Unveiling the Behavior of an Endangered Facultative Cuprophyte Coincya Species in an Abandoned Copper Mine (Southeast Portugal)
Abstract
1. Introduction
2. Results and Discussion
2.1. Soils Characterization
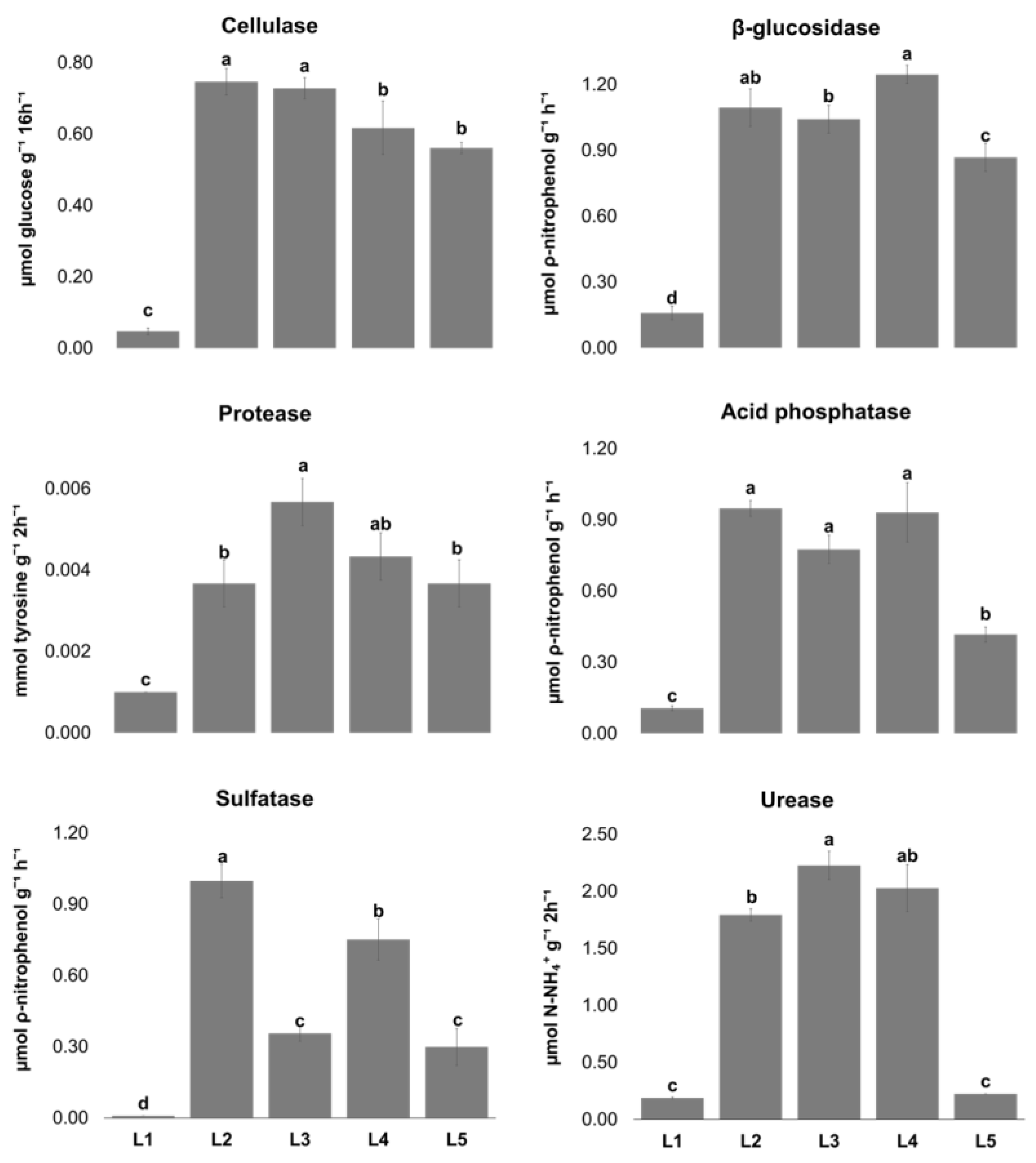
2.2. Enzymatic Activities
2.3. Plant Analysis
2.3.1. Flowers Characterization
2.3.2. Coincya transtagana Behavior
3. Materials and Methods
3.1. Study Species
3.2. Site Characterization
3.3. Soil and Plant Sampling, and Characterization
3.4. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Whiting, S.N.; Reeves, R.D.; Richards, D.; Johnson, M.S.; Cooke, J.A.; Malaisse, F.; Paton, A.; Smith, J.A.C.; Angle, J.S.; Chaney, R.L.; et al. Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor. Ecol. 2004, 12, 106–116. [Google Scholar] [CrossRef]
- Godefroid, S.; De Vyver, A.V.; Lebrun, J.; Kalenga, W.M.; Minengo, G.H.; Rose, C.; Mahy, G. Germination capacity and seed storage behaviour of threatened metallophytes from the Katanga copper belt (DR Congo): Implications for ex situ conservation. Plant Ecol. Evol. 2013, 146, 183–192. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; Van Der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef] [PubMed]
- del Real, A.E.P.; Garcia-Gonzalo, P.; Lobo, M.C.; Perez-Sanz, A. Chromium speciation modifies root exudation in two genotypes of Silene vulgaris. Environ. Exp. Bot. 2014, 107, 1–6. [Google Scholar] [CrossRef]
- Lombi, E.; Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol. 2000, 145, 11–20. [Google Scholar] [CrossRef]
- Poscic, F.; Fellet, G.; Vischi, M.; Casolo, V.; Schat, H.; Marchiol, L. Variation in heavy metal accumulation and genetic diversity at a regional scale among metallicolous and non-metallicolous populations of the facultative metallophyte Biscutella laevigata subsp laevigata. Int. J. Phytoremediat. 2015, 17, 464–475. [Google Scholar] [CrossRef]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.R.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. J. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Anacker, B.L.; Whittall, J.B.; Goldberg, E.E.; Harrison, S.P. Origins and consequences of serpentine endemism in the California Flora. Evolution 2011, 65, 365–376. [Google Scholar] [CrossRef]
- Kay, K.M.; Ward, K.L.; Watt, L.R.; Schemske, D.W. Plant speciation. In Serpentine: The Evolution and Ecology of a Model System; Harrison, S.P., Rajakaruna, N., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 71–96. [Google Scholar]
- Faucon, M.P.; Meersseman, A.; Shutcha, M.N.; Mahy, G.; Luhembwe, M.N.; Malaisse, F.; Meerts, P. Copper endemism in the Congolese flora: A database of copper affinity and conservational value of cuprophytes. Plant Ecol. Evol. 2010, 143, 5–18. [Google Scholar] [CrossRef]
- Faucon, M.P.; Le Stradic, S.; Boisson, S.; wa Ilunga, E.I.; Séleck, M.; Lange, B.; Guillaume, D.; Shutcha, M.N.; Pourret, O.; Meerts, P.; et al. Implication of plant-soil relationships for conservation and restoration of copper-cobalt ecosystems. Plant Soil 2016, 403, 153–165. [Google Scholar] [CrossRef]
- Cabezudo, B.; Rivera, J. Notas taxonómicas y corológicas sobre la Flora de Andalucía occidental, 2: Erica andevalensis Cabezudo y Rivera sp. Nov. Lagascalia 1980, 9, 223–226. [Google Scholar]
- Aparício, A. Erica andevalensis Cabezudo y Rivera. In Libro Rojo de la Flora Silvestre Amenzada de Andalucía, Tomo I.: Especies en Peligro de Extinción; Blanca, G., Cabezudo, B., Hernández-Bermejo, J.E., Herrera, C.M., Molero Mesa, J., Muñoz, J., Valdés, B., Eds.; Consejería de Medio Ambiente: Sevilla, Spain, 1999; pp. 119–122. [Google Scholar]
- Abreu, M.M.; Tavares, M.T.; Batista, M.J. Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos, Portugal. J. Geochem. Explor. 2008, 96, 210–222. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Abreu, M.M.; Leidi, E.O. A review of hazardous elements tolerance in a metallophyte model species: Erica andevalensis. Geoderma 2018, 319, 43–51. [Google Scholar] [CrossRef]
- Lange, B.; Van Der Ent, A.; Baker, A.J.M.; Echevarria, G.; Mahy, G.; Malaisse, F.; Faucon, M.P. Copper and cobalt accumulation in plants: A critical assessment of the current state of knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar] [CrossRef]
- Abreu, M.M.; Magalhães, M.C.F. Phytostabilization of soils in mining areas. Case studies from Portugal. In Soil Remediation; Aachen, L., Eichmann, P., Eds.; Nova science Publishers Inc.: New York, NY, USA, 2009; pp. 297–344. [Google Scholar]
- Abreu, M.M.; Santos, E.S.; Magalhães, M.C.F.; Fernandes, E. Trace elements tolerance, accumulation and translocation in Cistus populifolius, Cistus salviifolius and their hybrid growing in polymetallic contaminated mine areas. J. Geochem. Explor. 2012, 123, 52–60. [Google Scholar] [CrossRef]
- Pérez-López, R.; Márquez-García, B.; Abreu, M.M.; Nieto, J.M.; Córdoba, F. Erica andevalensis and Erica australis growing in the same extreme environments: Phytostabilization potential of mining areas. Geoderma 2014, 230–231, 194–203. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Santos, E.S.; Carvalho, L.C.; Abreu, M.M.; Andrade, M.L. Cistus monspeliensis L. as a potential species for rehabilitation of soils with multielemental contamination under Mediterranean conditions. Environ. Sci. Pollut. Res. 2018, 25, 6443–6455. [Google Scholar] [CrossRef]
- Carapeto, A.; Francisco, A.; Pereira, P.; Porto, M. Lista Vermelha da Flora Vascular de Portugal Continental; Sociedade Portuguesa de Botânica, Associação Portuguesa de Ciência da Vegetação—PHYTOS e Instituto da Conservação da Natureza e das Florestas (coord.). Coleção «Botânica em Português»; Imprensa Nacional: Lisboa, Portugal, 2020; Volume 7, p. 374. ISBN 978-972-27-2876-8. [Google Scholar]
- Van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Van der Bij, A.U.; Weijters, M.J.; Bobbink, R.; Harris, J.A.; Pawlett, M.; Ritz, K.; Benetková, P.; Moradi, J.; Frouz, J.; van Diggelen, R. Facilitating ecosystem assembly: Plant-soil interactions as a restoration tool. Biol. Conserv. 2018, 220, 272–279. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; ISBN 9798986245119. [Google Scholar]
- Natural Resources Conservation Service (USDA). Carbon to Nitrogen Ratios in Cropping Systems; USDA Natural Resources Conservation Service: Washington, DC, USA. Available online: https://Soils.usda.gov/sqi (accessed on 23 September 2022).
- Horta, M.C.; Torrent, J. Dinâmica do Fósforo no Solo, Perspectiva Agronómica e Ambiental; Instituto Politécnico de Castelo Branco, Ed.; Edições IPCB: Castelo Branco, Portugal, 2010; ISBN 978-989-8196-10-1. [Google Scholar]
- Veloso, A.; Sempiterno, C.; Calouro, F.; Rebelo, F.; Pedra, F.; Castro, I.V.; da Conceição Gonçalves, M.; da Encarnação Marcelo, M.; Pereira, P.; Fareleira, P.; et al. Manual de Fertilização Das Culturas, 3rd ed.; Calouro, F., Ed.; INIAV (Instituto Nacional de Investigação Agrária e Veterinária, I.P.): Lisbon, Portugal, 2022; ISBN 978-972-579-063-2. [Google Scholar]
- The Environmental Protection Agency of Portugal (APA). Valores de Referência Para o Solo. Solos Contaminados—Guia Técnico; Revision 3 September 2022; Agência Portuguesa do Ambiente: Amadora, Portugal, 2019; Available online: https://sniambgeoviewer.apambiente.pt/GeoDocs/geoportaldocs/AtQualSolos/Guia_Tecnico_Valores%20de%20Referencia_2019_01.pdf (accessed on 3 September 2022).
- Vicente, S.R.P. Caracterização Geoquímica, Mineralógica e Petrográfica da Mina de Mociços. Master’s Thesis, University of Évora, Évora, Portugal, 2020. [Google Scholar]
- Abreu, M.M.; Matias, M.J.; Magalhães, M.C.F.; Basto, M.J. Impacts on water, soil and plants from the abandoned Miguel Vacas copper mine, Portugal. J. Geochem. Explor. 2008, 96, 161–170. [Google Scholar] [CrossRef][Green Version]
- Freitas, H.; Prasad, M.N.V.; Pratas, J. Plant community tolerant to trace elements growing on the degraded soils of São Domingos mine in the southeast of Portugal: Environmental implications. Environ. Int. 2004, 30, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 247–271. [Google Scholar]
- Bandyopadhyay, S.; Maiti, S.K. Different soil factors influencing dehydrogenase activity in mine degraded lands—State-of-art review. Water Air Soil Pollut. 2021, 232, 360. [Google Scholar] [CrossRef]
- Telesiński, A.; Pawłowska, B.; Biczak, R.; Śnieg, M.; Wróbel, J.; Dunikowska, D.; Meller, E. Enzymatic activity and its relationship with organic matter characterization and ecotoxicity to Aliivibrio fischeri of soil samples exposed to tetrabutylphosphonium bromide. Sensors 2021, 21, 1565. [Google Scholar] [CrossRef] [PubMed]
- Emran, M.; Doni, S.; Macci, C.; Masciandaro, G.; Rashad, M.; Gispert, M. Susceptible soil organic matter, SOM, fractions to agricultural management practices in salt-affected soils. Geoderma 2020, 366, 114257. [Google Scholar] [CrossRef]
- Henríquez, C.; Uribe, L.; Valenciano, A.; Nogales, R. Actividad enzimática del suelo—Deshidrogenasa, β-glucosidasa, fosfatasa y ureasa- bajo diferentes cultivos. Agron. Costarric. 2014, 38, 43–54. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Dong, Y.; Jiao, R. Soil organic carbon fractions, C-cycling hydrolytic enzymes, and microbial carbon metabolism in Chinese fir plantations. Sci. Total Environ. 2020, 758, 143695. [Google Scholar] [CrossRef]
- Alvarenga, P.; Laneiro, C.; Palma, P.; De Varennes, A.; Cunha-Queda, C. A study on As, Cu, Pb and Zn (bio)availability in an abandoned mine area (São Domingos, Portugal) using chemical and ecotoxicological tools. Environ. Sci. Pollut. Res. 2013, 20, 6539–6550. [Google Scholar] [CrossRef]
- Siczek, A.; Frąc, M.; Gryta, A.; Kalembasa, S.; Kalembasa, D. Variation in soil microbial population and enzyme activities under faba bean as affected by pentachlorophenol. Appl. Soil Ecol. 2020, 150, 103466. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Lia, D.; Xiaoa, K.; Wang, K. Controls on soil arylsulfatase activity at a regional scale. Eur. J. Soil Biol. 2019, 90, 9–14. [Google Scholar] [CrossRef]
- Aponte, H.; Herrera, W.; Cameron, C.; Black, H.; Meier, S.; Paolini, J.; Tapiaf, Y.; Cornejo, P. Alteration of enzyme activities and functional diversity of a soil contaminated with copper and arsenic. Ecotoxicol. Environ. Saf. 2020, 192, 110264. [Google Scholar] [CrossRef]
- Wu, C.A.; Lowry, D.B.; Cooley, A.M.; Wright, K.M.; Lee, Y.W.; Willis, J.H. Mimulus is an emerging model system for the integration of ecological and genomic studies. Heredity 2008, 100, 220–230. [Google Scholar] [CrossRef] [PubMed]
- De Varennes, A. Produtividade dos Solos e Ambiente; Escolar Editora: Lisboa, Portugal, 2003; ISBN 972-592-156-9. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Srivastava, P.C.; Gupta, U.C. Trace Elements in Crop Production; Science Publishers, Inc.: Lebanon, PA, USA, 1996. [Google Scholar]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Vecino-Bueno, I.; Feldman, S.R. Accumulation and tolerance characteristics of chromium in a cordgrass Cr-hyperaccumulator, Spartina argentinensis. J. Hazard. Mater. 2011, 185, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.L.V.; Calouro, F.; Abreu, M.M. Application of chromium to soils at different rates and oxidation states. I. Effect on dry matter yield and chromium uptake by radish. Commun. Soil Sci. Plant Anal. 2022, 33, 2259–2268. [Google Scholar] [CrossRef]
- Ancuceanu, R.; Dinu, M.; Hovanet, M.V.; Anghel, A.I.; Popescu, C.V.; Negres, S. A survey of plant iron content—A semi-systematic review. Nutrients 2015, 7, 10320–10351. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Santos, E.S.; Abreu, M.M. Accumulation of Mn and Fe in aromatic plant species from the abandoned Rosalgar Mine and their potential risk to human health. Appl. Geochem. 2019, 104, 42–50. [Google Scholar]
- Santos, E.S.; Abreu, M.M.; Saraiva, J.A. Mutielemental concentration and physiological responses of Lavandula pedunculata growing in soils developed on different mine wastes. Environ. Pollut. 2016, 213, 43–52. [Google Scholar] [CrossRef]
- Anjos, C.; Magalhães, M.C.F.; Abreu, M.M. Metal (Al, Mn, Pb and Zn) soils extractable reagents for available fraction assessment: Comparison using plants, and dry and moist soils from the Braçal abandoned lead mine area, Portugal. J. Geochem. Explor. 2012, 113, 45–55. [Google Scholar] [CrossRef]
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Duris, M.; Gilucis, A. FOREGS Geochemical Atlas of Europe, Part 1: Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005; ISBN 951-690-921-3. [Google Scholar]
- Mengel, K.; Kirkby, E.A. ; Principles of Plant Nutrition, 5th ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Hussain, I.; Ashraf, M.A.; Rasheed, R.; Asghar, A.; Sajid, M.A.; Iqbal, M. Exogenous application of silicon at the boot stage decreases accumulation of cadmium in wheat (Triticum aestivum L.) grains. Braz. J. Bot. 2015, 32, 223–234. [Google Scholar] [CrossRef]
- Keller, C.; Rizwan, M.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Chaurand, P.; Meunier, J.D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 mM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Durães, N.; Bobos, I.; Ferreira da Silva, E.; Dekayir, A. Copper, zinc and lead biogeochemistry in aquatic and land plants from the Iberian Pyrite Belt (Portugal) and north of Morocco mining areas. Environ. Sci. Pollut. Res. 2015, 22, 2087–2105. [Google Scholar] [CrossRef]
- Leadlay, E.A.; Castroviejo, S.; Aedo, C.; Cirujano, S.; Laínz, M.; Montserrat, P.; Morales, R.; Munõz-Garmendia, F.; Nieto Feliner, G.; Rico, E.; et al. (Eds.) Flora iberica, Cruciferae-Monotropaceae; Consejo Superior de Investigaciones Científicas: Real Jardín Botánico, Madrid, Spain, 1993; Volume 4, pp. 400–411. [Google Scholar]
- Márquez, F.; García, D.; Martínez, M.C. Aportación sobre la corología de la flora amenazada en Extremadura. Folia Bot. Extrem. 2012, 6, 45–60. [Google Scholar]
- Vázquez, F.M.; Gutiérrez, M.; Blanco, J.; García, D.; Guerra, M.J.; Márquez, F.; Cabeza de Vaca, M.A.; López, J.L.; Sánchez, A.; Palacios, M.J.; et al. Catálogo Regional de Especies Vegetales Amenazadas de Extremadura. Actualizado con la Lista Roja de la Flora Vascular Española 2008; Consejería de Industria, Energía y Medio Ambiente, Gobierno de Extremadura: Mérida, Spain, 2010; p. 447. [Google Scholar]
- Porto, M. Mina de Aparis. In Sítios de Interesse Botânico de Portugal Continental, Coleção «Botânica em Português» Volume 5, Tomo 2; Farminhão, J., Ed.; Imprensa Nacional Casa da Moeda: Lisboa, Portugal, 2020. [Google Scholar]
- Brandão, J.M.; Lopes, C.S. Memórias da lavra—Mina de Aparis. In Patrimonio Geológico y Minero: Su Caracterización y Puesta en Valor. Cuadernos del Museo Geominero nº 6; Rábano, I., Mata-Perelló, M., Eds.; Instituto Geológico y Minero de España: Madrid, Spain, 2002; pp. 169–178. [Google Scholar]
- Piçarra, J.; Pereira, Z.; Oliveira, J.T. Breves Apontamentos sobre a Geologia da Região de Barrancos: Vol. I.; Câmara Municipal de Barrancos, Colecção Catálogo do Museu de Barrancos: Barrancos, Portugal, 2001; p. 39. ISBN 972-97409-5-X. [Google Scholar]
- Mateus, A.; Matos, J.X.; Rosa, C.J.P.; Oliveira, V.M.J. Cu-ores in quartz-carbonate veins at Estremoz-Alandroal and Barrancos-Santo Aleixo regions, Ossa Morena: A result of Late-Variscan hydrothermal activity. In Proceedings of the VI Congresso Nacional de Geologia, Monte de Caparica, Portugal, 4–6 June 2003; [Comunicações]. Universidade Nova de Lisboa, Faculdade de Ciências e Tecnologia: Monte de Caparica, Portugal, 2003. CD-ROM. pp. F90–F93. [Google Scholar]
- Mateus, A.; Munhá, J.; Inverno, C.; Matos, J.; Martins, L.; Oliveira, D.; Jesus, A.; Salgueiro, R. Mineralizações no sector português da Zona de Ossa-Morena. In Geologia de Portugal, Geologia Pré-mesozóica de Portugal; Dias, R., Araújo, A., Terrinha, P., Kullberg, J.C., Eds.; Escolar Editora: Lisboa, Portugal, 2013; Volume 1, pp. 577–620. [Google Scholar]
- Normal Climatológica—Beja—1981–2010. Available online: https://www.ipma.pt/bin/file.data/climate-normal/cn_81-10_BEJA.pdf (accessed on 1 May 2024).
- Mulvaney, R.L. Chemical methods: Nitrogen–inorganic forms. In Methods of Soil Analysis: Part 3—Chemical Methods, SSSA Book Series No. 5.; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische bodenanalyse als grundlage für die beurteilung des nährstoffzustandes der böden, II: Chemische extraction method zur phosphor-und kaliumbestimmung. Kungl. Lantbr. Högsk. Ann. 1960, 26, 199–215. [Google Scholar]
- Póvoas, I.; Barral, M.F. Métodos de Análise de Solos; Comunicações do Instituto de Investigação Científica Tropical, Serie Ciências Agrária: Lisboa, Portugal, 1992. [Google Scholar]
- Lakanen, E.; Erviö, R. A comparison of eight extractants for the determination of plant available micronutrients in soils. Acta Agralia Fennica 1971, 123, 223–232. [Google Scholar]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. Comparison of a rhizosphere-based method with other one-step extraction methods for assessing the bioavailability of soil metals to wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef]
- Activation Laboratories. Aqua Regia “Partial” Digestion. Available online: https://actlabs.com/geochemistry/exploration-geochemistry/aqua-regia-partial-digestion/ (accessed on 1 April 2024).
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analyses Part 2, Chemical and Microbiological Properties; Miller, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 903–947. [Google Scholar]
- Hope, C.F.A.; Burns, R.G. Activity, origins and location of cellulase in a silt loam soil. Biol. Fertil. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Tabatabai, M.; Bremner, J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. (Eds.) Protease activity. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 1995; pp. 313–315. ISBN 9780125138406. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Page, A.L., Ed.; The American Society of Agronomy: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Abreu, M.M.; Godinho, B.; Magalhães, M.C.F. Risk assessment of Arbutus unedo L. fruits from plants growing on contaminated soils in the Panasqueira mine area, Portugal. J. Soils Sediments 2014, 14, 744–757. [Google Scholar] [CrossRef]
- Bu-Olayan, A.H.; Thomas, B.V. Translocation and bioaccumulation of trace metals in desert plants of Kuwait Governorates. Res. J. Environ. Sci. 2009, 3, 581–587. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J. Phytoextraction of Metals and Metalloids from Contaminated Soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Perelman, A.I. The Geochemistry of Land Areas; Izd Vish Shk: Moscow, Russia, 1966; 155p. (In Russian) [Google Scholar]


| L1 | L2 | L3 | L4 | L5 | |
|---|---|---|---|---|---|
| pH (H2O) | 8.30 | 7.42 | 7.06 | 7.42 | 6.68 |
| EC (mS/cm) | 4.06 | 0.193 | 0.106 | 0.195 | 0.072 |
| CaCO3 (g/kg) | 28.1 | 2.50 | 4.20 | 4.10 | 3.00 |
| Corg (g/kg) | 5.17 | 25.91 | 32.68 | 25.96 | 17.32 |
| Ntotal (g/kg) | 0.37 | 2.81 | 2.51 | 2.79 | 2.47 |
| N-NO3 (mg/kg) | 2.20 | 13.00 | 5.25 | 15.40 | 4.20 |
| Pextractable (mg/kg) | 11.86 | 25.46 | 16.92 | 10.11 | 18.09 |
| Kextractable (mg/kg) | 58.76 | 108.53 | 124.75 | 180.72 | 82.52 |
| Cation Exchange Capacity (CEC) and exchangeable cations (cmolc/kg) | |||||
| CEC | 2.44 | 13.29 | 12.22 | 12.90 | 13.47 |
| Ca | 4.43 | 10.17 | 9.10 | 10.11 | 7.56 |
| K | 0.13 | 0.32 | 0.31 | 0.43 | 0.21 |
| Mg | 1.85 | 4.96 | 2.22 | 3.74 | 2.45 |
| Na | <LD | 0.04 | <LD | 0.07 | <LD |
| Micronutrients (mg/kg) | |||||
| Cu | 635.99 | 457.67 | 2024.94 | 647.56 | 1925.90 |
| Fe | 136.19 | 191.93 | 83.72 | 99.09 | 101.73 |
| Mn | 47.86 | 84.65 | 173.64 | 228.56 | 264.16 |
| Zn | 1.59 | 3.22 | 8.83 | 7.19 | 10.70 |
| Macronutrients (g/kg) | |||||
| Ca | 3.60 | 2.38 | 2.04 | 2.31 | 1.69 |
| K | 0.429 | 0.150 | 0.168 | 0.225 | 0.140 |
| Mg | 1.28 | 0.620 | 0.283 | 0.493 | 0.295 |
| Na | 0.010 | 0.016 | 0.017 | 0.027 | 0.011 |
| Samples | As | Co | Cr | Cu | Mn | Ni | Pb | S | Zn |
|---|---|---|---|---|---|---|---|---|---|
| Pseudototal (mg/kg) | |||||||||
| L1 | 92.7 | 13.7 | 14 | 1330 | 625 | 27.7 | 1.30 | 410 | 28.9 |
| L2 | 37.9 | 21.3 | 24 | 1460 | 469 | 39.8 | 6.80 | 1110 | 58.6 |
| L3 | 136 | 25.2 | 19 | 5940 | 755 | 39.1 | 12.20 | 1100 | 87.5 |
| L4 | 60 | 25.7 | 23 | 2670 | 857 | 39.6 | 10.10 | 1320 | 79.4 |
| L5 | 118 | 29 | 21 | 5720 | 1180 | 40.40 | 7.40 | 990 | 103 |
| MAVs * | 11 | 22 | 160 | 140 | - | 100 | 45 | - | 340 |
| Available fraction extracted with RHIZO solution (mg/kg) | |||||||||
| L1 | 4.1 | 1.32 | 0.3 | 220 | 19.9 | 1.22 | <DL | 30 | 0.93 |
| L2 | 2.7 | 1.61 | <0.2 | 48.8 | 31.8 | 1.12 | <DL | 20 | 1.36 |
| L3 | 1.7 | 1.23 | <0.2 | 502 | 62.8 | 0.83 | <DL | 50 | 5.26 |
| L4 | 1.1 | 1.5 | <0.2 | 160 | 70.5 | 0.85 | <DL | 30 | 3.57 |
| L5 | 1.7 | 0.74 | <0.2 | 491 | 69.6 | 0.81 | <DL | 30 | 5.50 |
| %, ŧ | 1.4–7.1 | 2.6–9.6 | ≤2.1 | 3.3–16.5 | 3.2–8.3 | 2.0–4.4 | - | 1.8–7.3 | 2.3–6.0 |
| L1 | L2 | L3 | L4 | L5 | ||
|---|---|---|---|---|---|---|
| As | aerial part | 28.74 | <DL | 4.74 | <DL | <DL |
| roots | 10.93 | <DL | <DL | <DL | <DL | |
| Co | aerial part | 4.39 | 0.96 | 0.71 | 1.01 | 0.57 |
| roots | 0.73 | 0.50 | 0.77 | 0.63 | 0.61 | |
| Cu | aerial part | 285.93 | 39.73 | 103.98 | 74.92 | 78.21 |
| roots | 66.89 | 26.54 | 151.61 | 70.85 | 188.39 | |
| Cr | aerial part | 39.33 | 10.68 | 10.67 | 10.14 | 4.25 |
| roots | 6.07 | 5.03 | 4.59 | 2.51 | 6.08 | |
| Ni | aerial part | 22.69 | 6.09 | 5.93 | 4.94 | 2.98 |
| roots | 3.64 | 2.89 | 3.37 | 2.51 | 3.80 | |
| Pb | aerial part | 3.03 | <DL | 1.19 | 1.27 | <DL |
| roots | <DL | <DL | <DL | <DL | <DL | |
| Zn | aerial part | 33.43 | 25.63 | 29.05 | 29.03 | 52.99 |
| roots | 19.79 | 22.64 | 35.68 | 30.28 | 51.66 | |
| Ca | aerial part | 23.6 | 20.72 | 14.70 | 14.45 | 21.39 |
| roots | 6.798 | 5.447 | 5.988 | 7.462 | 6.320 | |
| Fe | aerial part | 5.87 | 1.153 | 0.922 | 1.495 | 0.545 |
| roots | 0.51 | 0.252 | 0.479 | 0.541 | 0.735 | |
| K | aerial part | 14.21 | 15.16 | 12.57 | 12.07 | 12.84 |
| roots | 13.23 | 17.61 | 13.83 | 16.58 | 13.52 | |
| Mg | aerial part | 10.17 | 5.147 | 2.691 | 3.524 | 3.570 |
| roots | 3.144 | 3.044 | 2.098 | 2.739 | 3.723 | |
| Mn | aerial part | 149.77 | 27.77 | 30.83 | 44.37 | 34.00 |
| roots | 16.99 | 10.06 | 21.44 | 26.38 | 36.46 | |
| Mo | aerial part | 8.77 | 8.86 | 1.19 | 2.03 | 7.37 |
| roots | 6.92 | 7.17 | <DL | 1.13 | 7.60 | |
| P | aerial part | 2.18 | 3.91 | 2.88 | 3.37 | 3.26 |
| roots | 1.42 | 3.32 | 2.43 | 2.09 | 3.25 | |
| S | aerial part | 8.02 | 6.19 | 4.51 | 5.58 | 4.53 |
| roots | 4.86 | 3.77 | 2.91 | 4.90 | 2.73 |
| L1 | L2 | L3 | L4 | L5 | ||
|---|---|---|---|---|---|---|
| TranslC | 2.63 | <1.0 | 1.58 | <1.0 | <1.0 | |
| As | TransferC | 1.62 | <1.0 | <1.0 | <1.0 | <1.0 |
| BAC | 2.67 | <1.0 | <1.0 | <1.0 | <1.0 | |
| TranslC | 6.02 | 1.91 | 0.93 | 1.62 | 0.93 | |
| Co | TransferC | 0.32 | 0.05 | 0.03 | 0.04 | 0.02 |
| BAC | 0.55 | 0.31 | 0.62 | 0.42 | 0.82 | |
| TranslC | 4.28 | 1.50 | 0.69 | 1.06 | 0.42 | |
| Cu | TransferC | 0.22 | 0.03 | 0.02 | 0.03 | 0.01 |
| BAC | 0.30 | 0.54 | 0.30 | 0.44 | 0.38 | |
| TranslC | 6.48 | 2.12 | 2.32 | 4.04 | 0.70 | |
| Cr | TransferC | 2.81 | 0.45 | 0.56 | 0.44 | 0.20 |
| BAC | 20.23 | <20.0 | <20.0 | <20.0 | <20.0 | |
| TranslC | 8.81 | 2.76 | 1.44 | 1.68 | 0.93 | |
| Mn | TransferC | 0.24 | 0.06 | 0.04 | 0.05 | 0.03 |
| BAC | 0.85 | 0.32 | 0.34 | 0.37 | 0.52 | |
| TranslC | 1.27 | 1.24 | <1.0 | 1.79 | 0.97 | |
| Mo | TransferC | 10.45 | 14.77 | 1.77 | 3.50 | 9.69 |
| BAC | <0.15 | <0.15 | <0.15 | <0.15 | <0.15 | |
| TranslC | 6.23 | 2.10 | 1.76 | 1.97 | 0.78 | |
| Ni | TransferC | 0.82 | 0.15 | 0.15 | 0.13 | 0.07 |
| BAC | 2.99 | 2.58 | 4.06 | 2.96 | 4.69 | |
| TranslC | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 | |
| Pb | TransferC | 2.32 | <1.0 | 0.10 | 0.13 | <1.0 |
| BAC | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | |
| TranslC | 1.69 | 1.13 | 0.81 | 0.96 | 1.03 | |
| Zn | TransferC | 1.15 | 0.44 | 0.33 | 0.37 | 0.51 |
| BAC | 21.28 | 16.65 | 6.78 | 8.48 | 9.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caperta, A.D.; Couchinho, F.; Cortinhas, A.; Abreu, M.M. Unveiling the Behavior of an Endangered Facultative Cuprophyte Coincya Species in an Abandoned Copper Mine (Southeast Portugal). Plants 2024, 13, 2847. https://doi.org/10.3390/plants13202847
Caperta AD, Couchinho F, Cortinhas A, Abreu MM. Unveiling the Behavior of an Endangered Facultative Cuprophyte Coincya Species in an Abandoned Copper Mine (Southeast Portugal). Plants. 2024; 13(20):2847. https://doi.org/10.3390/plants13202847
Chicago/Turabian StyleCaperta, Ana Delaunay, Filipa Couchinho, Ana Cortinhas, and Maria Manuela Abreu. 2024. "Unveiling the Behavior of an Endangered Facultative Cuprophyte Coincya Species in an Abandoned Copper Mine (Southeast Portugal)" Plants 13, no. 20: 2847. https://doi.org/10.3390/plants13202847
APA StyleCaperta, A. D., Couchinho, F., Cortinhas, A., & Abreu, M. M. (2024). Unveiling the Behavior of an Endangered Facultative Cuprophyte Coincya Species in an Abandoned Copper Mine (Southeast Portugal). Plants, 13(20), 2847. https://doi.org/10.3390/plants13202847







