycles, or constant light, which rise to a peak of 300 nM, regulated by the circadian clock and light signaling [38,39,40,41,42]. Ca2+ oscillations of the cytosol are driven by the rhythmic production of cyclic ADP ribose [43] and are explicitly suppressed by the circadian oscillator gene CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) [44]. Daily and circadian oscillations of Ca2+ regulate sense via a Ca2+ sensor protein CALMODULIN-LIKE 24, which interacts genetically with the circadian oscillator protein TIMING OF CAB 1 (TOC1) [45]. As aspects of photosynthesis, the changes in Ca2+ in the chloroplastic regulate the import of nuclear-encoded proteins and organelle division [46,47,48]. Additionally, the environmental transition between light and darkness produces a prolonged and sustained increase in Ca2+ that depends on the photoperiod [41,49,50].
This research simulated the normal plant growth environment in the image machine [51]. We also analyzed the relationship between low pH conditions and the basal Ca2+ concentration of Arabidopsis under a normal light/dark cycle. The main objective of this study was to investigate the effects of pH on changes in intracellular calcium levels and the plant’s cyclic response over a medium- to long-term period.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
The Arabidopsis thaliana genotypes were used in the Col-0 background. Under the control of the Cauliflower Mosaic Virus 35S promoter, active aequorin (Ca2+ indicator) (pMAQ2) was obtained from Dr. Marc R. Knight [52]. Seeds were sterilized with 2.5% PPM (a plant preservative mixture; Caisson Laboratories), stratified at 4 °C for 3 days in the dark, and then transferred to a growth chamber. The growth chamber was configured with an LD light/dark cycle (16 h of light and 8 h of dark) at 22 ± 1 °C. Arabidopsis plants were grown in a 150 mm round Petri dish, which contained 1/2 Murashige and Skoog salts with 1.0% (w/v) sucrose (Sigma, St. Louis, MO, USA) and 1.2% (w/v) agar (Sigma) adjusted to pH 5.7 with KOH (Sigma).
2.2. Aequorin (AQ) Reconstitution and Plant Pretreatment
Arabidopsis plants expressing cytosolic apoaequorin were used for [Ca2+]i measurements [52,53]. Seedlings were grown on a half-strength MS medium after 8 days and transferred onto a new half-strength MS medium in a 90 mm round Petri dish, which contains 1/2 Murashige and Skoog salts and 1.2% (w/v) agar without MES, adjusted to pH 5.8 with KOH. The culture medium was cut into a specific shape: a 5 cm striped strip and another 7 cm slope-type medium. The reconstitution of aequorin was performed in vivo by spraying seedlings with 0.8 mL of 50 μM coelenterazine (from Prolume) per Petri dish, followed by incubation at 22 °C in the dark for 8 h.
2.3. Low pH Treatment
Low-pH stress treatments were adopted from the experimental procedure described by [54]. For stress treatments, 4 Petri dishes were placed individually into the ChemiPro HT chamber. Two samples were repeated each time, and three independent experiments were performed. The treatment solution of 10 mM 2-Morpholinoethanesulfonic acid monohydrate (MES) (Sigma), which was adjusted to pH 5.0, 5.8, 6.0 with Tris, and pH 3.0, pH 4.0 with HCl, was added to a Petri dish in the dark. Aequorin luminescence was recorded for 20 min every 2 h after a switch from the light to the dark for 5 min as described [53,55]. The first 5 min imaging contained the autofluorescence of chlorophylls and was discarded. For Ca2+ channel blocking, seedlings were co-incubated with a treatment buffer containing 1 mM LaCl3. The total aequorin was estimated by discharging with 0.9 M CaCl2 in 10% (v/v) ethanol [55]. Experiments were carried out at room temperature (22–24 °C).
2.4. Measurement of Cytosolic Free Calcium ([Ca2+]i)
Treatments and aequorin luminescence imaging were performed at room temperature using a ChemiPro HT system [51], which includes a cryogenically cooled and back-illuminated charge-coupled device (CCD) camera, a liquid nitrogen autofiller, a camera controller, and computer-equipped WinView/32 software (Roper Scientific, Acton, MA, USA) [53]. The CCD camera has a 1300 × 1340-pixel resolution and was cooled to −120 °C by the cryogenic cooling system before image recording. The relative luminescence intensities were calculated as the ratio of aequorin luminescence intensity and discharged aequorin luminescence intensity [53,54,56].
2.5. Statistical Analysis
Microsoft Excel was used for data processing. A two-way analysis of variance (ANOVA) was implemented using SAS 9.1 software (SAS Institute, Cary, NC, USA). Means were separated using the least significant difference test at the 5% level. Values of p < 0.05 were considered statistically significant.
3. Result
3.1. pH-Induced Modulations in the [Ca2+]i Oscillations Were Observed in Both the Leaves and Roots of Arabidopsis Seedlings
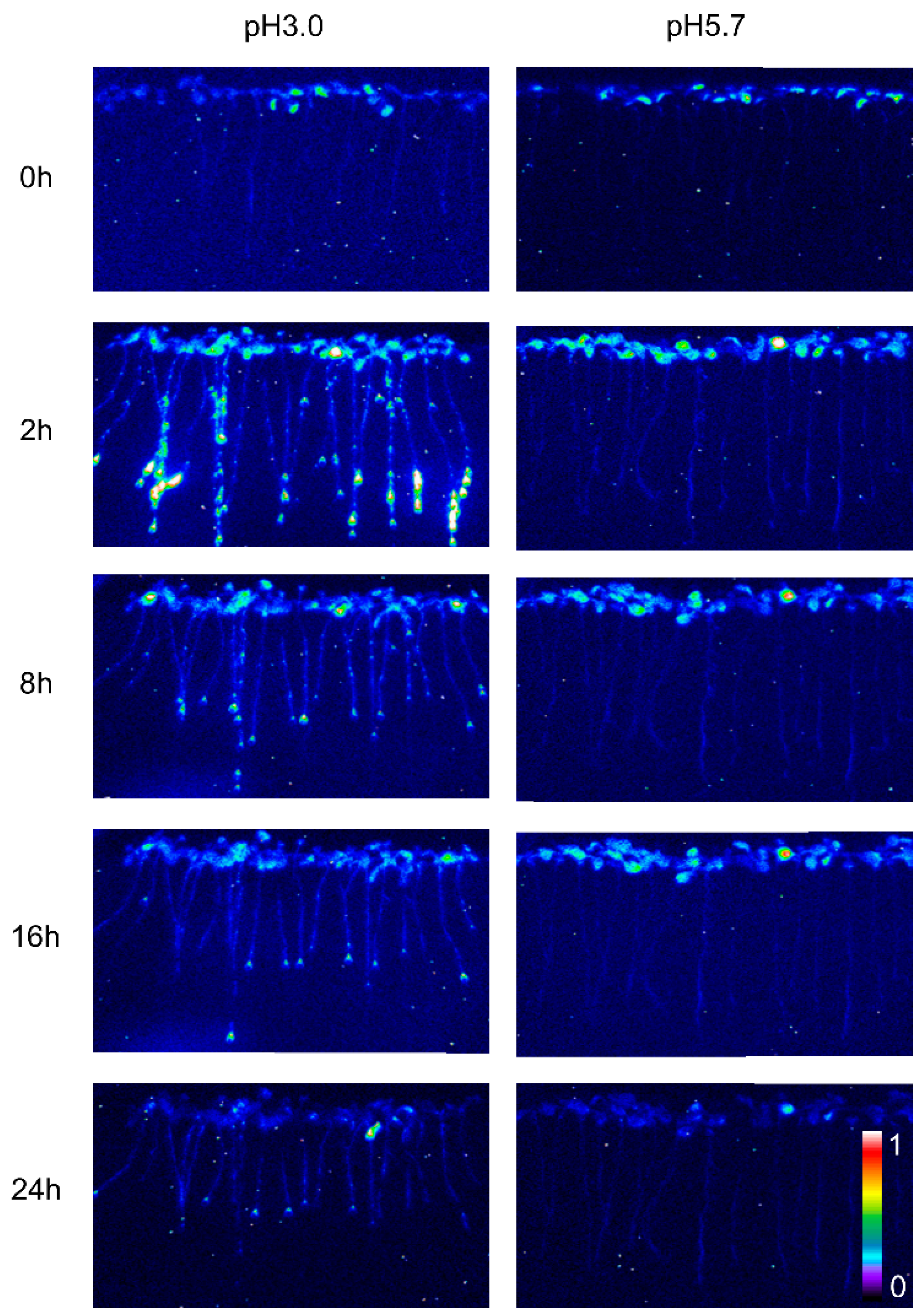
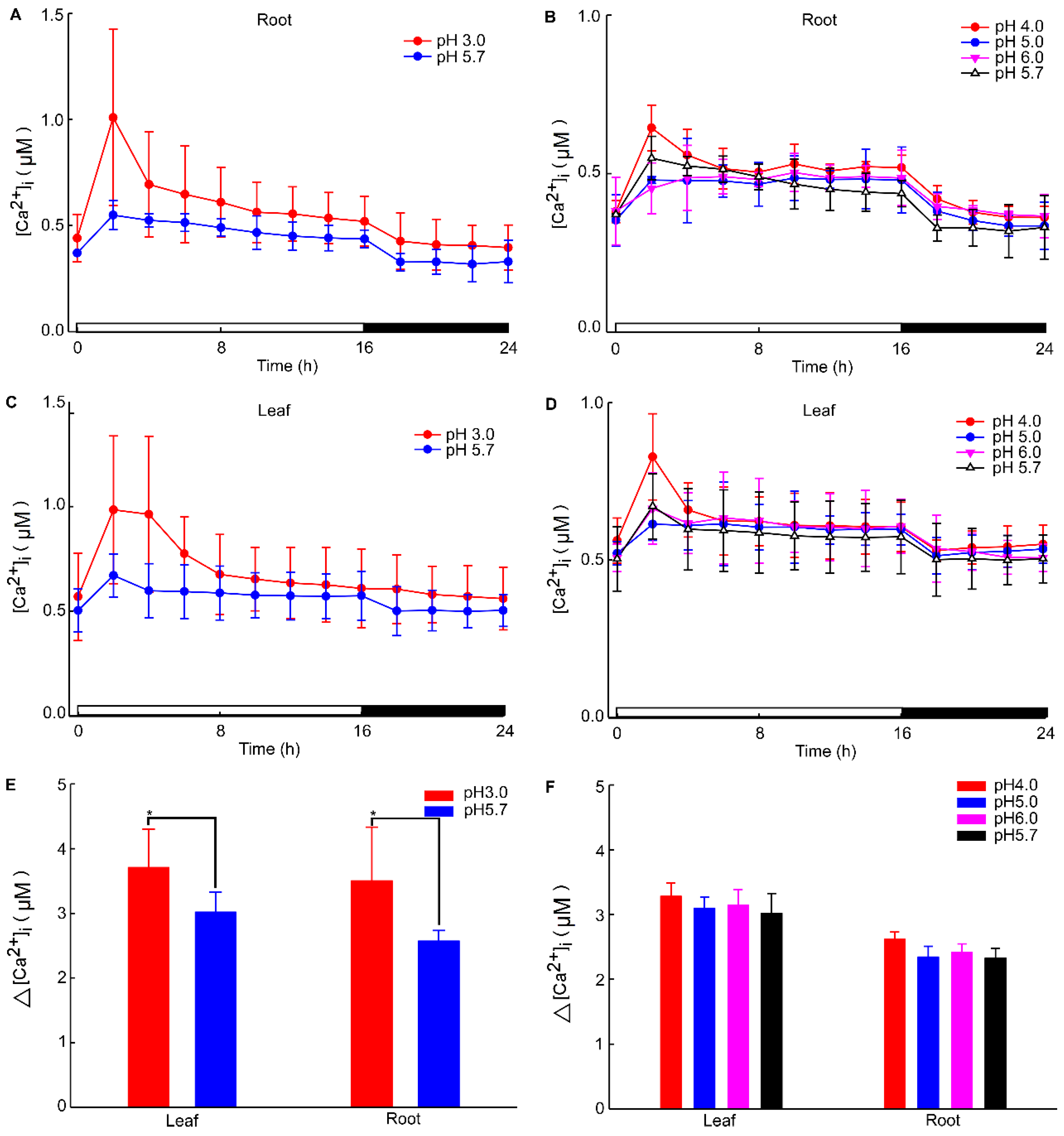
The calcium signal in the root was significantly influenced by different levels of stress. Specifically, when Arabidopsis roots were subjected to acidic stress (pH 3.0), the calcium signal in both root and leaf tissues increased within 2–4 h under light conditions and subsequently remained at elevated levels compared to dark conditions (Figure 1). Notably, under acid stress, the calcium signal was observed to migrate towards the upper part of the root relative to the control group (pH 5.7) (Figure 1). However, for the circadian rhythm under the natural light cycle, Arabidopsis exhibited a periodic oscillation of calcium signals in its roots and leaves, both at pH 3.0 and in the control group. No significant differences in the Ca2+ signal or circadian rhythm were detected between Arabidopsis plants undergoing water treatment and those in the control group (Figure A1). During the same oscillation period, the amplitude of Ca2+ signals induced by various pH treatments differed, with low pH inducing a more pronounced Ca2+ signal. Additionally, the Ca2+ content in leaves was higher than in the roots, although the overall trend of the Ca2+ variation remained similar. At pH 3.0 and pH 4.0, Arabidopsis initially increased the Ca2+ signal, which then gradually decreased, indicating that the intracellular Ca2+ concentration returned to equilibrium (Figure 2A–D). After 4–8 h, the Ca2+ signals induced by pH 3.0 and pH 4.0 recovered to a static state, albeit with a higher baseline Ca2+ concentration compared to pH 5.0 and pH 6.0 (Figure 2A–D). Under natural light conditions, both pH 3.0 and the control group (pH 5.7) induced constant changes in [Ca2+]i in the roots and leaves of Arabidopsis plants (Figure 2A,C). The leaf response to the Ca2+ signal recovered more slowly than the root response to acidic stimulation at pH 3.0 (Figure 2A,C). Nevertheless, the baseline [Ca2+]i in Arabidopsis under light conditions was higher than under dark conditions (Figure 2A–D). The total change in the calcium signal over 24 h reflected the alteration in the Ca2+ content in the roots and leaves under a low acid stimulation, indicating significant changes in [Ca2+]i in both leaves and roots at a pH of 3.0 (Figure 2E). Changes in the Ca2+ signal under pH 4.0 were less pronounced than those under pH 3.0 in the leaves while no significant difference was observed in the roots (Figure 2E,F).
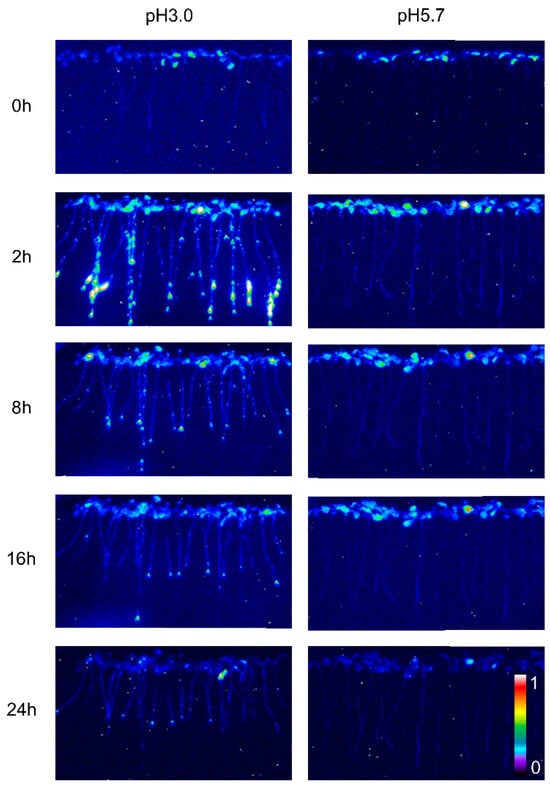
Figure 1.
Spatio-temporal Ca2+ responses of Arabidopsis seedlings to low pH. The luminescence emitted by Arabidopsis seedlings expressing aequorin was captured using pseudocolored photon-counting images. In these images, cold colors (blue and green) denote regions with low luminescence counts, which correspond to low intracellular calcium ion concentrations ([Ca2+]i). Conversely, warm colors (yellow and orange) indicate regions of more intense luminescence, reflecting higher [Ca2+]i levels. Prior to imaging, the seedlings were entrained to a 16 h light/8 h dark (16L/8D) photoperiod at an irradiance of approximately 90 μmol m−2 s−1 for a duration of 9 days. Sequential images were then acquired using a photon-counting camera, with integrations conducted every 20 min for a total of 24 h following exposure to low pH stimuli. The treatment solutions, consisting of a 1/2 MS medium without sugar, were adjusted to pH 3.0 (low pH) and pH 5.7 (control, CK) using a 1 M Tris base. Notably, only the roots of the Arabidopsis seedlings were subjected to these treatments, which were conducted on tilted media in each panel devoid of agar and EMS. During the treatment period, the photoperiod was maintained at 16L/8D, but each 1.5 h light and dark cycle was interrupted for 10 min for image acquisition, followed by a subsequent 20 min imaging session.
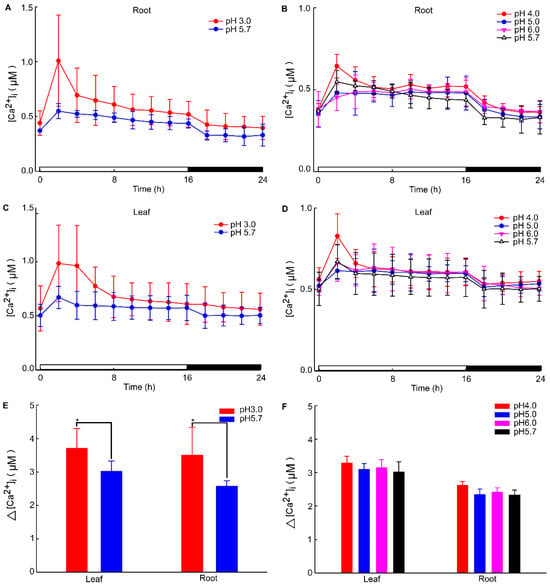
Figure 2.
Increases in [Ca2+]i in response to low pH treatments. Seedlings expressing aequorin and grown for a duration of 9 days were subjected to treatments with solutions whose pH levels were adjusted using a Tris base and EMS. Aequorin images were captured every 20 min within 2 h intervals for a total period of 24 h. The experiments aimed to investigate the increases in the intracellular calcium ion concentration ([Ca2+]i) induced by pH 3.0 and pH 5.7 treatments, specifically in the roots of Arabidopsis (A). Additionally, imaging was conducted to observe [Ca2+]i increases in response to treatments with pH 4.0, pH 5.0, pH 5.7, and pH 6.0 solutions in the roots of Arabidopsis (B). Furthermore, this study explored the induction of [Ca2+]i increases in Arabidopsis leaves by treating the roots with pH 3.0 and pH 5.7 solutions (C). Imaging was also performed to assess [Ca2+]i increases in the leaves of Arabidopsis in response to treatments with pH 4.0, pH 5.0, pH 5.7, and pH 6.0 solutions applied to the roots (D). The overall changes in [Ca2+]i in the roots and leaves of Arabidopsis under pH 3.0 and pH 5.7 conditions were analyzed (E). Similarly, the total change in [Ca2+]i in the roots and leaves of Arabidopsis under pH 4.0, pH 5.0, pH 5.7, and pH 6.0 conditions was evaluated (F). The presented data represent the results of four independent experiments, with mean values and standard deviations (mean ± SD) calculated from a sample size of 180 (mean ± SD; n = 180).
3.2. pH Stimulation-Elicited [Ca2+]i Oscillations in the Leaves and Roots of Arabidopsis Seedlings in the Presence of a Ca2+ Inhibitor
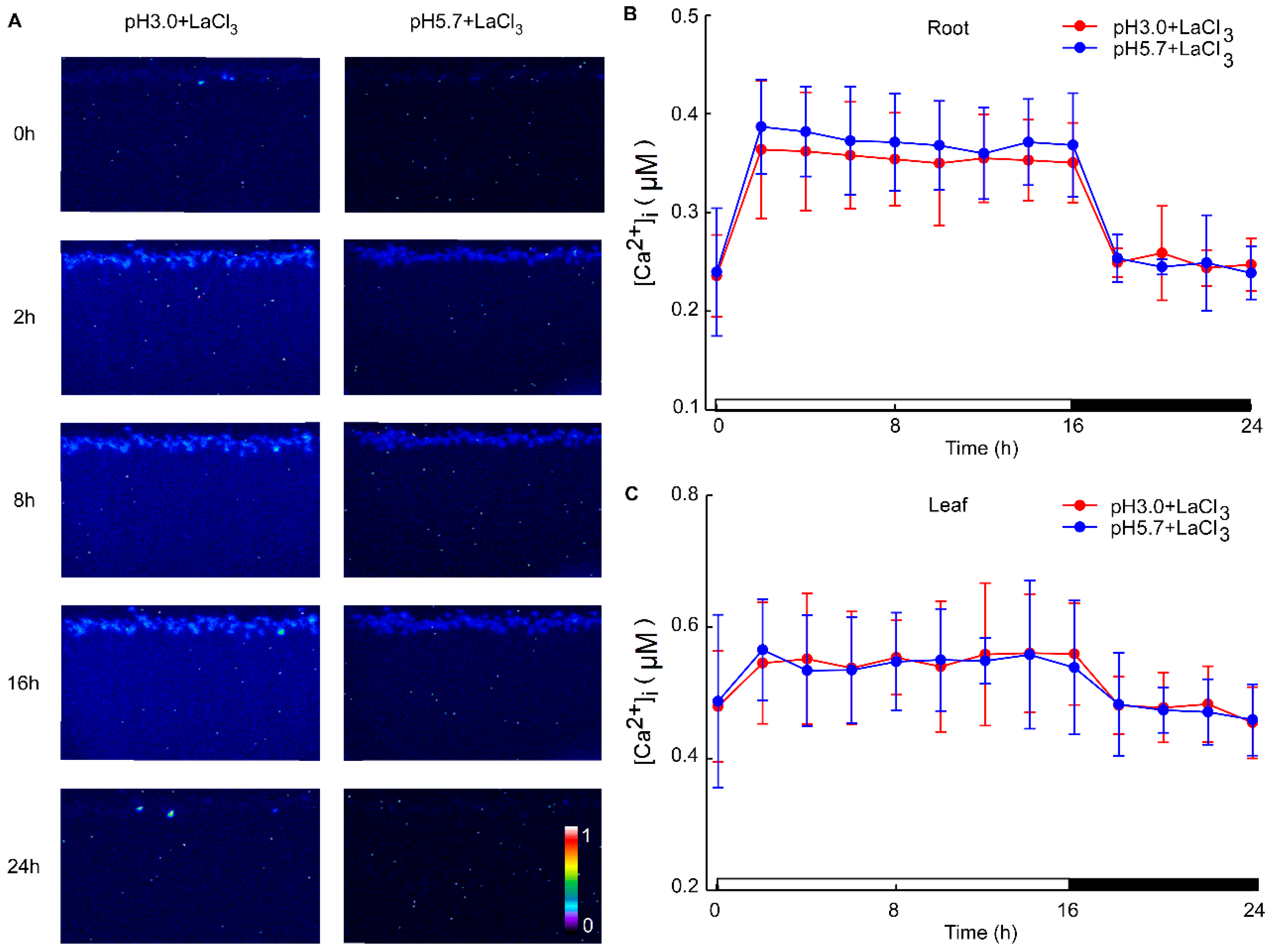
Under acidic stress conditions, the leaves exhibit the ability to sense acid stimulation originating from the roots. To investigate this phenomenon, we monitored alterations in calcium signals within the leaf while inhibiting the root with LaCl3 under acid stimulation. The La3+ ion, previously established as an agonist of Ca2+ channel blockers, was utilized to inhibit Ca2+ influx [57]. Despite the presence of the 1mM LaCl3 inhibitor, variations in pH did not alter the levels of Ca2+ in the leaves and roots of Arabidopsis. Notably, specific concentrations of LaCl3 demonstrated significant inhibitory effects on the low pH-triggered increase in [Ca2+]i (Figure A3). When comparing images at pH 3.0 and pH 5.7, no discernible differences in Ca2+ levels between the roots and leaves of Arabidopsis were observed, although variations in Ca2+ levels due to the photoperiod were present (Figure 3A). Across different pH treatments, there were no substantial changes in Ca2+ levels detected in the roots and leaves of Arabidopsis (Figure 3B,C, Figure A4A–D and Figure A5A–D). Distinct from the observations in Figure 1 and Figure 2, the pH did not significantly elicit alterations in Ca2+ levels in Arabidopsis when LaCl3 inhibition was present.
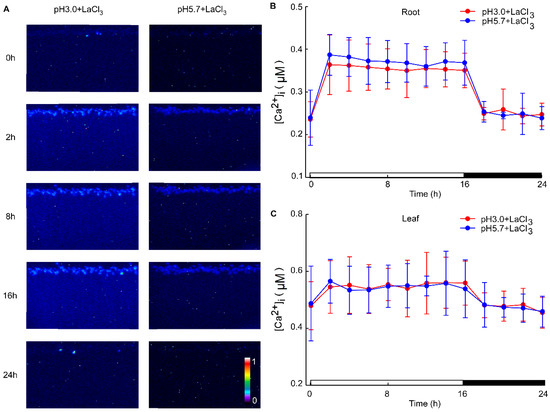
Figure 3.
The effects of Ca2+ inhibitors on low pH-induced spatio-temporal [Ca2+]i responses of Arabidopsis seedlings. The presented images show pseudocolored photons, counting the depictions of luminescence emitted by Arabidopsis seedlings expressing aequorin. These seedlings were entrained to a 16 h light/8 h dark (16L/8D) photoperiod at approximately 90 μmol m−2 s−1 for a duration of 9 days. Sequential images were captured using a photon-counting camera, employing a photon-counting integration interval of 20 min, with integrations conducted every 2 h over a 24 h period following exposure to low pH stimuli. The treatment solutions consisted of pH 3.0 (representing low pH) and pH 5.7 (serving as the control, denoted as CK), both containing 1 mM Ca2+ inhibitor (LaCl3), adjusted with a 1 M Tris base. Notably, these solutions were specifically applied to the roots of Arabidopsis seedlings, which were placed on a tilted medium in each panel devoid of agar and EMS. During the treatment phase, the photoperiod was maintained at 16L/8D, but with each 1.5 h light and dark cycle processed for 10 min, followed by imaging for 20 min (A). The administration of Ca2+ inhibitors (1 mM LaCl3) was observed to reduce pH 3.0 and pH 5.7-induced changes in the intracellular Ca2+ concentration ([Ca2+]i) in the roots of Arabidopsis (B). Similarly, treatment with Ca2+ inhibitors (1 mM LaCl3) mitigated [Ca2+]i changes in the leaves of Arabidopsis, which were elicited by exposure to pH 3.0 and pH 5.7 solutions applied to the roots of the seedlings (C). The data presented are derived from four independent experiments (mean ± SD; n = 180).
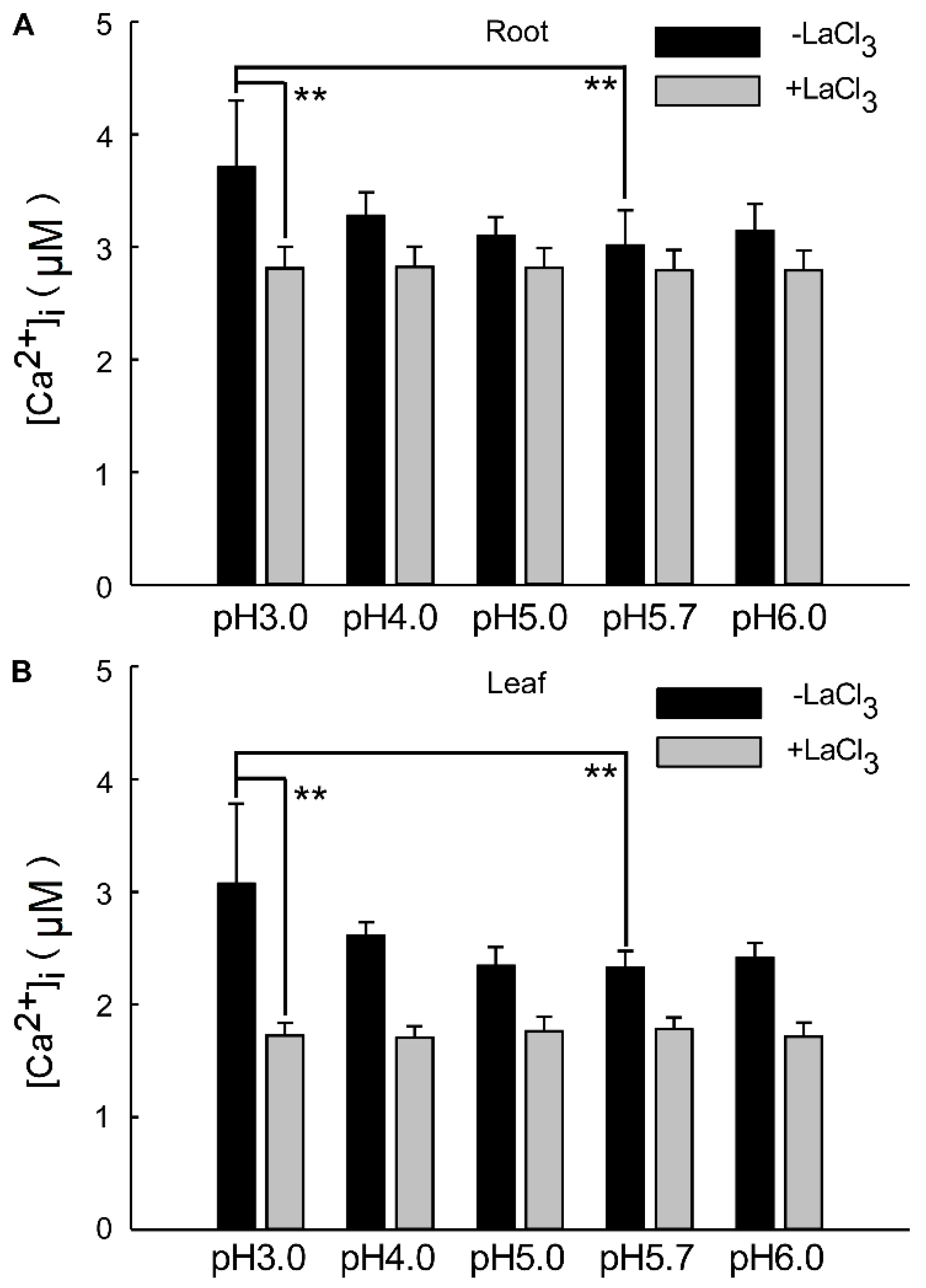
The overall change in calcium signals over a 24 h period reflects the alteration in Ca2+ content within the roots and leaves under a low-acid stimulation. This suggests that significant changes in [Ca2+]i occur in the leaves and roots of the plant, specifically under pH 3.0 conditions compared to the control. The alteration in Ca2+ signals under pH 4.0 was less pronounced than under pH 3.0 in both the leaf and root (Figure 4A,B). As depicted in Figure 1 and Figure 3, seedlings exhibited varying responses to different acidic treatments. Acid stimuli significantly induced Ca2+ signals, with notable differences in acidic treatments and signal amplitudes between the shoot and root. The amplitude of Ca2+ signals was significantly enhanced under pH 3.0, correlating with the intensity of the H+ stimulus and persisting for an extended period across all low pH conditions. The amplitudes of [Ca2+]i induced by the low pH 3.0 in Arabidopsis roots were 0.36 times higher than those induced by pH 5.7. However, under LaCl3 inhibition, the distinction between them was insignificant across all acidic treatments (Figure 4A). Similarly, the pH 3.0-induced [Ca2+]i amplitude was 0.51-fold higher than pH 5.7 in the leaves, but this difference became negligible under LaCl3 inhibition (Figure 4B).
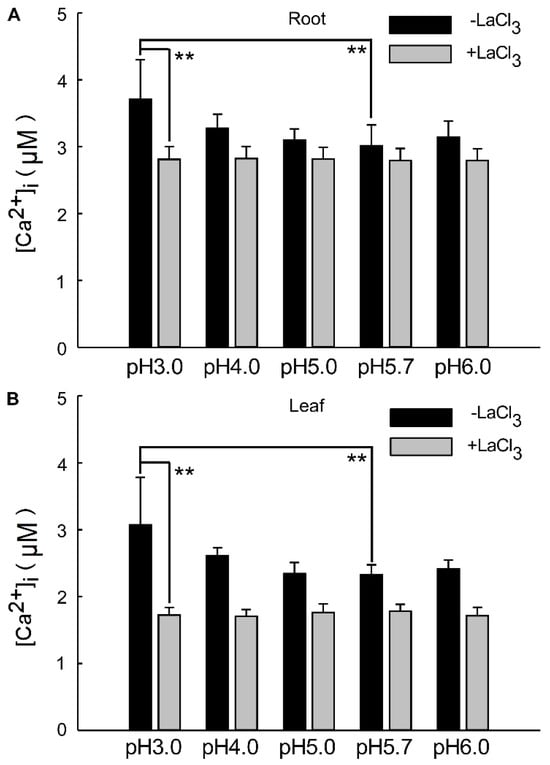
Figure 4.
Changes in [Ca2+]i in Arabidopsis under low pH in the presence of Ca2+ inhibitors. Under various pH conditions, specifically pH 3.0, pH 4.0, pH 5.0, pH 5.7, and pH 6.0 (as depicted in Figure 2 and Figure 4), the quantity of the intracellular Ca2+ concentration ([Ca2+]i) was elicited by acid stimulation in the roots of Arabidopsis exhibited alterations. However, in the presence of inhibitors, the [Ca2+]i levels in the roots remained unaltered (A). Similarly, under the aforementioned pH conditions (Figure 2 and Figure 4), the magnitude of [Ca2+]i in the leaves of Arabidopsis, which was induced by acid stimulation in the roots, also demonstrated changes. Nonetheless, when inhibitors were present, the [Ca2+]i levels in the leaves remained unchanged (B).
3.3. Prolonged Exposure to pH Stimulation-Induced [Ca2+]i Oscillations in the Leaves of Arabidopsis Seedlings
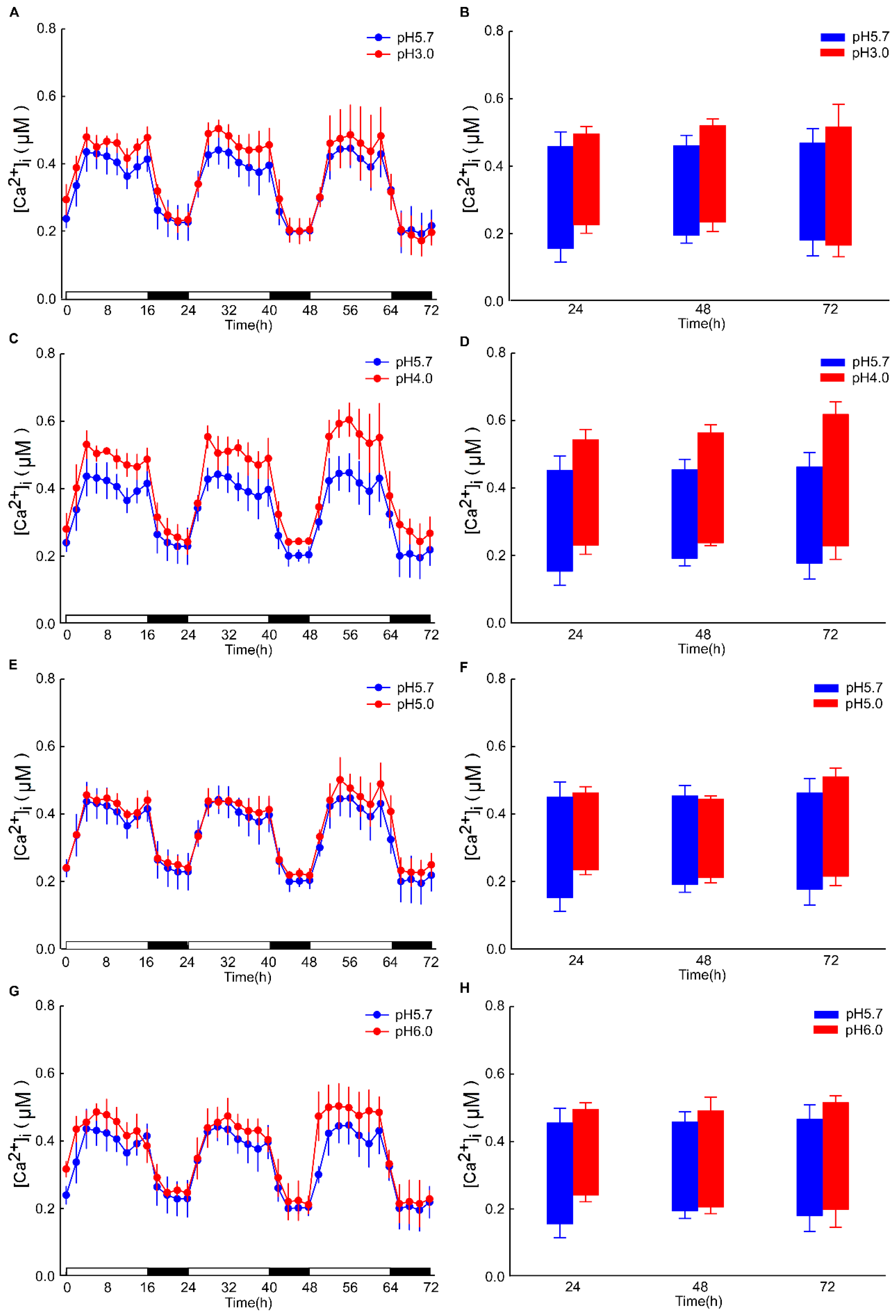
The intracellular Ca2+ ([Ca2+]i) changes in Arabidopsis after 4 h exhibited minimal significance under dark conditions. However, the alterations in the basal intracellular Ca2+ levels in Arabidopsis, induced by different pH conditions, are discernible (Figure A2). Specifically, under pH 3.0, the response to the Ca2+ signal is rapidly augmented in the leaf, whereas the response is retarded in the root. These results are analogous to those observed under light/dark (LD) conditions (Figure 2A,C). After 8 h of acid stimulation in the dark condition, the intracellular calcium signal resonance gradually stabilized. Nevertheless, under light conditions, pH 3.0 and 4.0 lead to an increase in Ca2+ levels in the leaves of Arabidopsis. Under prolonged conditions, at pH 4.0, the basal [Ca2+]i level of Arabidopsis over 72 h was sustained at a higher level relative to pH 3.0 (Figure 5A,C). Conversely, the basal [Ca2+]i level remained comparable to other pH conditions (Figure 5E,G). Notably, under pH 4.0, the amplitude of the Ca2+ signal was more pronounced than under other conditions. In contrast, under the remaining pH conditions, the amplitude of the Ca2+ signal remained unchanged (Figure 5B,D).
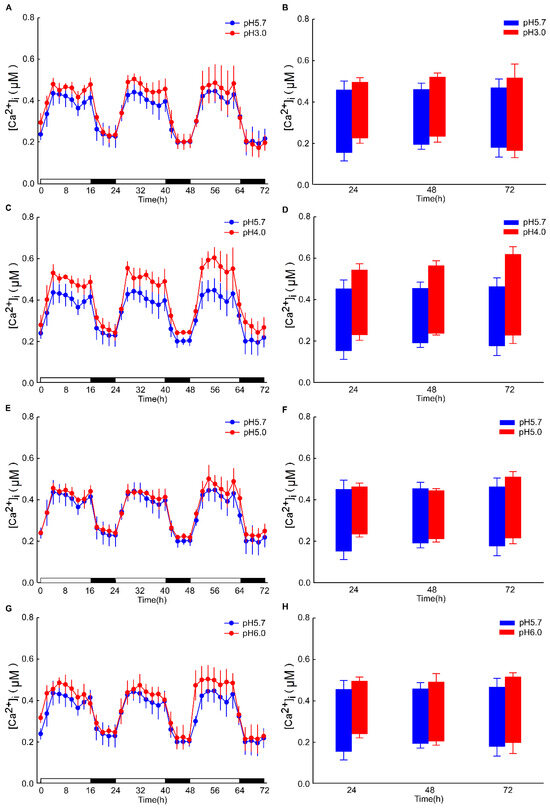
Figure 5.
Low pH modulates the amplitude of oscillations in [Ca2+]i. seedlings, which were entrained to a 16L/8D photoperiod at an irradiance of approximately 90 μmol m−2 s−1 for a duration of 9 days. Sequential images were captured utilizing a photon-counting camera, with a photon-counting integration interval set at 20 min. These integrations were performed every 2 h following low pH stimuli, which were applied 8 h into the dark period. In comparison to the pH 5.7 condition, notable differences were observed in the amplitude of [Ca2+]i oscillations elicited by the pH 3.0 (A), pH 4.0 (C), pH 5.0 (E), and pH 6.0 (G) stimulation in the leaves of Arabidopsis. The treatment solution consisted of a 1/2 MS medium without sugar, adjusted using a 1 M Tris base, and was applied solely to the roots of Arabidopsis seedlings placed on tilted media in each panel, devoid of agar and EMS. During the treatment phase, the photoperiod remained constant at 16L/8D, but each 1.5 h light and dark cycle was followed by a 10 min processing period, subsequent to which imaging was conducted for 20 min. A box plot is presented, illustrating the upper and lower limits of the primary [Ca2+]i oscillations induced by pH 3.0 (B), pH 4.0 (D), pH 5.0 (F), and pH 6.0 (H) stimulations in the leaves of Arabidopsis, relative to the pH 5.7 condition. The data presented are derived from four independent experiments (mean ± s.e.m; n = 180).
4. Discussion
Previous research on pH and calcium ions has primarily focused on the correlation of Ca2+ signals to the biological clock, indicating periodic oscillations of [Ca2+]i [40,58]. In this study, under the acid stimulation of pH 3.5 and 4.5, the Ca2+ signal was observed to be delayed. However, the more potent acid stimulation (pH 3.5) elicited a more evident response than the acid stimulation at pH 4.5, with a more considerable increase in Ca2+ levels initiated within a few minutes [59]. Building upon these two methods, we have refined the experimental approach, enabling us to use AQ to study the response of pH to the long-term calcium ion dynamics in the plant Arabidopsis. In this paper, we specifically focus on responses and variations in [Ca2+]i in Arabidopsis over an extended period. Given that the nature of the medium determines the limitations of the low pH conditions and the pH properties, we adopted a unique pH system that influences [Ca2+]i changes in Arabidopsis [51]. This system allowed us to study the changes in the [Ca2+]i under low pH stress over a prolonged duration. In our experiment, an overdose of coelenterazine was administered. We employed a recording system to compare various Ca2+ responses under a low pH in Arabidopsis seedlings. The bioluminescence emitted by each seedling cluster was recorded for 1200 s, every 2 h, over a 24 h period. The Ca2+ recordings revealed tissue-specific Ca2+ signals in the shoot and root associated with low pH acidic stress.
Calcium ions (Ca2+) have been recognized as a ubiquitous second messenger, playing a pivotal role in plant signal transduction [32,60]. Aequorin is an early version of the Ca2+ indicator, with the intensity of its bioluminescence contributing to the elucidation of the Ca2+ signature in plants [52]. The transformation of aequorin in plants has proven to be a valuable tool for the non-invasive investigation of [Ca2+]i signatures. It is known that specific stimuli, including abiotic and biotic stresses, hormones, and amino acids, can elicit unique spatio-temporal patterns of Ca2+ signatures in most aspects of whole seedlings [59,61,62,63]. The basal [Ca2+]i is maintained at a concentration of approximately 100 nM, which is about 20,000-fold lower than the extracellular Ca2+ concentration [32,64,65]. The stimulus may rapidly increase [Ca2+]i spikes or induce complex [Ca2+]i oscillations recurring within a period of 1 to 20 min [66,67,68]. However, long-term [Ca2+]i oscillations, characterized by a 24 h period, have been observed in the cytosol and chloroplast of plants, as well as in the cytosol of the suprachiasmatic nucleus of the mouse [40,69].
We conducted an investigation into the basal [Ca2+]i response to varying pH levels. Specifically, we observed a slow kurtosis in [Ca2+]i increases in response to pH 3.0 compared to pH 5.7 during the first 8 h (Figure 1 and Figure 2A). Notably, the peak [Ca2+]i increased when induced by pH 4.0 and was less pronounced than that elicited by pH 3.0 (Figure 2A). Moreover, other pH values (pH 5.0, pH 5.7, pH 6.0) did not significantly increase [Ca2+]i compared to the acidic stress induced by pH 3.0 and pH 4.0 (Figure 2A,B). To our knowledge, such a systematic comparison, which is essential for understanding Aequorin-recorded Ca2+ signals, has not been previously conducted in Arabidopsis. This recording system can serve as a valuable tool for identifying potentially novel components of the Ca2+ signal in response to acidic stress. Although only Arabidopsis roots were directly stimulated by acid stress, which led to an increase in Ca2+, the stimulation of the leaves also resulted in an increase in intracellular Ca2+, albeit with a prolonged response rate. These data are consistent with the transmission of calcium signals stimulated by other stresses [70], indicating that acid stress can increase [Ca2+]i signaling and that the [Ca2+]i signal can also be transmitted upwards as a systemic wound signal [71,72].
Auxin stimulates growth and exerts an influence on the PM H+-ATP complex, sustaining protons pumping phosphorylation and ultimately leading to proton efflux, potentially through apoplast acidification. Treatments with acid buffers have been observed to stimulate hypocotyl segment elongation [73,74,75]. The growth response is immediate, with a decrease in pH preceding growth but occurring after auxin treatment [76,77]. In response to acid buffer stimulation at pH 3.5 and pH 4.5, a Ca2+ signal is triggered within a few minutes. However, the more potent acid stimulation at pH 3.5 is initiated earlier, and the Ca2+ increase is more pronounced than that elicited by the acidic stimulus at pH 4.5 [59]. In this research, changes in [Ca2+]i were observed in the seedlings under acid stimulation during a light/dark (LD) cycle. Since light also causes an increase in calcium levels in plants during the LD growth cycle, the [Ca2+]i levels in plants decrease to lower levels in darkness [38,45]. When plants are transferred to dark conditions, the [Ca2+]i in the plant seedlings still maintain this periodic [Ca2+]i oscillation cycle [45,48]. Therefore, our results presented in Figure 2 demonstrate that the oscillation of [Ca2+]i is not only included by light but also induced by acid stress.
The increase in extracellular pH was inhibited by pretreatment with the Ca2+ channel blocker La3+ [29,30]. The La3+ ion has been used as an agonist of Ca2+ channel blockers to suppress Ca2+ influx [57,78]. No significant differences were observed between concentrations of 0.03 mM, 0.1 mM, and 1 mM, indicating that LaCl3 effectively blocked the saturation of Ca2+ channels (Figure A3). Specifically, 1 mM LaCl3 completely inhibited the increase in [Ca2+]i in response to acidic stress for 24 h. In the presence of LaCl3, after 1.5 h, the [Ca2+]i levels in Arabidopsis did not change across different acidic pH conditions, suggesting that LaCl3 could effectively inhibit the influx of [Ca2+]i in plants under acid stress (Figure 1 and Figure 3A). Although acid stress-induced changes in [Ca2+]i in plants were abrogated, inherent light-induced [Ca2+]i signals are still detectable (Figure 3B,C). These findings imply that the influx of extracellular Ca2+ primarily mediates the Ca2+ signal generated by acid stimulation in the roots. When Ca2+ channels are blocked, Ca2+ may not enter cells to form calcium signals and cannot be transmitted upward (Figure A3). Concurrently, due to the incomplete inhibition of H+ channels, excessive H+ could still enter cells. However, while the intracellular pH of root cells may change, the intracellular Ca2+ signal does not increase, and Ca2+ signals are not transmitted upward to the leaves. This may be attributed to the intracellular calcium store not releasing Ca2+ into the cytoplasm to form a detectable calcium signal response to low pH.
The activation of apoplast acidification occurs through the hyperpolarization of PM H+-ATPase, ultimately leading to cell wall loosening, solute and water uptake, elevated turgor pressure, and cell expansion [79]. α- and β-expansions are activated at a low pH of approximately 4.0, playing a crucial role in regulating the structure and relaxation of the cellulose–xyloglucan network within the cell walls [80]. The low pH environment modulates the stability of the PME3-PMEI7 complex and inhibits PME3 activity, although other PME complex formations may exhibit pH insensitivity [81]. Short galacturonic acid (GalA) fragments, specifically GalA3-4, have been found to stimulate hypocotyl growth in dark conditions, suggesting that specific cell wall components are involved in the cell-to-cell communications linked to expansion [82]. In this research, stimulation with pH 3.0 on the root resulted in an increase in [Ca2+]i in both the leaves and roots of Arabidopsis, particularly within the first 4 h under dark conditions. At pH 3.0, the leaf response to the Ca2+ signal was rapidly augmented, albeit with a slight response compared to the roots. These findings align with observations made under LD light conditions (Figure 2A,C). Notably, the changes in intracellular [Ca2+]i changes in the Arabidopsis leaf after 4 h were insignificant under dark conditions (Figure A2). These results imply that a low pH may exert an influence on cell wall structures and biophysical properties, which constitute a complex network orchestrating polar cell growth.
Under both light and dark conditions, the plant exhibits a prolonged and sustained increase in Ca2+ levels, which is dependent on the photoperiod [41,49,50]. This increase is driven by cyclic adenosine diphosphate ribose-mediated Ca2+ release from internal stores [44,83], serving to encode information regarding photoperiod, timing, and light intensity [40,84]. Tonoplast-localized Ca2+/H+ antiporters, such as CAX, elevate Ca2+ concentration in the apoplastic, which is concomitant with altered cell wall mechanical properties, the reduced expression of cell wall-modifying agent transcripts, an increase in apoplastic pH, and perturbed auxin transport [85,86]. Additionally, the vacuolar H+–pyrophosphatase AVP1 has been implicated in alterations in auxin efflux and changes in apoplastic pH [87,88]. Despite these findings, the specific functions regulated by circadian oscillations of [Ca2+]i remain unidentified. In this research, intracellular calcium signals were observed to gradually stabilize after 8 h of acid stimulation under dark conditions. We investigated the changes in intracellular calcium in Arabidopsis following 8 h of acid stimulation, particularly focusing on the alterations in basal [Ca2+]I levels. Our comparisons were made across pH levels of 5.0, 5.7, and 6.0, where no discernible differences in intracellular calcium in Arabidopsis were recorded. However, under pH 4.0 conditions, the level of intracellular basal calcium in Arabidopsis was significantly higher than that observed under pH 3.0 conditions. This result indicates that acid stimulation can continuously influence plants’ intracellular [Ca2+]i under light conditions and also that suitable low pH conditions may affect basal [Ca2+]i levels, as well as the growth and development of plants. Our study demonstrated that low pH stress triggers the [Ca2+]i signaling of calcium oscillation in the Arabidopsis cytoplasm, along with the [Ca2+]i influx from the apoplastic to the cytoplasm, independent of the [Ca2+]i efflux from calcium stored in the vacuole. It is plausible that a suitable low pH of 4.0 may serve as a spatial-temporal input into the circadian clock system.
5. Conclusions
In conclusion, our findings reveal that Arabidopsis roots exhibit variations in basal Ca2+ levels when subjected to low pH stress, with a notable increase observed in response to decreasing pH values. This elevation in basal Ca2+ levels was mirrored in the leaves. Interestingly, the induction of basal Ca2+ levels by a low pH exceeded normal levels under light conditions but returned to the baseline under dark conditions. Furthermore, the presence of La3+ inhibited extracellular Ca2+ influx, preventing significant increases in basal Ca2+ levels in both the roots and leaves. This suggests that H+ is unable to trigger Ca2+ efflux from the vacuoles to the cytoplasm, thereby sustaining relatively high basal Ca2+ levels. Notably, under the conditions of an extended photoperiod, the elevated basal Ca2+ levels in leaves, which were initially induced in the roots, remained consistently high when exposed to a specific pH stress. The benefits of these results for researchers are significant as they provide new insights into the mechanisms underlying plant responses to low pH stress, particularly in terms of calcium signaling. These findings open up new avenues for further research into the role of calcium signaling in plant responses to environmental stresses, such as low pH. The discovery that extended photoperiods maintain high basal Ca2+ levels in leaves under specific pH stress suggests a potential link between light signaling and calcium homeostasis, which warrants further investigation. Additionally, the study’s findings may have implications for crop improvement and stress tolerance, as understanding the mechanisms of calcium signaling in response to low pH stress could lead to the development of more resilient plant varieties.
Author Contributions
Z.-M.P.: designed the experiment; W.C. and S.Z.: performed the research; W.C., J.X. and J.C. collected and analyzed the data; W.C., S.Z. and Z.-M.P. wrote the manuscript; W.C., Z.-M.P., S.Z. and J.-F.W. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Zhejiang Natural Science Foundation (LZ16C020001), the project titled “Development of Multi-dimensional Visual Recognition and Precise Grasping Equipment for Seed Sprouts” (2023CXGC010702), and the “333” Talent Project of the Shandong Academy of Agricultural Sciences (CXGC2024F08), and the Shandong Province technology innovation guidance program (YDZX2023007).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Marc R. Knight, who is responsible for Arabidopsis seeds expressing aequorin. Members in the Pei lab were responsible for discussing and critically reading the manuscript, and Douglas M. Johnson and Gary B. Swift were responsible for maintaining the ChemiPro system.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
[Ca2+]i, cytosolic free Ca2+ concentration; AQ, aequorin.
Appendix A
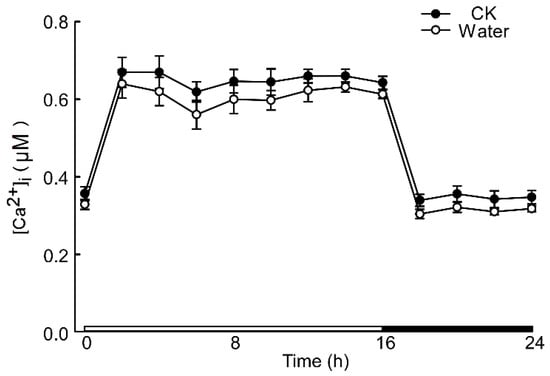
Figure A1.
Increases in [Ca2+]i in response to water. Aequorin-expressing seedlings grown for 9 days were treated with water and no treatment, and aequorin images were taken every 20 min for 2 h for 24 h—an increase in [Ca2+]i induced by water and no treatment in root of Arabidopsis is shown. Data from four independent experiments are shown (mean ± s.e.m; n = 180).
Figure A1.
Increases in [Ca2+]i in response to water. Aequorin-expressing seedlings grown for 9 days were treated with water and no treatment, and aequorin images were taken every 20 min for 2 h for 24 h—an increase in [Ca2+]i induced by water and no treatment in root of Arabidopsis is shown. Data from four independent experiments are shown (mean ± s.e.m; n = 180).
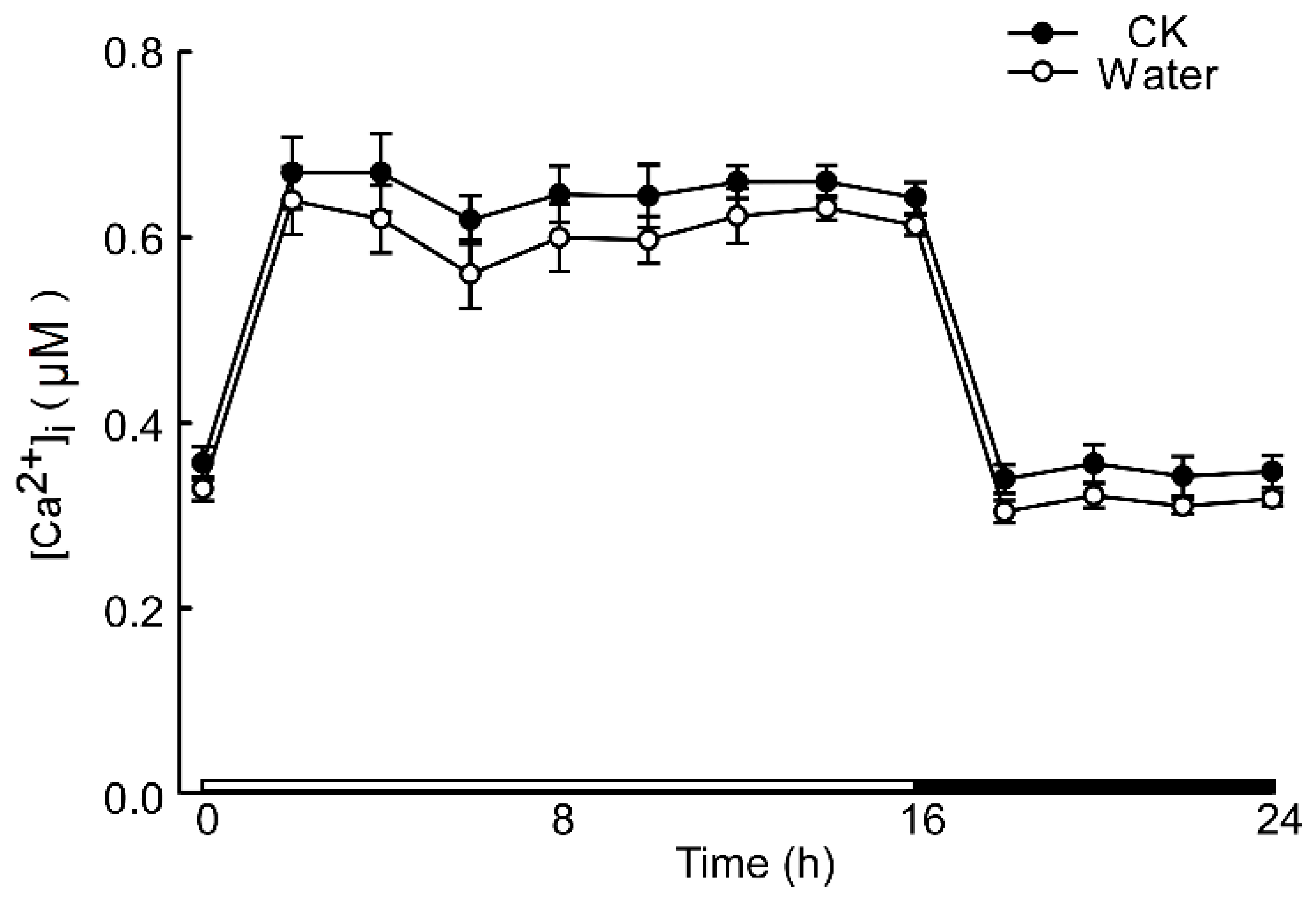
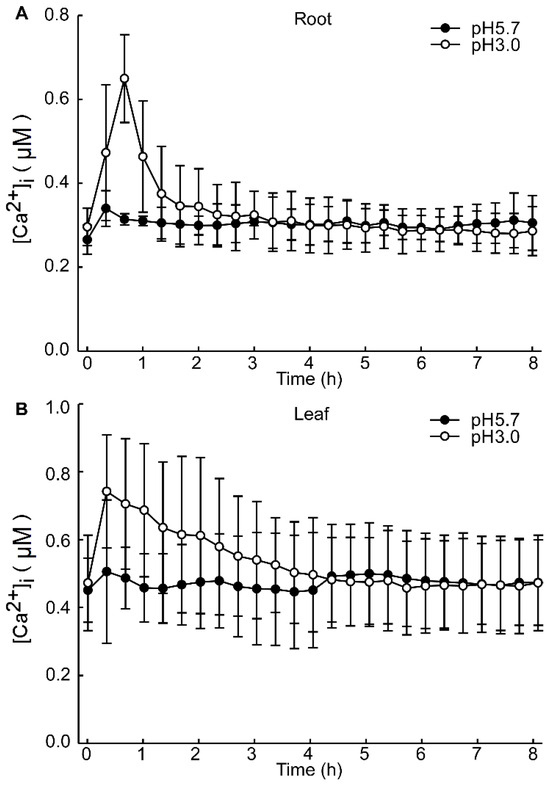
Figure A2.
Increases in [Ca2+]i in response to low pH after 8 h. Aequorin-expressing seedlings grown for 9 days were treated with solutions containing EMS-adjusted pH with Tris base, and aequorin images were taken every 20 min for 8 h. Increase in [Ca2+]i induced by pH 3.0 and pH 5.7 treatment in root of Arabidopsis (A). Increase in [Ca2+]i in Arabidopsis leaf, induced by pH 3.0 and pH 5.7 solution treated on root of Arabidopsis (B). Data from four independent experiments are shown (mean ± s.e.m; n = 180).
Figure A2.
Increases in [Ca2+]i in response to low pH after 8 h. Aequorin-expressing seedlings grown for 9 days were treated with solutions containing EMS-adjusted pH with Tris base, and aequorin images were taken every 20 min for 8 h. Increase in [Ca2+]i induced by pH 3.0 and pH 5.7 treatment in root of Arabidopsis (A). Increase in [Ca2+]i in Arabidopsis leaf, induced by pH 3.0 and pH 5.7 solution treated on root of Arabidopsis (B). Data from four independent experiments are shown (mean ± s.e.m; n = 180).
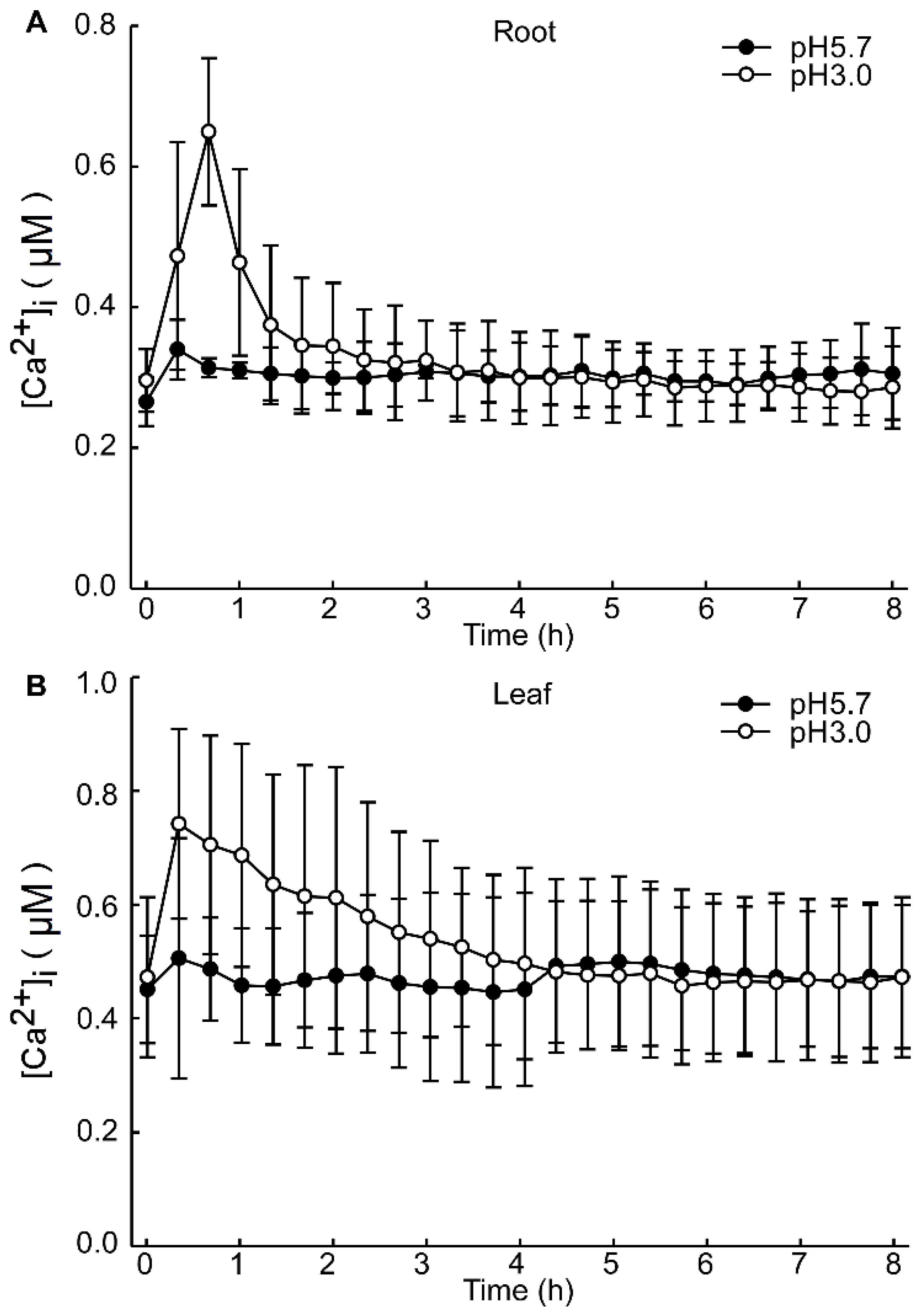
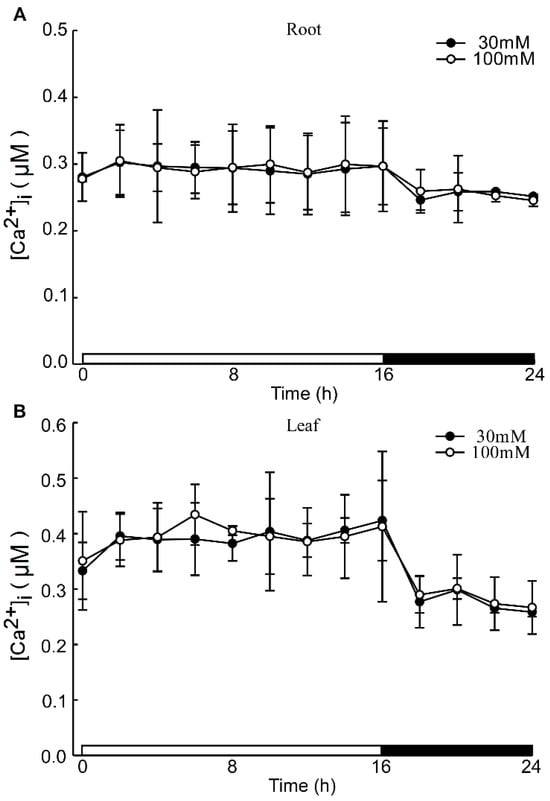
Figure A3.
The effects of Ca2+ inhibitors on low pH-induced [Ca2+]i increases. The effects of different concentrations of Ca2+ inhibitors in pH 3.0 induced [Ca2+]i increases. The concentration gradient of LaCl3 was 30 μM and 100 μM. Data from four independent experiments are shown (mean ± sd; n = 10; NS, insignificant p > 0.05; Student’s t-test).
Figure A3.
The effects of Ca2+ inhibitors on low pH-induced [Ca2+]i increases. The effects of different concentrations of Ca2+ inhibitors in pH 3.0 induced [Ca2+]i increases. The concentration gradient of LaCl3 was 30 μM and 100 μM. Data from four independent experiments are shown (mean ± sd; n = 10; NS, insignificant p > 0.05; Student’s t-test).
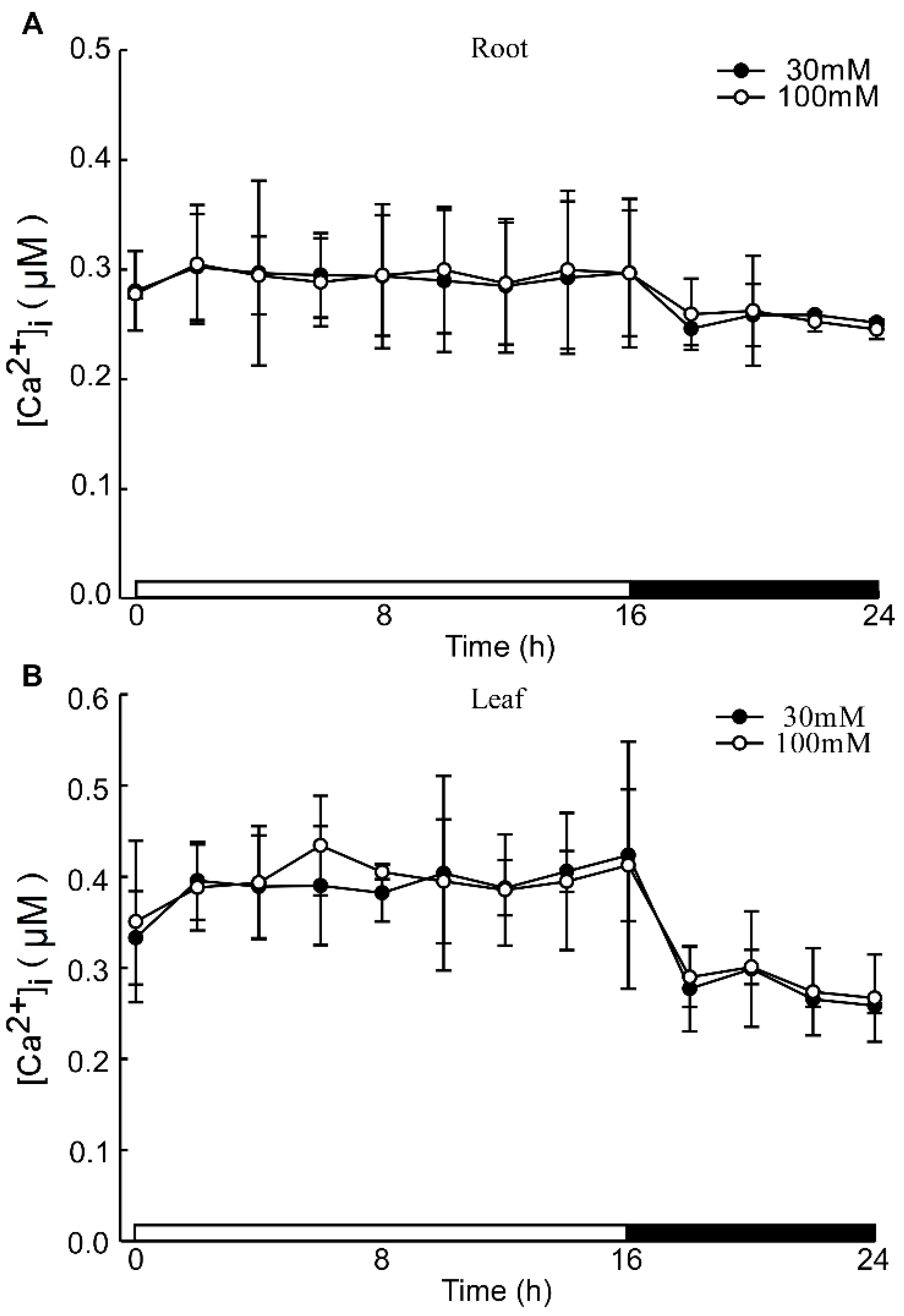
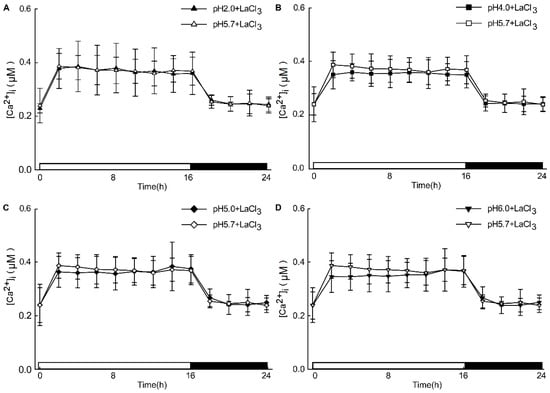
Figure A4.
The effects of Ca2+ inhibitors on low pH-induced [Ca2+]i increases in the root. Seedlings expressing aequorin and grown for 9 days were treated with solutions containing 1mM LaCl3 with an adjusted pH with Tris base, and aequorin images were taken every 20 min in 2 h for 24 h. The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced pH 2.0, and pH 5.7 induced [Ca2+]i changes in the Arabidopsis root (A). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 4.0 and treated with a pH 5.7 solution (B). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 5.0 and treated with a pH 5.7 solution (C). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 6.0 and treated with a pH 5.7 solution (D). Data from four independent experiments are shown (mean ± SD; n = 180).
Figure A4.
The effects of Ca2+ inhibitors on low pH-induced [Ca2+]i increases in the root. Seedlings expressing aequorin and grown for 9 days were treated with solutions containing 1mM LaCl3 with an adjusted pH with Tris base, and aequorin images were taken every 20 min in 2 h for 24 h. The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced pH 2.0, and pH 5.7 induced [Ca2+]i changes in the Arabidopsis root (A). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 4.0 and treated with a pH 5.7 solution (B). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 5.0 and treated with a pH 5.7 solution (C). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 6.0 and treated with a pH 5.7 solution (D). Data from four independent experiments are shown (mean ± SD; n = 180).
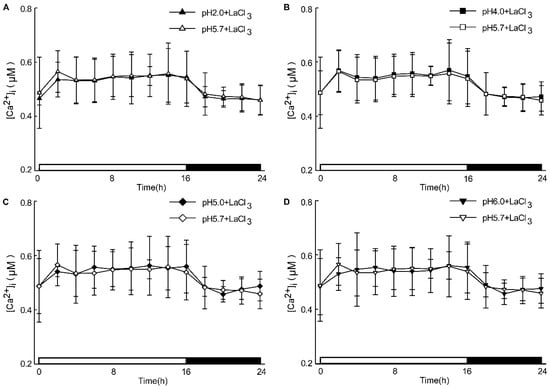
Figure A5.
The effects of Ca2+ inhibitors on low pH-induced [Ca2+]i increases in leaves. Seedlings expressing aequorin and grown for 9 days were treated with solutions containing 1 mM LaCl3 with an adjusted pH with Tris base, and aequorin images were taken every 20 min in 2 h for 24 h. The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced pH 2.0, and pH 5.7 induced [Ca2+]i changes in Arabidopsis leaves (A). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 4.0 and treated with a pH 5.7 solution (B). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 5.0 and treated with a pH 5.7 solution (C). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in Arabidopsis leaves, which was induced by pH 6.0 and treated with a pH 5.7 solution (D). Data from four independent experiments are shown (mean ± SD; n = 180).
Figure A5.
The effects of Ca2+ inhibitors on low pH-induced [Ca2+]i increases in leaves. Seedlings expressing aequorin and grown for 9 days were treated with solutions containing 1 mM LaCl3 with an adjusted pH with Tris base, and aequorin images were taken every 20 min in 2 h for 24 h. The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced pH 2.0, and pH 5.7 induced [Ca2+]i changes in Arabidopsis leaves (A). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 4.0 and treated with a pH 5.7 solution (B). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in the leaves of Arabidopsis, which were induced by pH 5.0 and treated with a pH 5.7 solution (C). The treatment of Ca2+ inhibitors (1 mM of LaCl3) reduced [Ca2+]i changes in Arabidopsis leaves, which was induced by pH 6.0 and treated with a pH 5.7 solution (D). Data from four independent experiments are shown (mean ± SD; n = 180).
References
- Abbasi, T.; Poornima, P.; Kannadasan, T.; Abbasi, S.A. Acid rain: Past, present, and future. Int. J. Environ. Eng. 2013, 5, 229–272. [Google Scholar] [CrossRef]
- Anita, S.; Madhoolika, A. Acid rain and its ecological consequences. J. Environ. Biol. 2008, 29, 15–24. [Google Scholar]
- Bashkin, V.N.; Radojevic, M. Acid Rain and Its Mitigation in Asia. Int. J. Environ. Stud. 2003, 60, 205–214. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, X.; Hao, T.; Zeng, M.; Shen, J.; Zhang, F.; de Vries, W. Cropland acidification increases risk of yield losses and food insecurity in China. Environ. Pollut. 2020, 256, 113145. [Google Scholar] [CrossRef]
- Odiyi, B.O.; Bamidele, J.J.F. Effects of Simulated Acid Rain on Growth and Yield of Cassava Manihot esculenta (Crantz). J. Agric. Sci. 2013, 6, 96. [Google Scholar] [CrossRef]
- Wyrwicka, A.; Skłodowska, M. Influence of repeated acid rain treatment on antioxidative enzyme activities and on lipid peroxidation in cucumber leaves. Environ. Exp. Bot. 2006, 56, 198–204. [Google Scholar] [CrossRef]
- Wyrwicka, A.; Skłodowska, M. Intercompartmental differences between cytosol and mitochondria in their respective antioxidative responses and lipid peroxidation levels in acid rain stress. Acta Physiol. Plant. 2014, 36, 837–848. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Y.; Wang, L.; Zhou, Q.; Huang, X.; Sun, Z. Combined Effects of Lanthanum (III) and Acid Rain on Antioxidant Enzyme System in Soybean Roots. PLoS ONE 2015, 10, e0134546. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Q.; de Vries, W.; Ros, G.H.; Chen, X.; Muneer, M.A.; Zhang, F.; Wu, L. Effects of soil amendments on soil acidity and crop yields in acidic soils: A world-wide meta-analysis. J. Environ. Manag. 2023, 345, 118531. [Google Scholar] [CrossRef]
- Velle, K.B.; Garner, R.M.; Beckford, T.K.; Weeda, M.; Liu, C.; Kennard, A.S.; Edwards, M.; Fritz-Laylin, L.K. A conserved pressure-driven mechanism for regulating cytosolic osmolarity. Curr. Biol. 2023, 33, 3325–3337. [Google Scholar] [CrossRef]
- Majdi, A.; Mahmoudi, J.; Sadigh-Eteghad, S.; Golzari, S.E.; Sabermarouf, B.; Reyhani-Rad, S. Permissive role of cytosolic pH acidification in neurodegeneration: A closer look at its causes and consequences. J. Neurosci. Res. 2016, 94, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Bu, Y.; Takano, T.; Zhang, X.; Liu, S. Conserved V-ATPase c subunit plays a role in plant growth by influencing V-ATPase-dependent endosomal trafficking. Plant Biotechnol. J. 2016, 14, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zeng, Y.; Zhuang, X.; Sun, L.; Yao, X.; Pimpl, P.; Jiang, L. Organelle pH in the Arabidopsis endomembrane system. Mol. Plant 2013, 6, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Scholl, S.; Doering, A.; Zhang, Y.; Irani, N.G.; Di Rubbo, S.; Neumetzler, L.; Krishnamoorthy, P.; Van Houtte, I.; Mylle, E.; et al. V-ATPase activity in the TGN/EE is required for exocytosis and recycling in Arabidopsis. Nat. Plants 2015, 1, 15094. [Google Scholar] [CrossRef]
- Krebs, M.; Beyhl, D.; Görlich, E.; Al-Rasheid, K.A.S.; Marten, I.; Stierhof, Y.-D.; Hedrich, R.; Schumacher, K. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 3251–3256. [Google Scholar] [CrossRef]
- Gao, D.; Knight, M.R.; Trewavas, A.J.; Sattelmacher, B.; Plieth, C. Self-Reporting Arabidopsis Expressing pH and [Ca2+] Indicators Unveil Ion Dynamics in the Cytoplasm and in the Apoplast under Abiotic Stress. Plant. Physiol. 2004, 134, 898–908. [Google Scholar] [CrossRef]
- Calderón Villalobos, L.I.; Lee, S.; De Oliveira, C.; Ivetac, A.; Brandt, W.; Armitage, L.; Sheard, L.B.; Tan, X.; Parry, G.; Mao, H.; et al. A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat. Chem. Biol. 2012, 8, 477–485. [Google Scholar] [CrossRef]
- Salehin, M.; Bagchi, R.; Estelle, M. SCFTIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. Plant Cell 2015, 27, 9–19. [Google Scholar] [CrossRef]
- Sun, N.; Wang, J.; Gao, Z.; Dong, J.; He, H.; Terzaghi, W.; Wei, N.; Deng, X.W.; Chen, H. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. USA 2016, 113, 6071–6076. [Google Scholar] [CrossRef]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR Inhibition of PP2C-D Phosphatases Activates Plasma Membrane H+-ATPases to Promote Cell Expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef]
- Ren, H.; Park, M.Y.; Spartz, A.K.; Wong, J.H.; Gray, W.M. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis. PLoS Genet. 2018, 14, e1007455. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, K.; Vissenberg, K. Roles of the XTH Protein Family in the Expanding Cell. In The Expanding Cell; Verbelen, J.-P., Vissenberg, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 89–116. [Google Scholar]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 1992, 4, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting Homogalacturonan-Type Pectin Remodeling to Acid Growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, K.; Suzuki, T.; Kuruma, M.; Nishiyama, K.; Hayashi, K.I.; Hagihara, S.; Seto, Y. Radicle Growth Regulation of Root Parasitic Plants by Auxin-related Compounds. Plant Cell Physiol. 2024, 65, 1377–1387. [Google Scholar] [CrossRef]
- Pacheco-Villalobos, D.; Díaz-Moreno, S.M.; van der Schuren, A.; Tamaki, T.; Kang, Y.H.; Gujas, B.; Novak, O.; Jaspert, N.; Li, Z.; Wolf, S.; et al. The Effects of High Steady State Auxin Levels on Root Cell Elongation in Brachypodium. Plant Cell 2016, 28, 1009–1024. [Google Scholar] [CrossRef]
- Barbez, E.; Dünser, K.; Gaidora, A.; Lendl, T.; Busch, W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E4884–E4893. [Google Scholar] [CrossRef]
- Gjetting, K.S.; Ytting, C.K.; Schulz, A.; Fuglsang, A.T. Live imaging of intra- and extracellular pH in plants using pHusion, a novel genetically encoded biosensor. J. Exp. Bot. 2012, 63, 3207–3218. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Miller, N.D.; Murphy, A.S.; Gilroy, S. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J. 2011, 65, 309–318. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Bibikova, T.N.; Weisenseel, M.H.; Gilroy, S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 2009, 21, 2341–2356. [Google Scholar] [CrossRef]
- Kudla, J.; Batistič, O.; Hashimoto, K. Calcium Signals: The Lead Currency of Plant Information Processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Y.; Zhong, M.; Hussian, J.; Tang, Y.; Liu, S.; Qi, G. Calcium signaling-mediated transcriptional reprogramming during abiotic stress response in plants. TAG Theor. Appl. Genet. 2023, 136, 210. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 1996, 8, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.P. Calcium Signalling in Plant Biotic Interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.F.; Breton, G.; Harmon, A. Decoding Ca2+ signals through plant protein kinases. Annu. Rev. Plant Biol. 2004, 55, 263–288. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2010, 425, 27–40. [Google Scholar] [CrossRef]
- Kmiecik, P.; Leonardelli, M.; Teige, M. Novel connections in plant organellar signalling link different stress responses and signalling pathways. J. Exp. Bot. 2016, 67, 3793–3807. [Google Scholar] [CrossRef]
- Dalchau, N.; Hubbard, K.E.; Robertson, F.C.; Hotta, C.T.; Briggs, H.M.; Stan, G.B.; Gonçalves, J.M.; Webb, A.A. Correct biological timing in Arabidopsis requires multiple light-signaling pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 13171–13176. [Google Scholar] [CrossRef]
- Johnson, C.H.; Knight, M.R.; Kondo, T.; Masson, P.; Sedbrook, J.; Haley, A.; Trewavas, A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 1995, 269, 1863–1865. [Google Scholar] [CrossRef]
- Love, J.; Dodd, A.N.; Webb, A.A. Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 2004, 16, 956–966. [Google Scholar] [CrossRef]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef]
- Abdul-Awal, S.M.; Hotta, C.T.; Davey, M.P.; Dodd, A.N.; Smith, A.G.; Webb, A.A. NO-Mediated [Ca2+]cyt Increases Depend on ADP-Ribosyl Cyclase Activity in Arabidopsis. Plant Physiol. 2016, 171, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.C.; Stancombe, M.A.; Webb, A.A. Cell- and stimulus type-specific intracellular free Ca2+ signals in Arabidopsis. Plant Physiol. 2013, 163, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Gardner, M.J.; Hotta, C.T.; Hubbard, K.E.; Dalchau, N.; Love, J.; Assie, J.M.; Robertson, F.C.; Jakobsen, M.K.; Gonçalves, J.; et al. The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 2007, 318, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Martí Ruiz, M.C.; Hubbard, K.E.; Gardner, M.J.; Jung, H.J.; Aubry, S.; Hotta, C.T.; Mohd-Noh, N.I.; Robertson, F.C.; Hearn, T.J.; Tsai, Y.C.; et al. Circadian oscillations of cytosolic free calcium regulate the Arabidopsis circadian clock. Nat. Plants 2018, 4, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.G.; Vothknecht, U.C. The role of calcium in chloroplasts--an intriguing and unresolved puzzle. Protoplasma 2012, 249, 957–966. [Google Scholar] [CrossRef]
- Nomura, H.; Shiina, T. Calcium signaling in plant endosymbiotic organelles: Mechanism and role in physiology. Mol. Plant 2014, 7, 1094–1104. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta 2015, 1847, 993–1003. [Google Scholar] [CrossRef]
- Sello, S.; Perotto, J.; Carraretto, L.; Szabò, I.; Vothknecht, U.C.; Navazio, L. Dissecting stimulus-specific Ca2+ signals in amyloplasts and chloroplasts of Arabidopsis thaliana cell suspension cultures. J. Exp. Bot. 2016, 67, 3965–3974. [Google Scholar] [CrossRef]
- Loro, G.; Wagner, S.; Doccula, F.G.; Behera, S.; Weinl, S.; Kudla, J.; Schwarzländer, M.; Costa, A.; Zottini, M. Chloroplast-Specific in Vivo Ca2+ Imaging Using Yellow Cameleon Fluorescent Protein Sensors Reveals Organelle-Autonomous Ca2+ Signatures in the Stroma. Plant Physiol. 2016, 171, 2317–2330. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Q.R.; Liu, L.L.; Zhang, H.M.; Gao, J.W.; Pei, Z.M. Osmotic stress alters circadian cytosolic Ca2+ oscillations and OSCA1 is required in circadian gated stress adaptation. Plant Signal. Behav. 2020, 15, 1836883. [Google Scholar] [CrossRef]
- Knight, M.R.; Campbell, A.K.; Smith, S.M.; Trewavas, A.J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 1991, 352, 524. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.H.; Han, S.; Zheng, H.; Cook, C.W.; Choi, C.S.; Woerner, T.E.; Jackson, R.B.; Pei, Z.-M. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science 2007, 315, 1423–1426. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Knight, M.R. Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant Sci. 2001, 6, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Ranf, S.; Grimmer, J.; Pöschl, Y.; Pecher, P.; Chinchilla, D.; Scheel, D.; Lee, J. Defense-Related Calcium Signaling Mutants Uncovered via a Quantitative High-Throughput Screen in Arabidopsis thaliana. Mol. Plant 2012, 5, 115–130. [Google Scholar] [CrossRef]
- Tracy, F.E.; Gilliham, M.; Dodd, A.N.; Webb, A.A.; Tester, M. NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell Environ. 2008, 31, 1063–1073. [Google Scholar] [CrossRef]
- Wacquier, B.; Voorsluijs, V.; Combettes, L.; Dupont, G. Coding and decoding of oscillatory Ca2+ signals. Semin. Cell Dev. Biol. 2019, 94, 11–19. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, Y.; Liang, G.; Liu, N.; Zhu, J.K. Aequorin-based luminescence imaging reveals stimulus- and tissue-specific Ca2+ dynamics in Arabidopsis plants. Mol. Plant 2013, 6, 444–455. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The Language of Calcium Signaling. Annu. Rev. Plant. Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Zhu, S.; Ye, R.; Xue, Y.; Chen, A.; An, L.Z.; Pei, Z.M. Relationship between NaCl- and H2O2-Induced Cytosolic Ca2+ Increases in Response to Stress in Arabidopsis. PLoS ONE 2013, 8, e76130. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Mohapatra, S.K.; Rout, G.R. Plant membrane transporters function under abiotic stresses: A review. Planta 2024, 260, 125. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.J.; Choi, W.G.; Chanoca, A.; Gilroy, S. In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu. Rev. Plant. Biol. 2011, 62, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Tao, Q.; Li, W.; Qi, G.; Wang, B.; Wang, Y.; Dai, S.; Shen, Q.; Wang, X.; Wu, X.; et al. Osmosensor-mediated control of Ca2+ spiking in pollen germination. Nature 2024, 629, 1118–1125. [Google Scholar] [CrossRef]
- Bergner, A.; Sanderson, M.J. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1271–L1279. [Google Scholar] [CrossRef]
- Schuster, S.; Marhl, M.; Hofer, T. Modelling of simple and complex calcium oscillations. From single-cell responses to intercellular signalling. Eur. J. Biochem. 2002, 269, 1333–1355. [Google Scholar] [CrossRef]
- Ruan, H.; Wang, T.; Ren, H.; Zhang, Y. AtFH5-labeled secretory vesicles-dependent calcium oscillation drives exocytosis and stepwise bulge during pollen germination. Cell Rep. 2023, 42, 113319. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef]
- Holzheu, P.; Krebs, M.; Larasati, C.; Schumacher, K.; Kummer, U. An integrative view on vacuolar pH homeostasis in Arabidopsis thaliana: Combining mathematical modeling and experimentation. Plant J. 2021, 106, 1541–1556. [Google Scholar] [CrossRef]
- Shao, Q.; Gao, Q.; Lhamo, D.; Zhang, H.; Luan, S. Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci. Signal. 2020, 13, eaba1453. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.; Menzle, H.; Krauss, A. Versuche und Hypothese zur Primärwirkung des Auxins beim Streckungswachstum [Experiments and hypothesis concerning the primary action of auxin in elongation growth]. Planta 1971, 100, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Rayle, D.L. Auxin-induced hydrogen-ion secretion in Avena coleoptiles and its implications. Planta 1973, 114, 63–73. [Google Scholar] [CrossRef]
- Qiu, T.; Qi, M.; Ding, X.; Zheng, Y.; Zhou, T.; Chen, Y.; Han, N.; Zhu, M.; Bian, H.; Wang, J. The SAUR41 subfamily of SMALL AUXIN UP RNA genes is abscisic acid inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann. Bot. 2020, 125, 805–819. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. Evidence that Auxin-induced Growth of Soybean Hypocotyls Involves Proton Excretion. Plant Physiol. 1980, 66, 433–437. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, S.; Haq, S.I.U.; Zheng, D.; Qiu, Q.S. Regulation of pollen tube growth by cellular pH and ions. J. Plant Physiol. 2022, 277, 153792. [Google Scholar] [CrossRef]
- Giridhar, M.; Meier, B.; Imani, J.; Kogel, K.H.; Peiter, E.; Vothknecht, U.C.; Chigri, F. Comparative analysis of stress-induced calcium signals in the crop species barley and the model plant Arabidopsis thaliana. BMC Plant Biol. 2022, 22, 447. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, Y.; Qiu, T.; Wang, J. The SAUR41 subfamily of cell expansion-promoting genes modulates abscisic acid sensitivity and root touch response: A possible connection to ion homeostasis regulation. Plant Signal. Behav. 2020, 15, 1702239. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef]
- Sénéchal, F.; Habrylo, O.; Hocq, L.; Domon, J.-M.; Marcelo, P.; Lefebvre, V.; Pelloux, J.; Mercadante, D. Structural and dynamical characterization of the pH-dependence of the pectin methylesterase–pectin methylesterase inhibitor complex. J. Biol. Chem. 2017, 292, 21538–21547. [Google Scholar] [CrossRef]
- Sinclair, S.A.; Larue, C.; Bonk, L.; Khan, A.; Castillo-Michel, H.; Stein, R.J.; Grolimund, D.; Begerow, D.; Neumann, U.; Haydon, M.J.; et al. Etiolated Seedling Development Requires Repression of Photomorphogenesis by a Small Cell-Wall-Derived Dark Signal. Curr. Biol. 2017, 27, 3403–3418.e7. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.P.; Duque, P.; Chua, N.H. ABA activates ADPR cyclase and cADPR induces a subset of ABA-responsive genes in Arabidopsis. Plant J. 2004, 38, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hotta, C.T.; Dodd, A.N.; Love, J.; Sharrock, R.; Lee, Y.W.; Xie, Q.; Johnson, C.H.; Webb, A.A. Distinct light and clock modulation of cytosolic free Ca2+ oscillations and rhythmic CHLOROPHYLL A/B BINDING PROTEIN2 promoter activity in Arabidopsis. Plant Cell 2007, 19, 3474–3490. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.J.; Gilliham, M.; Athman, A.; Schreiber, A.W.; Baumann, U.; Moller, I.; Cheng, N.H.; Stancombe, M.A.; Hirschi, K.D.; Webb, A.A.; et al. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 2011, 23, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Villiers, F.; Kroniewicz, L.; Lee, S.; Seo, Y.J.; Hirschi, K.D.; Leonhardt, N.; Kwak, J.M. Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol. 2012, 160, 1293–1302. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Peer, W.A.; Richter, G.; Blakeslee, J.; Bandyopadhyay, A.; Titapiwantakun, B.; Undurraga, S.; Khodakovskaya, M.; Richards, E.L.; et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 2005, 310, 121–125. [Google Scholar] [CrossRef]
- Serre, N.B.C.; Wernerová, D.; Vittal, P.; Dubey, S.M.; Medvecká, E.; Jelínková, A.; Petrášek, J.; Grossmann, G.; Fendrych, M. The AUX1-AFB1-CNGC14 module establishes a longitudinal root surface pH profile. eLife 2023, 12, e85193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).