Analysis of Petrogenic Hydrocarbons in Plant Tissues: A Simple GC-MS-Based Protocol to Distinguish Biogenic Hydrocarbons from Diesel-Derived Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Growth Measurements
2.2. Analytical Method for Diesel-Derived Hydrocarbon Quantification in Soil and Plant Material
2.3. Statistical Analysis
3. Results
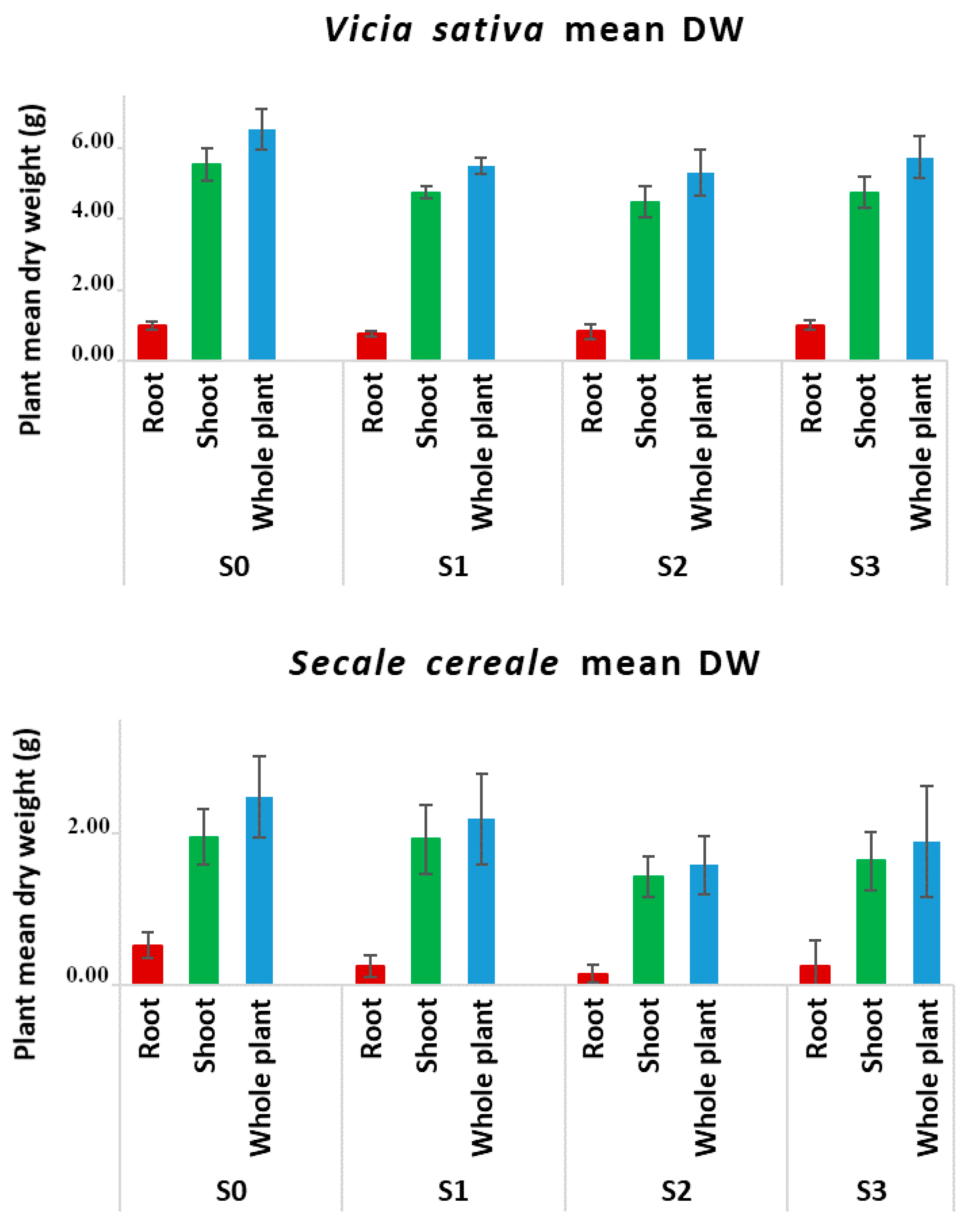
3.1. Production of Plants Containing Diesel-Derived Hydrocarbons: Survival and Growth
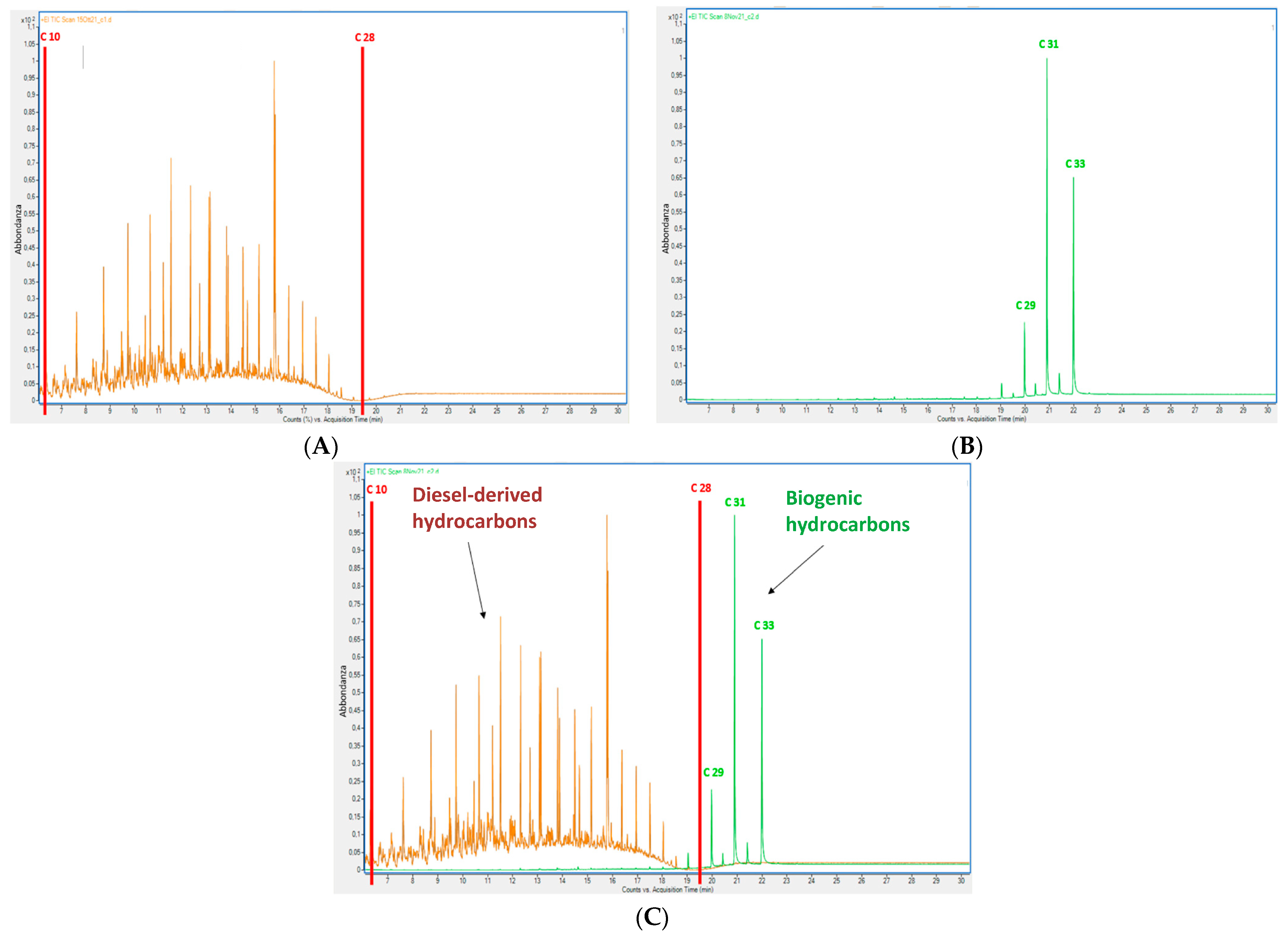
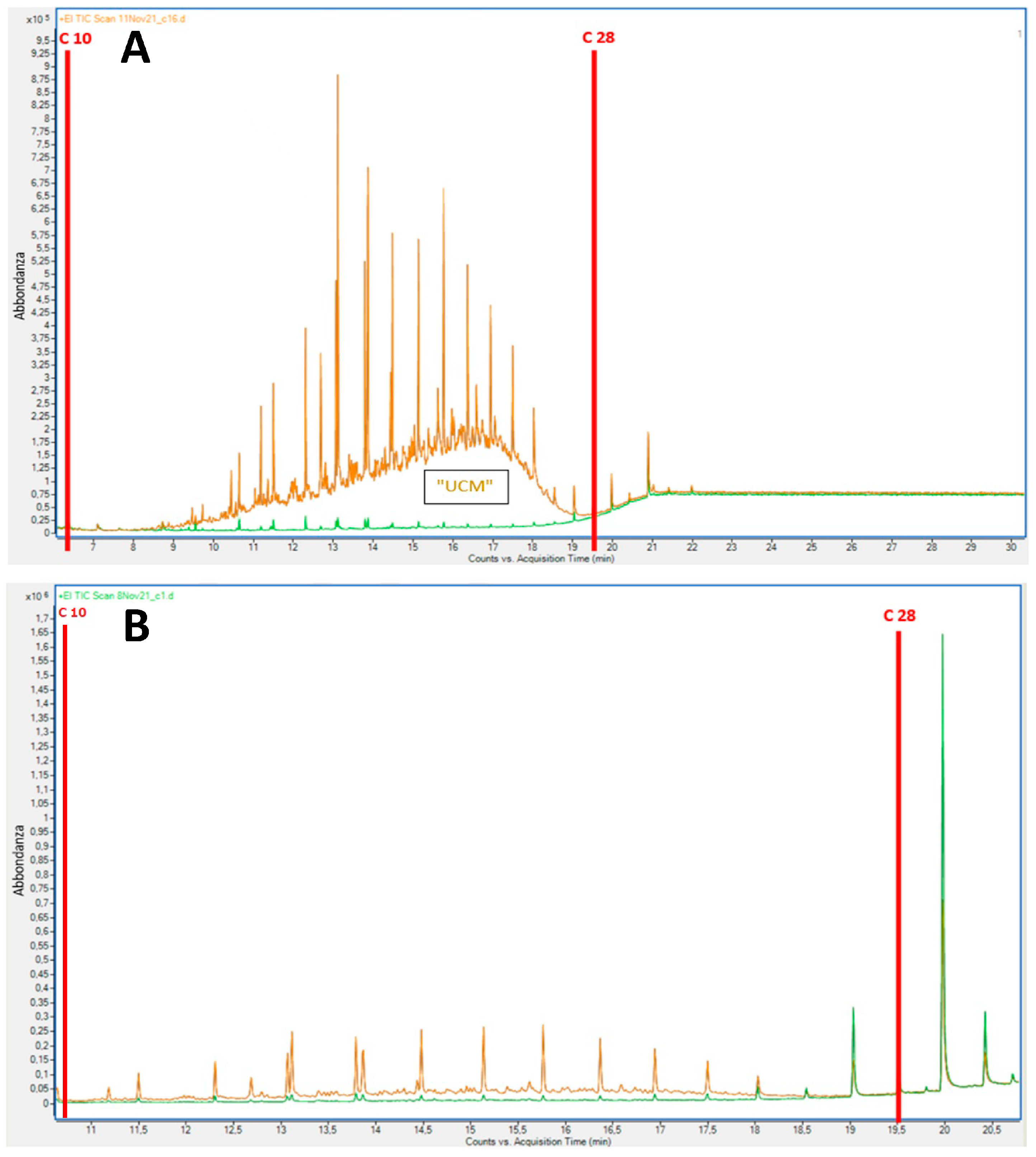
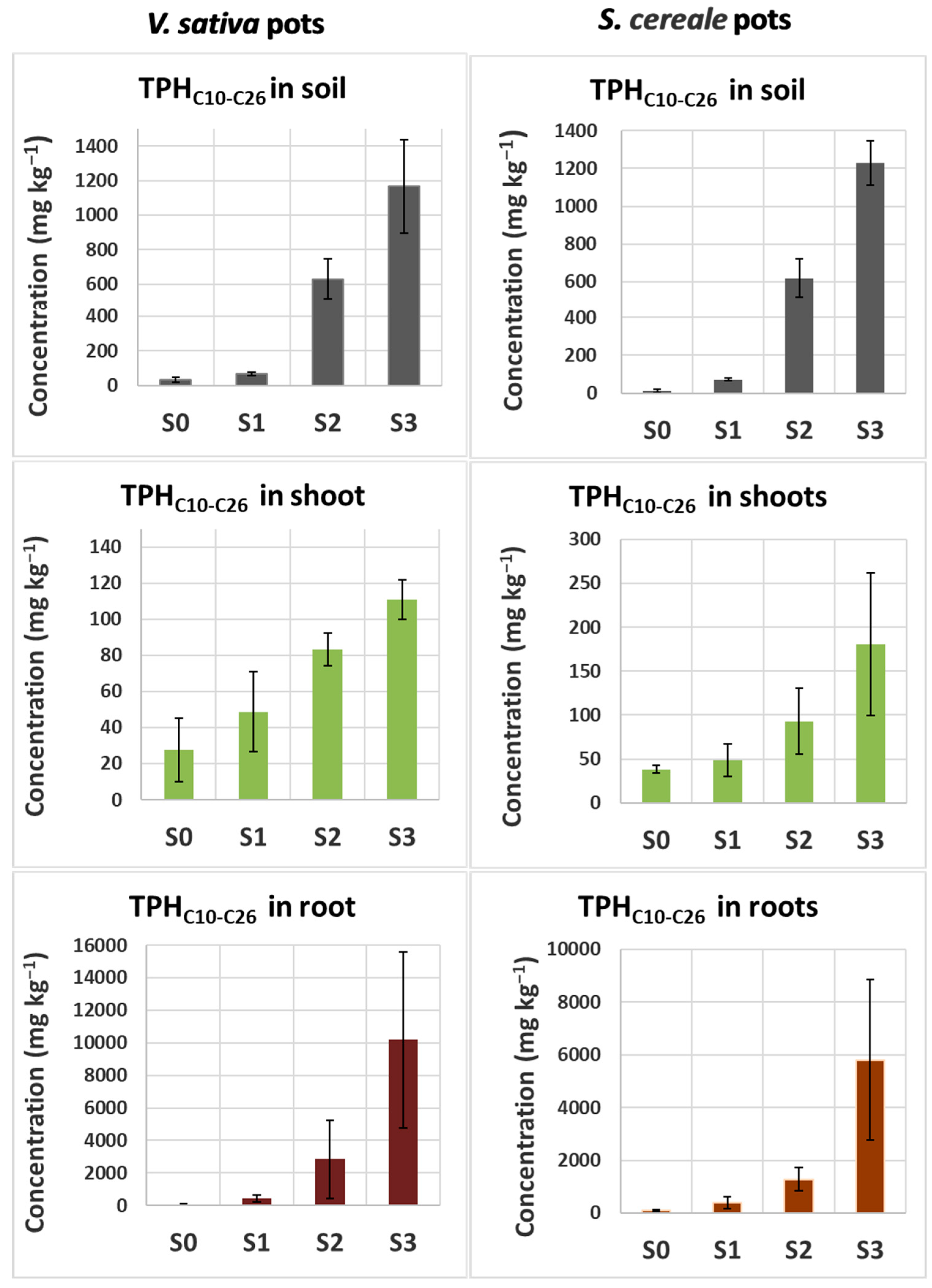
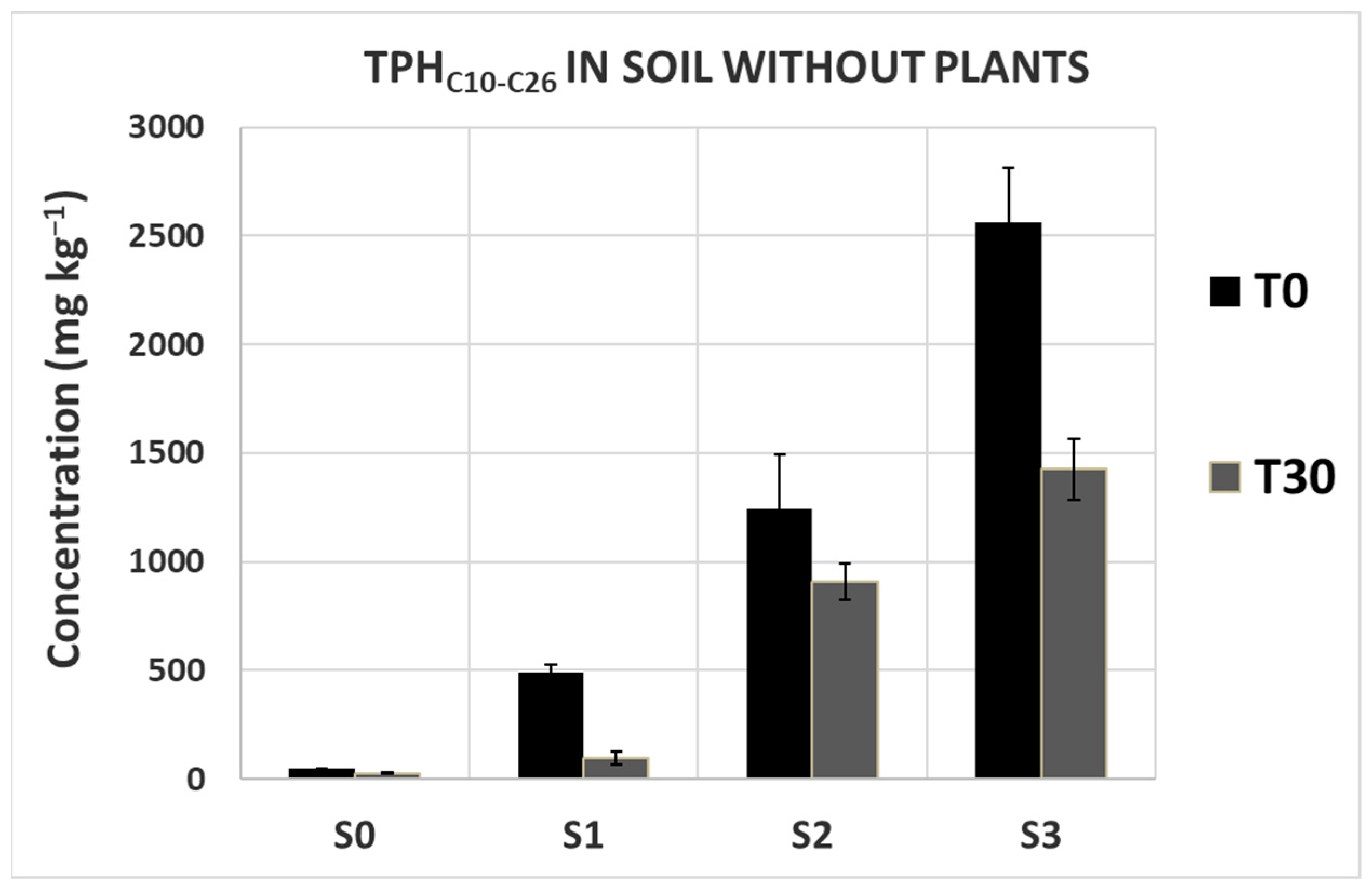
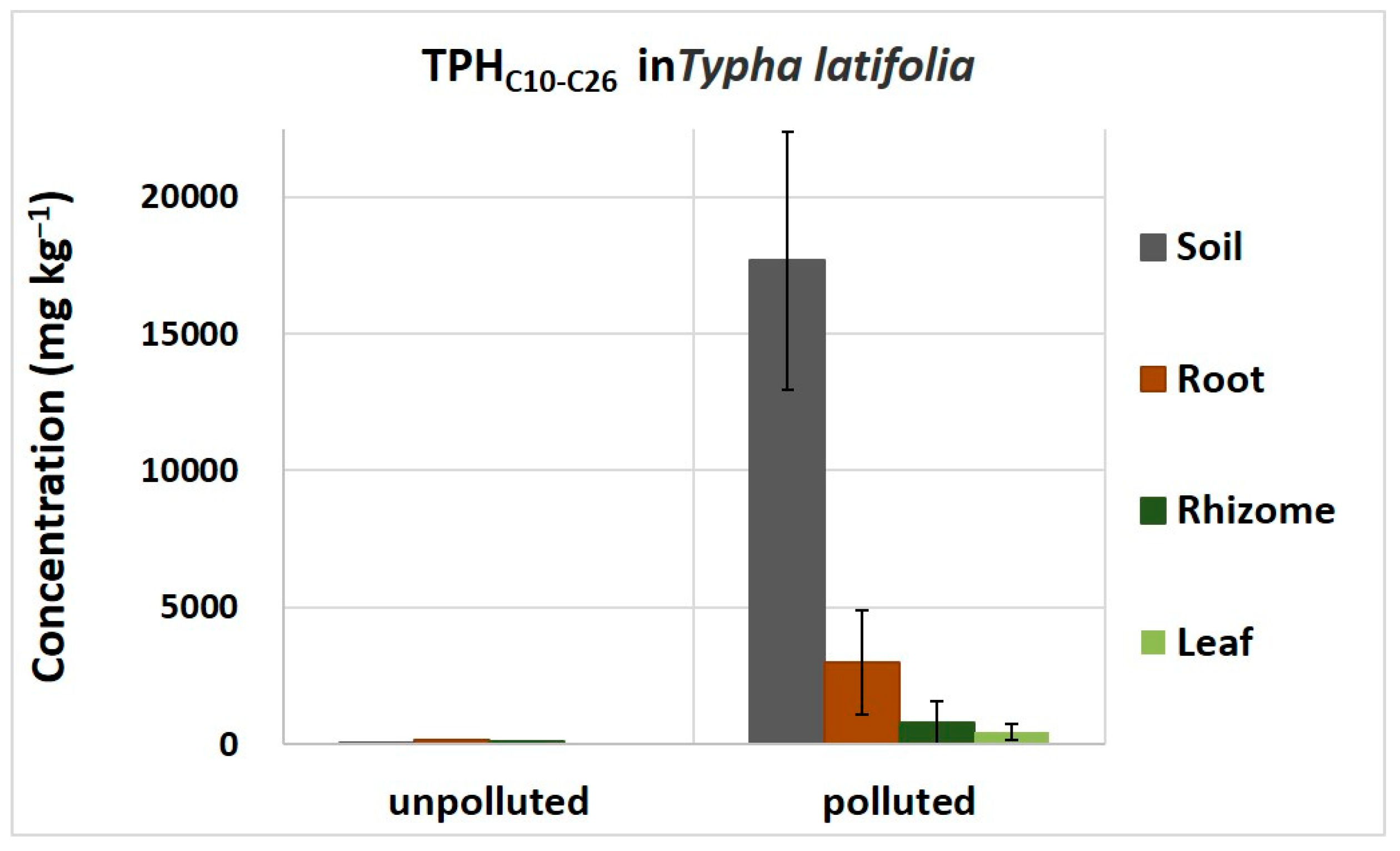
3.2. Measurements of Diesel-Derived Hydrocarbons in Soil and Plant Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogunlaja, A.; Ogunlaja, O.O.; Okewole, D.M.; Morenikeji, O.A. Risk assessment and source identification of heavy metal contamination by multivariate and hazard index analyses of a pipeline vandalised area in Lagos State, Nigeria. Sci. Total Environ. 2019, 651, 2943–2952. [Google Scholar] [CrossRef]
- Grifoni, M.; Rosellini, I.; Angelini, P.; Petruzzelli, G.; Pezzarossa, B. The effect of residual hydrocarbons in soil following oil spillages on the growth of Zea mays plants. Environ. Pollut. 2020, 265, 114950. [Google Scholar] [CrossRef] [PubMed]
- Unione Petrolifera. Annual Report 2019; Unione Petrolifera: Roma, Italy, 2019. [Google Scholar]
- Masy, T.; Demanèche, S.; Tromme, O.; Thonart, P.; Jacques, P.; Hilgsmann, S.; Vogel, T.M. Hydrocarbon biostimulation and bioaugmentation in organic carbon and clay-rich soils. Soil Biol. Biochem. 2016, 99, 66–74. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Aina, R.; Palin, L.; Citterio, S. Molecular evidence for benzo [a] pyrene and naphthalene genotoxicity in Trifolium repens L. Chemosphere 2006, 65, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Moubasher, H.A.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.M.; Kabiel, H.F.; Hamad, A.A. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhu, Y.G. Uptake of selected PAHs from contaminated soils by rice seedlings (Oryza sativa) and influence of rhizosphere on PAH distribution. Environ. Pollut. 2008, 155, 359–365. [Google Scholar] [CrossRef]
- Fismes, J.; Perrin-Ganier, C.; Empereur-Bissonnet, P.; Morel, J.L. Soil-to-Root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils. J. Environ. Qual. 2002, 31, 1649. [Google Scholar] [CrossRef]
- Bakker, M.I.; Casado, B.; Koerselman, J.W.; Tolls, J.; Kolloffel, C. Polycyclic aromatic hydrocarbons in soil and plant samples from the vicinity of an oil refinery. Sci. Total Environ. 2000, 263, 91–100. [Google Scholar] [CrossRef]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown inanindustrial area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef]
- Hunt, L.J.; Duca, D.; Dan, T.; Knopper, L.D. Petroleum Hydrocarbon (PHC) Uptake in Plants: A Literature Review. Environ. Pollut. 2019, 245, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, G.; Naidoo, K. Uptake of polycyclic aromatic hydrocarbons and their cellular effects in the mangrove Bruguiera gymnorrhiza. Mar. Pollut. Bull. 2016, 113, 193199. [Google Scholar] [CrossRef] [PubMed]
- Wild, E.; Dent, J.; Thomas, G.O.; Jones, K.C. Direct observation of organic contaminant uptake, storage, and metabolism within plant roots. Environ. Sci. Technol. 2015, 39, 3695–3702. [Google Scholar] [CrossRef]
- ISO 16703:2004; Soil Quality—Determination of Content of Hydrocarbon in the Range C10 to C40 by Gas Chromatography. International Organization for Standardization (ISO): Geneva, Switzerland, 2004.
- Nisa, W.; Rashid, A. Potential of vetiver (Vetiveria Zizanioides L.) grass in removing selected pahs from diesel contaminated soil. Pak. J. Bot. 2015, 47, 291–296. [Google Scholar] [CrossRef]
- Patowary, R.; Patowary, K.; Devi, A.; Kalita, M.C.; Deka, S. Uptake of total petroleum hydrocarbon (TPH) and polycyclic aromatic hydrocarbons (PAHs) by Oryza sativa L. Grown in soil contaminated with crude oil. Bull. Environ. Contam. Toxicol. 2017, 98, 120–126. [Google Scholar] [CrossRef]
- Al-Ali, B.S.; Al-Aradi, H.J.; Al-Khion, D.D.; Al-Saad, H.T. Petroleum hydrocarbons in water, soil and tomato plant (Lycopersican esculentum L.) at Basra City, Iraq. J. Biol. Agric. Healthc. 2016, 6, 55–64. [Google Scholar]
- Anyasi, R.O.; Atagana, H.I. Profiling of plants at petroleum contaminated site for phytoremediation. Int. J. Phytoremediat. 2018, 20, 352–361. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Muratova, A.Y.; Golubev, S.N.; Dubrovskaya, E.V.; Pozdnyakova, N.N.; Panchenko, L.V.; Pleshakova, E.V.; Chernyshova, M.P.; Turkovskaya, O.V. Remediating abilities of different plant species grown in diesel-fuel-contaminated leached chernozem. Appl. Soil. Ecol. 2012, 56, 51–57. [Google Scholar] [CrossRef]
- Kaur, N.; Erickson, T.E.; Ball, A.S.; Ryan, M.H. A review of germination and early growth as a proxy for plant fitness under petrogenic contamination—Knowledge gap sand recommendations. Sci. Total Environ. 2017, 603, 728–744. [Google Scholar] [CrossRef]
- Bush, R.T.; Mcinerney, F.A. Leaf wax N-alkane distributions in andacross modern plants: Implications for paleoecology and chemot-axonomy. Geochim. Cosmochim. Acta 2013, 117, 161–179. [Google Scholar] [CrossRef]
- Albers, P.H. Petroleum and Individual Polycyclic Aromatic Hydrocarbons. In Handbook of Ecotoxicology; Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., Cairns, J., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2002; Chapter 14; p. 365. [Google Scholar]
- Doucette, W.J.; Shunthirasingham, C.; Dettenmaier, E.M.; Zaleski, R.T.; Fantke, P.; Arnot, J.A. A review of measured bioaccumulation data on terrestrial plants for organic chemicals: Metrics, variability, and the need for standardized measurement protocols. Environ. Toxicol. Chem. 2018, 37, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Ite, A.E.; Ibok, U.J. Role of plants and microbes in bioremediation of petroleum hydrocarbons contaminated soils. Int. J. Environ. Bioremediat. Biodegrad. 2019, 7, 1–19. [Google Scholar] [CrossRef]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of hydrophobic organic contaminants in soils: Fundamental concepts and techniques for analysis. Eur. J. Soil. Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Schwab, A.P.; Su, J.; Wetzel, S.; Pekarek, S.; Banks, M.K. Extraction of petroleum hydrocarbons from soil by mechanical shaking. Environ. Sci. Technol. 1999, 33, 1940–1945. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Yang, Z.; Hollebone, B.; Brown, C.E.; Landriault, M.; Sun, J.; Mudge, S.M.; Kelly-Hooper, F.; Dixon, D.G. Fingerprinting of petroleum hydrocarbons (PHC) and other biogenic organic compounds (BOC) in oil-contaminated and background soil samples. J. Environ. Monit. 2012, 14, 2367–2381. [Google Scholar] [CrossRef] [PubMed]
- Vecchiato, M.; Bonato, T.; Bertin, A.; Argiriadis, E.; Barbante, C.; Piazza, R. Plant Residues as Direct and Indirect Sources of Hydrocarbons in Soils: Current Issues and Legal Implications. Environ. Sci. Technol. 2017, 4, 512–517. [Google Scholar] [CrossRef]
- Shepherd, T.; Griffiths, D.W. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Eglinton, G.; Hamilton, R.J. The distribution of alkanes. In Chemical Plant Taxonomy; Swain, T., Ed.; Academic Press: London, UK, 1963; Chapter 8; p. 187. [Google Scholar]
- Eglinton, G.; Hamilton, R.J. Leaf epicuticular waxes. Science 1967, 156, 1322–1335. [Google Scholar] [CrossRef]

| S0 | S1 | S2 | S3 | |
|---|---|---|---|---|
| (0 mg kg−1) | (1000 mg kg−1) | (5000 mg kg−1) | (10,000 mg kg−1) | |
| No plants | 3 pots | 3 pots | 3 pots | 3 pots |
| Vicia sativa | 3 pots | 3 pots | 3 pots | 3 pots |
| Secale cereale | 3 pots | 3 pots | 3 pots | 3 pots |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collina, E.; Casati, E.; Franzetti, A.; Caronni, S.; Gentili, R.; Citterio, S. Analysis of Petrogenic Hydrocarbons in Plant Tissues: A Simple GC-MS-Based Protocol to Distinguish Biogenic Hydrocarbons from Diesel-Derived Compounds. Plants 2024, 13, 298. https://doi.org/10.3390/plants13020298
Collina E, Casati E, Franzetti A, Caronni S, Gentili R, Citterio S. Analysis of Petrogenic Hydrocarbons in Plant Tissues: A Simple GC-MS-Based Protocol to Distinguish Biogenic Hydrocarbons from Diesel-Derived Compounds. Plants. 2024; 13(2):298. https://doi.org/10.3390/plants13020298
Chicago/Turabian StyleCollina, Elena, Enrico Casati, Andrea Franzetti, Sarah Caronni, Rodolfo Gentili, and Sandra Citterio. 2024. "Analysis of Petrogenic Hydrocarbons in Plant Tissues: A Simple GC-MS-Based Protocol to Distinguish Biogenic Hydrocarbons from Diesel-Derived Compounds" Plants 13, no. 2: 298. https://doi.org/10.3390/plants13020298
APA StyleCollina, E., Casati, E., Franzetti, A., Caronni, S., Gentili, R., & Citterio, S. (2024). Analysis of Petrogenic Hydrocarbons in Plant Tissues: A Simple GC-MS-Based Protocol to Distinguish Biogenic Hydrocarbons from Diesel-Derived Compounds. Plants, 13(2), 298. https://doi.org/10.3390/plants13020298








