HSCCC Straightforward Fast Preparative Method for Isolation of Two Major Cytotoxic Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann
Abstract
1. Introduction
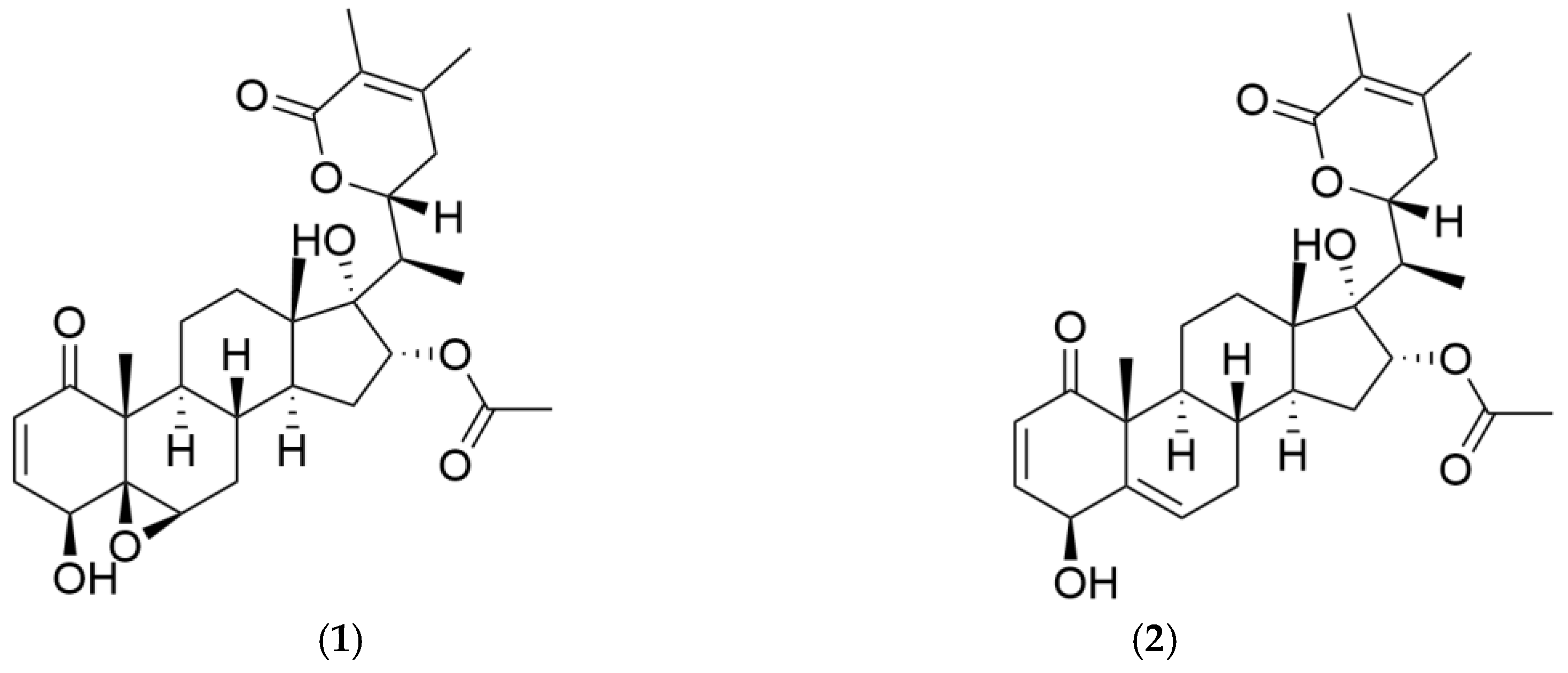
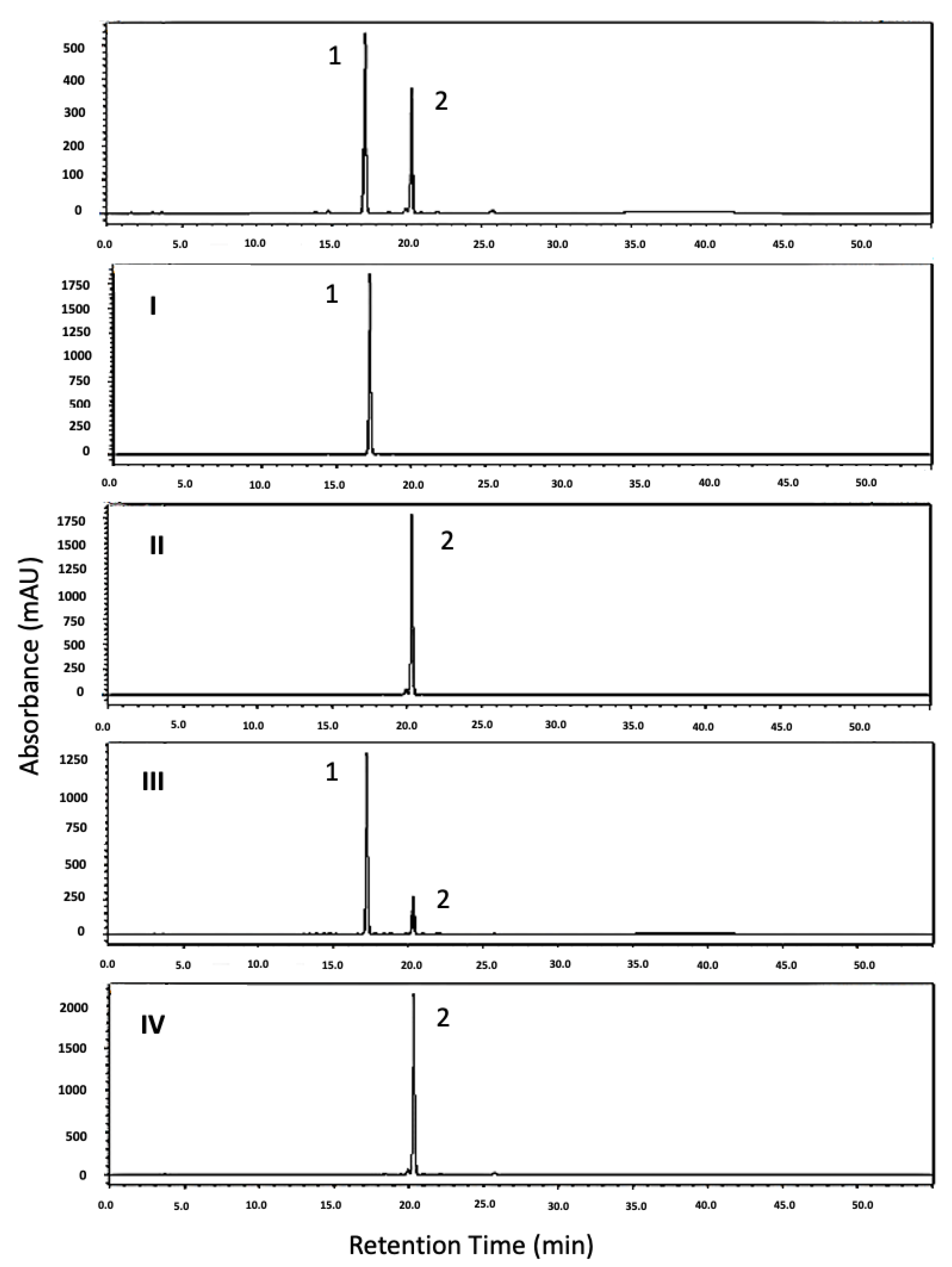
2. Results and Discussion
Separation Procedure and Scale-Up
3. Materials and Methods
3.1. Reagents and Materials
3.2. Botanical Material
3.3. Plant Extraction
3.4. HPLC Analysis and Identification of HSCCC Peak Fractions
3.5. AFFD Chlorophyll Cleanup
3.6. Aurelianolide A and B’s Pre-Concentration
3.7. High-Speed Countercurrent Chromatography (HSCCC)
3.7.1. Selection of Two-Phase Solvent System
3.7.2. HSCCC Equipment and Separation Procedure
3.8. Scale-Up and Separation Procedure
Optimization of Separation and Purification Conditions
3.9. NMR Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IARC, International Agency for Research on Cancer. Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype (accessed on 11 March 2024).
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines 2024, 12, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Tauro, S.; Dhokchawle, B.; Mohite, P.; Nahar, D.; Nadar, S.; Coutinho, E. Natural Anticancer Agents: Their Therapeutic Potential, Challenges and Promising Outcomes. Curr. Med. Chem. 2023, 31, 848–870. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- White, P.T.; Subramanian, C.; Motiwala, H.F.; Cohen, M.S. Natural Withanolides in the Treatment of Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 329–373. [Google Scholar]
- Singh, A.; Raza, A.; Amin, S.; Damodaran, C.; Sharma, A.K. Recent Advances in the Chemistry and Therapeutic Evaluation of Naturally Occurring and Synthetic Withanolides. Molecules 2022, 27, 886–916. [Google Scholar] [CrossRef]
- Xia, G.; Cao, S.; Chen, L.; Qiu, F. Natural withanolides, an update. Nat. Prod. Rep. 2022, 39, 784–813. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Cao, S.; Kang, N.; Qiu, F. Withanolides: Promising candidates for cancer therapy. Phytother. Res. 2024, 38, 1104–1158. [Google Scholar] [CrossRef]
- Rodrigues, I.M.D.C.; Knapp, S.; Stehmann, J.R. Two new species of Athenaea Sendtn. (Solanaceae) from the Atlantic forests of south-eastern Brazil. PhytoKeys 2021, 178, 1–15. [Google Scholar] [CrossRef]
- Silva, S.G.W.; Marques, A.M.; Fontão, A.P.G.A.; DE Moura Lima, S.C.; Kaplan, M.A.C.; Figueiredo, M.R.; Sampaio, A.L.F. Aurelianolides from Aureliana fasciculata var. fasciculata Trigger Apoptosis with Caspase Activation in Human Leukemia Cells. Anticancer Res. 2023, 43, 1245–1253. [Google Scholar] [CrossRef]
- Peres, R.B.; Fiuza, L.F.A.; da Silva, P.B.; Batista, M.M.; Camillo, F.D.C.; Marques, A.M.; Brito, L.; Figueiredo, M.R.; Soeiro, M.N.C. In Vitro Phenotypic Activity and In Silico Analysis of Natural Products from Brazilian Biodiversity on Trypanosoma cruzi. Molecules 2021, 26, 5676. [Google Scholar] [CrossRef]
- Lima, S.C.M.; Pacheco, J.D.S.; Marques, A.M.; Veltri, E.R.P.; Almeida-Lafetá, R.C.; Figueiredo, M.R.; Kaplan, M.A.C.; Torres-Santos, E.C. Leishmanicidal Activity of Withanolides from Aureliana fasciculata var. fasciculata. Molecules 2018, 23, 3160. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Smetanska, I. Special Issue “Bioactive Compounds from Natural Sources (2020, 2021)”. Molecules 2022, 27, 1929. [Google Scholar] [CrossRef] [PubMed]
- Mello, R.F.A.; Pinheiro, W.B.S.; Benjamim, J.K.F.; Siqueira, F.C.; Chisté, R.C.; Santos, A.S. A fast and efficient preparative method for separation and purification of main bioactive xanthones from the waste of Garcinia mangostana L. by high-speed countercurrent chromatography. Arab. J. Chem. 2021, 14, 103252. [Google Scholar] [CrossRef]
- Ponphaiboon, J.; Krongrawa, W.; Aung, W.W.; Chinatangkul, N.; Limmatvapirat, S.; Limmatvapirat, C. Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review. Molecules 2023, 28, 5163. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Bioassay-guided isolation and evaluation of herbal drugs. Qual Control. Eval. Herb. Drugs. J. 2019, 14, 515–537. [Google Scholar]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Trujillo-Roldán, M.A. Factors that influence the extraction methods of terpenes from natural sources. Chem. Pap. 2024, 78, 2783–2810. [Google Scholar] [CrossRef]
- Fontana, A.; Schieber, A. Preparative Fractionation of Phenolic Compounds and Isolation of an Enriched Flavonol Fraction from Winemaking Industry By-Products by High-Performance Counter-Current Chromatography. Plants 2023, 12, 2242. [Google Scholar] [CrossRef]
- Lima, A.M.; Siani, A.C.; Nakamura, M.J.; D’Avila, L.A. Selective and cost-effective protocol to separate bioactive triterpene acids from plant matrices using alkalinized ethanol: Application to leaves of Myrtaceae species. Pharmacogn. Mag. 2015, 11, 470–476. [Google Scholar]
- Zuo, G.; Je, K.-H.; Guillen Quispe, Y.N.; Shin, K.-O.; Kim, H.Y.; Kim, K.H.; Arce, P.H.G.; Lim, S.S. Separation and Identification of Antioxidants and Aldose Reductase Inhibitors in Lepechinia meyenii (Walp.) Epling. Plants 2021, 10, 2773. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Yang, T.; Sun, B. High-speed countercurrent chromatography as an efficient technique for large separation of plant polyphenols: A review. Food Res Int. 2022, 153, 110956. [Google Scholar] [CrossRef]
- Marques, A.M.; Fingolo, C.E.; Kaplan, M.A.C. HSCCC separation and enantiomeric distribution of key volatile constituents of Piper claussenianum (Miq.) C. DC. (Piperaceae). Food Chem. Toxicol. 2017, 109, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- de Mello, L.L.O.; Leitão, G.G. Solvent Systems Used in Countercurrent Chromatography for the Purification of Diterpene Compounds. Rev. Bras. Farmacogn. 2024, 34, 23–34. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Feng, X.; Ibrahim, S.A.; Liu, Y.; Huang, W. Anti-Inflammatory Activity of Four Triterpenoids Isolated from Poriae cutis. Foods 2021, 10, 3155. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Woźniak, K.; Garrard, I. Counter-current chromatography for the separation of terpenoids: A comprehensive review with respect to the solvent systems employed. Phytochem. Rev. 2014, 13, 547–572. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Lafetá, R.; Ferreira, M.J.P.; Emerenciano, V.P.; Kaplan, M.A.C. Withanolides from Aureliana fasciculata var. fasciculata. Helv. Chim. Acta. 2010, 93, 2478–2487. [Google Scholar] [CrossRef]
- Kostanyan, A.E. On influence of sample loading conditions on peak shape and separation efficiency in preparative isocratic counter-current chromatography. J Chromatogr A. 2012, 1254, 71–77. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Liu, C.; Zhang, Y.; Li, S. Total Triterpenoid Extraction from Inonotus obliquus Using Ionic Liquids and Separation of Potential Lactate Dehydrogenase Inhibitors via Ultrafiltration High-Speed Countercurrent Chromatography. Molecules 2021, 26, 2467. [Google Scholar] [CrossRef]
- Zhu, L.; Li, B.; Liu, X.; Meng, X. Purification of two triterpenoids from Schisandra chinensis by macroporous resin combined with high-speed counter-current chromatography. J. Chromatogr. Sci. 2014, 52, 1082–1088. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhao, S.; Sun, W.; Tong, S. Preparative separation of structural isomeric pentacyclic triterpene oleanolic acid and ursolic acid from natural products by pH-zone-refining countercurrent chromatography. RSC Adv. 2019, 9, 38860–38866. [Google Scholar] [CrossRef]
- Kou, P.; Wang, S.; Pan, H.; Wan, N.; Wang, X.; Liu, Z.; Zhao, C.; Jiang, S.; Fu, Y. Preparative separation of specific triterpenoids from Inonotus obliquus based on negative-pressure cavitation extraction and high-speed counter-current chromatography. J. Taiwan Inst. Chem. Eng. 2021, 125, 69–77. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Chen, P.; Winterhalter, P. Preparation of Ursane Triterpenoids from Centella asiatica Using High Speed Countercurrent Chromatography with Step-Gradient Elution. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2201–2215. [Google Scholar] [CrossRef]




| Two-Phase Solvents | Volume Ratio | Partition Coefficient (KD) | ||
|---|---|---|---|---|
| KD1 | KD2 | |||
| 1 | n-Hex/AcoEt/MeOH/H2O | 3:6:2:1 | 1.5 | 1.3 |
| 2 | n-Hex/MeOH | 2:1 | 12.0 | 15.0 |
| 3 | CHCl3/MeOH/H2O | 7:13:8 | 4.4 | 5.8 |
| 4 | CHCl3/BuOH/MeOH | 7:3:6:4 | 13.0 | 17.0 |
| 5 | CHCl3/MeOH/H2O | 2:2:1 | 6.6 | 7.5 |
| 6 | AcoEt/BuOH/H2O | 1:1:2 | 14.0 | 17.0 |
| 7 | n-Hex/BuOH/H2O | 1:10:5 | 6.5 | 14.5 |
| 8 | n-Hex/BuOH/MeOH/H2O | 1:1:1:1 | 12.0 | 4.0 |
| 9 | n-Hex/EtOH/H2O | 6:5:1 | 15.0 | 16.0 |
| 10 | n-Hex/AcoEt/ACN | 1:1:1 | 18.0 | 18.0 |
| HEMWat Two-Phase Solvent System | ||||
|---|---|---|---|---|
| Volume Ratio | VUP/VLP | Partition Coefficient (KD) | ||
| K1 | K2 | |||
| I | 3:6:2:1 | 1/1 | 1.1 | 1.3 |
| 1 | 10:5:2.5:1 | 6/1 | 5.0 | 7.0 |
| 2 | 3:8:4:2 | 2/3 | 1.4 | 1.6 |
| 3 | 4:10:5:2 | 1/3 | 1.1 | 1.2 |
| 4 | 4:8:1:2 | 6/1 | 3.7 | 1.9 |
| 5 | 4:8:4:3 | 1/1 | 1.3 | 1.2 |
| 6 | 4:8:2:4 | 3/2 | 1.5 | 2.6 |
| 7 | 4:8:1:5 | 3/2 | 1.7 | 1.9 |
| 8 | 4:8:0.5:5 | 3/2 | 5.0 | 2.3 |
| 9 | 4:8:5:5 | 1/1 | 1.3 | 1.1 |
| 10 | 10:8:1:2 | 6/1 | 1.3 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.M.; Brito, L.d.C.; Figueiredo, M.R. HSCCC Straightforward Fast Preparative Method for Isolation of Two Major Cytotoxic Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann. Plants 2024, 13, 3039. https://doi.org/10.3390/plants13213039
Marques AM, Brito LdC, Figueiredo MR. HSCCC Straightforward Fast Preparative Method for Isolation of Two Major Cytotoxic Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann. Plants. 2024; 13(21):3039. https://doi.org/10.3390/plants13213039
Chicago/Turabian StyleMarques, André Mesquita, Lavínia de Carvalho Brito, and Maria Raquel Figueiredo. 2024. "HSCCC Straightforward Fast Preparative Method for Isolation of Two Major Cytotoxic Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann" Plants 13, no. 21: 3039. https://doi.org/10.3390/plants13213039
APA StyleMarques, A. M., Brito, L. d. C., & Figueiredo, M. R. (2024). HSCCC Straightforward Fast Preparative Method for Isolation of Two Major Cytotoxic Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann. Plants, 13(21), 3039. https://doi.org/10.3390/plants13213039






