Abstract
Formins or formin homology 2 (FH2) proteins, evolutionarily conserved multi-domain proteins in eukaryotes, serve as pivotal actin organizers, orchestrating the structure and dynamics of the actin cytoskeleton. However, a comprehensive investigation into the formin family and their plausible involvement in abiotic stress remains undocumented in soybean (Glycine max). In the current study, 34 soybean FH (GmFH)family members were discerned, their genomic distribution spanning the twenty chromosomes in a non-uniform pattern. Evolutionary analysis of the FH gene family across plant species delineated five discernible groups (Group I to V) and displayed a closer evolutionary relationship within Glycine soja, Glycine max, and Arabidopsis thaliana. Analysis of the gene structure of GmFH unveiled variable sequence lengths and substantial diversity in conserved motifs. Structural prediction in the promoter regions of GmFH gene suggested a large set of cis-acting elements associated with hormone signaling, plant growth and development, and stress responses. The investigation of the syntenic relationship revealed a greater convergence of GmFH genes with dicots, indicating a close evolutionary affinity. Transcriptome data unveiled distinctive expression patterns of several GmFH genes across diverse plant tissues and developmental stages, underscoring a spatiotemporal regulatory framework governing the transcriptional dynamics of GmFH gene. Gene expression and qRT–PCR analysis identified many GmFH genes with a dynamic pattern in response to abiotic stresses, revealing their potential roles in regulating plant stress adaptation. Additionally, protein interaction analysis highlighted an intricate web of interactions among diverse GmFH proteins. These findings collectively underscore a novel biological function of GmFH proteins in facilitating stress adaptation in soybeans.
1. Background
Soybean (Glycine max L.) represents one of the major crops in the world, extensively cultivated in the United States, South America, and East Asia [1]. Renowned for its remarkable biological nitrogen-fixing capability achieved through a symbiotic relationship with soil rhizobacteria, soybean has long been acknowledged as a pivotal nitrogen source, supplying essential feed proteins for animals and oils for human consumption [2,3]. Nevertheless, the escalation of severe environmental adversities on a global scale, including water scarcity and soil salinization, has significantly impeded crop growth and agricultural productivity in recent years, exerting a detrimental impact on grain yield and food quality [4,5,6]. To cope with these unfavorable growth conditions caused by drought and salt stress, soybean has evolved complex regulatory mechanisms, particularly during vegetative and reproductive growth stages, enabling its survival and maximizing its achievable yield production. Consequently, extensive efforts in recent decades have been dedicated to elucidating the potential effects of drought and salt stress on various growth stages of soybeans, encompassing impediments to seed germination and seedling growth [7,8,9,10]. Concurrently, the altered expression of numerous genes responsive to drought and salt stress has been observed, attributable to physiological and metabolic alterations. As transcriptome sequencing techniques have rapidly advanced, the acquisition of genome-wide genetic information for soybeans has been realized through the whole-genome shotgun approach. Additionally, the generation of a soybean gene atlas using RNA-Seq has yielded valuable genetic resources, facilitating the exploration of the genetic basis underlying soybean traits [11,12]. Furthermore, bioinformatic analysis based on soybean genomic data has made more contributions to the systematic study of evolutionary relationships and molecular characterization of soybean gene families, offering a potential possibility for the elucidation of molecular biological functions inherent in such genes.
Actin cytoskeleton in eukaryotes plays a pivotal role in cell homeostasis, growth and development, signal perception, and cellular immunity, and its dynamic rearrangement is coordinately regulated by several types of actin-binding proteins (ABPs), functionally categorized into multiple distinct classes, including formins, or formin homology 2 (FH2) proteins [13,14,15,16,17]. Plant formins are relatively evolutionarily conserved in the plant kingdom and generally defined by the conserved FH2 domain. Based on the sequence similarity and domain composition, formins derived from angiosperm are phylogenetically grouped into two distinct clades (Class I and Class II). Members of Class I typically possess two conserved domains: a repetitive, profilin-binding FH1 domain and an actin-nucleating and -capping FH2 domain. On the other hand, Class II members comprise an N-terminal PTEN (Phosphatase and Tensin)-like domain, intricately associated with its phosphatase and fold [18,19,20]. In the model plant Arabidopsis thaliana genome, more than 20 formin-encoding proteins have been found, and many of them have been documented the engagement in regulating actin nucleation and dynamics as an important molecular regulator for nucleating apical actin assembly [18,20,21]. Moreover, a polygenetic analysis based on multiple sequence alignment in eukaryotes suggested that formin paralogs exist not only in plant genomes but also in fungi and metazoans. For example, a detailed comparative analysis examined 10 formins in the genome of the Dictyostelium discoideum [22]. Beyond the above-implicated formins on fungi and plants, a total of nine distinct formin subtypes were identified in metazoans based on an evolutionary analysis, suggesting a wide existence of the formin family in eukaryotes [23].
Over the past decades, research endeavors have extensively documented the regulatory role of formins in many aspects through control of actin filaments, microfilament, and membrane dynamics, ultimately affecting numerous physiological and cellular processes, especially for cell division, organogenesis, cell-to-cell trafficking, hormone response, and interaction with pathogens. The Arabidopsis plants expressing formin AFH1 showed an excessive actin cable formation, resulting in tube broadening, growth depolarization, and growth arrest, which emphasizes their crucial role in controlling the normal developmental process of pollen tubes through regulation of actin polymerization [24]. FH3 and FH5, two members of Arabidopsis formins, have been functionally characterized as a regulator of actin dynamics to coordinate actin polymerization and actin filament (F-actin) array construction and finally contribute to proper pollen tube growth [25,26]. Moreover, Arabidopsis FH14, classified as a Class II formin, was reported to impact cell division through its interaction with microtubules and microfilaments. Knock-out mutants of afh14 showed an abnormal development during the microspore formation process and led to a defective microspore generation, while overexpressing AFH14 could enhance the resistance of microtubules to oryzalin and cause an imbalance of turnover of spindles and phragmoplasts [27]. In addition, FH13 has recently emerged as a negative regulator of pollen tube growth, as evidenced that mutants lacking FH13 grow faster than Wild Type (WT) during pollen tube growth, displaying a longer pollen tube, while overexpression of FH13 leads to a pronounced inhibition of pollen tube elongation [28]. In a parallel context, the actin organizing protein RMD, a type II formin in rice (Oryza sativa), was identified as a key component controlling auxin polar transport, auxin distribution gradients, and subcellular localization of auxin transporters through an auxin–RMD regulatory loop to determine the growth and morphogenesis in root cell [15,16,29].
While an increasing body of evidence underscores the significance of plant formins in numerous developmental, physiological processes, and defense responses, there remains a dearth of experimental evidence characterizing their regulatory roles under novel abiotic stress conditions. Moreover, research regarding the plant formin family and its members mainly focuses on Arabidopsis and rice. However, relatively little research on the formins gene family and its members’ functions from other plant species has been conducted so far. Therefore, systematic identification and classification of members of the formins gene family in the soybean need to be conducted further. In the present study, we performed a genome-wide identification of the GmFH gene family, encompassing an analysis of physicochemical property, evolutionary relationships, chromosome distribution, gene replication, promoter cis-acting elements, and expression profiles under drought and salt stress conditions, revealing a potential regulatory role of GmFH in response to distinct stress conditions to orchestrate plant growth and development. The findings from our study on the GmFH gene family not only contribute to a deeper understanding of their novel biological functions in response to abiotic stress in soybeans but also highlight the functional diversity inherent in plant formins.
2. Result
2.1. Identification and Characterization of FH Gene Family from Soybean
A comprehensive investigation at the genome-wide level revealed the presence of 34 GmFH genes in the soybean (Table 1). The anticipated polypeptides encoded by GmFH genes exhibited a substantial variation in length, ranging from 653 to 1464 amino acid residues, with predicted relative molecular weights (RMW) spanning from 72.5 to 162.72 kDa. The isoelectric point (pI) values exhibited a spectrum from 5.42 to 9.43. Notably, all proteins displayed an instability index surpassing 40, indicating their generally unstable nature. The aliphatic index, within the range of 71.48 to 83.73, suggested a notable thermostability among these proteins. The calculated grand average of the hydrophilic index (GRAVY) ranged from −0.378 to −0.61, signifying the hydrophilic nature of the 34 GmFH proteins. Subcellular localization predictions predominantly placed most GmFH proteins within the nucleus, with exceptions observed for GmFH18, GmFH19, GmFH24, GmFH25, GmFH26, and GmFH32, which were predicted to localize in the cytoplasm. These findings collectively contribute to a nuanced understanding of the structural and functional attributes of the identified GmFH genes in the soybean.

Table 1.
Analysis of the DUF668 gene family members in soybean. ORF, Open Reading Length. PL, Protein Length. RMW, Relative Molecular Weights. TIP, Theoretical Isoelectric Point. GRAVY, Grand Average of the Hydrophilic Index.
2.2. Evolutionary Comparison of the FH Gene Family from Diverse Plant Species
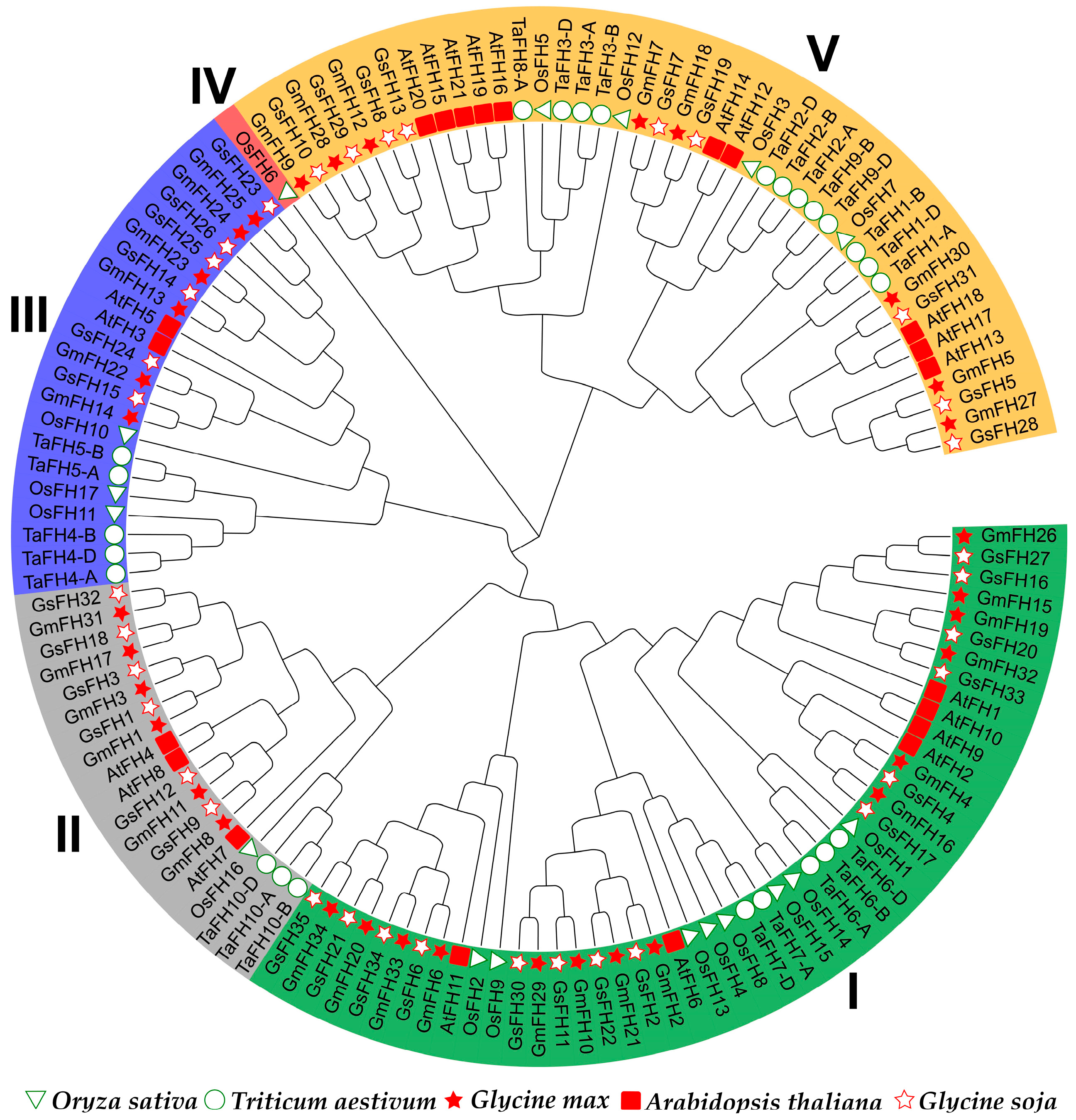
In an endeavor to deepen our understanding of the evolutionary interconnections within the GmFH gene family, FH proteins from diverse plant species, namely Arabidopsis thaliana, Triticum aestivum, Oryza sativa, Glycine soja, and Glycine max, were incorporated for the construction of an evolutionary tree (Figure 1). The FH family members employed for this evolutionary reconstruction are meticulously delineated in Table S1. The outcomes of the evolutionary analysis delineate five discernible groups, denoted as Groups I through V. Groups I and V emerged as the most populous, with Group I encapsulating eleven GmFH members and Group V accommodating eight GmFH, collectively constituting 55.8 percent of the entire GmFH repertoire. In contrast, Group IV exclusively harbored a singular OsFH member. This clustering phenomenon implies homology among FH proteins within the same species, suggesting potential functional similarities.
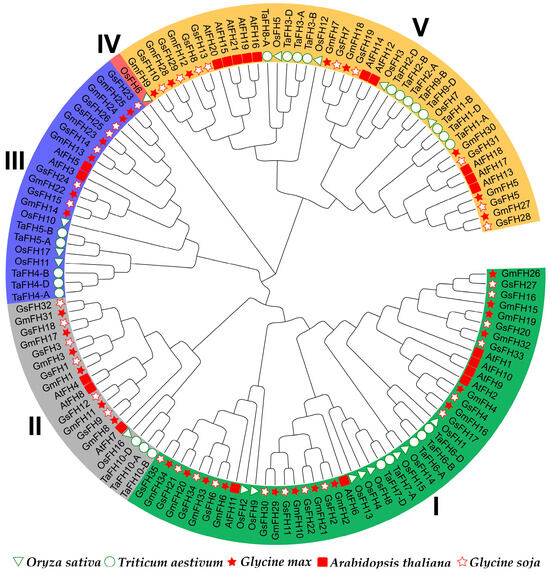
Figure 1.
The evolutionary analysis of the FH gene family from diverse species groups. The full-length protein sequences were utilized for tree construction. At represents Arabidopsis thaliana, Gm represents Glycine max, Os represents Oryza sativa, Ta represents Triticum aestivum, and Gs represents Glycine soja. Different groups were presented with different colors.
2.3. Analysis of Evolutionary Relationship, Gene Structure, and Conservative Motif of FH Family Members in Soybean
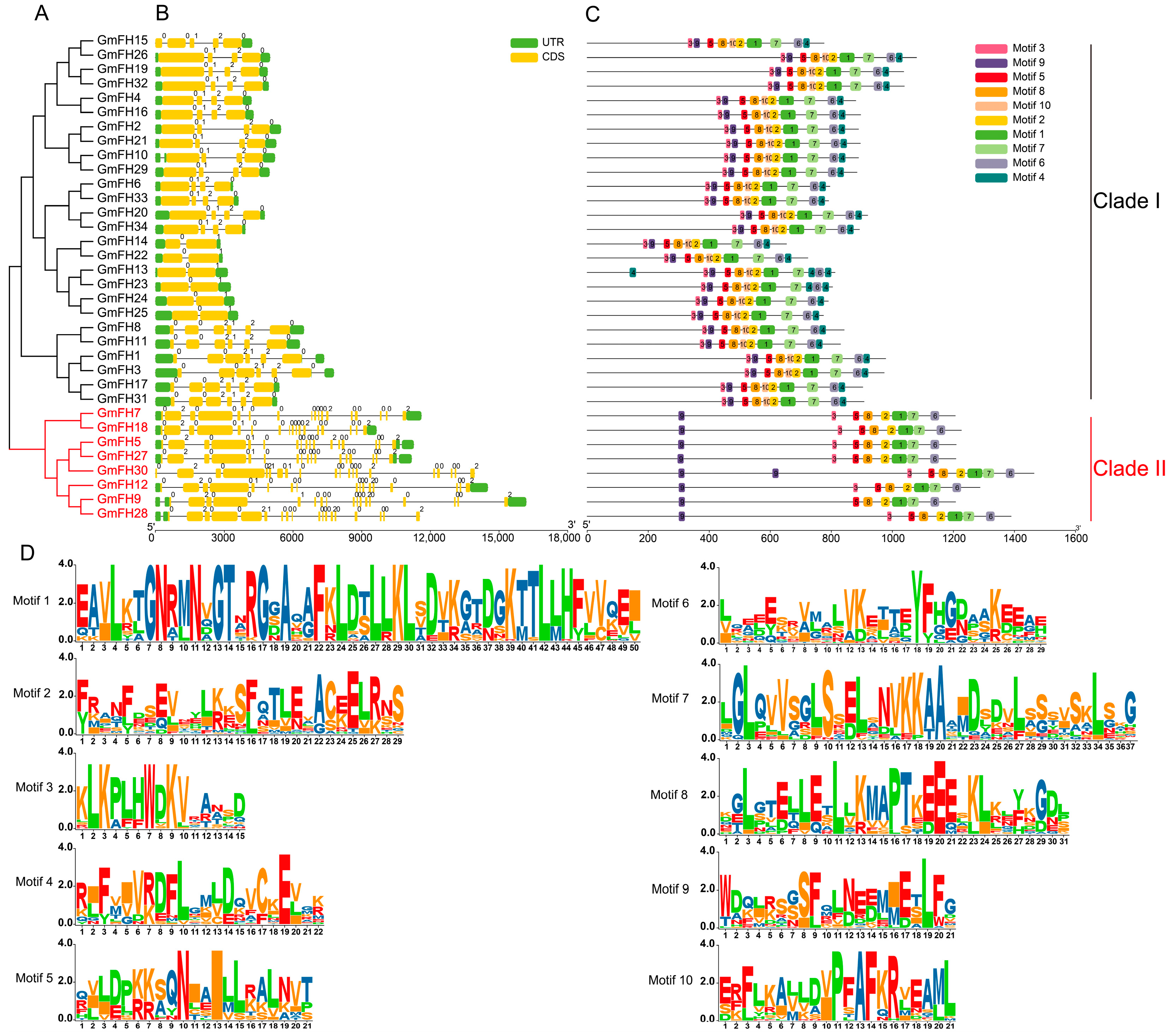
To comprehensively scrutinize the structural attributes of GmFH gene, an exhaustive examination encompassing conserved domains and exon–intron configurations was conducted on the cohort of 34 GmFH genes (Figure 2). The findings elucidate a prevailing presence of conserved domains and untranslated regions (UTRs) across almost all GmFH genes, with GmFH30 constituting the exception (Figure 2B). The intricate composition of GmFH proteins was delineated by identifying various motifs (Figure 2C), where the amino acid sequences surrounding conserved residues manifested as distinct motifs. The overall height of the letters illustrated the graphical representation of sequence conservation at each position proportionate to the relative frequency of the corresponding amino acid. Furthermore, an in-depth analysis of the gene structures of GmFH was undertaken (Figure 2D). Results disclosed that GmFH genes exhibited variable sequence lengths, with GmFH9 representing the longest, while GmFH14 and GmFH22 featured as the shortest. A predominant inclusion of both 5′ and 3′ untranslated regions (UTRs), exons, and introns was observed in most GmFH genes. However, noteworthy exceptions were identified, such as GmFH13 exclusively possessing 3′-UTRs, GmFH28 featuring only 5′-UTR, and GmFH30 lacking any UTRs. Furthermore, the analysis of the conservative motifs of GmFH revealed that all GmFH family members were clustered into two major subgroups (Clade I and II). Most of the GmFH members in Clade I comprised 10 conserved motifs, whereas all members in Clade II contained 8 conserved motifs absent in GmFH30. Collectively, the structural landscape of GmFH genes showcased considerable diversity; certain GmFH genes within the same clades demonstrated a propensity for comparable exon–intron lengths and numbers. These observations underscore a nuanced evolutionary differentiation within the GmFH gene family, concomitant with the retention of relatively similar biological functions among homologous.
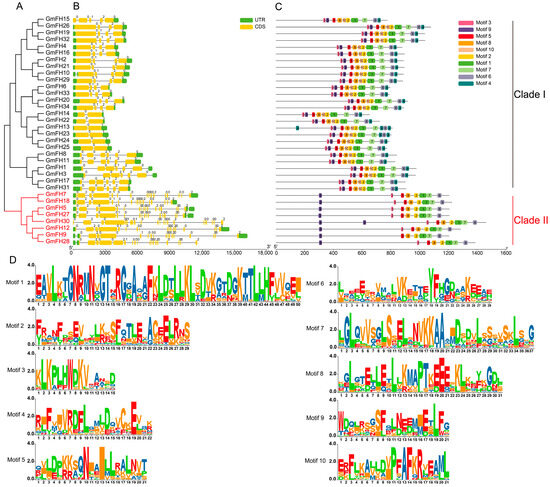
Figure 2.
Evolutionary classification, protein domains, and gene structures of GmFH. (A) Evolutionary analysis of GmFH proteins was performed using MEGA7.0 based on their full-length protein sequences. The resulting evolutionary tree provides insights into the evolutionary relationships among GmFH gene family members. (B) Gene structures of GmFH are depicted, where the scale bar at the bottom indicates the genomic length. Yellow boxes represent exons (CDS), while green boxes denote the 5′ and 3′ untranslated regions (UTRs). Black lines visually represent introns. This representation offers a detailed overview of the genomic organizations of GmFH gene. (C) The distribution of conserved domains within GmFH proteins is illustrated, emphasizing the various protein lengths and domains. The color-coded scale bar at the bottom facilitates the visualization of distinct domains, contributing to a comprehensive understanding of the structural features of GmFH proteins. (D) Amino acid sequences surrounding conserved amino acids are presented as motifs. The letter height in each position reflects the degree of sequence conservation, with taller letters indicating higher relative frequencies of the corresponding amino acids.
2.4. Chromosomal Distribution and Duplication Events
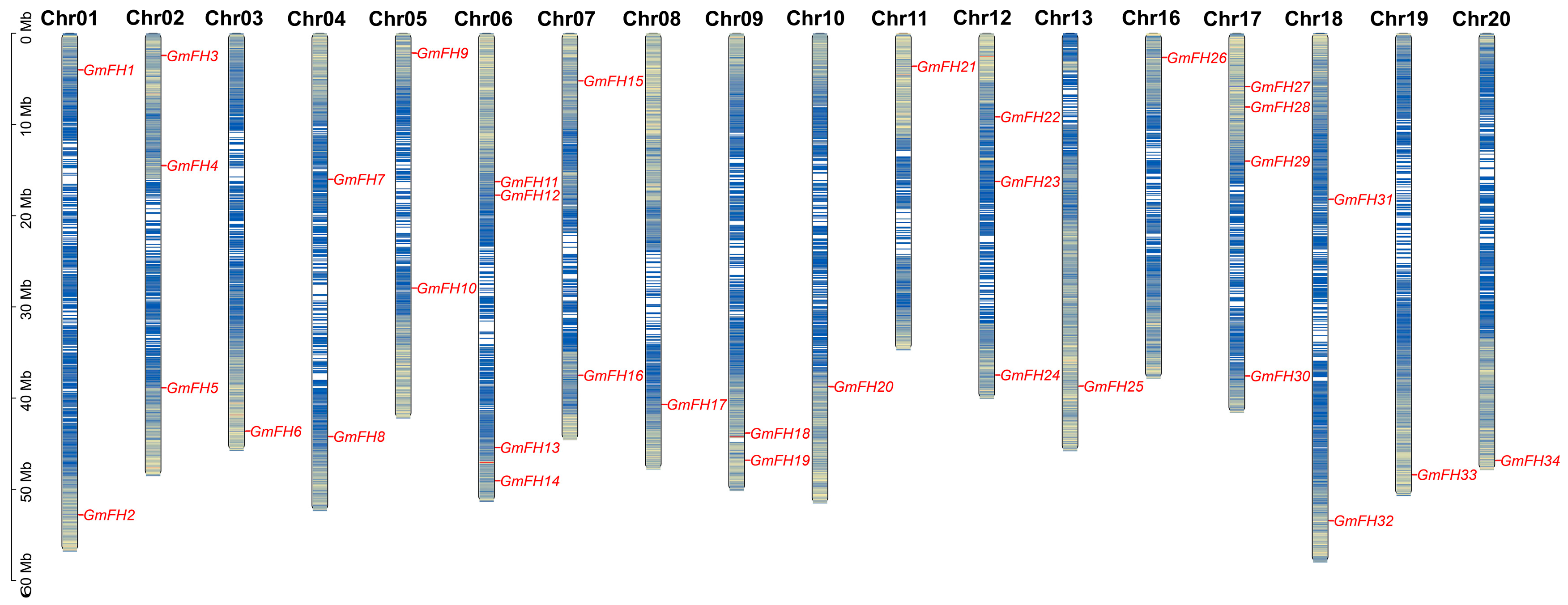
The chromosomal disposition and synteny of GmFH genes were systematically scrutinized based on their respective genome sequences. The allocation of 34 GmFH genes was mapped across the twenty chromosomes of soybeans, revealing a non-uniform distribution pattern (Figure 3). The spatial arrangement of these GmFH genes exhibited a dispersed configuration, with varying counts ranging from one to four genes on each chromosome. The spatial arrangement of these GmFH genes exhibited a dispersed configuration, with varying counts ranging from one to four genes on each chromosome. Notably, chromosome 06 (Chr06) and chromosome 07 (Chr07) harbored four GmFH genes, representing the highest count on all chromosomes, while certain chromosomes harbored only a single member of the GmFH family. These findings contribute valuable insights into the chromosomal landscape and synteny patterns characterizing the GmFH family, thereby enriching our understanding of their genomic context within soybeans.
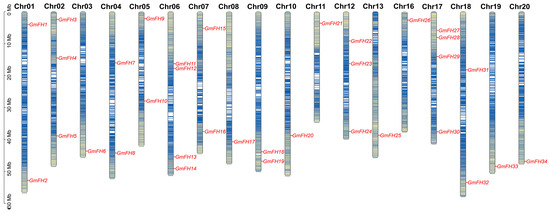
Figure 3.
Chromosomal Localization of GmFH genes in Soybean. The spatial distribution of GmFH genes on soybean chromosomes is illustrated. Each chromosome is labeled at the top with its corresponding chromosome number, providing a clear identification of individual chromosomes. The left-side ruler serves as a scale, representing the length of each chromosome. The gene abundance were indicated by blue colors on chromosome. This depiction offers a visual representation of the specific genomic positions of GmFH, facilitating an understanding of the gene’s chromosomal localization within the soybean genome.
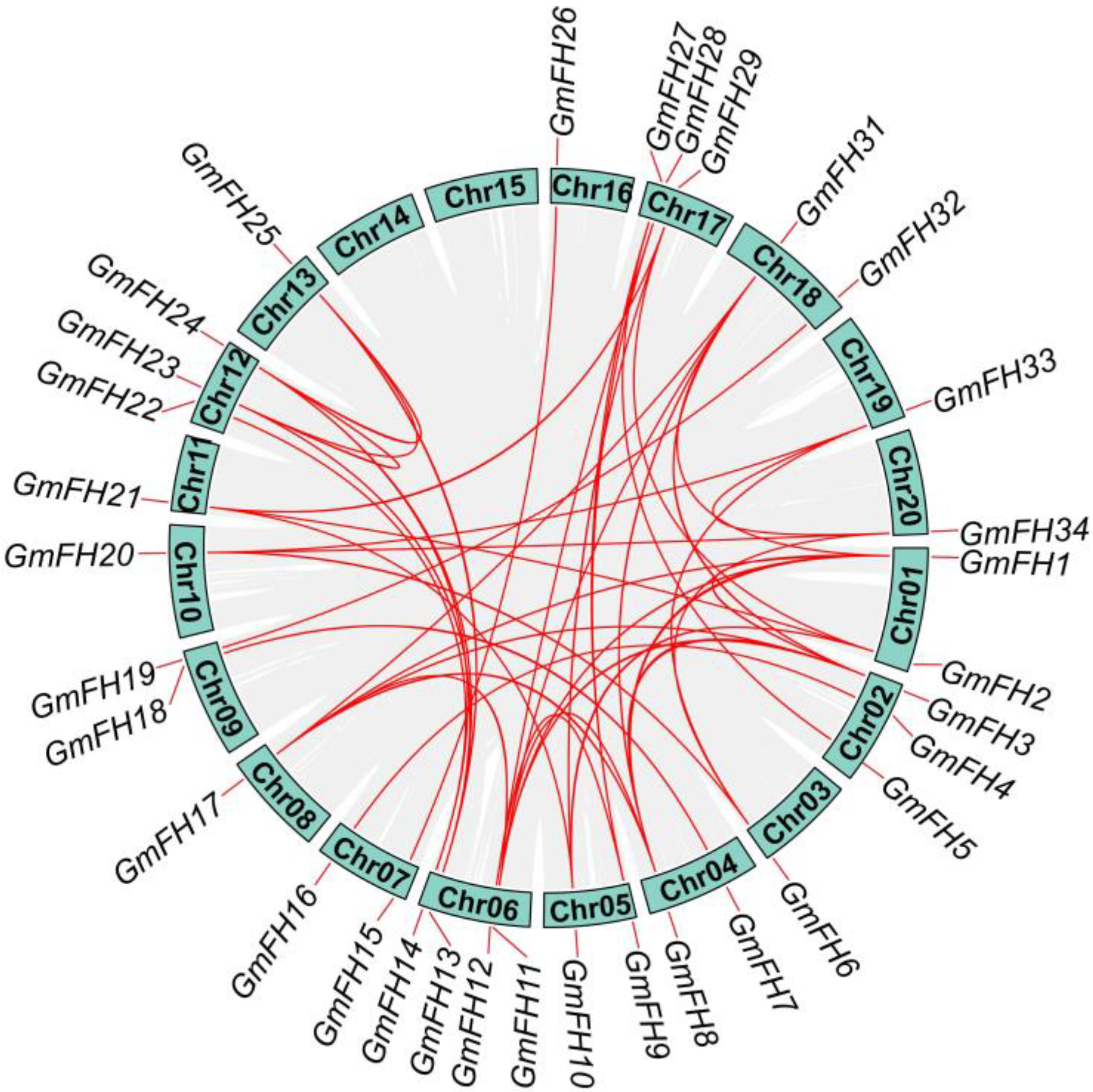
To further enhance our comprehension of the arrangement of genes on each chromosome, an investigation into gene duplication events focused on the GmFH family was conducted. Such duplications arise through diverse mechanisms, including tandem and segmental duplication. In this analysis, we discovered 25 segmental duplication pairs existing in the 33 GmFH genes but no tandem duplications during this evolutionary investigation (Figure 4). The Ka and Ks ratios were calculated for the identified gene pairs to delve deeper into the evolutionary dynamics (Table 2). Most gene pairs exhibited Ka/Ks ratios less than 1, indicative of robust purifying selection acting upon these GmFH genes during their evolutionary trajectory. This pervasive purifying selection implies a concerted effort to preserve the functional integrity of the GmFH gene family, underscoring its biological significance and highlighting the evolutionary pressures that have shaped its molecular evolution within the soybean genome.
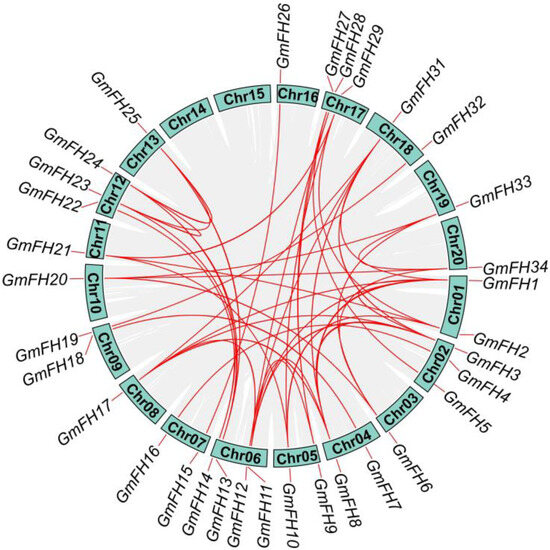
Figure 4.
Syntenic relationship among FH gene family in soybean. The chromosome numbers are displayed in green boxes. The red lines indicate the syntenic relationship of GmFH genes among different gene pairs. The grey lines indicated the syntenic relationship among GmFH genes in the soybean genome.

Table 2.
The ratio of Non-synonymous substitution (Ka) and Synonymous substitution (Ks) of GmFH segment duplication pairs.
2.5. Investigation of Syntenic Relationships between Soybeans and Other Plant Species
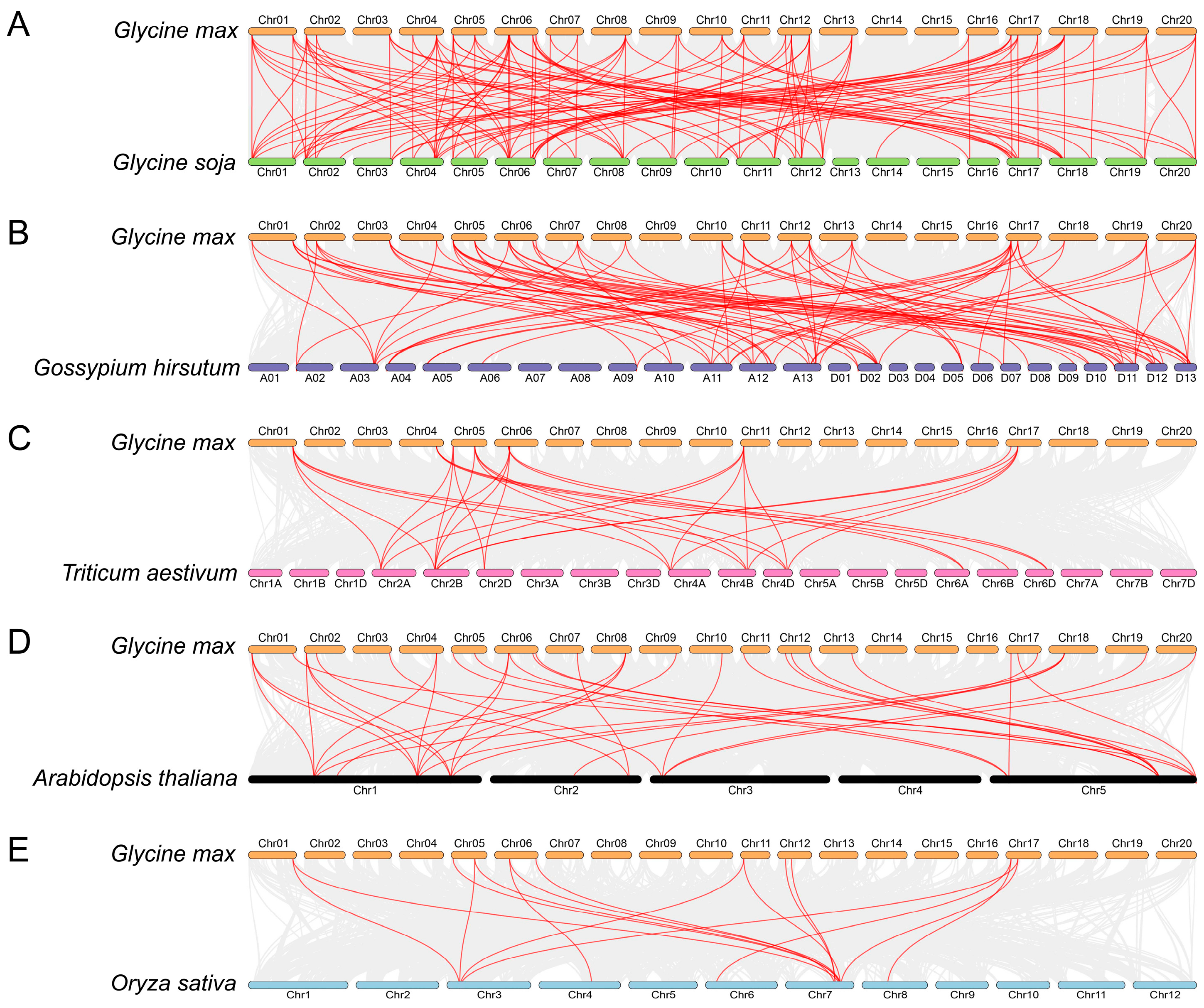
The study of syntenic relationships was extended beyond soybean to encompass five distinct plant species, as shown in Figure 5. The outcomes exhibited a total of 32, 25, 9, 27, and 10 GmFH genes constituting syntenic gene pairs with FH genes in Glycine soja, Gossypium hirsutum, Triticum aestivum, Arabidopsis thaliana, and Oryza sativa, respectively. Correspondingly, we also found a certain number of collinear gene pairs between these plant species (wild soybean (120), cotton (86), wheat (30), Arabidopsis (37), and rice (16)) and soybean. The comparative analysis unveiled a particularly noteworthy syntenic relationship between Glycine max and Glycine soja, wherein both species shared an identical set of 20 chromosomes, and their genes exhibited a close syntenic association. This observation not only underscores a high degree of genomic conservation but also implies a close evolutionary relationship, suggesting potential shared physiological functions. Similarly, the syntenic relationship between Glycine max and Gossypium hirsutum indicated a close evolutionary affinity, with certain genes occupying corresponding chromosomal positions in both plant species (Figure 5B). This alignment further supports the notion of shared ancestry and potential functional similarities.
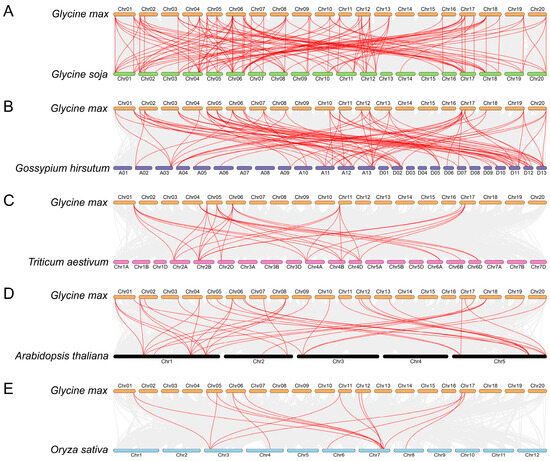
Figure 5.
Syntenic relation of the GmFH family with the other five plant species, including Glycine soja (A), Gossypium hirsutum (B), Triticum aestivum (C), Arabidopsis thaliana (D), and Oryza sativa (E). The highlighted red lines indicate the syntenic gene pairs with GmFH. The gray lines indicate the syntenic gene pairs within and other plant genomes. The label next to each chromosome indicates the chromosome number.
2.6. Analysis of Promoter Cis-Acting Elements in the FH Family Genes
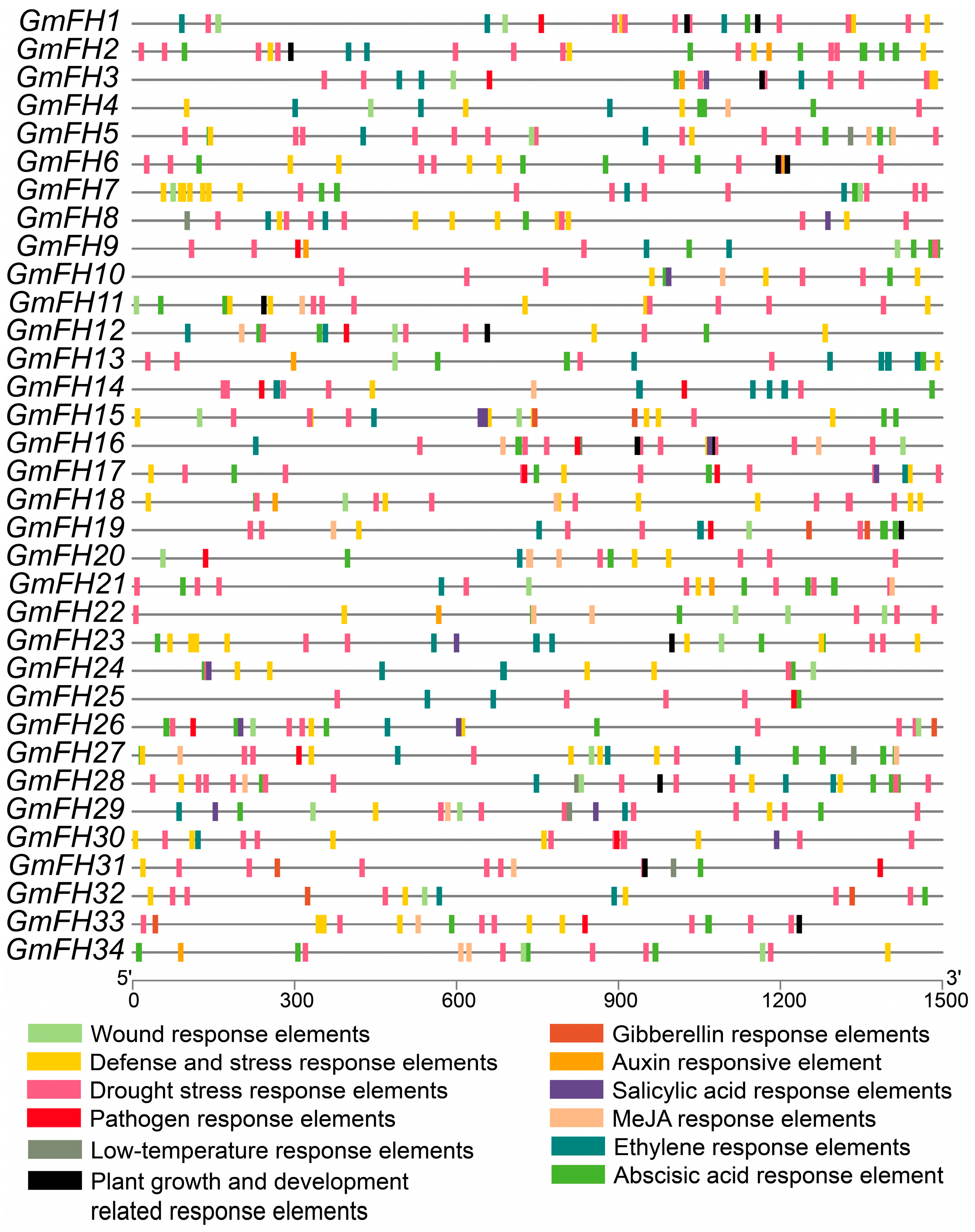
The scrutiny of cis-elements within gene promoter regions is pivotal for elucidating their biological functions. To unravel the functional underpinnings of GmFH, an in-depth analysis of the cis-elements within these genes was conducted utilizing the PlantCARE databases, and a comprehensive listing is provided in Table S2. These cis-elements were stratified into four overarching categories: hormone-responsive, growth- and development-related, stress-responsive, and wound-responsive elements. Notably, among the hormone-responsive elements, the (MeJA-responsive element) and ABRE (ABA-responsive element) featured prominently, underscoring their significance in the hormonal regulatory milieu (Figure 6).
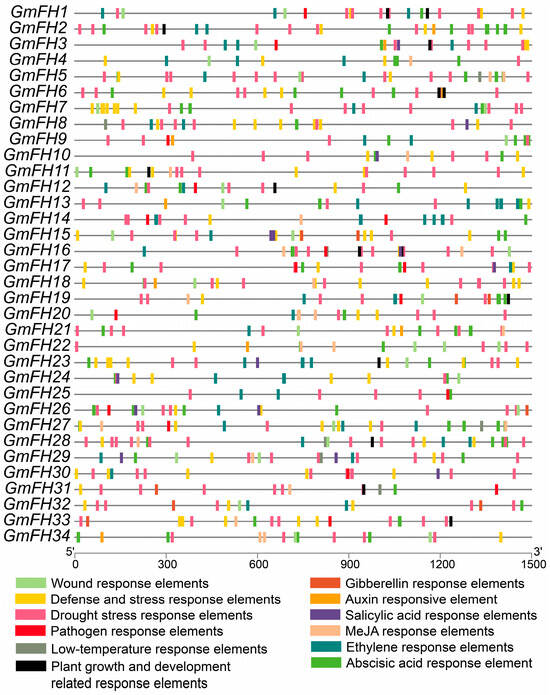
Figure 6.
Examination of cis-elements in GmFH promoter. The figure presents a comprehensive analysis of cis-elements within the promoters of GmFH. The numerical annotations within grids, accompanied by distinct colors, signify the presence of different cis-elements. The spatial arrangement of these elements is specifically focused on those associated with hormonal responses, growth and development, and stress. The variety of colors employed serves to distinguish between different cis-elements, thereby providing a nuanced portrayal of their distribution within the GmFH promoter regions.
Similarly, stress-responsive elements, particularly the wound response element, LTR low-temperature-responsive element, and drought stress response element, exhibited substantial representation. The prevalence of these hormone- and stress-responsive elements suggests that GmFH may play integral roles in mediating soybean responses to hormonal cues and environmental stresses. Furthermore, several GmFH genes harbor cis-elements associated with pathogen response, such as GmFH26, GmFH20, and GmFH31. This observation suggests a potential involvement of these genes in orchestrating soybean’s response to pathogenic challenges. The meticulous exploration of cis-elements within GmFH genes provides a foundation for understanding the regulatory landscape governing their biological functions, with implications for their roles in hormonal signaling, stress responses, and pathogen interactions in the soybean.
2.7. Investigation of Networks Depicting Protein-Protein Interactions
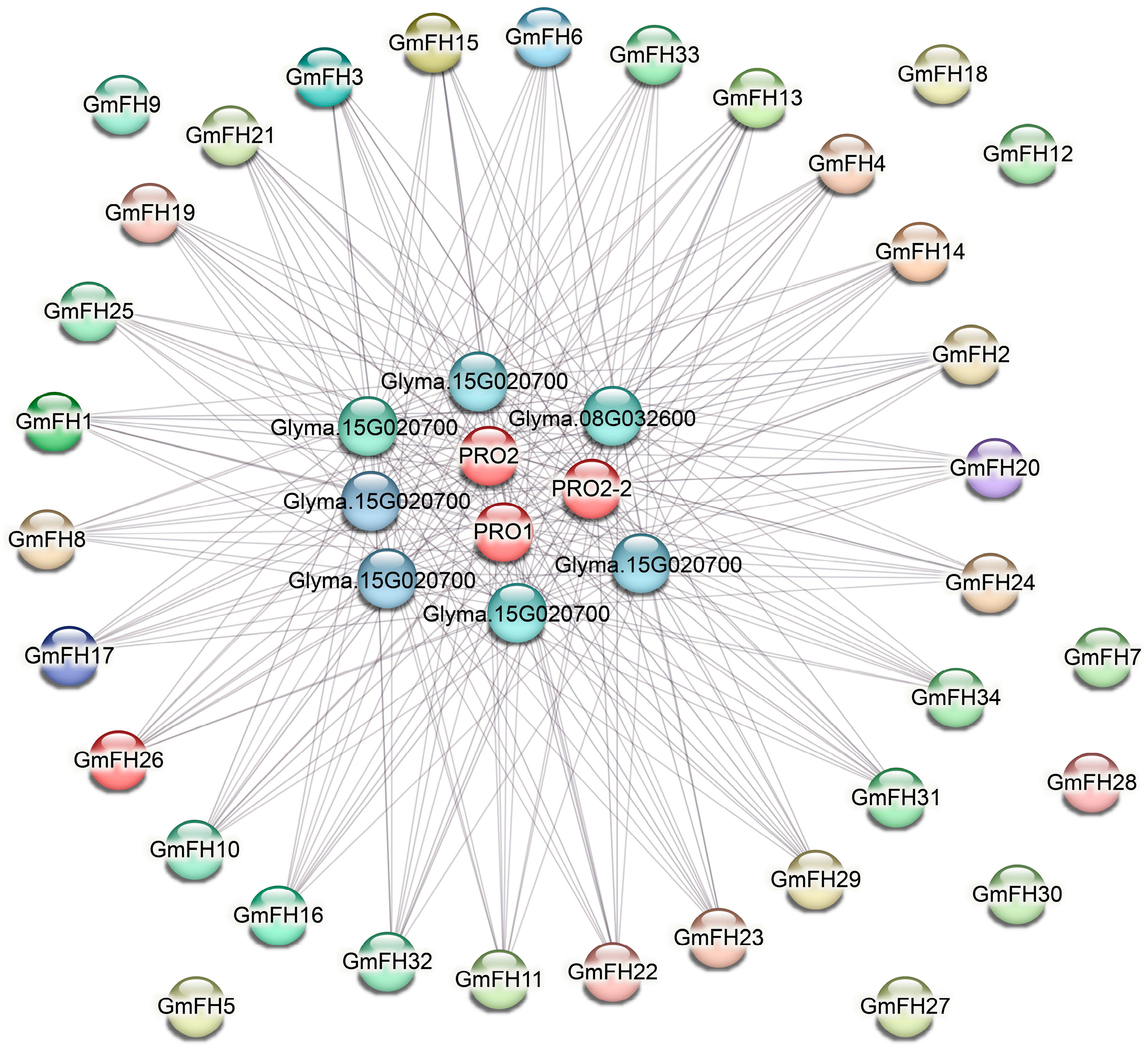
The protein-protein interaction (PPI) network analysis of the GmFH gene family was executed, and the resultant network is illustrated in Figure 7. Comprising 40 nodes interconnected by edges, this network delineates the intricate web of interactions among diverse GmFH proteins. Notably, the PPI network underscores robust associations among most GmFH proteins, suggesting a cohesive interplay within this gene family. However, a subset of proteins, including GmFH9, GmFH18, GmFH12, GmFH7, GmFH28, GmFH30, and GmFH27, exhibited an absence of interactions. The observed network architecture implies that FH proteins may function as core entities central to the collaborative network of protein interactions. The distinctive clustering of these genes with strong associations hints at the prospect of their concerted involvement in regulating various biological events. Notably, the absence of interactions for specific GmFH proteins may signify unique functional roles or regulatory independence. These findings provide a comprehensive insight into the protein interaction dynamics of the GmFH family and offer a promising avenue for future investigations into the functional roles and regulatory mechanisms of these proteins. The strong associations identified in the PPI network serve as a guidepost for shaping the direction of subsequent functional studies, shedding light on the intricacies of GmFH protein interactions and their implications in the soybean.
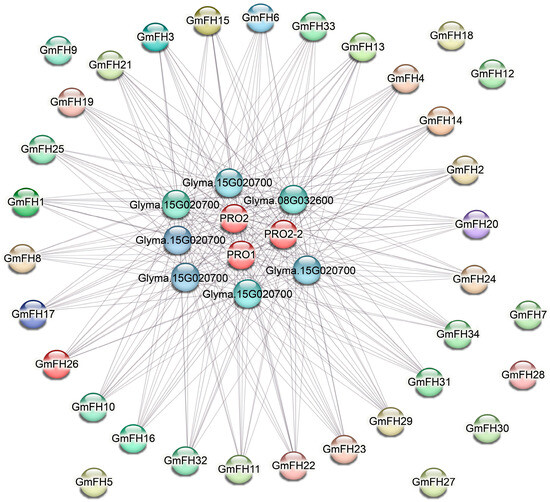
Figure 7.
Protein interaction network of the GmFH family. Nodes denote proteins, while edges depict the connections between two proteins. The diversity of edge colors serves to differentiate between types of evidence, thereby indicating distinct forms of functional links. This visual portrayal elucidates the intricate network of protein-protein associations, offering insights into the various functional relationships supported by diverse types of evidence.
2.8. Expression Patterns of FH Genes in Soybean Specific to Different Tissues
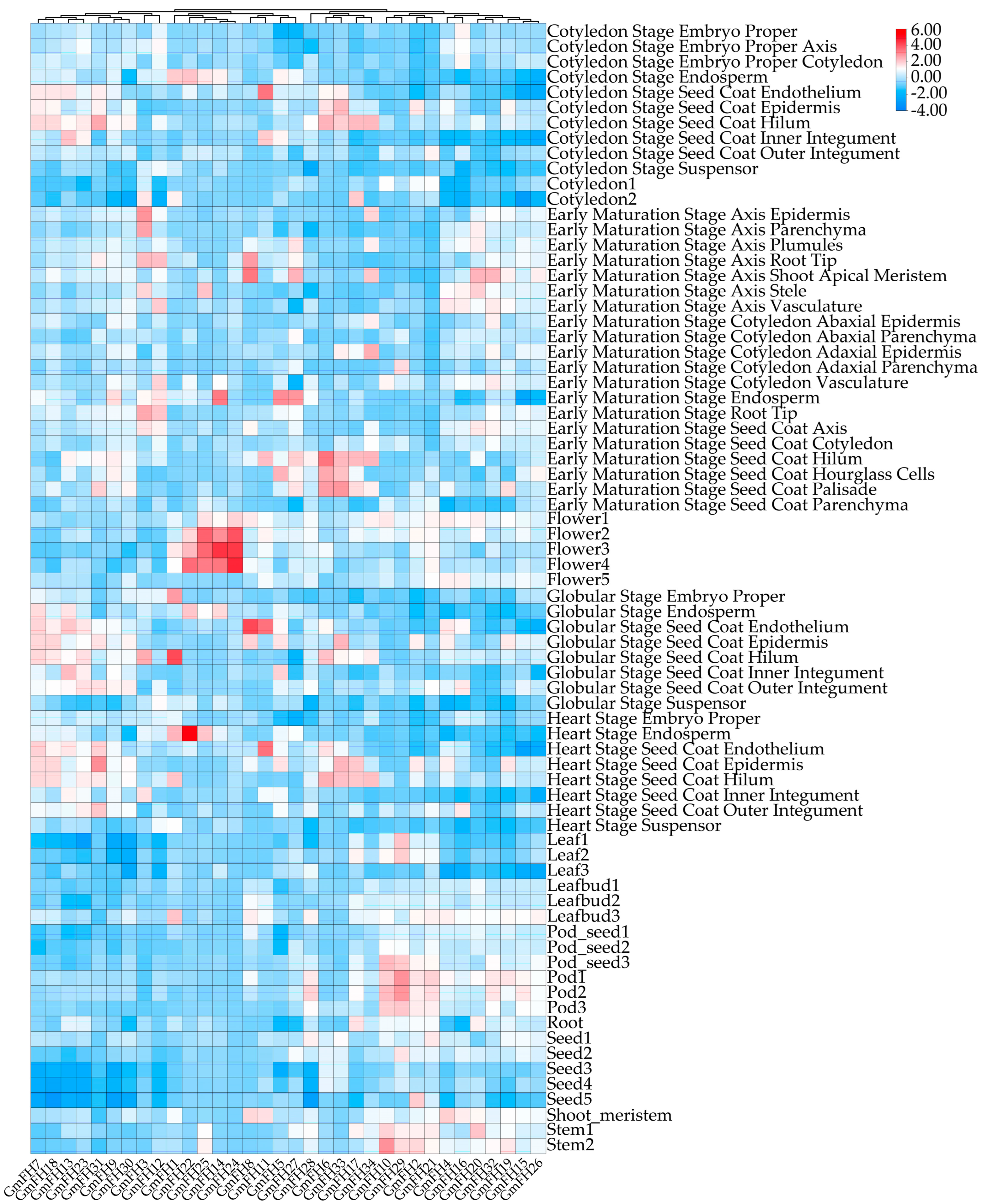
The expression profiles of the GmFH family gene were systematically evaluated across diverse plant tissues, revealing distinct and nuanced expression patterns for each gene within the GmFH family. This comprehensive analysis encompassed various developmental stages, including the cotyledon stage, early maturation stage, pod stage, root, and shoot. Notably, several GmFH family members, including GmFH24, GmFH11, GmFH8, and GmFH5, exhibited heightened expression with considerable intensity at the flowering stage. In addition, GmFH22 demonstrated elevated expression levels in endosperm tissues during the heart stage, while GmFH1 showcased heightened expression, specifically in the globular stage of the seed coat. Conversely, specific genes exhibited no discernible expression in the diverse tissues under investigation, as indicated by the white coloration in the graph (Figure 8). Specifically, GmFH7, GmFH18, GmFH13, and GmFH23 manifested suppressed expression levels at the seed stage. The graph’s color-coded bars on the right side visually represented the varying expression intensities of the respective genes across different soybean plant tissues.
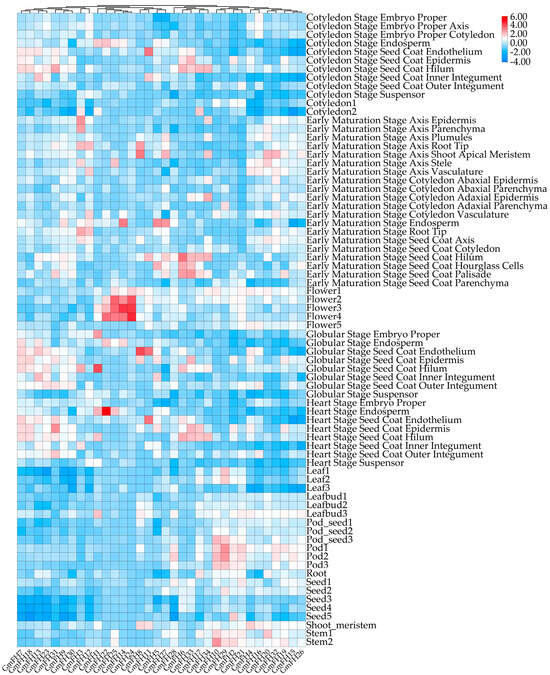
Figure 8.
Expression patterns of GmFH gene in different plant tissues. Bar indicates different intensities of gene expression in different tissues.
2.9. Expression Analysis of the FH Family Genes in Soybean in Response to Abiotic Stresses
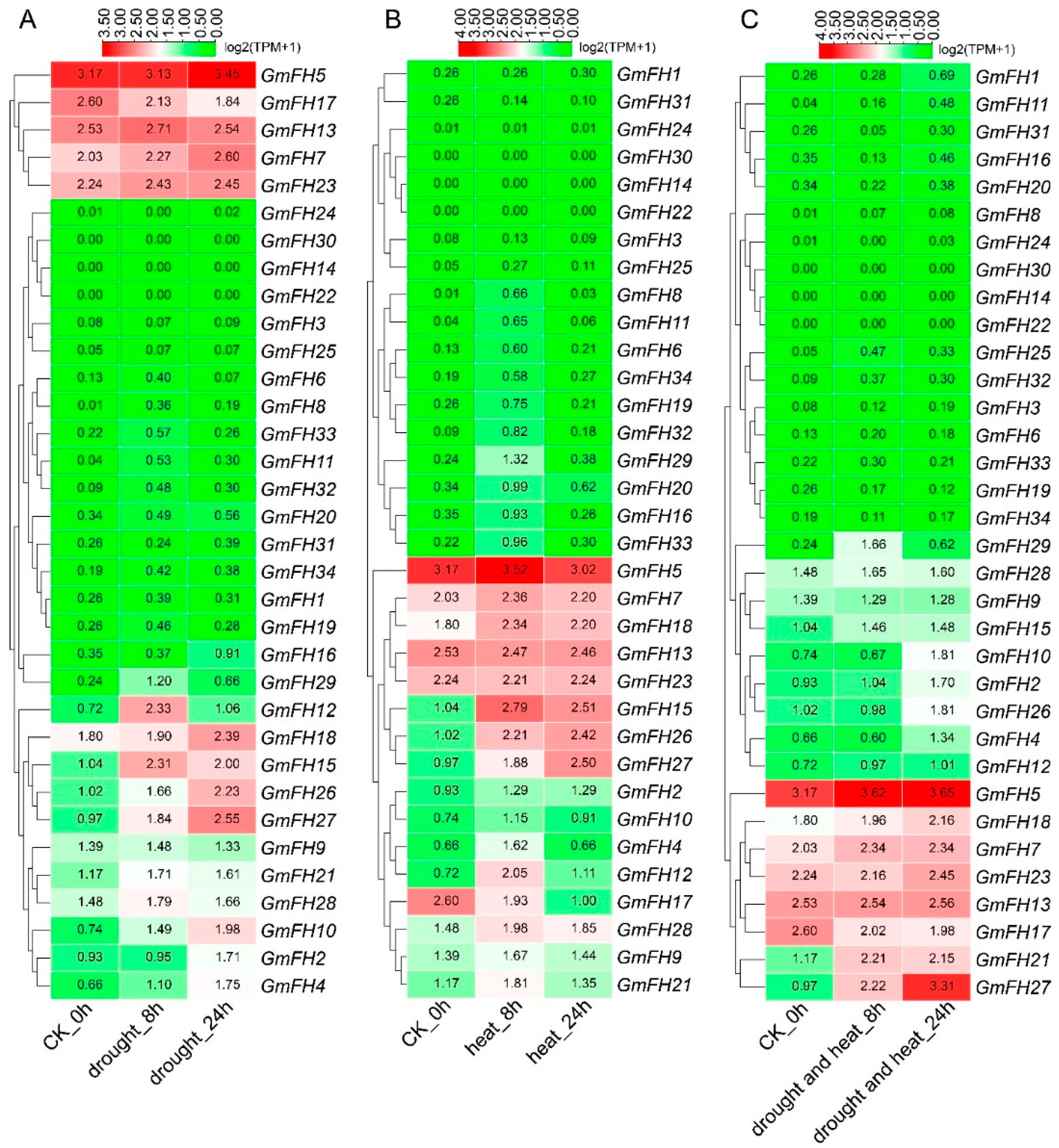
To evaluate the response of the FH gene family in the soybean to abiotic stress, we conducted a comprehensive analysis of the expression levels of each FH gene under varying stress conditions. The expression profiles revealed distinct responses of individual FH genes to specific stressors. Notably, GmFH5 exhibited heightened expression levels under drought conditions, with a comparable high expression observed in control plants (Figure 9). GmFH12 and GmFH15 displayed relatively elevated expression after 8 h (h) of drought treatment, while GmFH10 and GmFH27 exhibited a continuous induction upon drought treatment, indicating a potential role in stress responsiveness. Conversely, GmFH14, GmFH22, and GmFH30 demonstrated no detectable expression during both drought treatment and in control plants. In addition, most genes exhibited minimal expression under drought-stress conditions. Under heat stress, GmFH15 demonstrated significant upregulation after 8 h. Intriguingly, GmFH17 exhibited elevated expression levels under control conditions but experienced downregulation during heat stress at both 8 h and 24 h. Moreover, GmFH26 was significantly induced by heat stress in 24 h, but no remarkable difference under both heat and drought stress. The combined heat and drought stress presented distinctive outcomes in the expression profiles. However, GmFH27 showcased heightened expression under stress conditions, implying a potential role in stress tolerance. Additionally, several GmFH genes exhibited consistently high expression levels across control and stress conditions.
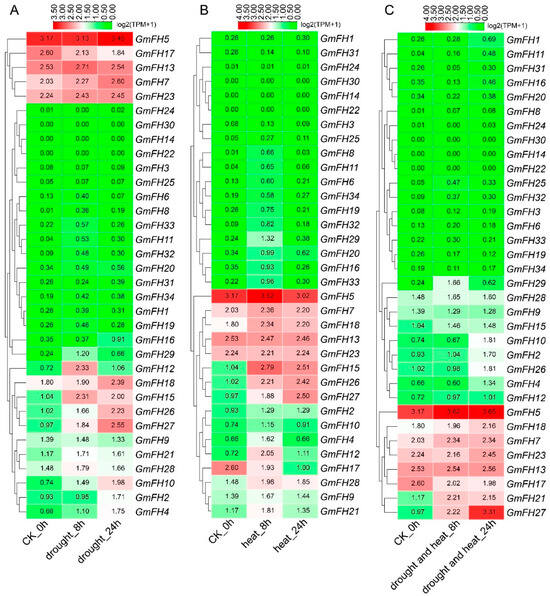
Figure 9.
Expression analysis of GmFH genes in soybean in response to drought and heat stress. The expression of different genes was examined under drought, heat, cumulative drought, and heat stress. (A) Expression profiles of different genes under drought stress after 8 h and 24 h stress treatment. (B) Expression profiles of different GmFH gene under heat stress treatment of 8 h and 24 h. (C) Expression patterns of GmFH gene under cumulative heat and drought stress after 8 h and 24 h, respectively.
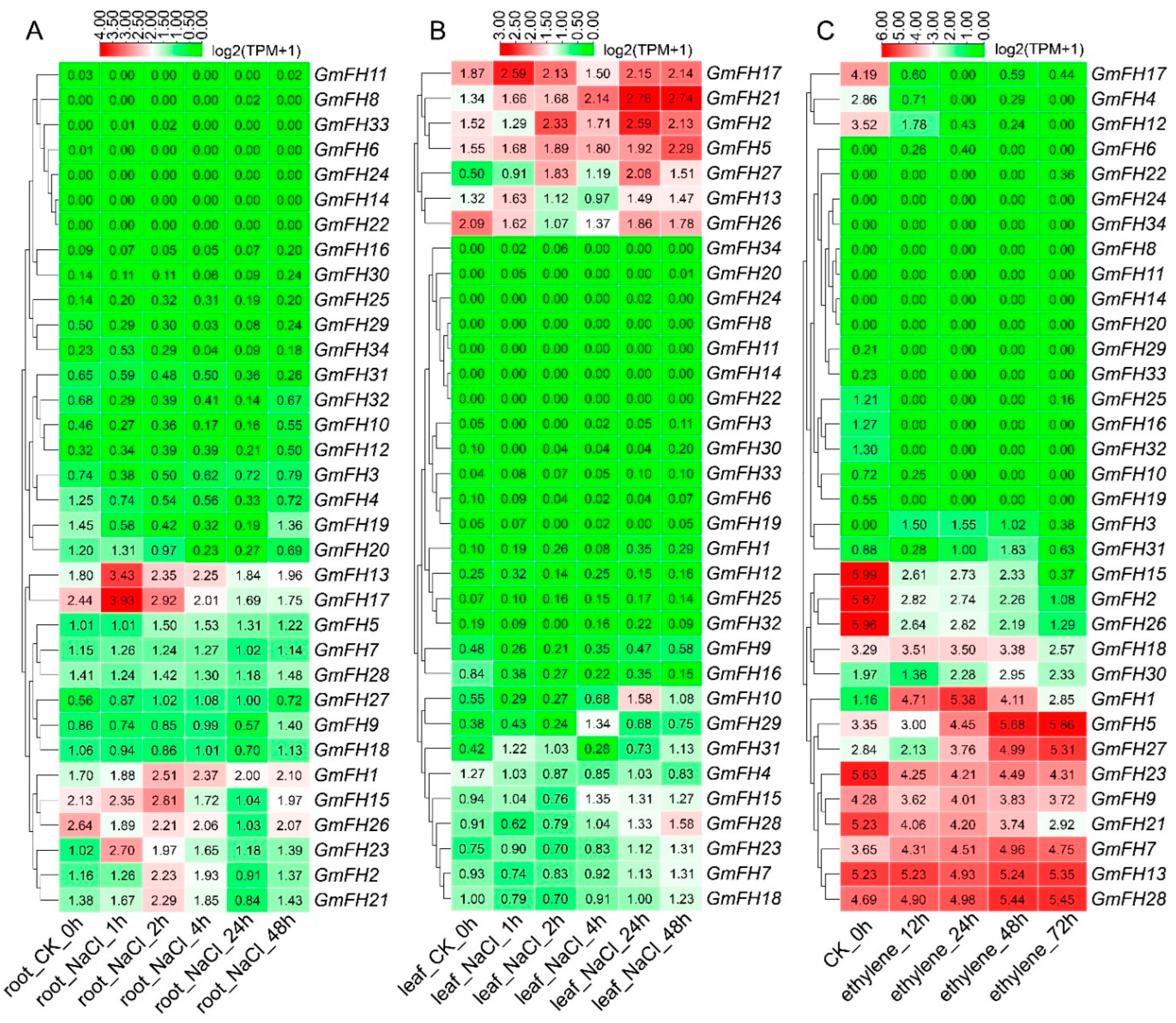
Examining the FH gene family expression dynamics in soybean extended to responses under salt and ethylene stress conditions (Figure 10). We systematically analyzed the expression levels of each GmFH gene across diverse tissues subjected to different stress durations, as depicted in Figure 9. Notably, GmFH13 and GmFH17 demonstrated heightened expression in roots under salt stress after a 1 h treatment. Conversely, GmFH17, GmFH21 (after 24 h and 48 h treatment), and GmFH2 exhibited elevated expression levels in leaves under the same stress condition. Upon exposure to ethylene treatment, GmFH15, GmFH2, and GmFH26 showcased elevated expression levels in control plants. In contrast, GmFH23, GmFH9, GmFH21, GmFH7, GmFH13, and GmFH28 exhibited expression across all ethylene treatments, albeit not very high. Intriguingly, GmFH5 manifested high expression, specifically in the 48 h and 72 h treatments. Despite the differential responses observed, a predominant trend emerged wherein most genes exhibited low expression levels under salt and ethylene stress conditions. This nuanced exploration of the GmFH expression profiles provides insights into their varied responsiveness to distinct abiotic stressors, underscoring the intricate regulatory mechanisms governing stress adaptation in the soybean.
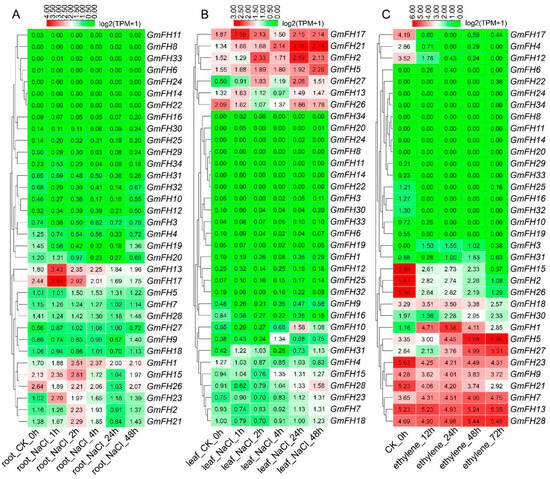
Figure 10.
Expression profiles of GmFH family gene under salt stress in root and leaf. (A) Gene expression patterns of GmFH in roots under salt stress treatment after 1 h, 2 h, 4 h, 24 h, and 48 h. (B) Gene expression patterns of GmFH in leaf under salt stress treatment after 1 h, 2 h, 4 h, 24 h, and 48 h. (C) Gene expression profiles of GmFH under ethylene treatment after 12 h, 24 h, 48 h, and 72 h.
2.10. Go Enrichment Analysis of GmFH Family Members
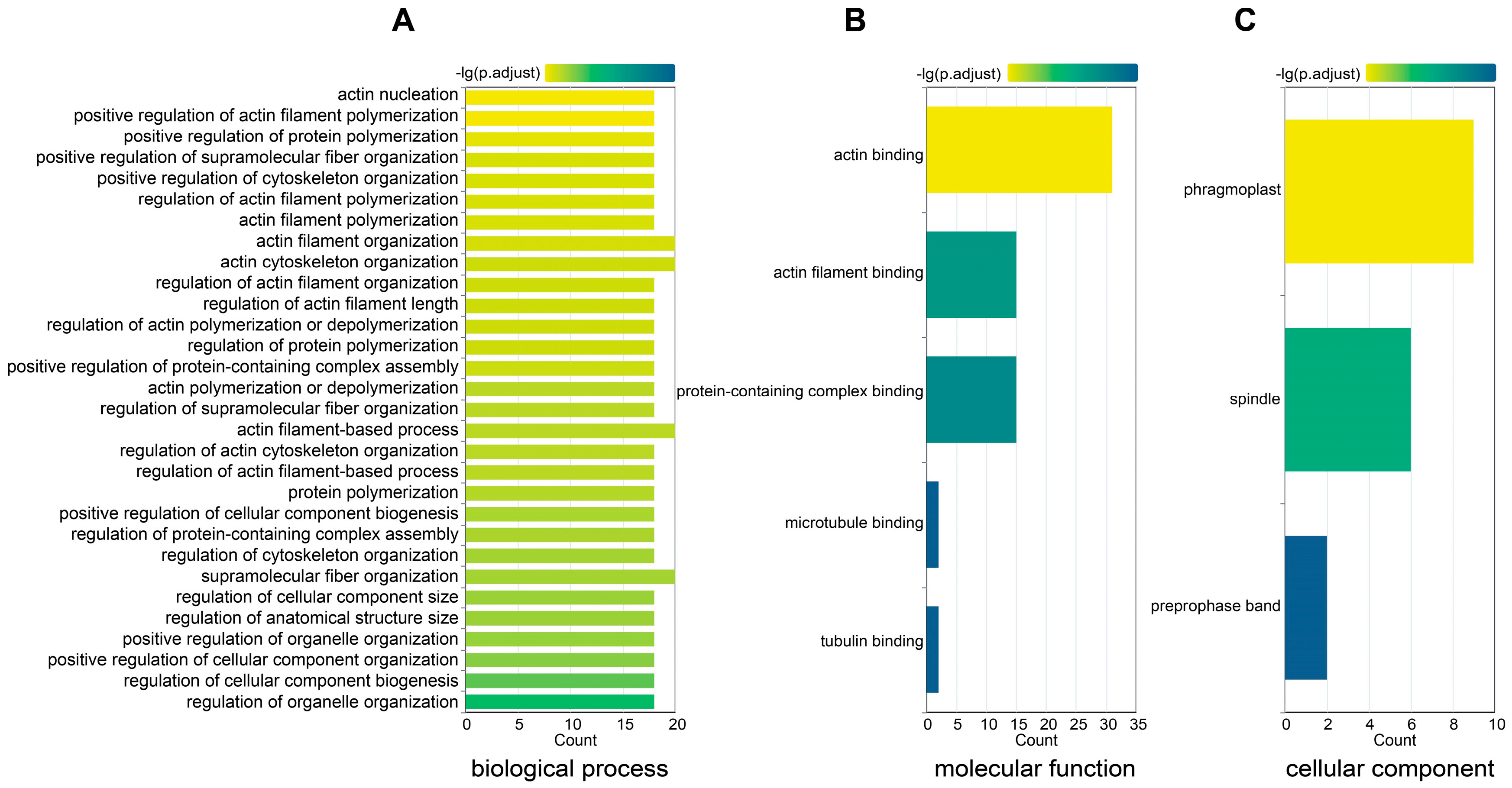
In-depth functional characterization of the GmFH gene family members was pursued to enhance our comprehension of their roles. Functional annotation delineated three distinct categories, each representing different functional dimensions (Figure 11). In the biological process category, the predominant involvement of GmFH was identified in actin filament organization (GO: 0007015), polymerization (GO: 0030838), and nucleation (GO: 0045010). In the molecular function domain, a substantial proportion of GmFH genes demonstrated engagement in protein binding activities, specifically with tubulin (GO: 0015631) and actin filament (GO: 0051015). Within the cellular component category, the prevalent localization of GmFH was observed in the phragmoplast (GO: 0009524), with an additional presence in the spindle (GO: 0005819) and select instances in the preprophase band (GO: 0009574). This systematic functional categorization provides valuable insights into the diverse roles played by GmFH gene family members, shedding light on their functional attributes across various biological processes, molecular interactions, and cellular components within the soybean genome.
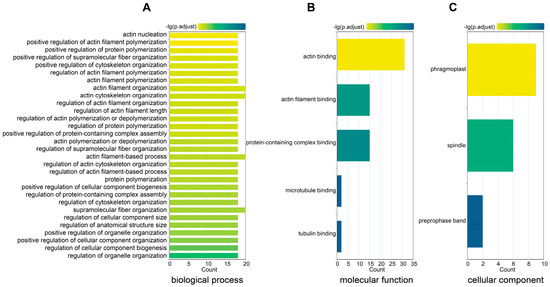
Figure 11.
Gene ontology (GO) enrichment for the GmFH gene family. (A) GO annotation of GmFH in biological process. (B) GO annotation of molecular function. (C) GO annotation of a cellular component. The labels on the left represent the GO terms and categories. Different color of boxes indicates different properties.
2.11. Verification of Expression Patterns of the GmFH Genes by qRT-PCR
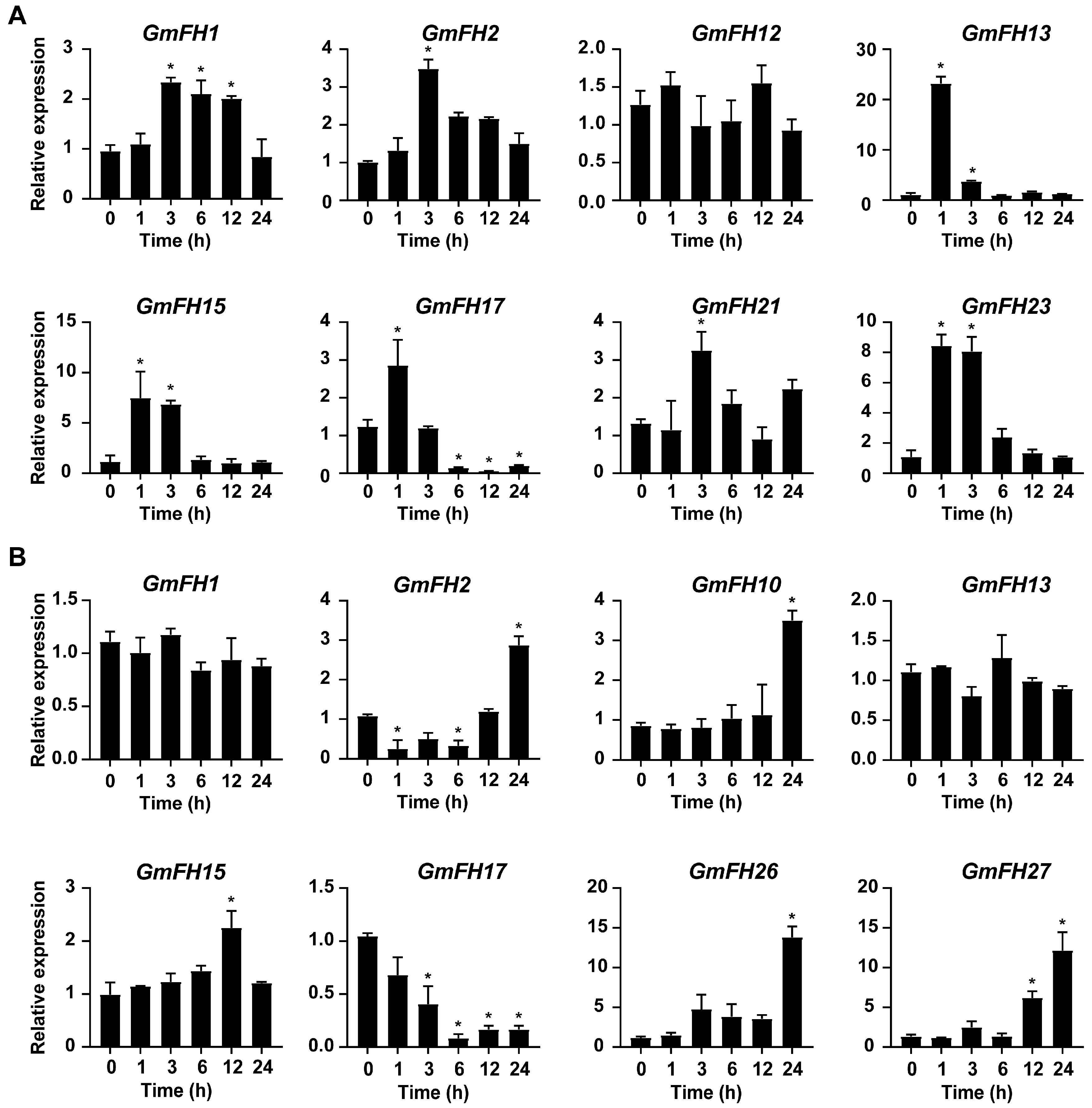
In the present study, several GmFH genes exhibiting significant changes in transcriptional levels under salt and drought stress were selected for qRT-PCR analysis, utilizing samples collected at various time points. As a result, the expression patterns of GmFH candidate genes examined by qRT-PCR were basically consistent with previous transcriptome data, thereby validating the reliability of existing transcriptome sequencing results. Notably, the majority of GmFH genes exhibited a significant increase in expression level at early stages upon salt treatment (Figure 12). Interestingly, GmFH2 and GmFH21 were only induced at 3 h after the initiation of salt stress treatment, while the expression of GmFH17 was dramatically inhibited after 6 h. In contrast, some candidate genes, such as GmFH10, GmFH26, and GmFH27, exhibited up-regulated expression upon drought treatment. In summary, the GmFH candidate genes selected for qRT-PCR exhibited diverse expression patterns under salt and drought stress.
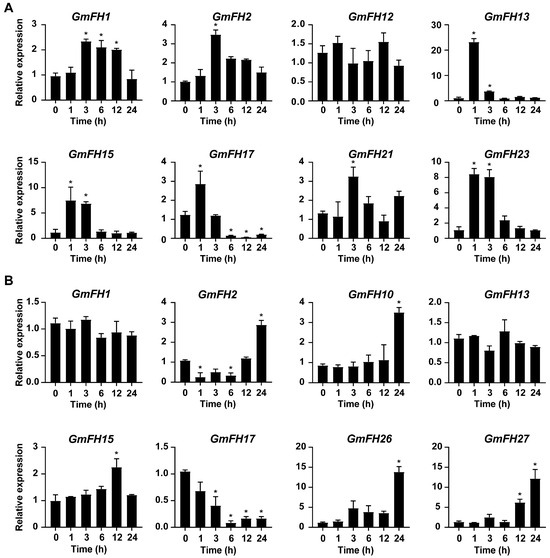
Figure 12.
Expression analysis of GmFH candidate genes at indicated time points under salt (A) and drought (B) stress conditions. The analysis was performed with 3 technical replicas for each experiment, and asterisks indicated significant differences, examined by Student’s t-test (* 0.01 < p < 0.05).
3. Discussion
The cytoskeleton in eukaryotes primarily consists of two highly conserved polymers known as microfilaments and microtubules. The coordinated reconfiguration and dynamic behaviors of these structures are regulated by various Actin-Binding Proteins (ABPs) [30]. Different ABPs play a distinct but critical role in almost all stages of plant growth and development through the regulation of assembly and disassembly of actin filaments. In plant species, ABPs mainly comprise six kinds, among which formins have been well-characterized actin-bundling proteins defined as a nucleation factor to initiate the microfilament polymerization, afterward participating in the structural organization of the actin cytoskeleton [17]. Previously, formin family proteins have successfully been identified in model plant Arabidopsis and rice, comprising 21 and 16 members, respectively, and an increasing number of formin-encoded genes were recently identified from angiosperm, including tomato, tobacco, rice, pea, sorghum, and wheat. However, a comprehensive study on the identification of putative formin-related genes in the soybean remains missing. Therefore, a genome-wide investigation of formin-encoded genes was conducted by referring to the existing public soybean genome database [18,19,31].
In this analysis, we identified 34 GmFH genes distributed non-uniformly across 20 soybean chromosomes (Table 1 and Figure 3), ranging from one to four genes on each chromosome. However, no discernible correlation was observed between the abundance of GmFH genes and the respective lengths of the chromosomes, emphasizing a nuanced and intricate genomic organization within the Glycine max genome. Formin proteins structurally contain two conserved domains: the profilin-rich FH1 domain, required for the elongation of actin filaments, and the microfilament-nucleating FH2 domain, responsible for actin filament nucleation [25]. Many formin proteins have been functionally documented as nucleation factors guiding transmembrane anchorage of the formins or sometimes localizing formins in endosomes and the tonoplast [32,33,34,35]. Consistent with this, our results to predicate the subcellular localization indicated that all GmFH proteins exhibited either nucleus or chloroplast localization (Table 1), suggesting a putative function as nucleation factors responsible for actin filament nucleation. However, many formin proteins, such as AtFH14, were predominantly localized to the microtubules, strongly suggesting a functional difference between the two kinks of formin proteins.
Formins, characterized by the presence of FH2-containing domain proteins, constitute an evolutionarily conserved family widely distributed in eukaryotes. Evolutionary comparison among five diverse plant species revealed that the FH gene family primarily clustered into five main classes according to their evolutionary relationships (Figure 1). FH proteins from discots, including Arabidopsis thaliana, Glycine soja, and Glycine max, demonstrated closer evolutionary conservation, compared with monocots Triticum aestivum and Oryza sativa, consistent with the previous discoveries, suggesting a relatively conserved mechanism during the evolution of FH gene family. A noteworthy clustering pattern was observed wherein FH proteins from O. sativa, A. thaliana, G. soja, G. max, and T. aestivum were predominantly coalesced within species-specific groups. Specific FH proteins from these five distinct species exhibited clustering, indicative of a closely entwined evolutionary relationship within the FH family. These findings contribute valuable insights into the intricate evolutionary dynamics of the GmFH gene family and its evolutionary affinities across diverse plant species. Furthermore, analysis of the syntenic relationship among five species pairs highlighted the closest evolutionary affinity between Glycine max and Gossypium hirsutum during FH family evolution except for Glycine soja, again contributing to the high degree of evolutionary conservation and underscoring the similar biological feathers in homologous gene pairs. Intriguingly, the results also revealed a distinctive pattern wherein soybeans exhibited greater syntenic convergence with dicots compared to monocots, elucidating a nuanced aspect of evolutionary divergence between these plant lineages. Additionally, distribution events suggested that the FH family mainly undergoes segment duplication as a primary driving force contributing to gene expansion. These findings provide valuable insights into the genomic dynamics and evolutionary relationships between soybeans and diverse plant species, thereby enriching our understanding of soybeans’ genetic landscape and evolutionary history.
Analyzing the expression profiles based on previous transcriptome data provides insights into the potential biological functions of GmFH across diverse plant tissues. The examination unveiled dynamic variations in GmFH gene expression across different plant tissues and developmental stages (Figure 8). Notably, the distinctive expression patterns of each GmFH gene underscored remarkable heterogeneity, with some genes exhibiting robust expression in specific plant parts while displaying no expression in others. For example, expression levels of GmFH24 and its homolog GmFH25 were significantly induced at the flowering stage, implying their association with flower organ development. In contrast, GmFH22 exhibited dramatic increases during the heart stage, especially in endosperm tissues, strongly suggesting a functional involvement during endosperm development. This variability in gene expression within the GmFH family across various plant tissues suggests a finely tuned and tissue-specific regulatory framework governing the transcriptional dynamics of these genes in the soybean. To gain an in-depth understanding of the biological functions involved by GmFH in diverse biological processes, a comprehensive analysis focused on their promoter cis-acting elements was performed (Figure 6). The examination identified a large body of hormone-responsive elements, with ABA- and ethylene-responsive elements prominently enriched in the promoter region of the majority of GmFH genes. The regulatory roles of ABA in modulating osmotic and drought stress through ABA-dependent or -independent strategies have been well-known in multiple plant species [36]. This result indicates a possible biological process involved by GmFH in responding to ABA-mediated drought stress tolerance. Correspondingly, ethylene is generally regarded as an important signaling molecule modulating salinity tolerance in plants through a fine-tuning of ROS production and removal [37,38]. Salinity can trigger the biosynthesis of ethylene in a plant by regulating the expression of key enzyme genes, and the production of ethylene, in turn, counteracts the salt-induced inhibition in multiple biological processes. The observation of an abundance of ethylene cis-acting elements predicted on the GmFH promoter strongly suggested a regulatory role of GmFH in response to salinity stress through a potential ethylene signaling pathway. Furthermore, a certain number of stress-responsive elements specifically respond to drought and low-temperature stresses, such as anaerobic induction element (ARE), stress-responsive element (TC-rich repeats), and drought-inducibility (MYB, MYC, Myb-binding site, MYB-like sequence), were also found on the promoter of some GmFH genes, underscoring their biological significance on the enhancement of plant adaptation to adverse conditions.
Formins, being evolutionarily conserved proteins, play crucial roles in nucleation, capping, and bundling of actin filaments, thereby influencing the organization and dynamics of the actin cytoskeleton. Previous research has extensively documented the roles of plant formin proteins in remodeling the actin cytoskeleton to coordinate physiological and cellular changes. In Arabidopsis, AtFH1 has been suggested to play a regulatory role in the normal development of pollen tubes, as overexpression of AFH1 resulted in growth depolarization and growth inhibin [24]. AtFH8 is speculated to regulate the growth of polarized cells, affecting the root hair cell development [39]. In rice, OsFH5, a type II formin nucleating factor, was involved in plant morphology through reorganization and dynamics of the actin cytoskeleton, while OsFH15, encoded by a class I formin, affects grain size by regulating cell expansion [16,40]. In moss Physcomitrella patens, For2 (Group II formins) demonstrated the necessity for maintaining polarized plant cell growth since silencing For2 in plants absence caused severe growth retardation [41]. To further probe the potential biological function of formin proteins in the soybean, a comprehensive analysis regarding gene expression of GmFH under diverse abiotic stresses was performed based on the previous transcriptome data. Several GmFH genes, like GmFH12 and GmFH15, exhibited a relatively higher expression level upon drought or heat treatment for 8 h (Figure 9), while GmFH26 and GmFH27 showed a persistent induction after the same treatment for 24 h, suggesting a nuanced regulatory response to heat stress. GmFH17, on the other hand, exhibited an inhibitory effect on its expression level under drought or heat stress conditions, potentially implying a positive regulatory role modulated by GmFH17 in response to drought or heat stress. In contrast with drought or heat treatment, we also identified certain GmFH genes displaying heightened expression under salt stress. Among them, GmFH17 demonstrated an elevated expression level in both root and leaf tissues upon salt treatment, while GmFH13 and GmFH23 were significantly induced only in root tissue after salt treatment. Meanwhile, we noticed that many GmFH genes, such as GmFH10 and GmFH29, showed the specific induced expression at 24 and 4 h in root under salt stress, strongly suggesting a dynamic spatial and temporal regulation. Considering that ethylene is a stress-related plant hormone that regulates plant salt tolerance responses, it was observed that several GmFH genes were specifically induced by ethylene, including GmFH5 and GmFH27. At the same time, the expression level of GmFH2, GmFH15, and GmFH26 is higher in control plants than in treated plants when exposed to ethylene (Figure 10), implying their probable functional distinctions in modulating the ethylene signaling pathway. Finally, qRT-PCR results verified the reliability of existing transcriptome data (Figure 1). These findings illuminate the nuanced and context-dependent regulatory dynamics of the GmFH gene family in response to abiotic stressors, providing valuable insights into their putative functions in stress adaptation in the soybean.
4. Methods and Materials
4.1. Plant Growth Conditions and Stress Treatment
Soybean Williams 82 (Glycine max) seeds were obtained from the Institute of Crop Science of the Chinese Academy of Agricultural Sciences. Soybean seeds were soaked with 1% sodium hypochlorite solution (v/v) for 15 min, followed by washing with deionized water to remove residual solution. To promote seed germination, the surface-sterilized seeds were first incubated on the wet filter papers at 25 °C for 3 days. Next, seedlings with consistent growth were selected and cultivated in half-strength modified Hoagland solution supplied with a 16-h light period and an 8-h dark period at 26 °C for growth. For the salt and drought treatment assay, plants growing to the first trifoliate leaf were placed into a newer half-strength modified Hoagland solution, followed by 150 mM NaCl or 20% PEG6000. Samples from different plant tissues were collected at indicated time points (0, 1, 3, 6, 12, 24 h). Three biological replicates for each sample were mixed at least for RNA extraction, and samples were -frozen with liquid nitrogen and then removed into the refrigerator at −80 °C for further use.
4.2. Identification and Physicochemical Analysis of the GmFH Family Members
The genome and its corresponding protein sequence of soybean Williams 82 (Glycine max) were downloaded from SoyBase (https://www.soybase.org/, (accessed on 14 December 2009)) (Wm82.a2.v1). The hidden Markov models (HMMs) of the FH domain (PF02181) were downloaded from the InterPro database (https://www.ebi.ac.uk/interpro/, (accessed on 6 January 2023)), and candidate genes of GmFH family were searched by simple HMM program (TBtools, (version 1.120), E-value < 1 × 10−5) [42]. Subsequently, validation of the GmFH protein domains was implemented in NCBI-CDD (https://www.ncbi.nlm.nih.gov/cdd/, (accessed on 4 January 2017)) and SMART (http://smart.embl-heidelberg.de/, (accessed on 8 January 2021)) database. The physical and chemical properties, including relative molecular weight, theoretical isoelectric point, instability index, aliphatic index, and grand average of hydropathicity, of GmFH proteins were analyzed through the ExPASy program (https://www.expasy.org/, (accessed on 1 January 2022)) [43]. Subcellular localization of the GmFH proteins was predicted on Cell-PLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/, (accessed on 17 January 2008)) online tools [44].
4.3. Evolutionary Analysis between Glycine Max and Other Plants
The protein sequences of wild soybean W05 (Glycine soja) were collected from SoyBase (https://www.soybase.org/, (accessed on 14 December 2009)) (Gsoja_W05.v1). The Arabidopsis thaliana genome database was extracted from TAIR (https://www.arabidopsis.org/, (accessed on 17 November 2010)). The genomic data and its annotation of wheat (Triticum aestivum) and rice (Oryza sativa) were obtained from EnsemblPlants (http://plants.ensembl.org/index.html, (accessed on 7 January 2022)). The FH family members from Arabidopsis, wild soybean, wheat, and rice were identified using the same methods described above, and the protein sequences of identified FHs in different plant species were extracted to construct the evolutionary tree on MEGA 7.0 software (Jone-Taylor-Thornton model) based on the multiple sequence alignment results [45]. All parameters are set as the maximum likelihood method, and the bootstrap replicates were 1000 times. Sequence information is presented in Table S3.
4.4. Evolutionary Relationships, Conserved Domain, and Gene Structure of GmFH Family Genes
The evolutionary relationship of the FH gene family in the soybean was analyzed using the same methods described above, and the corresponding protein sequences of identified GmFH genes were extracted to construct the evolutionary tree by MEGA 7.0 as described above [45]. The conserved motifs of GmFH protein were predicted using MEME Suite 5.4.1 (https://meme-suite.org/meme/doc/meme-format.html?man_type=web, (accessed on 1 July 2015)), and TBtools was employed to visualize the conserved boxes of each GmFH gene [46]. The gene structures of GmFH were constructed with the online website GSDS 2.0 (http://gsds.gao-lab.org/, (accessed on 12 October 2014)), referring to the soybean annotation file [47].
4.5. Chromosomal Mapping and Gene Duplication
The chromosome locations of GmFH genes were analyzed through a gene location visualization program of TBtools based on the physical positions of the GmFHs in the soybean genome [48]. MCScanX program package was utilized to determine gene duplication events of GmFH genes, and the collinearity between soybean and other species was analyzed by TBtools (version 1.120) [49]. Next, Ka (non-synonymous rate)/Ks (synonymous rate) values of syntenic pairs of GmFH genes were calculated using Kaks Calculator 2.0 software [50].
4.6. Promoter Elements, GO Enrichment Analysis, and Protein Interaction
Promoter sequence information (2000 bp upstream of coding sequences (CDS)) of GmFH genes was extracted from the soybean genome database, and then its corresponding homeopathic response elements were analyzed by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, (accessed on 1 January 2002)) [51]. Subsequently, TBtools were utilized to present the obtained cis-acting regulatory elements [48]. The GO enrichment analysis of the GmFH proteins was conducted using the SoyMD database (https://yanglab.hzau.edu.cn/SoyMD/#/tools/go, (accessed on 9 October 2023)) [52]. The interaction network of GmFH proteins was generated through analysis of GmFH protein information on the online website STRING 11.5 (https://cn.string-db.org/, (accessed on 12 August 2021)).
4.7. qRT-PCR Analysis
The RNA extraction of soybean root tissues was performed referring to the manufacturer’s protocol provided from TRIZOL reagent (Invitrogen, Burlington, CA, USA), and cDNA synthesis was performed using PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) provided from TaKaRa, Tokyo, Japan. Gene-specific primers used in present studies were designed using primer 5 software and then verified by the NCBI database (https://www.ncbi.nlm.nih.gov/tools/primer-blast/, (accessed on 18 June 2012)). The quantitative expression level was examined on an ABI 7500 real-time PCR detection system referring to the manufacturer’s protocol. Soybean gene GmActin (F: CGGTGGTTCTATCTTGGCATC, R: GTCTTTCGCTTCAATAACCCTA) was utilized as the control to normalize the relative expression levels examined using the 2−ΔΔCT method. All primers used for the examination of gene expression were present in Table S4.
5. Conclusions
To summarize, 34 GmFH genes were identified in this study, and their structural elements, subcellular locations, and evolutionary connections were disclosed. The evolutionary analysis showed a close evolutionary relationship with other species, pointing to possible functional similarities. Segmental duplication became apparent as a plausible mechanism for the gene family’s expansion despite its uneven chromosomal distribution. The GmFH promoters showed characteristics linked to different hormones, stress reactions, and developmental processes. The network of protein-protein interactions revealed a robust correlation between their proteins, suggesting a cooperative control of various biological processes. Its importance for additional research is indicated by expression patterns under heat and salt stress, especially the strong response of GmFH1, GmFH15, and GmFH27 to abiotic stress. All things considered, this thorough investigation offers insightful information about the possible functions of GmFH gene in biotic and abiotic stress responses, laying the groundwork for further studies to improve crop quality and reduce production losses in challenging circumstances.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13020276/s1, Table S1: Sequence Information on the FH gene family in diverse plants. Table S2: Primer sequence. Table S3: Gene ID and its responding FH gene number in different plants. Table S4: Analysis of GmFH primer elements.
Author Contributions
Y.G. conceived and designed the research, helped in revising the final manuscript. Z.Z. (Zhenbiao Zhang) and Z.Z. (Zhongqi Zhang) designed the experiments, performed the research and data analysis. Z.Z. (Zenglin Zhang) and M.S. drafted and revised the manuscript. Z.A., J.X. and J.W. and help with data analysis and figure organization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Fundamental Research Funds for the Chinese Academy of Agricultural Sciences (2013ZL024), the Agricultural Science and Technology Innovation Program (ASTIP-TRIC02), the National Natural Science Foundation of China (31970204), and the Technical system of coarse cereals industry in Shandong Province (SDAIT-15-02).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank Chao Qi at Institute of Crop Science of Chinese Academy of Agricultural Sciences (CAAS) for soybean Williams 82 seeds. Also, we are grateful to the current Guo lab members at the Tobacco Research Institute (TRI) from CAAS for the helpful comments on the revision of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Qi, X.; Li, M.; Xie, M.; Gao, Y.; Cheung, M.; Wong, F.; Lam, H. Improvement in nitrogen fixation capacity could be part of the domestication process in soybean. Heredity 2016, 117, 84–93. [Google Scholar] [CrossRef]
- De Borja Reis, A.F.; Moro Rosso, L.; Purcell, L.C.; Naeve, S.; Casteel, S.N.; Kovács, P.; Archontoulis, S.; Davidson, D.; Ciampitti, I.A. Environmental factors associated with nitrogen fixation prediction in soybean. Front. Plant Sci. 2021, 12, 27–39. [Google Scholar] [CrossRef] [PubMed]
- El Haddad, N.; En-Nahli, Y.; Choukri, H.; Aloui, K.; Mentag, R.; El-Baouchi, A.; Hejjaoui, K.; Rajendran, K.; Smouni, A.; Maalouf, F. Metabolic Mechanisms Underlying Heat and Drought Tolerance in Lentil Accessions: Implications for Stress Tolerance Breeding. Plants 2023, 12, 3962. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl– ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Isayenkov, S.V. Genetic sources for the development of salt tolerance in crops. Plant Growth Regul. 2019, 89, 1–17. [Google Scholar] [CrossRef]
- Tiwari, R.; Singh, A.K.; Rajam, M.V. GmPARPs differentially regulate the drought and heat stress tolerance in soybean. Plant Growth Regul. 2023, 101, 643–661. [Google Scholar] [CrossRef]
- Singh, A.K.; Raina, S.K.; Kumar, M.; Aher, L.; Ratnaparkhe, M.B.; Rane, J.; Kachroo, A. Modulation of GmFAD3 expression alters abiotic stress responses in soybean. Plant Mol. Biol. 2022, 110, 199–218. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H.; Fang, Y.; Guo, W.; Chen, H.; Zhang, X.; Dai, W.; Chen, S.; Hao, Q.; Yuan, S. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol. J. 2021, 19, 702–716. [Google Scholar] [CrossRef]
- Hao, Y.J.; Wei, W.; Song, Q.X.; Chen, H.W.; Zhang, Y.Q.; Wang, F.; Zou, H.F.; Lei, G.; Tian, A.G.; Zhang, W.K. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 2011, 68, 302–313. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.S.; Lyu, Z.; Schmutz, J.; Stacey, G.; Xu, D.; Joshi, T. SoyCSN: Soybean context-specific network analysis and prediction based on tissue-specific transcriptome data. Plant Direct 2019, 3, e00167. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, X.; Ren, H. New insights into the role of plant formins: Regulating the organization of the actin and microtubule cytoskeleton. Protoplasma 2012, 249, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Staiger, C.J. Signaling to the actin cytoskeleton in plants. Annu. Rev. Plant Biol. 2000, 51, 257–288. [Google Scholar] [CrossRef]
- Li, G.; Liang, W.; Zhang, X.; Ren, H.; Hu, J.; Bennett, M.J.; Zhang, D. Rice actin-binding protein RMD is a key link in the auxin–actin regulatory loop that controls cell growth. Proc. Natl. Acad. Sci. USA 2014, 111, 10377–10382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Tan, H.; Wang, Y.; Li, G.; Liang, W.; Yuan, Z.; Hu, J.; Ren, H.; Zhang, D. RICE MORPHOLOGY DETERMINANT encodes the type II formin FH5 and regulates rice morphogenesis. Plant Cell 2011, 23, 681–700. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Xie, M.; Liu, J.; Kong, Z.; Su, H. Actin bundles in the pollen tube. Int. J. Mol. Sci. 2018, 19, 3710. [Google Scholar] [CrossRef]
- Deeks, M.J.; Hussey, P.J.; Davies, B. Formins: Intermediates in signal-transduction cascades that affect cytoskeletal reorganization. Trends Plant Sci. 2002, 7, 492–498. [Google Scholar] [CrossRef]
- Cvrčková, F.; Novotný, M.; Pícková, D.; Žárský, V. Formin homology 2 domains occur in multiple contexts in angiosperms. BMC Genom. 2004, 5, 44. [Google Scholar] [CrossRef]
- Grunt, M.; Žárský, V.; Cvrčková, F. Roots of angiosperm formins: The evolutionary history of plant FH2 domain-containing proteins. BMC Evol. Biol. 2008, 8, 115. [Google Scholar] [CrossRef]
- Kovar, D.R. Molecular details of formin-mediated actin assembly. Curr. Opin. Cell Biol. 2006, 18, 11–17. [Google Scholar] [CrossRef]
- Rivero, F.; Muramoto, T.; Meyer, A.-K.; Urushihara, H.; Uyeda, T.Q.; Kitayama, C. A comparative sequence analysis reveals a common GBD/FH3-FH1-FH2-DAD architecture in formins from Dictyostelium, fungi and metazoa. BMC Genom. 2005, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Pruyne, D. Probing the origins of metazoan formin diversity: Evidence for evolutionary relationships between metazoan and non-metazoan formin subtypes. PLoS ONE 2017, 12, e0186081. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Wu, H.-M. Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell 2004, 16, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Niroomand, S.; Zou, Y.; Wu, H.-M. A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc. Natl. Acad. Sci. USA 2010, 107, 16390–16395. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zheng, Y.; Yan, A.; Chen, N.; Wang, Z.; Huang, S.; Yang, Z. Arabidopsis formin3 directs the formation of actin cables and polarized growth in pollen tubes. Plant Cell 2009, 21, 3868–3884. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Cai, C.; Zhong, C.; Zhu, L.; Yuan, M.; Ren, H. The type II Arabidopsis formin14 interacts with microtubules and microfilaments to regulate cell division. Plant Cell 2010, 22, 2710–2726. [Google Scholar] [CrossRef]
- Kollarova, E.; Baquero Forero, A.; Cvrčková, F. The Arabidopsis thaliana class II formin FH13 modulates pollen tube growth. Front. Plant Sci. 2021, 12, 599961. [Google Scholar] [CrossRef]
- Li, G.; Yang, X.; Zhang, X.; Song, Y.; Liang, W.; Zhang, D. Rice morphology determinant-mediated actin filament organization contributes to pollen tube growth. Plant Physiol. 2018, 177, 255–270. [Google Scholar] [CrossRef]
- Hussey, P.J.; Ketelaar, T.; Deeks, M.J. Control of the actin cytoskeleton in plant cell growth. Annu. Rev. Plant Biol. 2006, 57, 109–125. [Google Scholar] [CrossRef]
- Duan, W.-J.; Liu, Z.-H.; Bai, J.-F.; Yuan, S.-H.; Li, Y.-M.; Lu, F.-K.; Zhang, T.-B.; Sun, J.-H.; Zhang, F.-T.; Zhao, C.-P. Comprehensive analysis of formin gene family highlights candidate genes related to pollen cytoskeleton and male fertility in wheat (Triticum aestivum L.). BMC Genom. 2021, 22, 570. [Google Scholar] [CrossRef]
- Oulehlová, D.; Kollárova, E.; Cifrová, P.; Pejchar, P.; Žársky, V.; Cvrčková, F. Arabidopsis class I formin FH1 relocates between membrane compartments during root cell ontogeny and associates with plasmodesmata. Plant Cell Physiol. 2019, 60, 1855–1870. [Google Scholar] [CrossRef]
- Cvrčková, F. Formins and membranes: Anchoring cortical actin to the cell wall and beyond. Front. Plant Sci. 2013, 4, 436. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Ren, S.; Wang, Q.; Qian, L.; Shen, J.; Liu, Y.; Huang, S. Arabidopsis formin 2 regulates cell-to-cell trafficking by capping and stabilizing actin filaments at plasmodesmata. eLife 2018, 7, e36316. [Google Scholar] [CrossRef]
- Van Gisbergen, P.A.; Bezanilla, M. Plant formins: Membrane anchors for actin polymerization. Trends Cell Biol. 2013, 23, 227–233. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef]
- Xue, X.-H.; Guo, C.-Q.; Du, F.; Lu, Q.-L.; Zhang, C.-M.; Ren, H.-Y. AtFH8 is involved in root development under effect of low-dose latrunculin B in dividing cells. Mol. Plant 2011, 4, 264–278. [Google Scholar] [CrossRef]
- Sun, T.; Li, S.; Ren, H. OsFH15, a class I formin, interacts with microfilaments and microtubules to regulate grain size via affecting cell expansion in rice. Sci. Rep. 2017, 7, 6538. [Google Scholar] [CrossRef]
- Vidali, L.; van Gisbergen, P.A.; Guérin, C.; Franco, P.; Li, M.; Burkart, G.M.; Augustine, R.C.; Blanchoin, L.; Bezanilla, M. Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc. Natl. Acad. Sci. USA 2009, 106, 13341–13346. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, 200–204. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Li, W.; Cheng, H.; Zhao, W.; Li, T.; Sun, B.; You, Q.; Zhou, D. Genome-Wide Analysis Revealed NBS-LRR Gene Candidates Associated with Bacterial Wilt Resistance in Eggplant (Solanum melongena L.). Agronomy 2023, 13, 2583. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Cell-PLoc: A package of Web servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Zhang, Z. KaKs_calculator 3.0: Calculating selective pressure on coding and non-coding sequences. Genom. Proteom. Bioinform. 2022, 20, 536–540. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, C.; Pei, X.; Wang, S.; Huang, Y.; Li, J.; Liu, B.; Kong, F.; Yang, Q.-Y.; Fang, C. SoyMD: A platform combining multi-omics data with various tools for soybean research and breeding. Nucleic Acids Res. 2024, 52, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).