Water-Retaining Polymer and Planting Pit Size on Chlorophyll Index, Gas Exchange and Yield of Sour Passion Fruit with Deficit Irrigation
Abstract
1. Introduction
2. Materials and Methods
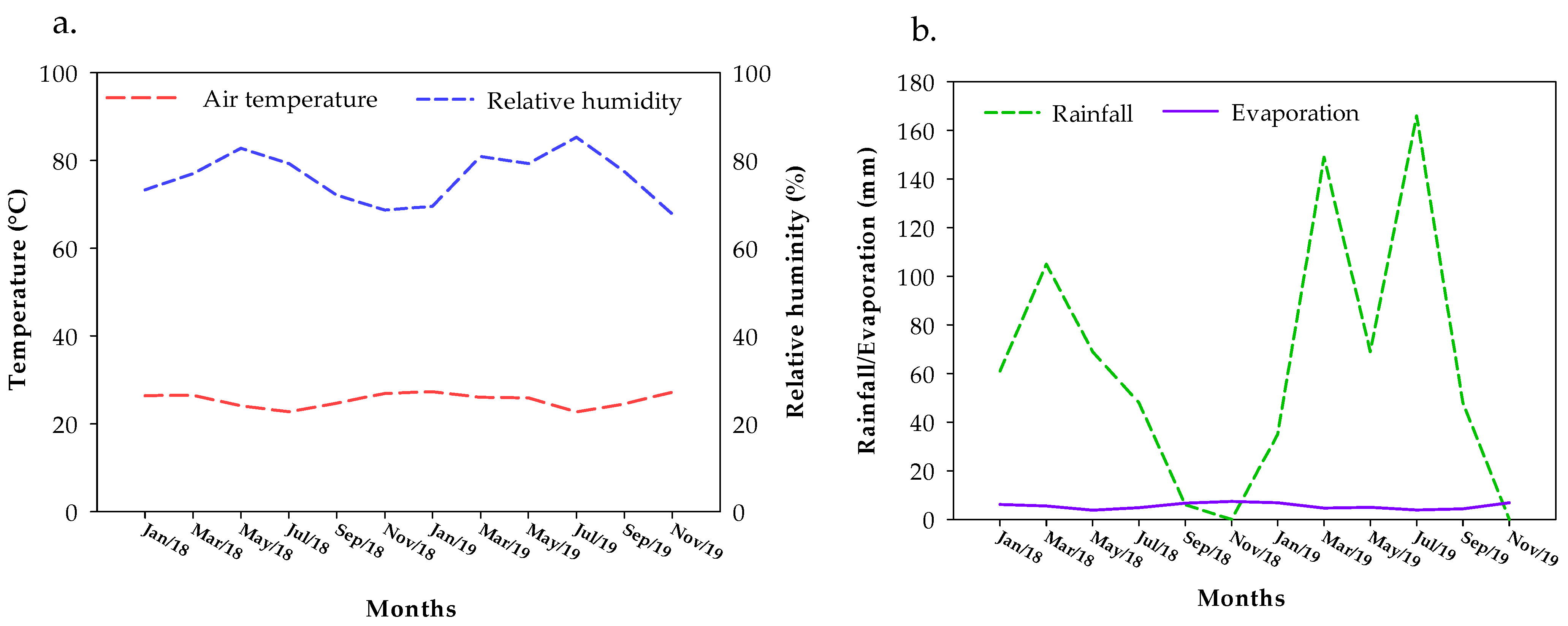
2.1. Characterization of the Experimental Area
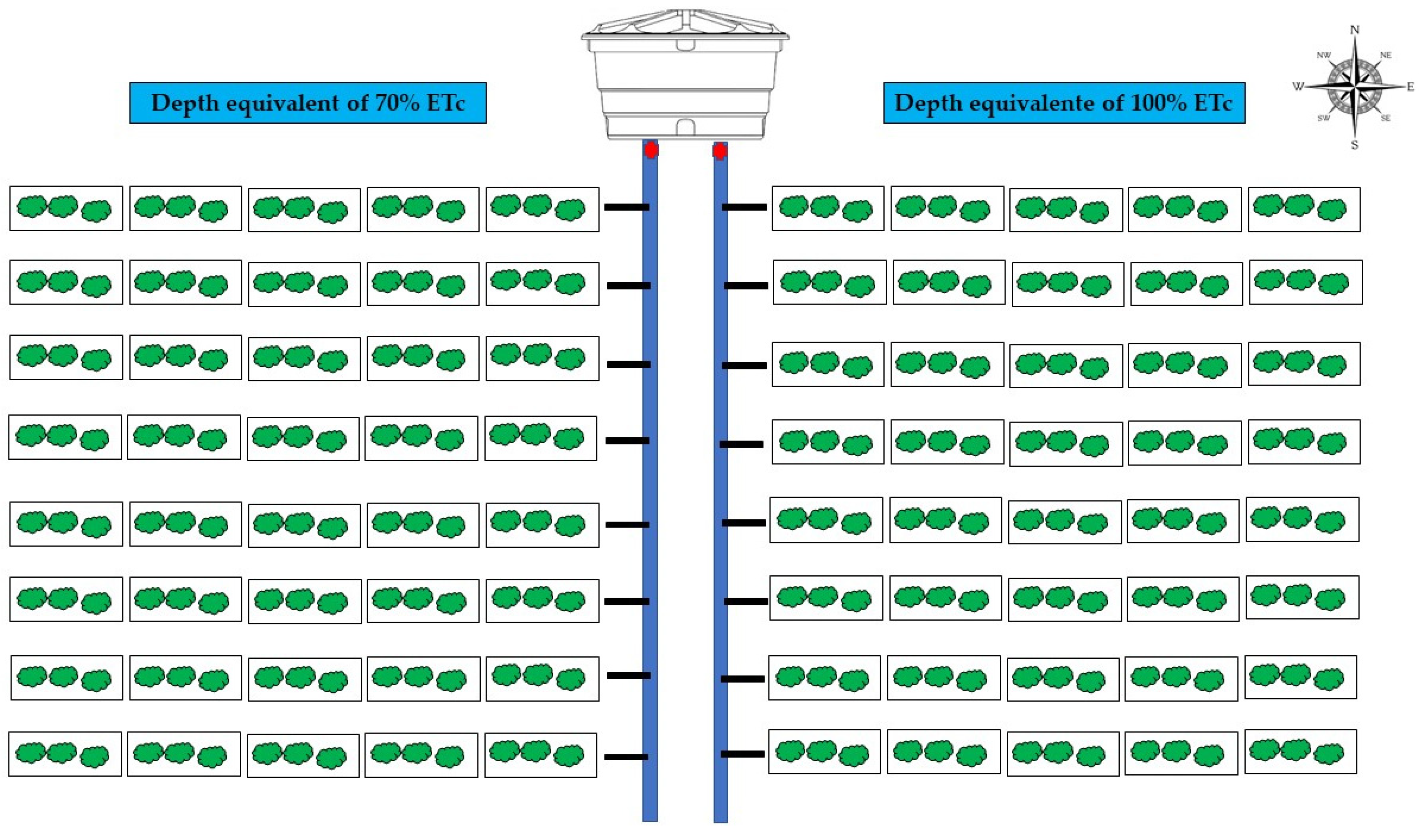
2.2. Experimental Design and Plant Material
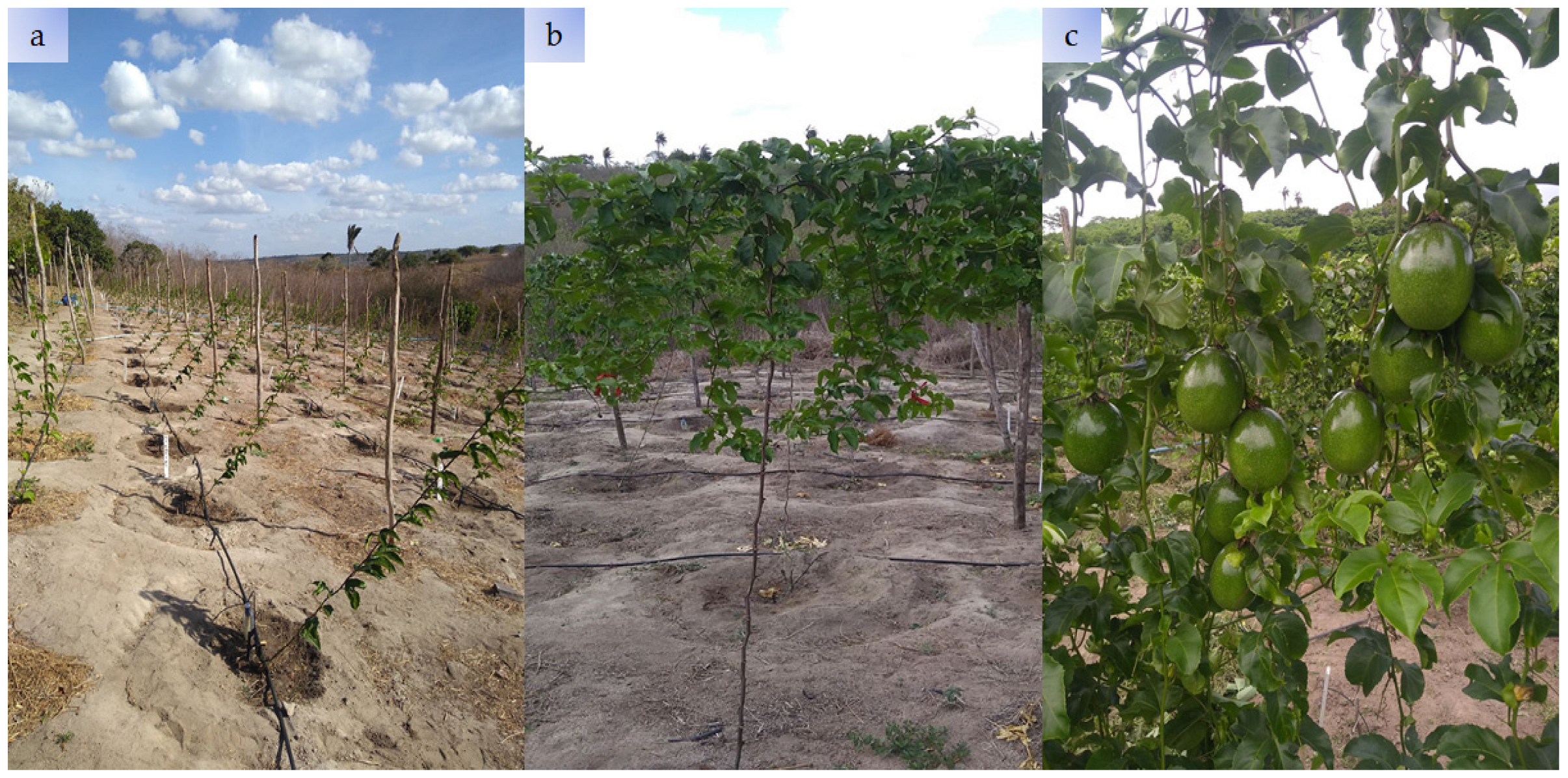
2.3. Conducting the Experiment
2.4. Traits Analyzed
2.5. Statistical Analysis
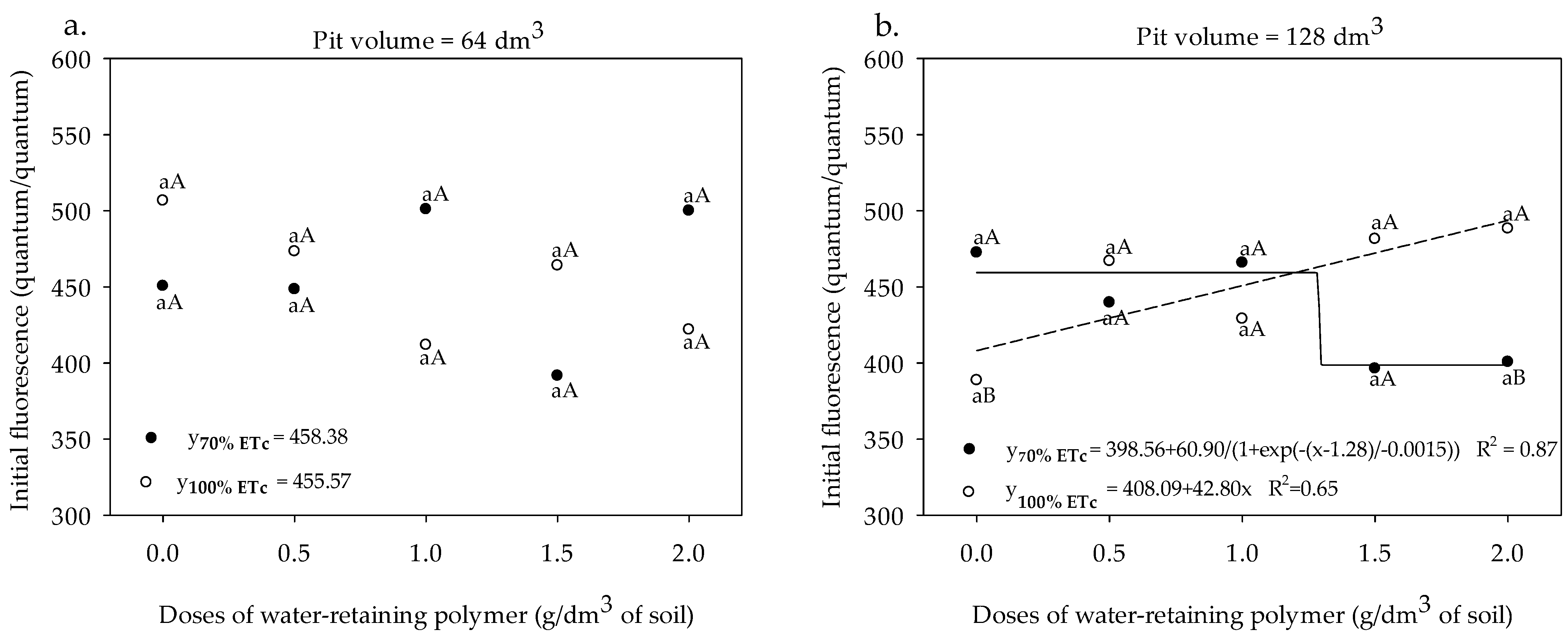
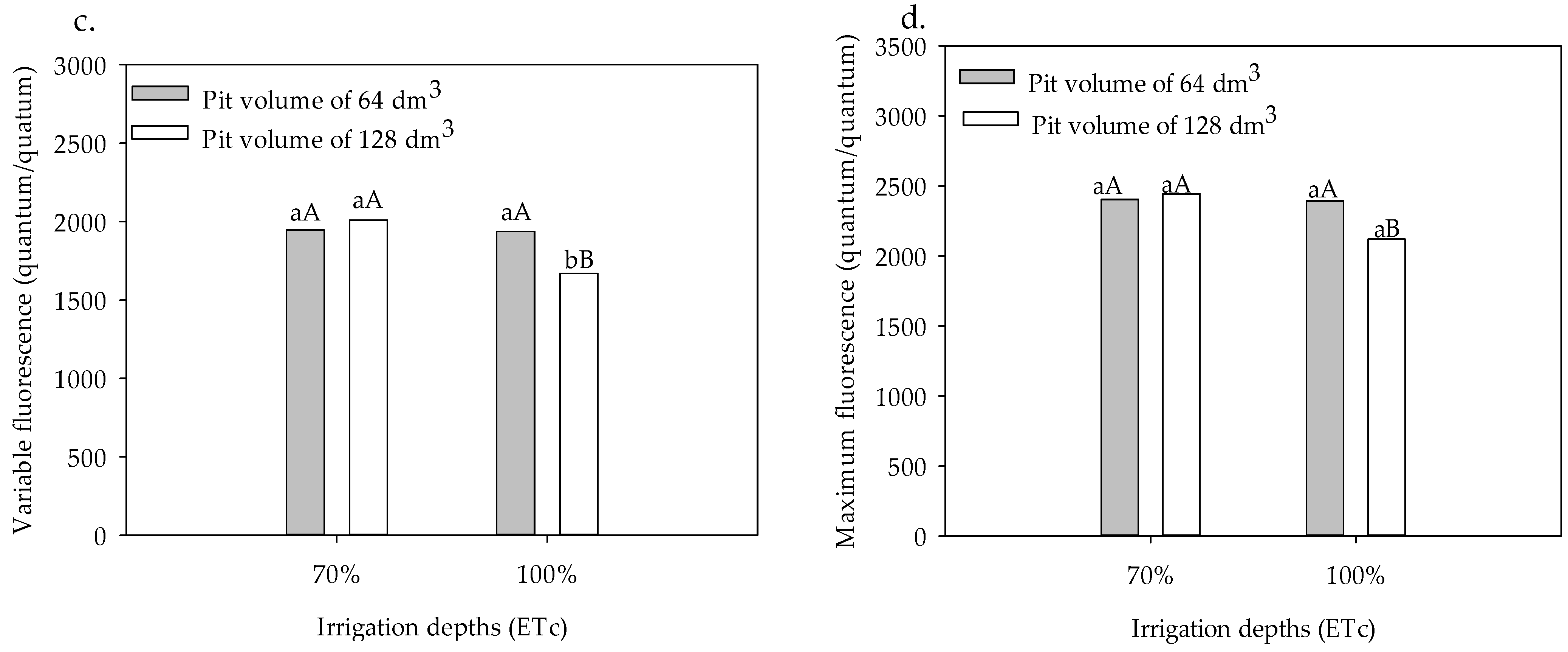
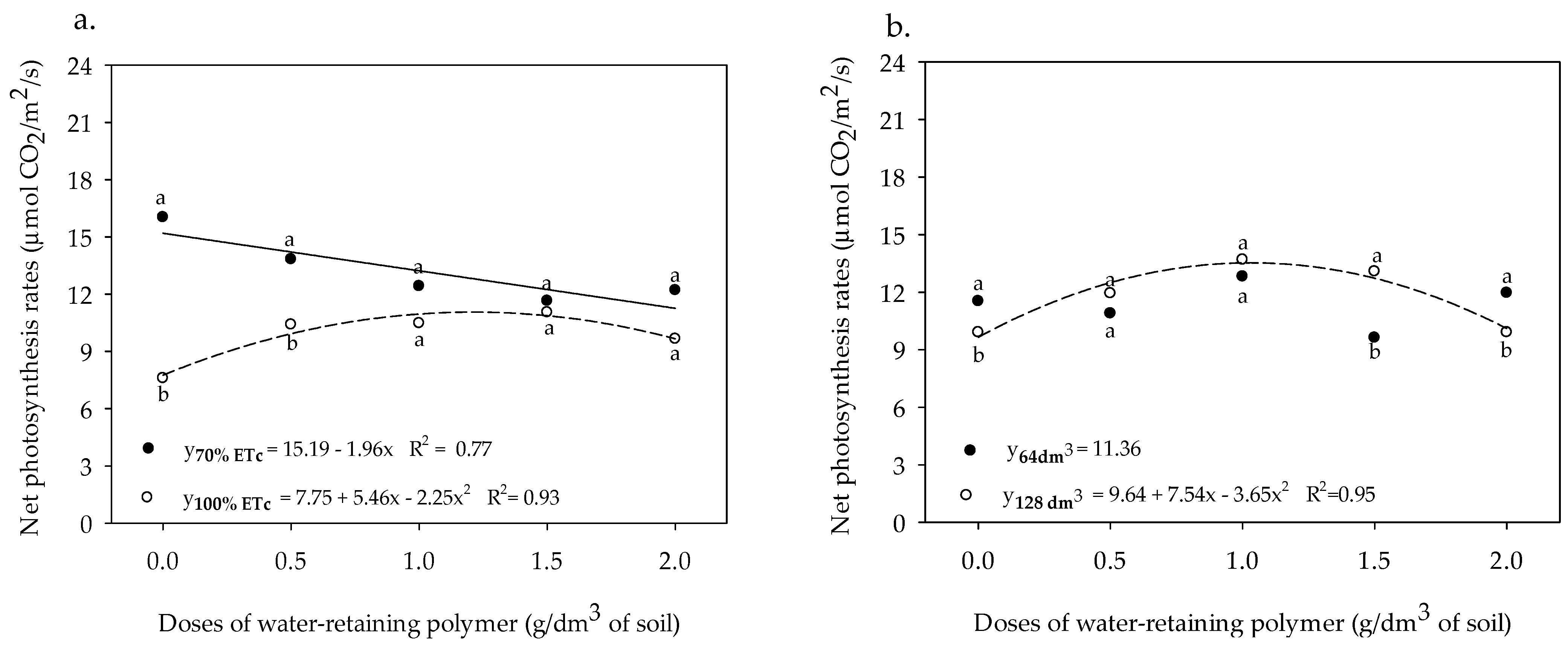
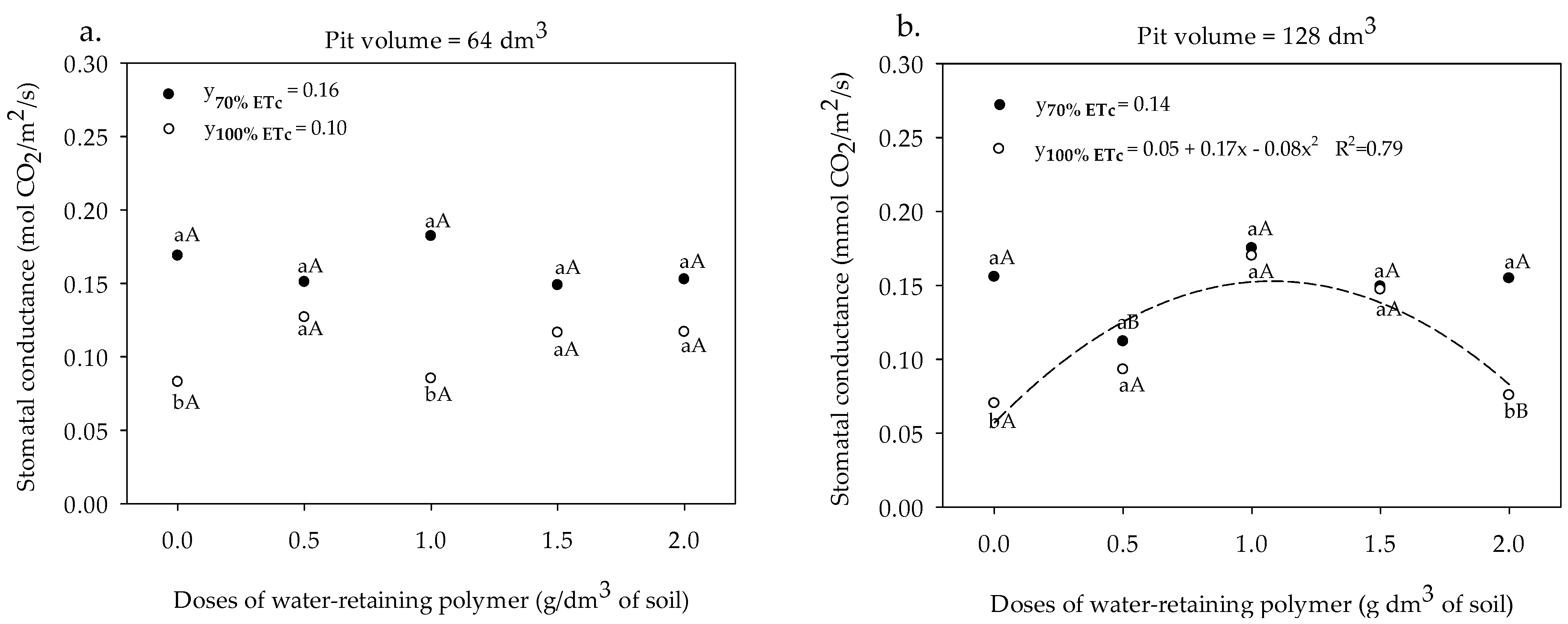
3. Results
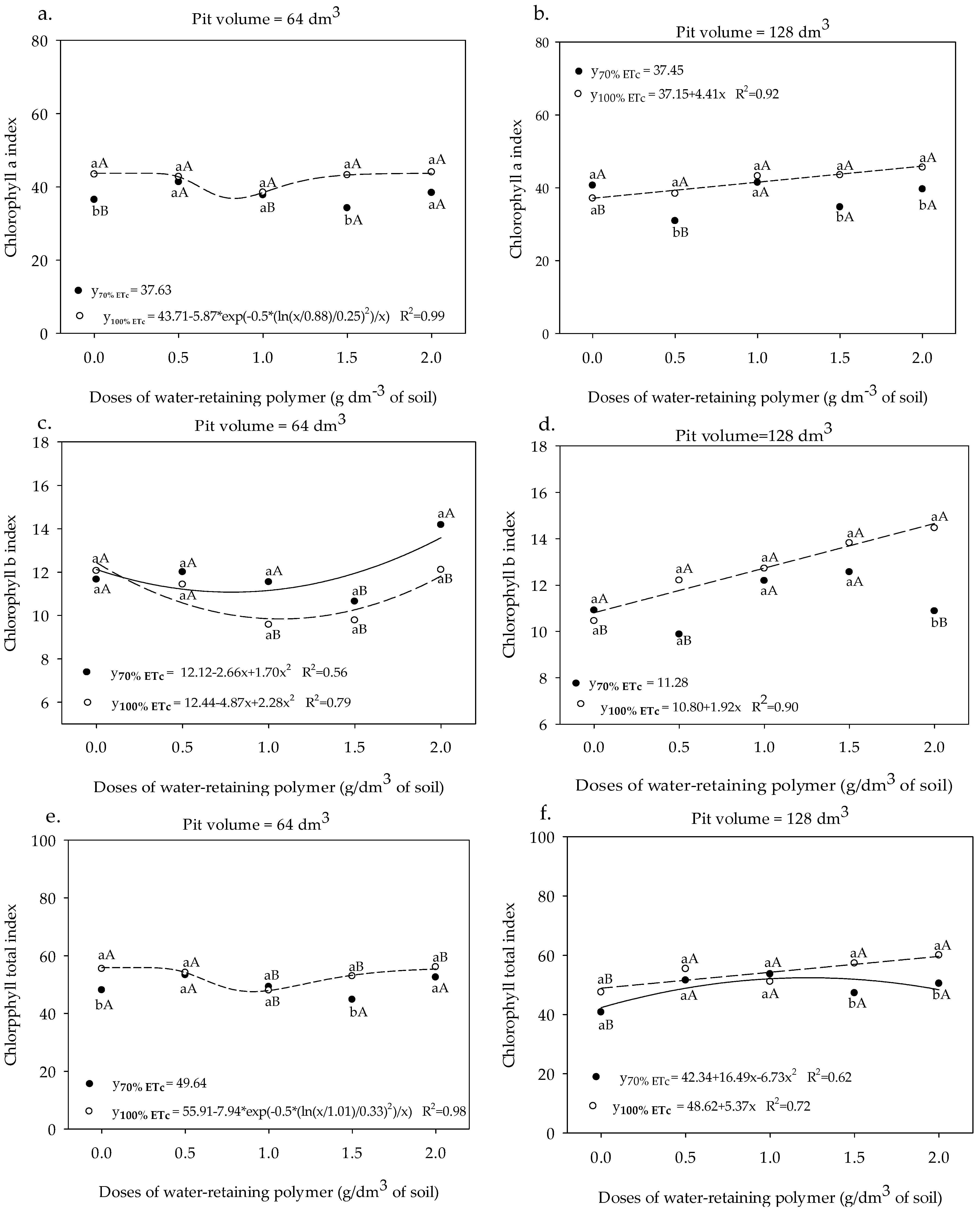
3.1. Chlorophyll Indices and Fluorescence
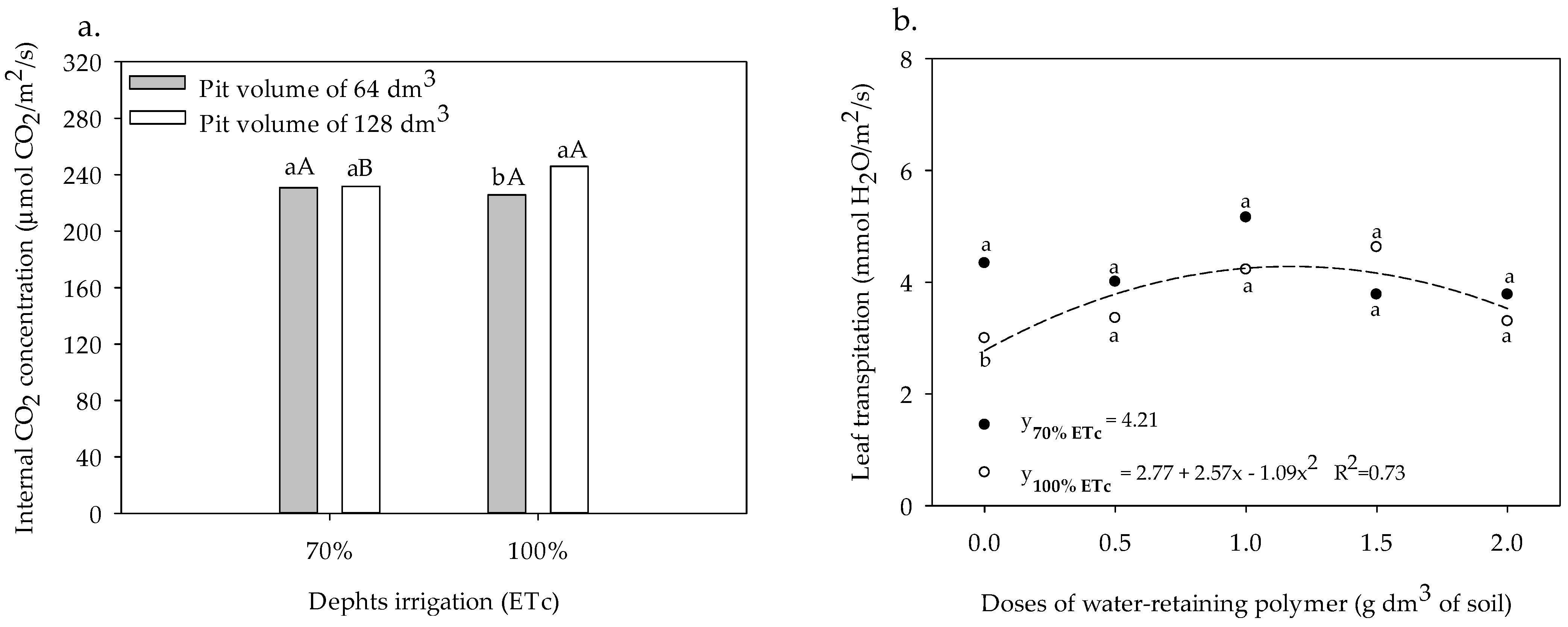
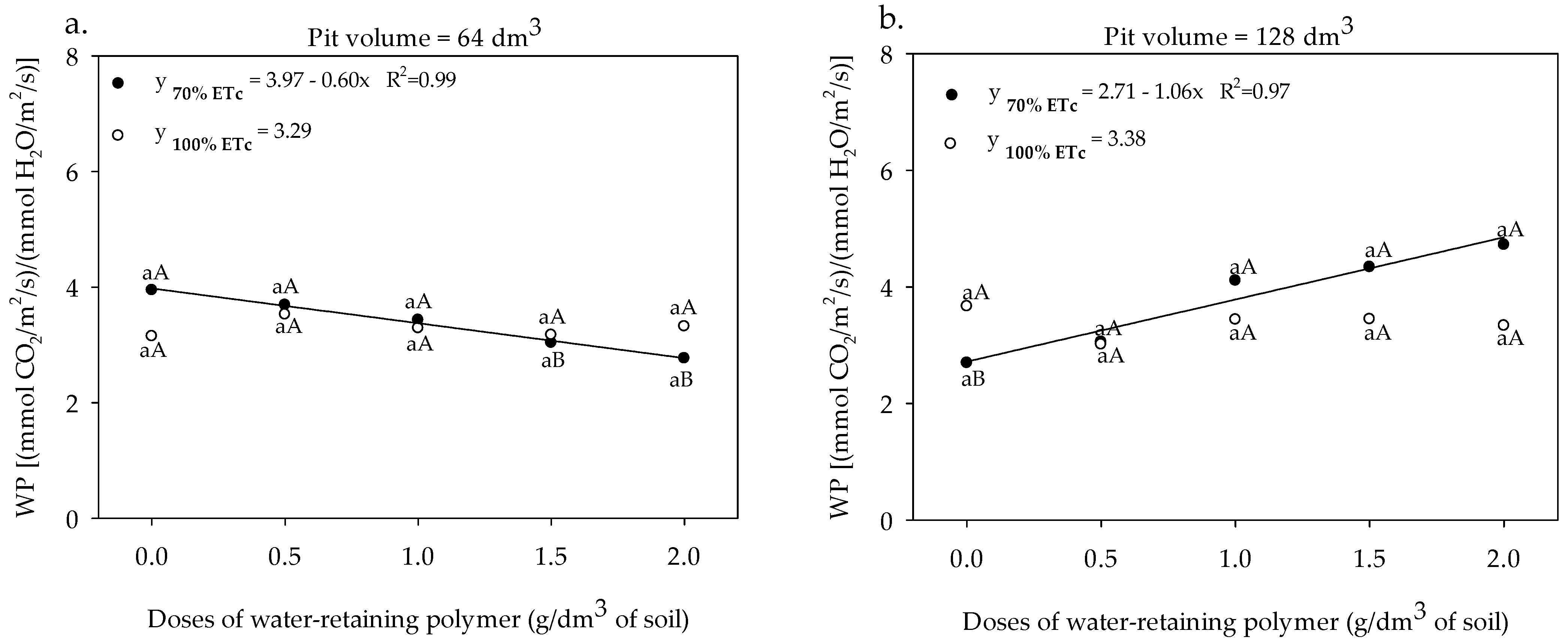
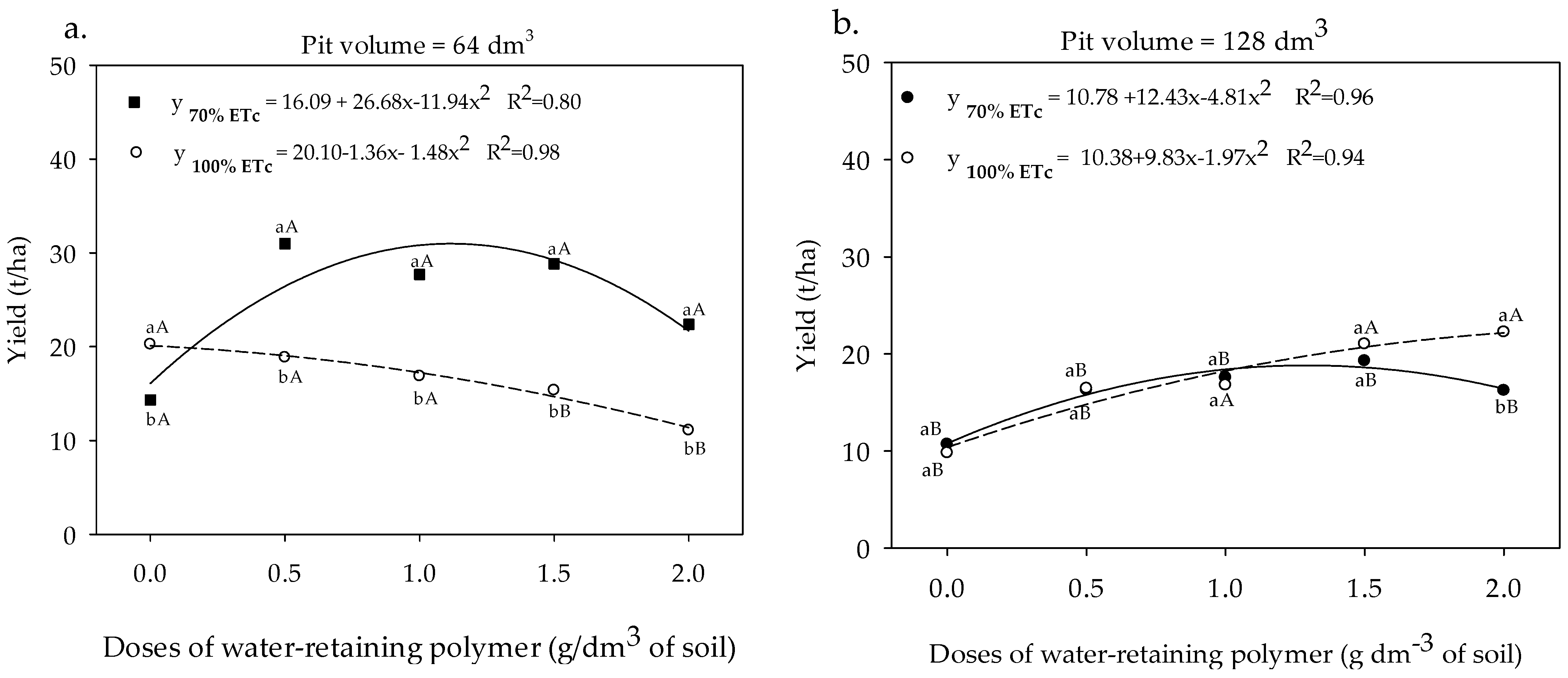
3.2. Gas Exchange and Yield
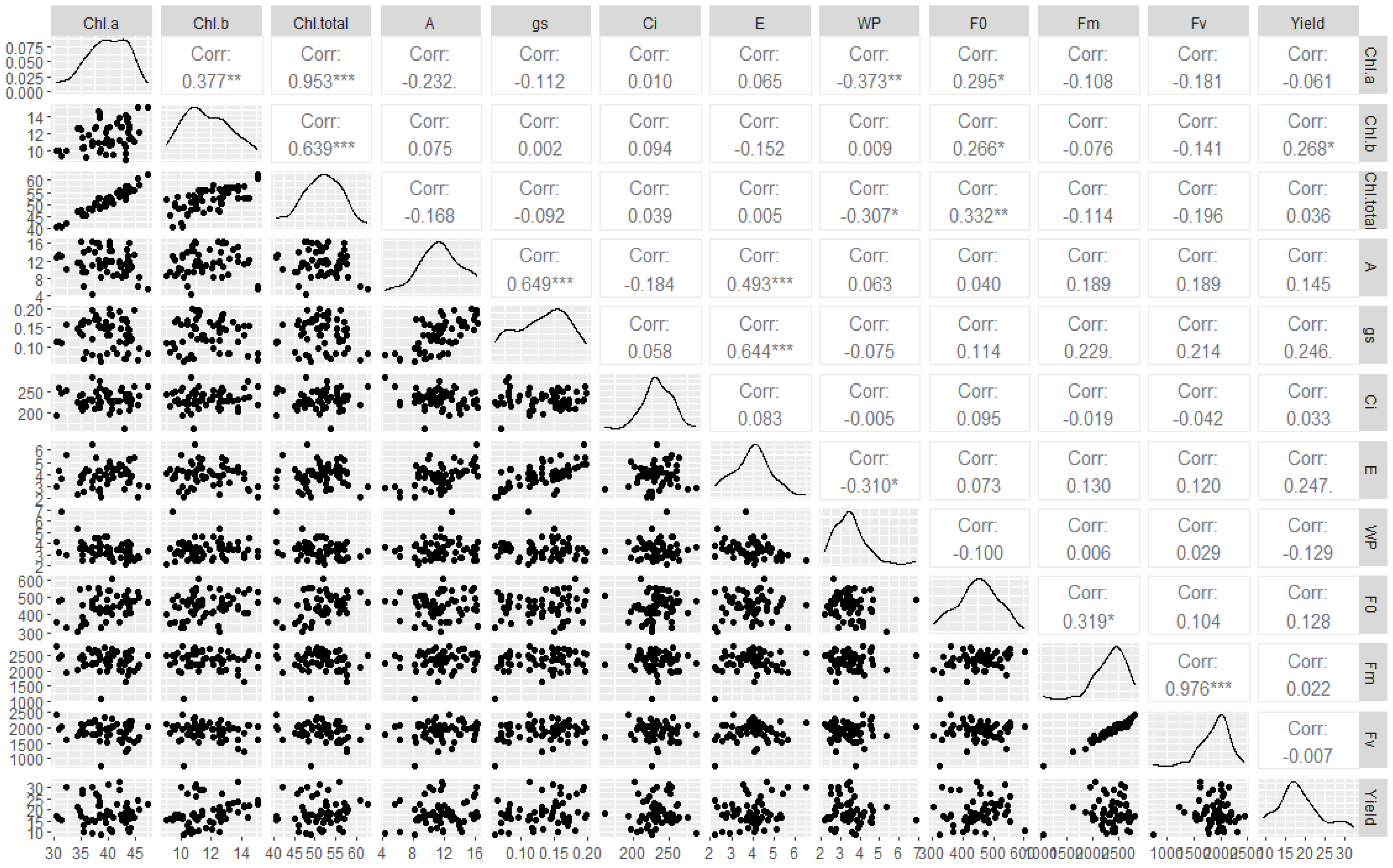
3.3. Correlation Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thokchom, R.; Mandal, G. Production preference and importance of passion fruit (Passiflora edulis): A Review. J. Agric. Eng. Food Technol. 2017, 4, 27–30. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística [IBGE]. Brazilian Production of Passion Fruit; IBGE: Rio de Janeiro, Brazil, 2021.
- Freitas, G.C.d.O.; dos Santos, W.F.; da Paz, J.C.; da Silva, E.R. O cultivo do maracujazeiro no Centro—Oeste do Brasil. Rev. Agrotecnol. 2020, 11, 45–53. [Google Scholar]
- Cavalcante, A.G.; Cavalcante, L.F.; Souto, A.G.d.L.; Cavalcante, A.C.P.; de Araújo, D.L.; do Nascimento, A.P.P.; Zanuncio, J.C. Physiology and production of yellow passion fruit with hydroabsorbent polymer and different irrigation depths. Rev. Ceres 2020, 67, 365–373. [Google Scholar] [CrossRef]
- Araújo, D.L.; Souto, A.G.d.L.; Cavalcante, A.G.; Cavalcante, L.F.; Pereira, W.E.; de Melo, A.S. Physiological aspects of yellow passion fruit with use of hydrogel and mulching. Rev. Caatinga 2022, 35, 382–392. [Google Scholar] [CrossRef]
- Lira, R.M.; dos Santos, A.N.; da Silva, J.S.; Barnabé, J.M.C.; de Barros, M.S.; Soares, H.R. A utilização de águas de qualidade inferior na agricultura irrigada. Rev. Geama 2015, 1, 341–362. [Google Scholar]
- Qi, Y.; Ma, L.; Ghani, M.I.; Peng, Q.; Fan, R.; Hu, X.; Chen, X. Effects of Drought Stress Induced by Hypertonic Polyethylene Glycol (PEG-6000) on Passiflora edulis Sims Physiological Properties. Plants 2023, 12, 2296. [Google Scholar] [CrossRef]
- da Silva, F.G.; Dutra, W.F.; Dutra, A.F.; de Oliveira, I.M.; Filgueiras, L.M.B.; de Melo, A.S. Gas exchange and chlorophyll fluorescence in eggplant plants under irrigation depths. Rev. Bras. Eng. Agríc. Ambien. 2015, 10, 946–952. [Google Scholar] [CrossRef]
- da Silva, A.R.A.; Bezerra, F.M.L.; de Lacerda, C.F.; de Sousa, C.H.C.; Bezerra, M.A. Physiological responses of dwarf coconut plants under water deficit in salt-affected soils. Rev. Caatinga 2017, 30, 447–457. [Google Scholar] [CrossRef]
- dos Anjos Veimrober Júnior, L.A.; da Silva, A.J.P.; Gheyi, H.R.; do Nascimento, F.A.L.; da Silva, M.G.; Vellame, L.M. Water productivity of passion fruit under different forms of propagation and soil-based irrigation management criteria. Irrig. Sci. 2022, 40, 423–433. [Google Scholar] [CrossRef]
- Navroski, M.C.; Araújo, M.M.; de Oliveira Pereira, M.; Fior, C.S. Influência do polímero hidroretentor nas características do substrato comercial para produção de mudas florestais. Interciência 2016, 41, 357–361. [Google Scholar]
- Pontes Filho, R.A.; Gondim, F.A.; Costa, M.C.G. Seedling growth of tree species under doses of hydrogel and two levels of luminosity. Rev. Árvore 2018, 42, e420112. [Google Scholar] [CrossRef]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Malik, S.; Chaudhary, K.; Malik, A.; Punia, H.; Sewhag, M.; Berkesia, N.; Nagora, M.; Kalia, S.; Malik, K.; Kumar, D.; et al. Superabsorbent Polymers as a Soil Amendment for Increasing Agriculture Production with Reducing Water Losses under Water Stress Condition. Polymers 2023, 15, 161. [Google Scholar] [CrossRef]
- Carvalho, R.P.; Cruz, M.d.C.M.; Martins, L.M. Irrigation frequency using hydro absorbent polymer in the production of yellow passion fruit seedlings. Rev. Bras. Frut. 2013, 35, 518–526. [Google Scholar] [CrossRef][Green Version]
- Fernandes, D.A.; Araujo, M.M.V.; Camili, E.C. Formation of yellow passion fruit seedlings under different irrigation depths and use of hydrogel. Rev. Agric. 2015, 90, 229–236. [Google Scholar] [CrossRef]
- Khalil, G.A.; Ali, M.A.; Harhash, M.M.; ELSegieny, A.M. Impact of super absorbent polymers (hydrogels) on water use parameters of plum trees under water stress conditions. J. Adv. Agric. Res. 2022, 27, 791–803. [Google Scholar] [CrossRef]
- Alshallash, K.S.; Sharaf, M.; Hmdy, A.E.; Khalifa, S.M.; Abdel-Aziz, H.F.; Sharaf, A.; Ibrahim, M.T.S.; Alharbi, K.; Elkelish, A. Hydrogel improved growth and productive performance of mango trees under semi-arid condition. Gels 2022, 8, 602. [Google Scholar] [CrossRef]
- Lucas, A.A.T.; Frizzone, J.Á.; Coelho Filho, M.A. Characteristics of the root distribution of passion fruit under irrigation. Irriga 2012, 17, 245–250. [Google Scholar] [CrossRef][Green Version]
- Junghans, T.G.; de Jesus, O.N. Maracujá do Cultivo à Colheita, 1st ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Tavares, O.C.H.; Lima, E.; Zonta, E. Growth and productivity of sugarcane plant grown in different soil preparation and harvesting systems. Acta Sci. Agron. 2010, 32, 61–68. [Google Scholar] [CrossRef]
- Moraes, M.T.; Debiasi, H.; Franchini, J.; da Silva, V.R. Benefits of cover crops on the physical properties of the soil. In Soil and Water Management and Conservation in Small Rural Properties in Southern Brazil: Alternative Management Practices Aimed at Soil and Water Conservation; Tiecher, T., Ed.; UFRGS: Porto Alegre, Brazil, 2016; pp. 34–48. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- US Soil Survey Staff. Keys to Soil Taxonomy; United States Department of Agriculture and Natural Resources Conservation Service: Lincoln, NE, USA, 2014; 332p.
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A. Soil Analysis Methods Manual, 3rd ed.; Embrapa: Brasília, Brazil, 2017; 573p. [Google Scholar]
- Borges, A.L.; Coelho, E.F. Fertigation in Tropical Fruit Trees. In Portuguese with English Summary, 2nd ed.; Embrapa: Cruzdas Almas, Brazil, 2009; 179p. [Google Scholar]
- Souza, J.T.A.; Nunes, J.C.; Cavalcante, L.F.; Nunes, J.A.d.S.; Pereira, W.E.; Freire, J.L.d.O. Effects of water salinity and organomineral fertilization on leaf composition and production in Passiflora edulis. Rev. Bras. Eng. Agríc. Ambien. 2018, 22, 235–240. [Google Scholar] [CrossRef]
- Nogueira, E.; Gomes, E.R.; de Sousa, V.F.; da Silva, L.R.A.; Broetto, F. Crop coefficient and irrigation depths of yellow passion fruit under semi-arid conditions. In Proceedings of the II Inovagri International Meeting, Fortaleza, Brazil, 13–16 April 2014. [Google Scholar]
- Freire, J.L.d.O.; Dias, T.J.; Cavalcantee, L.F.; Fernandes, P.D.; Lima Neto, A.J.d. Rendimento quântico e trocas gasosas em maracujazeiro amarelo sob salinidade hídrica, biofertilização e cobertura morta. Rev. Ciênc. Agron. 2014, 45, 82–91. [Google Scholar] [CrossRef][Green Version]
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects split plots type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- de Carvalho, J.M.; Caldeira, D.S.A.; Vieira, C.L.; da Silva, G.V.B.; Rocha, R.R.; de Campos, I. Crescimento inicial de mudas de Sapindus saponária com uso de hidrogel e lâminas de água. Sci. Elec. Arch. 2022, 15, 7–12. [Google Scholar] [CrossRef]
- Gomes Do Ó, L.M.; Cova, A.M.W.; Silva, P.C.C.; Gheyi, H.R.; de Azevedo Neto, A.D.; Ribas, R.F. Aspectos bioquímicos e fluorescência da clorofila a em plantas de minimelancia hidropônica sob estresse salino. Irriga 2021, 26, 221–239. [Google Scholar] [CrossRef]
- Felippe, D.; Navroski, M.C.; Pereira, M.d.O.; Baptista, K.R.S.d. Hydrogel in the seedling growth of Eucalyptus dunnii Maiden under different irrigation management. Rev. Ambient. Água 2021, 16, e2582. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Cremon, T.; Scalon, S.d.P.Q.; Rosa, D.B.C.J.; Dresch, D.M. How does Copaifera langsdorffii respond to flooding under different irradiance levels? Plant Biosyst. 2022, 156, 68–78. [Google Scholar] [CrossRef]
- Mendonça, T.G.; Querido, D.M.; Souza, C.F. Eficiência do polímero hidroabsorvente na manutenção da umidade do solo no cultivo de alface. Rev. Bras. Agri. Irri. 2015, 9, 239–245. [Google Scholar] [CrossRef][Green Version]
- Azizi, P.; Rafii, M.Y.; Maziah, M.; Abdullah, S.N.A.; Hanafi, M.M.; Latif, M.A.; Rashid, A.A.; Sahebi, M. Understanding the shoot apical meristem regulation: A study of the phytohormones, auxin and cytokinin, in rice. Mech Dev. 2015, 135, 1–15. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Abiotic Stress, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; pp. 732–761. [Google Scholar]
- Gomes, M.M.A.; Ramos, M.J.M.; Torres Netto, A.; Rosa, R.C.C.; Campostrini, E. Water relations, photosynthetic capacity, and growth in passion fruit (Passiflora edulis Sims f. flavicarpa Deg). Rev. Ceres 2018, 65, 135–143. [Google Scholar] [CrossRef]
- Cajazeira, J.P.; Correa, M.C.M.; Almeida, E.I.B.; Queiroz, R.F.; Mesquita, R.O. Growth and gas exchange in white pitaya under different concentrations of potassium and calcium. Rev. Ciênc. Agron. 2018, 49, 112–121. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Applications of Absorbent Polymers for Sustainable Plant Protection and Crop Yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Mansour, H.A.; Jiandong, H.; Hongjuan, R.; Kheiry, A.N.O.; Abd-Elmabod, S.K. Influence of using automatic irrigation system and organic fertilizer treatments on faba bean water productivity. Int. J. GEOMATE 2019, 17, 250–259. [Google Scholar] [CrossRef]
- Mansour, H.A.; Abdel-Hady, M.; Eldardiry, E.I.; Bralts, V.F. Performance of automatic control different localized irrigation systems and lateral lengths for:1-Emitters clogging and maize (Zea mays L.) growth and yield. Int. J. GEOMATE 2015, 9, 1545–1552. [Google Scholar] [CrossRef]
| Physical Attributes | Chemical Attributes | ||
|---|---|---|---|
| Coarse Sand (g kg−1) | 602 | pH in water (1:2.5) | 5.37 |
| Fine Sand (g kg−1) | 194 | SOM (g dm−3) | 4 |
| Silt (g kg−1) | 132 | P–Rem (mg dm−3) | 45.6 |
| Clay (g kg−1) | 72 | P (mg dm−3) | 5.5 |
| Dispersed clay (g kg−1) | 10.5 | S (mg dm−3) | 6.55 |
| Bulk density (kg dm−3) | 1.54 | K+ (cmolc dm−3) | 0.13 |
| Particle density (kg dm−3) | 2.77 | Ca2+ (cmolc dm−3) | 1.18 |
| Total porosity (%) | 44.5 | Mg2+(cmolc dm−3) | 0.37 |
| Degree of flocculation (%) | 86 | Na+ (cmolc dm−3) | Dash |
| Dispersion index (%) | 14 | SB (cmolc dm−3) | 1.68 |
| Flocculation/dispersion ratio | 7.75 | H+ + Al3+ (cmolc dm−3) | 1.8 |
| Field capacity (%-v) * | 8 | Al3+ (cmolc dm−3) | 0.18 |
| Wilting point (%-v) ** | 3.75 | CEC (cmolc dm−3) | 3.48 |
| Available water (%-v) | 4.25 | V (%) | 47.85 |
| Textural class | Sandy loam | Fertility class | Eutrophic |
| Attribute | Value | Attribute | Value |
|---|---|---|---|
| pH (H2O) | 8.81 | Mg2+ (g kg−1) | 5.0 |
| C (g kg−1) | 159.0 | S (g kg−1) | 1.8 |
| C/N | 19:1 | B (mg kg−1) | 21.3 |
| Na+ (mg kg−1) | 790 | Cu (mg kg−1) | 8.0 |
| N (g kg−1) | 8.3 | Fe (mg kg−1) | 991 |
| P (g kg−1) | 2.8 | Mn (mg kg−1) | 250 |
| K (g kg−1) | 10.4 | Zn (mg kg−1) | 58 |
| Ca2+ (g kg−1) | 8.2 |
| SV | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| Chl a | Chl b | Chl Total | F0 | Fm | Fv | ||
| Blocks | 2 | 3.09 ns | 0.11 ns | 4.37 ns | 13,952.06 ns | 384,103.26 ns | 378,388.86 ns |
| Irrigation depths (ID) | 1 | 293.22 * | 0.71 ns | 322.75 * | 640.26 ns | 417,333.60 ns | 449,800.41 ns |
| Error 1 | 2 | 4.64 | 0.42 | 0.003 | 17,617.86 | 63,411.80 | 49,449.26 |
| Pit volume (PV) | 1 | 3.66 ns | 3.89 * | 5.50 | 2912.06 ns | 203,933.40 ns | 157,388.81 ns |
| Water-retaining polymer (WRP) | 4 | 18.09 ** | 5.28 ** | 41.03 ** | 1069.93 ns | 42,117.66 ns | 33,767.85 ns |
| ID × PV | 1 | 1.47 ns | 22.81 ** | 12.69 * | 1288.06 ns | 363,793.06 * | 409,200.41 * |
| ID × WRP | 4 | 49.89 ** | 1.24 ns | 63.32 ** | 8125.26 * | 50,025.68 ns | 38,505.37 ns |
| PV × WRP | 4 | 22.62 ** | 9.95 ** | 47.32 ** | 1398.40 ns | 31,285.06 ns | 31,029.52 ns |
| ID × PV × WRP | 4 | 44.44 ** | 4.04 ** | 64.84 ** | 9042.06 * | 94,404.98 ns | 74,372.04 ns |
| Error 2 | 36 | 1.67 | 0.84 | 3.02 | 3010.46 | 71,006.90 | 62,160.80 |
| Total | 59 | ||||||
| CV1 | 5.42 | 5.56 | 4.56 | 29.50 | 10.76 | 11.77 | |
| CV2 | 3.25 | 7.83 | 3.37 | 12.19 | 11.39 | 13.19 | |
| Mean | 39.75 | 11.75 | 51.51 | 449,93 | 2339.83 | 1889.78 | |
| SV | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| A | Gs | Ci | E | WP | Yield | ||
| Blocks | 2 | 2.86 ns | 0.0011 ** | 2908.71 ** | 0.0001 ns | 5.00 ns | 194.40 ** |
| Irrigation depths (ID) | 1 | 180.26 * | 0.0325 ** | 317.40 | 6.01 * | 1.66 ns | 1.61 ns |
| Error 1 | 2 | 1.86 | 0.0001 | 1.55 | 0.06 | 0.46 | 0.65 |
| Pit volumes (PV) | 1 | 1.86 ns | 0.0001 ns | 1643.26 * | 2.81 * | 0.60 ns | 248.06 ** |
| Water-retaining polymer (WRP) | 4 | 12.73 ** | 0.0010 ns | 605.94 ns | 1.77 * | 0.62 ns | 107.80 ** |
| ID × PV | 1 | 0.06 ns | 0.0010 ns | 1401.66 * | 0.01 ns | 0.06 ns | 345.60 ** |
| ID × WRP | 4 | 17.85 ** | 0.0063 ** | 504.60 ns | 2.89 ** | 0.70 ns | 38.85 ** |
| PV × WRP | 4 | 14.50 ** | 0.0005 ns | 348.97 ns | 0.27 ns | 0.55 ns | 59.44 ** |
| ID × PV × WRP | 4 | 2.15 ns | 0.0015 ** | 255.54 ns | 1.39 ns | 1.77 * | 68.39 ** |
| Error 2 | 36 | 2.29 | 0.0003 | 290.52 | 0.45 | 0.58 | 2.39 |
| Total | 59 | ||||||
| CV1 | 11.85 | 2.48 | 0.53 | 6.54 | 19.52 | 4.34 | |
| CV2 | 13.13 | 15.10 | 7.30 | 17.16 | 21.86 | 8.33 | |
| Mean | 11.53 | 0.131 | 233.63 | 3.95 | 3.50 | 18.56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Luna Souto, A.G.; de Melo, E.N.; Cavalcante, L.F.; do Nascimento, A.P.P.; Cavalcante, Í.H.L.; de Lima, G.S.; Batista, R.O.; Gheyi, H.R.; de Fátima, R.T.; de Mesquita, E.F.; et al. Water-Retaining Polymer and Planting Pit Size on Chlorophyll Index, Gas Exchange and Yield of Sour Passion Fruit with Deficit Irrigation. Plants 2024, 13, 235. https://doi.org/10.3390/plants13020235
de Luna Souto AG, de Melo EN, Cavalcante LF, do Nascimento APP, Cavalcante ÍHL, de Lima GS, Batista RO, Gheyi HR, de Fátima RT, de Mesquita EF, et al. Water-Retaining Polymer and Planting Pit Size on Chlorophyll Index, Gas Exchange and Yield of Sour Passion Fruit with Deficit Irrigation. Plants. 2024; 13(2):235. https://doi.org/10.3390/plants13020235
Chicago/Turabian Stylede Luna Souto, Antônio Gustavo, Edinete Nunes de Melo, Lourival Ferreira Cavalcante, Ana Paula Pereira do Nascimento, Ítalo Herbert Lucena Cavalcante, Geovani Soares de Lima, Rafael Oliveira Batista, Hans Raj Gheyi, Reynaldo Teodoro de Fátima, Evandro Franklin de Mesquita, and et al. 2024. "Water-Retaining Polymer and Planting Pit Size on Chlorophyll Index, Gas Exchange and Yield of Sour Passion Fruit with Deficit Irrigation" Plants 13, no. 2: 235. https://doi.org/10.3390/plants13020235
APA Stylede Luna Souto, A. G., de Melo, E. N., Cavalcante, L. F., do Nascimento, A. P. P., Cavalcante, Í. H. L., de Lima, G. S., Batista, R. O., Gheyi, H. R., de Fátima, R. T., de Mesquita, E. F., de Souza, G. L. F., Silva, G. R., Silva, D. V., de Oliveira Mesquita, F., & de Oliveira, P. V. C. (2024). Water-Retaining Polymer and Planting Pit Size on Chlorophyll Index, Gas Exchange and Yield of Sour Passion Fruit with Deficit Irrigation. Plants, 13(2), 235. https://doi.org/10.3390/plants13020235











