Nitrogen Deficiency Enhances Eggplant Defense against Western Flower Thrips via the Induction of the Jasmonate Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. WFT Treatment
2.3. RNA Sequencing
2.4. Quantitative Real-Time PCR Analysis
2.5. Plant Hormone Analysis
2.6. MeJA Treatment
2.7. Determination of Polyphenol Oxidase and Peroxidase Activities
2.8. Data Analysis
3. Results
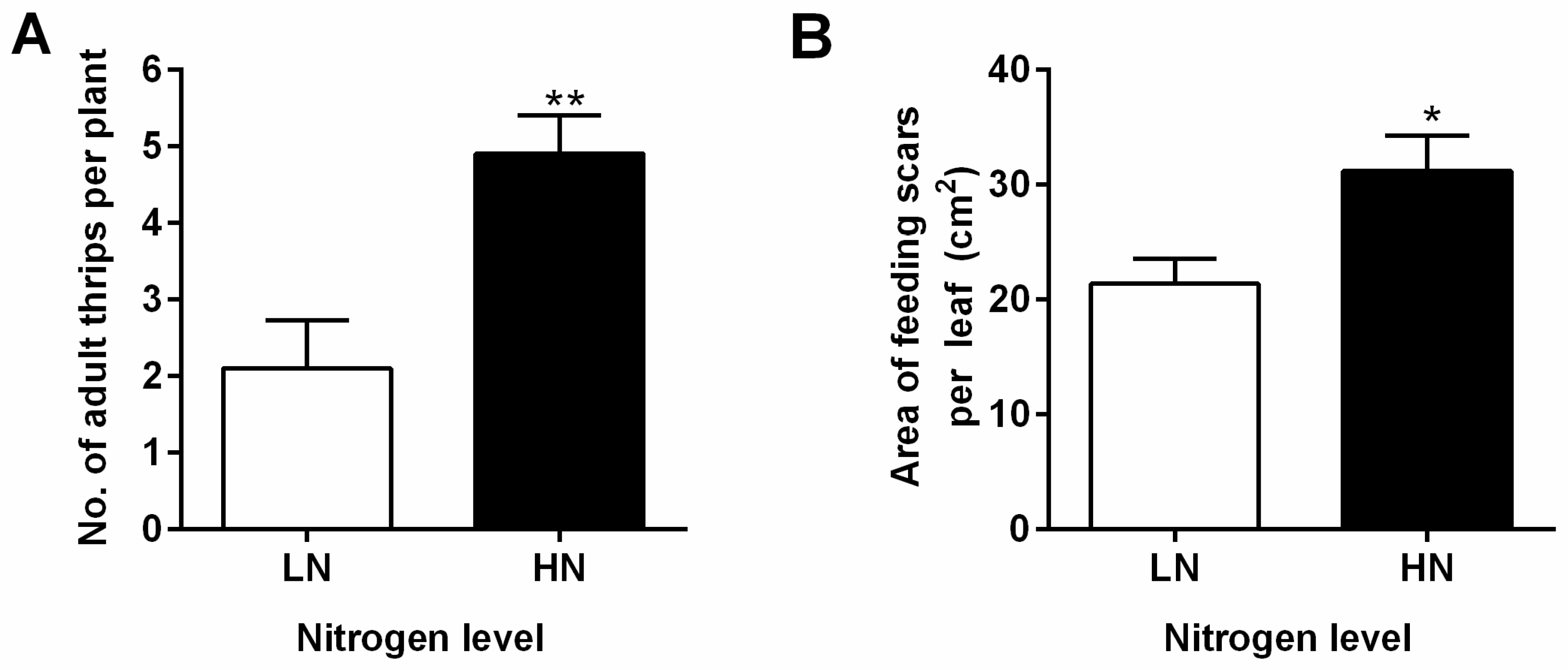
3.1. Low Nitrate Augments Eggplant Anti-Herbivore Defense
3.2. Effect of Nitrate Levels on the Eggplant Phytohormone Response to WFT Infestation
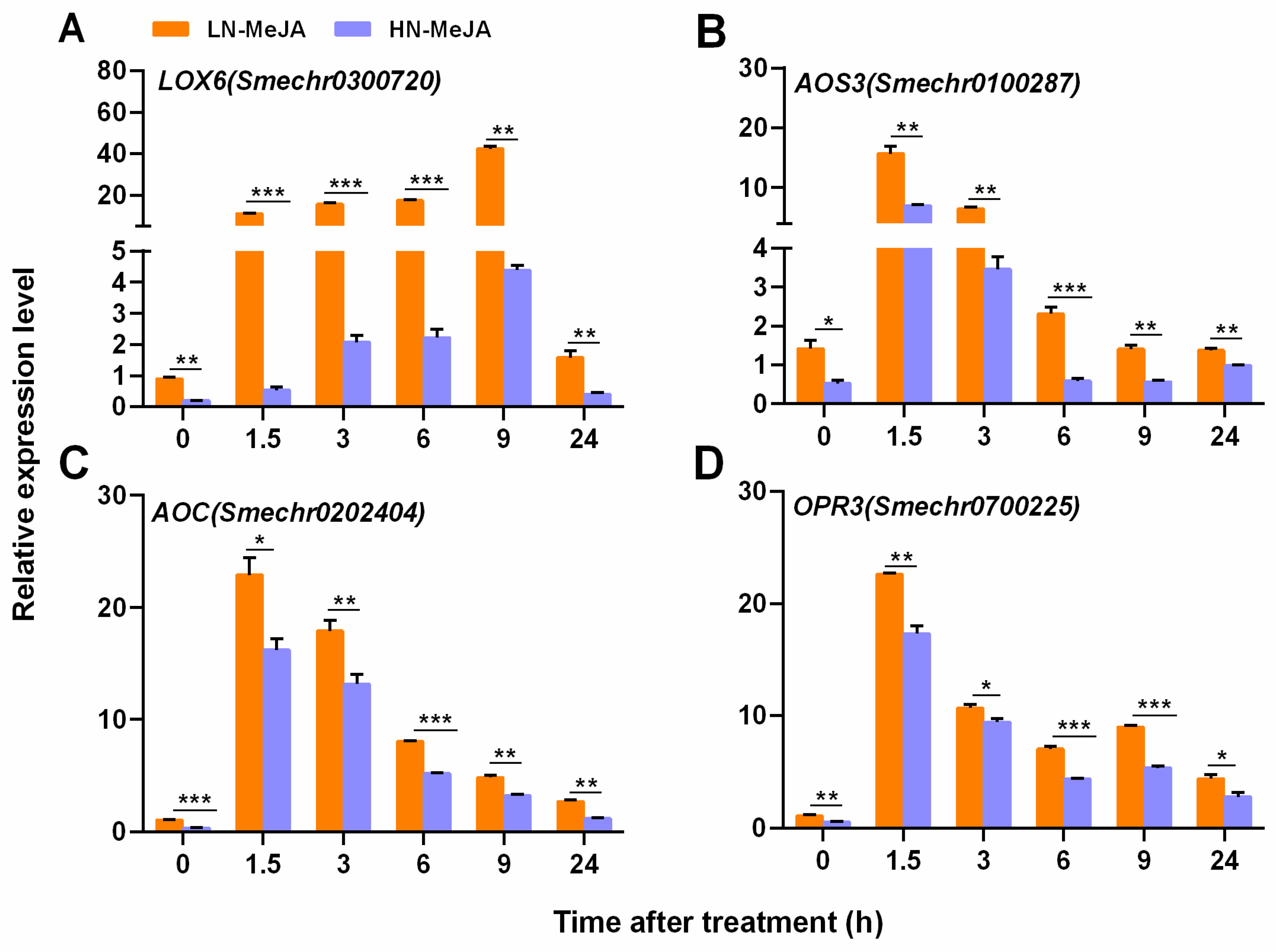
3.3. Effect of Nitrate Supply on JA Biosynthesis Pathway Genes under Exogenous MeJA Application
3.4. Low Nitrate-Mediated Enhancement of MAPK and WRKY Transcriptional Responses
3.5. Low Nitrate-Mediated Enhancement of Defense-Related Enzyme Induction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Z.X.; Yu, X.P.; Heong, K.L.; Cui, H.U. Effect of nitrogen fertilizer on herbivores and its stimulation to major insect pests in rice. Rice Sci. 2007, 14, 56–66. [Google Scholar] [CrossRef]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty years from transport to signaling networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Vega, A.; O’Brien, J.A.; Gutiérrez, R.A. Nitrate and hormonal signaling crosstalk for plant growth and development. Curr. Opin. Plant Biol. 2019, 52, 155–163. [Google Scholar] [CrossRef]
- Comadira, G.; Rasool, B.; Karpinska, B.; Morris, J.; Verrall, S.R.; Hedley, P.E.; Foyer, C.H.; Hancock, R.D. Nitrogen deficiency in barley (Hordeum vulgare) seedlings induces molecular and metabolic adjustments that trigger aphid resistance. J. Exp. Bot. 2015, 66, 3639–3655. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Canessa, P.; Hoppe, G.; Retamal, I.; Moyano, T.C.; Canales, J.; Gutiérrez, R.A.; Rubilar, J. Transcriptome analysis reveals regulatory networks underlying differential susceptibility to Botrytis cinerea in response to nitrogen availability in Solanum lycopersicum. Front. Plant Sci. 2015, 6, 911. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, X.; Liu, X.; Qin, N.; Xu, K.; Zeng, R.; Liu, J.; Song, Y. Nitrogen supply alters rice defenses against the striped stem borer Chilo suppressalis. Front. Plant Sci. 2021, 12, 691292. [Google Scholar] [CrossRef]
- Mattson, W.J. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Evol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Prudic, K.L.; Oliver, J.C.; Bowers, M.D. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 2005, 143, 578–587. [Google Scholar] [CrossRef]
- Lu, Z.X.; Heong, K.L.; Yu, X.P.; Hu, C. Effects of nitrogen on the tolerance of brown planthopper, Nilaparvata Lugens, to adverse environmental factors. Insect Sci. 2005, 12, 121–128. [Google Scholar] [CrossRef]
- Larbat, R.; Adamowicz, S.; Robin, C.; Han, P.; Desneux, N.; Le Bot, J. Interrelated responses of tomato plants and the leaf miner Tuta absoluta to nitrogen supply. Plant Biol. 2016, 18, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.F.; Traw, M.B.; Bergelson, J. On testing for a tradeoff between constitutive and induced resistance. Oikos 2006, 112, 102–110. [Google Scholar] [CrossRef]
- Zhang, P.J.; Shu, J.P.; Fu, C.X.; Zhou, Y.; Hu, Y.; Zalucki, M.P.; Liu, S.S. Trade-offs between constitutive and induced resistance in wild crucifers shown by a natural, but not an artificial, elicitor. Oecologia 2008, 157, 83–92. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Ohnishi, J.; Narusaka, M.; Seo, S.; Narusaka, Y.; Tsuda, S.; Kobayashi, M. Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol. 2008, 49, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Song, Y.Y.; Baerson, S.R.; Long, J.; Wang, J.; Pan, Z.; Lin, W.X.; Zeng, R.S. Ratoon rice generated from primed parent plants exhibit enhanced herbivore resistance. Plant Cell Environ. 2017, 40, 779–787. [Google Scholar] [CrossRef]
- Steenbergen, M.; Abd-el-Haliem, A.; Bleeker, P.; Dicke, M.; Escobar-Bravo, R.; Cheng, G.; Haring, M.A.; Kant, M.R.; Kappers, I.; Klinkhamer, P.G.; et al. Thrips advisor: Exploiting thrips-induced defences to combat pests on crops. J. Exp. Bot. 2018, 69, 1837–1848. [Google Scholar] [CrossRef]
- Mouden, S.; Leiss, K.A. Host plant resistance to thrips (Thysanoptera: Thripidae)—Current state of art and future research avenues. Curr. Opin. Insect Sci. 2021, 45, 28–34. [Google Scholar] [CrossRef]
- Du, B.; Zhang, W.; Liu, B.; Hu, J.; Wei, Z.; Shi, Z.; He, R.; Zhu, L.; Chen, R.; Han, B.; et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lou, Y.R.; Tzin, V.; Jander, G. Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S.; et al. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Kalloo, G. Eggplant: Solanum melongena L. In Genetic Improvement of Vegetable Crops; Kallo, G., Bergh, B.O., Eds.; Pergamon Press: Oxford, UK, 1993; pp. 587–604. [Google Scholar]
- Mauceri, A.; Bassolino, L.; Lupini, A.; Badeck, F.; Rizza, F.; Schiavi, M.; Toppino, L.; Abenavoli, M.R.; Rotino, G.L.; Sunseri, F. Genetic variation in eggplant for nitrogen use efficiency under contrasting NO3- supply. J. Integr. Plant Biol. 2020, 62, 487–508. [Google Scholar] [CrossRef]
- Yadav, R.; Chang, N.T. Effects of temperature on the development and population growth of the melon thrips, Thrips palmi, on eggplant, Solanum melongena. J. Insect Sci. 2014, 14, 78. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Luo, S.; Ma, L.; Zhang, W.; Xuan, S.; Wang, Y.; Zhao, J.; Shen, S.; Ma, W.; et al. Metabolomic and transcriptomic analyses identify quinic acid protecting eggplant from damage caused by western flower thrips. Pest Manag. Sci. 2022, 78, 5113–5123. [Google Scholar] [CrossRef]
- Wu, S.; Tang, L.; Zhang, X.; Xing, Z.; Lei, Z.; Gao, Y. A decade of a thrips invasion in China: Lessons learned. Ecotoxicology 2018, 27, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Maris, P.C.; Joosten, N.N.; Peters, D.; Goldbach, R.W. Thrips resistance in pepper and its consequences for the acquisition and inoculation of tomato spotted wilt virus by the western flower thrips. Phytopathology 2003, 93, 96–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarde, S.J.; Bouwmeester, K.; Venegas-Molina, J.; David, A.; Boland, W.; Dicke, M. Involvement of sweet pepper CaLOX2 in jasmonate-dependent induced defence against western flower thrips. J. Integr. Plant Biol. 2019, 61, 1085–1098. [Google Scholar] [CrossRef]
- Chen, G.; Kim, H.K.; Klinkhamer, P.G.; Escobar-Bravo, R. Site-dependent induction of jasmonic acid-associated chemical defenses against western flower thrips in Chrysanthemum. Planta 2020, 251, 8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, Y.; Zhao, L.; Chen, Y.; Zheng, L.; Zheng, K.; Mu, Y.; Zhao, X.; Gao, Y.; Zhang, J. Tripartite interactions between jasmonic/salicylic acid pathways, western flower thrips, and thrips-transmitted tomato zonate spot virus infection in Capsicuum annuum. Arthropod Plant Interact. 2019, 13, 289–297. [Google Scholar] [CrossRef]
- Mauceri, A.; Abenavoli, M.R.; Toppino, L.; Panda, S.; Mercati, F.; Aci, M.M.; Aharoni, A.; Sunseri, F.; Rotino, G.L.; Lupini, A. Transcriptomics reveal new insights into molecular regulation of nitrogen use efficiency in Solanum melongena. J. Exp. Bot. 2021, 72, 4237–4253. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Lu, C.; Tian, H.; Lin, S.; Wang, L.; Linghu, T.; Zheng, X.; Wei, H.; Fan, X.; et al. Chemosensory protein regulates the behavioural response of Frankliniella intonsa and Frankliniella occidentalis to tomato zonate spot virus-infected pepper (Capsicum annuum). PLoS Pathog. 2023, 19, e1011380. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, J.; Wang, W.; Hu, T.; Hu, H.; Bao, C. A high-quality chromosome-level genome assembly reveals genetics for important traits in eggplant. Hortic. Res. 2020, 7, 153. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, C.; Yan, X.; Zhang, J.; Xu, J. Simultaneous analysis of ten phytohormones in Sargassum horneri by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Floková, K.; Tarkowská, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novák, O. UHPLC-MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, Y.Q.; Song, W.M.; Chen, D.Y.; Chen, F.Y.; Chen, X.Y.; Chen, Z.W.; Ge, S.X.; Wang, C.Z.; Zhan, S.; et al. An effector from cotton bollworm oral secretion impairs host plant defense signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 14331–14338. [Google Scholar] [CrossRef] [PubMed]
- Zauberman, G.; Ronen, R.; Akerman, M.; Weksler, A.; Rot, I.; Fuchs, Y. Post-harvest retention of the red colour of litchi fruit pericarp. Sci. Hortic. 1991, 47, 89–97. [Google Scholar] [CrossRef]
- Kraus, T.E.; Fletcher, R.A. Paclobutrazol protects wheat seedlings from heat and paraquat injury. Is detoxification of active oxygen involved? Plant Cell Physiol. 1994, 35, 45–52. [Google Scholar]
- Rodriguez, M.C.S.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar]
- Jia, Z.; Giehl, R.F.H.; von Wirén, N. Nutrient-hormone relations: Driving root plasticity in plants. Mol. Plant. 2022, 15, 86–103. [Google Scholar] [CrossRef]
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutiérrez, R.A. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482. [Google Scholar] [CrossRef]
- Parker, J.L.; Newstead, S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 2014, 507, 68–72. [Google Scholar] [CrossRef]
- Landrein, B.; Formosa-Jordan, P.; Malivert, A.; Schuster, C.; Melnyk, C.W.; Yang, W.; Turnbull, C.; Meyerowitz, E.M.; Locke, J.C.; Jönsson, H. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc. Natl. Acad. Sci. USA 2018, 115, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zha, M.; Li, Y.; Ding, Y.; Chen, L.; Ding, C.; Wang, S. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant Cell Rep. 2015, 34, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Trivellini, A.; Fatma, M.; Masood, A.; Francini, A.; Iqbal, N.; Ferrante, A.; Khan, N.A. Role of ethylene in responses of plants to nitrogen availability. Front. Plant Sci. 2015, 6, 927. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Hanada, A.; Chiba, Y.; Ichikawa, T.; Nakazawa, M.; Matsui, M.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc. Natl. Acad. Sci. USA 2012, 109, 9653–9658. [Google Scholar] [CrossRef]
- Ondzighi-Assoume, C.A.; Chakraborty, S.; Harris, J.M. Environmental nitrate stimulates abscisic acid accumulation in Arabidopsis root tips by releasing it from inactive stores. Plant Cell 2016, 28, 729–745. [Google Scholar] [CrossRef]
- Tal, I.; Zhang, Y.; Jørgensen, M.E.; Pisanty, O.; Barbosa, I.C.; Zourelidou, M.; Regnault, T.; Crocoll, C.; Erik Olsen, C.; Weinstain, R.; et al. The Arabidopsis NPF3 protein is a GA transporter. Nat. Commun. 2016, 7, 11486. [Google Scholar] [CrossRef]
- Gupta, K.J.; Brotman, Y.; Segu, S.; Zeier, T.; Zeier, J.; Persijn, S.T.; Cristescu, S.M.; Harren, F.J.; Bauwe, H.; Fernie, A.R.; et al. The form of nitrogen nutrition affects resistance against Pseudomonas syringae pv. phaseolicola in tobacco. J. Exp. Bot. 2013, 64, 553–568. [Google Scholar] [CrossRef]
- Zhang, G.B.; Yi, H.Y.; Gong, J.M. The Arabidopsis ethylene/jasmonic acid-NRT signaling module coordinates nitrate reallocation and the trade-off between growth and environmental adaptation. Plant Cell 2014, 26, 3984–3998. [Google Scholar] [CrossRef]
- Chiba, Y.; Shimizu, T.; Miyakawa, S.; Kanno, Y.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of Arabidopsis thaliana NRT1/PTR FAMILY(NPF) proteins capable of transporting plant hormones. J. Plant Res. 2015, 128, 679–686. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef]
- Wu, X.; Ding, C.; Baerson, S.R.; Lian, F.; Lin, X.; Zhang, L.; Wu, C.; Hwang, S.Y.; Zeng, R.; Song, Y. The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L.). Plant Cell Environ. 2019, 42, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, F.; Cuyas, L.; Jouhet, J.; Gros, V.; Chiarenza, S.; Secco, D.; Whelan, J.; Seddiki, K.; Block, M.A.; Nussaume, L.; et al. Interplay between jasmonic acid, phosphate signaling and the regulation of glycerolipid homeostasis in Arabidopsis. Plant Cell Physiol. 2019, 60, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Du, J.; Han, X.; Li, H.; Kui, M.; Zhang, J.; Huang, Z.; Fu, Q.; Jiang, Y.; Hu, Y. PHOSPHATE STARVATION RESPONSE1 (PHR1) interacts with JASMONATE ZIM-DOMAIN (JAZ) and MYC2 to modulate phosphate deficiency-induced jasmonate signaling in Arabidopsis. Plant Cell 2023, 35, 2132–2156. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.A.; Vogiatzaki, E.; Glauser, G.; Poirier, Y. Phosphate deficiency induces the jasmonate pathway and enhances resistance to insect herbivory. Plant Physiol. 2016, 171, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Val-Torregrosa, B.; Bundó, M.; Martín-Cardoso, H.; Bach-Pages, M.; Chiou, T.J.; Flors, V.; Segundo, B.S. Phosphate-induced resistance to pathogen infection in Arabidopsis. Plant J. 2022, 110, 452–469. [Google Scholar] [CrossRef]
- Shikha, D.; Jakhar, P.; Satbhai, S.B. Role of jasmonate signaling in the regulation of plant responses to nutrient deficiency. J. Exp. Bot. 2023, 74, 1221–1243. [Google Scholar] [CrossRef]
- Armengaud, P.; Breitling, R.; Amtmann, A. Coronatine-insensitive 1 (COI1) mediates transcriptional responses of Arabidopsis thaliana to external potassium supply. Mol. Plant 2010, 3, 390–405. [Google Scholar] [CrossRef]
- Bala, K.; Sood, A.K.; Pathania, V.S.; Thakur, S. Effect of plant nutrition in insect pest management: A review. J. Pharmacogn. Phytochem. 2018, 7, 2737–2742. [Google Scholar]
- de Lange, E.S.; Kyryczenko-Roth, V.; Johnson-Cicalese, J.; Davenport, J.; Vorsa, N.; Rodriguez-Saona, C. Increased nutrient availability decreases insect resistance in cranberry. Agric. For. Entomol. 2019, 21, 326–335. [Google Scholar] [CrossRef]
- Huang, W.Q.; Zeng, G.; Zhi, J.R.; Qiu, X.Y.; Yin, Z.J. Exogenous calcium suppresses the oviposition choices of Frankliniella occidentalis (Thysanoptera: Thripidae) and promotes the attraction of Orius similis (Hemiptera: Anthocoridae) by altering volatile blend emissions in kidney bean plants. Insects 2022, 13, 1127. [Google Scholar] [CrossRef]
- Abe, H.; Tomitaka, Y.; Shimoda, T.; Seo, S.; Sakurai, T.; Kugimiya, S.; Tsuda, S.; Kobayashi, M. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 2012, 53, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, J.; Xing, D. Low concentrations of salicylic acid delay methyl jasmonate-induced leaf senescence by up-regulating nitric oxide synthase activity. J. Exp. Bot. 2016, 67, 5233–5245. [Google Scholar] [CrossRef]
- Ndamukong, I.; Abdallat, A.A.; Thurow, C.; Fode, B.; Zander, M.; Weigel, R.; Gatz, C. SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J. 2007, 50, 128–139. [Google Scholar] [CrossRef]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef]
- Beckers, G.J.; Jaskiewicz, M.; Liu, Y.; Underwood, W.R.; He, S.Y.; Zhang, S.; Conrath, U. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 2009, 21, 944–953. [Google Scholar] [CrossRef]
- O’Shaughnessy, E.C.; Palani, S.; Collins, J.J.; Sarkar, C.A. Tunable signal processing in synthetic MAP kinase cascades. Cell 2011, 144, 119–131. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Eulgem, T. Regulation of the Arabidopsis defense transcriptome. Trends Plant Sci. 2005, 10, 71–78. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Hastings, A.P.; Johnson, M.T.; Maron, J.L.; Salminen, J.P. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 2012, 338, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, M.; Conrath, U.; Peterhansel, C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011, 12, 50–55. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Liu, Q.; Shi, S.; Zhu, X.; Chen, Y.; Lin, S.; Tian, H.; Huang, L.; Wei, H. Nitrogen Deficiency Enhances Eggplant Defense against Western Flower Thrips via the Induction of the Jasmonate Pathway. Plants 2024, 13, 273. https://doi.org/10.3390/plants13020273
Zheng Y, Liu Q, Shi S, Zhu X, Chen Y, Lin S, Tian H, Huang L, Wei H. Nitrogen Deficiency Enhances Eggplant Defense against Western Flower Thrips via the Induction of the Jasmonate Pathway. Plants. 2024; 13(2):273. https://doi.org/10.3390/plants13020273
Chicago/Turabian StyleZheng, Yueqin, Qianxia Liu, Shuang Shi, Xiaowen Zhu, Yong Chen, Shuo Lin, Houjun Tian, Lanyan Huang, and Hui Wei. 2024. "Nitrogen Deficiency Enhances Eggplant Defense against Western Flower Thrips via the Induction of the Jasmonate Pathway" Plants 13, no. 2: 273. https://doi.org/10.3390/plants13020273
APA StyleZheng, Y., Liu, Q., Shi, S., Zhu, X., Chen, Y., Lin, S., Tian, H., Huang, L., & Wei, H. (2024). Nitrogen Deficiency Enhances Eggplant Defense against Western Flower Thrips via the Induction of the Jasmonate Pathway. Plants, 13(2), 273. https://doi.org/10.3390/plants13020273





