Comparative Phytotoxicity of Metallic Elements on Duckweed Lemna gibba L. Using Growth- and Chlorophyll Fluorescence Induction-Based Endpoints
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials and Experimental Design
2.2. In Vivo Chlorophyll Fluorescence Induction Measurements
2.3. Measurement of Growth Inhibition
- X = area or number of fronds;
- Xi = initial value of the respective growth parameter;
- Xf = final value of the respective growth parameter;
- ti = starting day of treatments (i.e., 0);
- tf = final day of treatments (i.e., 3).
2.4. Data Analysis and Statistics
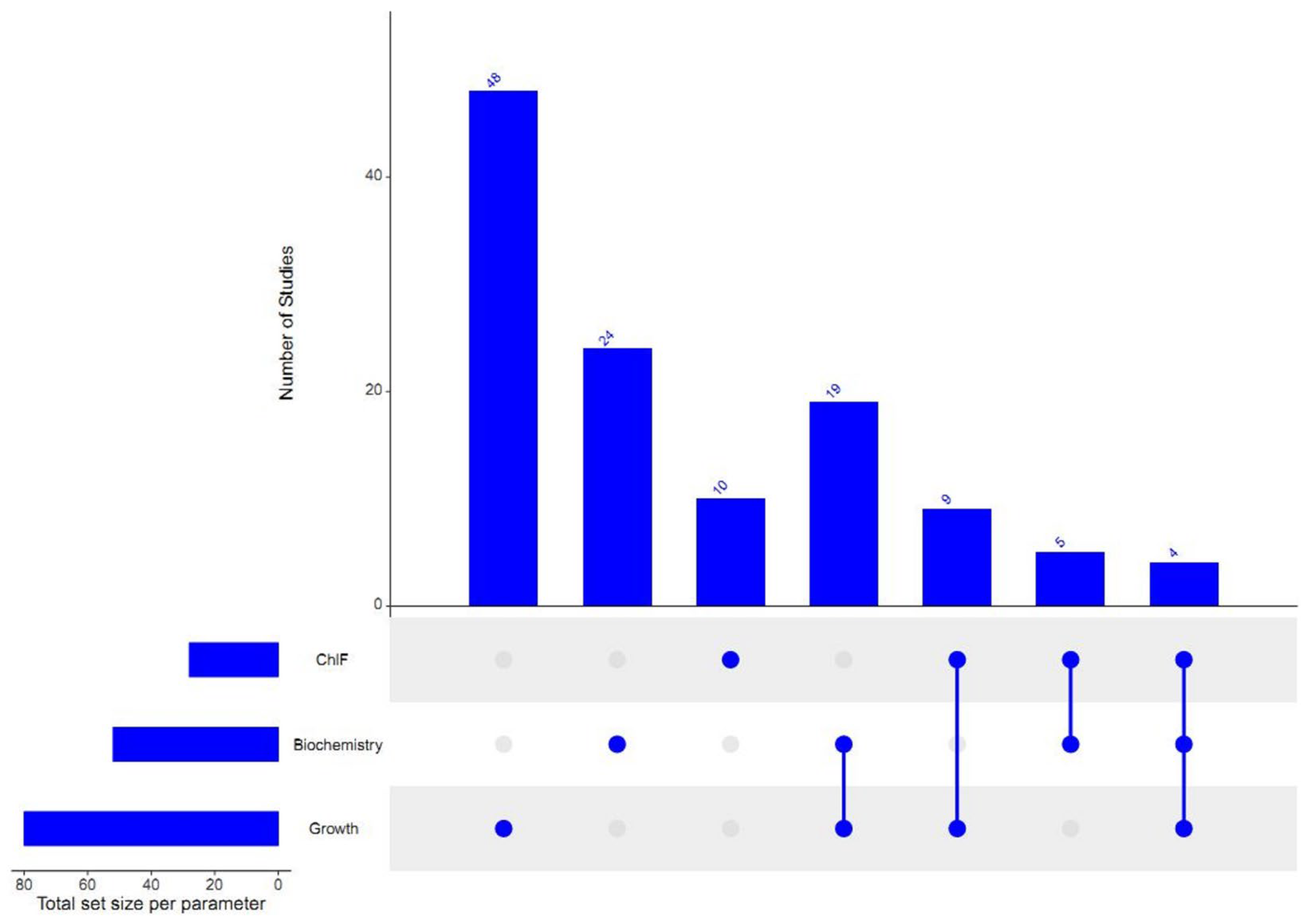
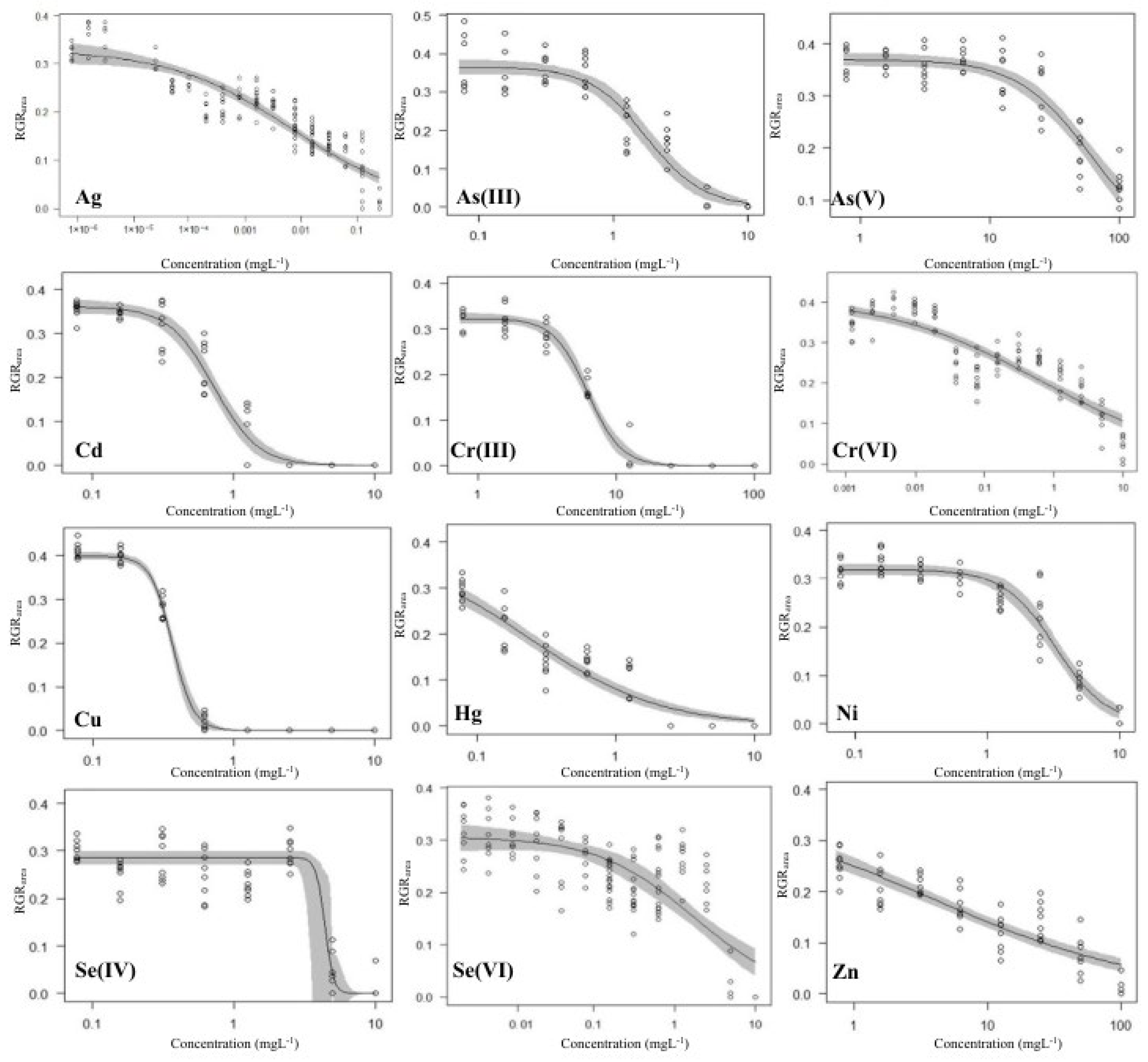
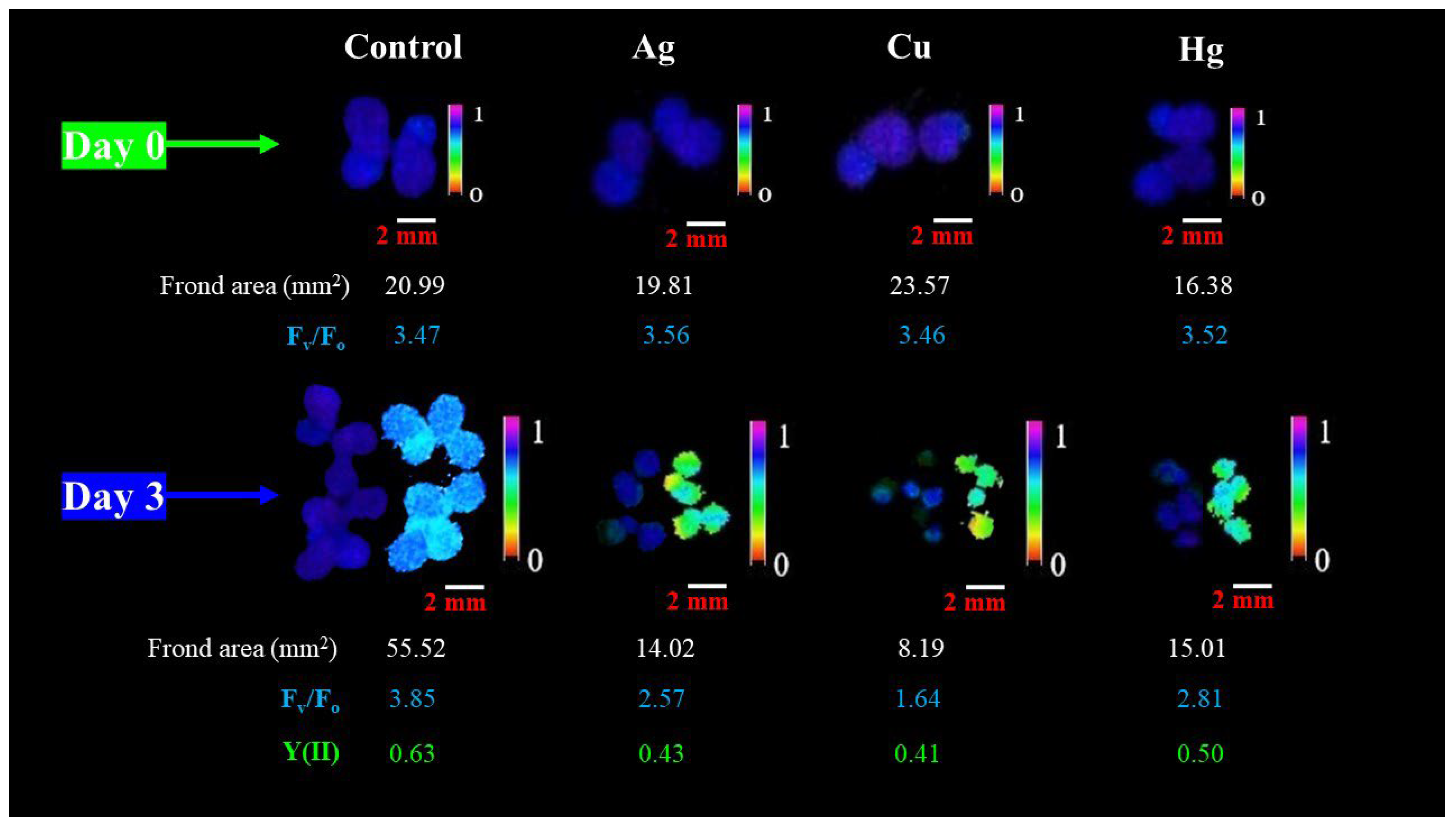
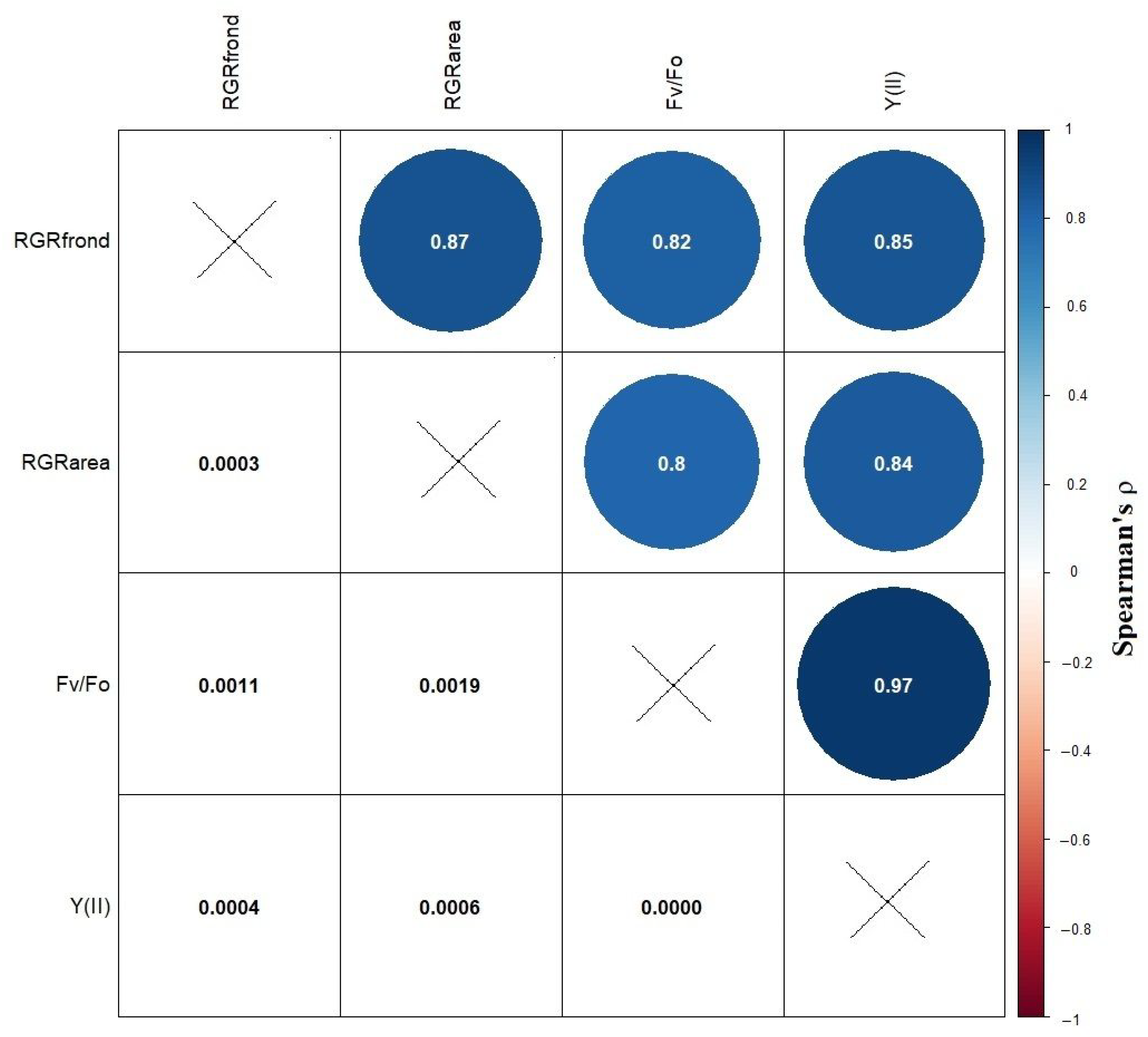
3. Results and Discussion
3.1. Growth-Inhibition-Based Endpoints
3.2. Chlorophyll-Fluorescence-Induction-Based Endpoints
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radić, S.; Stipaničev, D.; Cvjetko, P.; Marijanović Rajčić, M.; Širac, S.; Pevalek-Kozlina, B.; Pavlica, M. Duckweed Lemna minor as a Tool for Testing Toxicity and Genotoxicity of Surface Waters. Ecotoxicol. Environ. Saf. 2011, 74, 182–187. [Google Scholar] [CrossRef]
- Yahaya, N.; Hamdan, N.H.; Zabidi, A.R.; Mohamad, A.M.; Suhaimi, M.L.H.; Johari, M.A.A.M.; Yahya, H.N.; Yahya, H. Duckweed as a Future Food: Evidence from Metabolite Profile, Nutritional and Microbial Analyses. Future Foods 2022, 5, 100128. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, K.; Li, W.; Yan, B.; Yu, Y.; Li, J.; Zhang, Y.; Xia, S.; Cheng, Z.; Lin, F.; et al. A Review on Bioenergy Production from Duckweed. Biomass Bioenergy 2022, 161, 106468. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, J.J.; Cui, C.W. Growing Duckweed for Biofuel Production: A Review. Plant Biol. 2015, 17, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Paolacci, S.; Stejskal, V.; Toner, D.; Jansen, M.A.K. Wastewater Valorisation in an Integrated Multitrophic Aquaculture System; Assessing Nutrient Removal and Biomass Production by Duckweed Species. Environ. Pollut. 2022, 302, 119059. [Google Scholar] [CrossRef] [PubMed]
- Petersen, F.; Demann, J.; Restemeyer, D.; Olfs, H.-W.; Westendarp, H.; Appenroth, K.-J.; Ulbrich, A. Influence of Light Intensity and Spectrum on Duckweed Growth and Proteins in a Small-Scale, Re-Circulating Indoor Vertical Farm. Plants 2022, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, Y.; Huang, J.; Zhao, Y.; Li, Q.; Lai, F.; Xu, Y.; Tian, X.; He, K.; Jin, Y.; et al. Duckweed Systems for Eutrophic Water Purification through Converting Wastewater Nutrients to High-Starch Biomass: Comparative Evaluation of Three Different Genera (Spirodela polyrhiza, Lemna minor and Landoltia punctata) in Monoculture or Polyculture. RSC Adv. 2018, 8, 17927–17937. [Google Scholar] [CrossRef]
- Golob, A.; Vogel-Mikuš, K.; Brudar, N.; Germ, M. Duckweed (Lemna minor L.) Successfully Accumulates Selenium from Selenium-Impacted Water. Sustainability 2021, 13, 13423. [Google Scholar] [CrossRef]
- Iqbal, J.; Javed, A.; Baig, M.A. Growth and Nutrient Removal Efficiency of Duckweed (Lemna minor) from Synthetic and Dumpsite Leachate under Artificial and Natural Conditions. PLoS ONE 2019, 14, e0221755. [Google Scholar] [CrossRef]
- Szabó, S.; Zavanyi, G.; Koleszár, G.; del Castillo, D.; Oláh, V.; Braun, M. Phytoremediation, Recovery and Toxic Effects of Ionic Gadolinium Using the Free-Floating Plant Lemna gibba. J. Hazard. Mater. 2023, 458, 131930. [Google Scholar] [CrossRef]
- OECD Guidelines for the Testing of Chemicals, Revised Proposal for a New Guideline 221, Lemna sp. Growth Inhibition Test. 2006. Available online: https://www.oecd-ilibrary.org/environment/test-no-221-lemna-sp-growth-inhabition-test_9789264016194-en (accessed on 14 September 2023).
- ISO 20079:2005; ISO Water Quality—Determination of the Toxic Effect of Water Constituents and Waste Water on Duckweed (Lemna minor)—Duckweed Growth Inhibition Test. ISO: Geneva, Switzerland, 2005.
- Yang, J.; Li, G.; Xia, M.; Chen, Y.; Chen, Y.; Kumar, S.; Sun, Z.; Li, X.; Zhao, X.; Hou, H. Combined Effects of Temperature and Nutrients on the Toxicity of Cadmium in Duckweed (Lemna aequinoctialis). J. Hazard. Mater. 2022, 432, 128646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, H.; Duan, D.; Li, H.; Lei, T.; Wang, M.; Zhao, H.; Zhao, Y. The Influence of Duckweed Species Diversity on Ecophysiological Tolerance to Copper Exposure. Aquat. Toxicol. 2015, 164, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Braglia, R.; Harren, F.J.M.; Cristescu, S.M. Stress Responses of Duckweed (Lemna minor L.) and Water Velvet (Azolla filiculoides Lam.) to Anionic Surfactant Sodium-Dodecyl-Sulphate (SDS). Aquat. Toxicol. 2012, 110–111, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, M.; Schröder, C.A.; Helmreich, B.; Schröder, P. The Enzymatic and Antioxidative Stress Response of Lemna minor to Copper and a Chloroacetamide Herbicide. Environ. Sci. Pollut. Res. Int. 2015, 22, 18495–18507. [Google Scholar] [CrossRef] [PubMed]
- Henke, R.; Eberius, M.; Appenroth, K.-J. Induction of Frond Abscission by Metals and Other Toxic Compounds in Lemna minor. Aquat. Toxicol. 2011, 101, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Oláh, V.; Hepp, A.; Mészáros, I. Assessment of Giant Duckweed (Spirodela polyrhiza L. Schleiden) Turions as Model Objects in Ecotoxicological Applications. Bull. Environ. Contam. Toxicol. 2016, 96, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; De Saeger, J.; Bae, S.; Kim, M.; Depuydt, S.; Heynderickx, P.M.; Wu, D.; Han, T.; Park, J. Giant Duckweed (Spirodela polyrhiza) Root Growth as a Simple and Sensitive Indicator of Copper and Chromium Contamination. Toxics 2023, 11, 788. [Google Scholar] [CrossRef]
- Zhang, L.M.; Jin, Y.; Yao, S.M.; Lei, N.F.; Chen, J.S.; Zhang, Q.; Yu, F.H. Growth and Morphological Responses of Duckweed to Clonal Fragmentation, Nutrient Availability, and Population Density. Front. Plant Sci. 2020, 11, 618. [Google Scholar] [CrossRef]
- Chen, M.; Yin, G.; Zhao, N.; Gan, T.; Feng, C.; Gu, M.; Qi, P.; Ding, Z. Rapid and Sensitive Detection of Water Toxicity Based on Photosynthetic Inhibition Effect. Toxics 2021, 9, 321. [Google Scholar] [CrossRef]
- Gan, T.; Yin, G.; Zhao, N.; Tan, X.; Wang, Y. A Sensitive Response Index Selection for Rapid Assessment of Heavy Metals Toxicity to the Photosynthesis of Chlorella pyrenoidosa Based on Rapid Chlorophyll Fluorescence Induction Kinetics. Toxics 2023, 11, 468. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-Stage Detection of Biotic and Abiotic Stress on Plants by Chlorophyll Fluorescence Imaging Analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Oláh, V.; Hepp, A.; Irfan, M.; Mészáros, I. Chlorophyll Fluorescence Imaging-Based Duckweed Phenotyping to Assess Acute Phytotoxic Effects. Plants 2021, 10, 2763. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Depuydt, S.; Shin, K.; Choi, S.; Kim, G.; Lee, Y.H.; Park, J.T.; Han, T.; Park, J. Assessment of Various Toxicity Endpoints in Duckweed (Lemna minor) at the Physiological, Biochemical, and Molecular Levels as a Measure of Diuron Stress. Biology 2021, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.M.; Laurich, J.; Lash, E.; Frederickson, M.E. Mutualistic Outcomes Across Plant Populations, Microbes, and Environments in the Duckweed Lemna minor. Microb. Ecol. 2020, 80, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Yang, B.; Ma, F.; Lu, P.; Li, L.; Wan, C.; Rayner, S.; Chen, S. Duckweed (Lemna minor) as a Model Plant System for the Study of Human Microbial Pathogenesis. PLoS ONE 2010, 5, e13527. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, F.; Pietrini, F.; Zacchini, M.; Scarascia Mugnozza, G.; Macagnano, A. Catechol-Loading Nanofibrous Membranes for Eco-Friendly Iron Nutrition of Plants. Nanomaterials 2019, 9, 1315. [Google Scholar] [CrossRef] [PubMed]
- Baudo, R.; Foudoulakis, M.; Arapis, G.; Perdaen, K.; Lanneau, W.; Paxinou, A.-C.; Kouvdou, S.; Persoone, G. History and Sensitivity Comparison of the Spirodela polyrhiza Microbiotest and Lemna Toxicity Tests. Knowl. Manag. Aquat. Ecosyst. 2015, 416, 23. [Google Scholar] [CrossRef]
- Kalčíková, G.; Marolt, G.; Kokalj, A.J.; Gotvajn, A.Ž. The Use of Multiwell Culture Plates in the Duckweed Toxicity Test—A Case Study on Zn Nanoparticles. New Biotechnol. 2018, 47, 67–72. [Google Scholar] [CrossRef]
- Kose, T.; Lins, T.F.; Wang, J.; O’Brien, A.M.; Sinton, D.; Frederickson, M.E. Accelerated High-Throughput Imaging and Phenotyping System for Small Organisms. PLoS ONE 2023, 18, e0287739. [Google Scholar] [CrossRef]
- Pietrini, F.; Zacchini, M. A New Ecotoxicity Assay for Aquatic Plants: Eco-Tox Photosystem Tool (ETPT). Trends Plant Sci. 2020, 25, 1266–1267. [Google Scholar] [CrossRef]
- Pietrini, F.; Passatore, L.; Fischetti, E.; Carloni, S.; Ferrario, C.; Polesello, S.; Zacchini, M. Evaluation of Morpho-Physiological Traits and Contaminant Accumulation Ability in Lemna minor L. Treated with Increasing Perfluorooctanoic Acid (PFOA) Concentrations under Laboratory Conditions. Sci. Total Environ. 2019, 695, 133828. [Google Scholar] [CrossRef] [PubMed]
- Roháček, K. Chlorophyll Fluorescence Parameters: The Definitions, Photosynthetic Meaning, and Mutual Relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Mallakin, A.; Babu, T.S.; Dixon, D.G.; Greenberg, B.M. Sites of Toxicity of Specific Photooxidation Products of Anthracene to Higher Plants: Inhibition of Photosynthetic Activity and Electron Transport in Lemna gibba L. G-3 (Duckweed). Environ. Toxicol. 2002, 17, 462–471. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II Quantum Yields Calculated from Simple Fluorescence Parameters Measured by PAM Fluorometry and the Saturation Pulse Method. PAM Appl. Notes 2008, 1, 201–247. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Landini, G. Novel Context-Based Segmentation Algorithms for Intelligent Microscopy. 2020. Available online: https://blog.bham.ac.uk/intellimic/g-landini-software/ (accessed on 14 September 2023).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio Team RStudio Desktop IDE (Version 2023.06.0-421) [Computer Software]; PBC: Boston, MA, USA, 2023; Available online: http://www.rstudio.com/ (accessed on 14 September 2023).
- Wei, T.; Simko, V.R. Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.90). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 14 September 2023).
- Li, H.; Mo, F.; Li, Y.; Wang, M.; Li, Z.; Hu, H.; Deng, W.; Zhang, R. Effects of Silver(I) Toxicity on Microstructure, Biochemical Activities, and Genic Material of Lemna minor L. with Special Reference to Application of Bioindicator. Environ. Sci. Pollut. Res. 2020, 27, 22735–22748. [Google Scholar] [CrossRef]
- Jiang, H.-S.; Li, M.; Chang, F.-Y.; Li, W.; Yin, L.-Y. Physiological Analysis of Silver Nanoparticles and AgNO3 Toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 2012, 31, 1880–1886. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Q.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Mercury Induced Oxidative Stress, DNA Damage, and Activation of Antioxidative System and Hsp70 Induction in Duckweed (Lemna minor). Ecotoxicol. Environ. Saf. 2017, 143, 46–56. [Google Scholar] [CrossRef]
- Hou, W.; Chen, X.; Song, G.; Wang, Q.; Chi Chang, C. Effects of Copper and Cadmium on Heavy Metal Polluted Waterbody Restoration by Duckweed (Lemna minor). Plant Physiol. Biochem. 2007, 45, 62–69. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-Induced Morphogenic Responses: Growing out of Trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Naumann, B.; Eberius, M.; Appenroth, K.J. Growth Rate Based Dose-Response Relationships and EC-Values of Ten Heavy Metals Using the Duckweed Growth Inhibition Test (ISO 20079) with Lemna minor L. Clone St. J. Plant Physiol. 2007, 164, 1656–1664. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M. Growth Response of the Duckweed Lemna minor to Heavy Metal Pollution. J. Environ. Health Sci. Eng. 2009, 6, 161–166. [Google Scholar]
- Lahive, E.; O’Callaghan, M.J.A.; Jansen, M.A.K.; O’Halloran, J. Uptake and Partitioning of Zinc in Lemnaceae. Ecotoxicol. Lond. Engl. 2011, 20, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Lanthemann, L.; van Moorsel, S.J. Species Interactions in Three Lemnaceae Species Growing along a Gradient of Zinc Pollution. Ecol. Evol. 2022, 12, e8646. [Google Scholar] [CrossRef] [PubMed]
- Khellaf, N.; Zerdaoui, M. Growth Response of the Duckweed Lemna Gibba L. to Copper and Nickel Phytoaccumulation. Ecotoxicology 2010, 19, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Oláh, V.; Hepp, A.; Mészáros, I. Comparative Study on the Sensitivity of Turions and Active Fronds of Giant Duckweed (Spirodela polyrhiza (L.) Schleiden) to Heavy Metal Treatments. Chemosphere 2015, 132, 40–46. [Google Scholar] [CrossRef]
- Hepp, A.; Vaca, N.Y.G.; Kovács, F.; Tamás, M.; Oláh, V.; Mészáros, I. Effects of Hg on Growth of Active and Resting (Turions) Fronds of Giant Duckweed (Spirodela polyrhiza (L.) Schleiden). In Proceedings of the XII. Environmental Scientific Conference of the Carpathian Basin, Berehove, Ukraine, 1–4 June 2016; Kiss, I., Pincehelyi, Z.É., Eds.; PTE TTK Szentágothai János Protestant College: Pécs, Hungary, 2016; Volume 126, pp. 94–103. ISBN 9789634290506. [Google Scholar]
- Markovic, M.; Neale, P.A.; Nidumolu, B.; Kumar, A. Combined Toxicity of Therapeutic Pharmaceuticals to Duckweed, Lemna minor. Ecotoxicol. Environ. Saf. 2021, 208, 111428. [Google Scholar] [CrossRef]
- Oláh, V.; Hepp, A.; Gaibor Vaca, N.Y.; Tamás, M.; Mészáros, I. Retrospective Analyses of Archive Phytotoxicity Test Data Can Help in Assessing Internal Dynamics and Stability of Growth in Laboratory Duckweed Cultures. Aquat. Toxicol. 2018, 201, 40–46. [Google Scholar] [CrossRef]
- Heinz Walz GmbH. IMAGING-PAM M-Series Chlorophyll Fluorometer: Instrument Description and Information for Users, 5th ed.; Heinz Walz GmbH: Effeltrich, Germany, 2019. [Google Scholar]
- Ziegler, P.; Appenroth, K.J.; Sree, K.S. Survival Strategies of Duckweeds, the World’s Smallest Angiosperms. Plants 2023, 12, 2215. [Google Scholar] [CrossRef]
- Park, J.; Brown, M.T.; Depuydt, S.; Kim, J.K.; Won, D.-S.; Han, T. Comparing the Acute Sensitivity of Growth and Photosynthetic Endpoints in Three Lemna Species Exposed to Four Herbicides. Environ. Pollut. 2017, 220, 818–827. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, D.; Soni, V. Performance of Chlorophyll a Fluorescence Parameters in Lemna minor under Heavy Metal Stress Induced by Various Concentration of Copper. Sci. Rep. 2022, 12, 10620. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Xie, P.; Liu, C.; Yu, L.; Ma, X. Dualistic Effects of Bisphenol A on Growth, Photosynthetic and Oxidative Stress of Duckweed (Lemna minor). Environ. Sci. Pollut. Res. Int. 2022, 29, 87717–87729. [Google Scholar] [CrossRef]
- Pietrini, F.; Passatore, L.; Carloni, S.; Zacchini, M. Non-Standard Physiological Endpoints to Evaluate the Toxicity of Emerging Contaminants in Aquatic Plants: A Case Study on the Exposure of Lemna minor L. and Spirodela polyrhiza (L.) Schleid. to Dimethyl Phthalate (DMP). In Emerging Contaminants and Plants: Interactions, Adaptations and Remediation Technologies; Emerging Contaminants and Associated Treatment Technologies; Aftab, T., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 87–108. ISBN 978-3-031-22269-6. [Google Scholar]
- Rodrigues, S.; Pinto, I.; Martins, F.; Formigo, N.; Antunes, S.C. An Ecotoxicological Approach Can Complement the Assessment of Natural Waters from Portuguese Reservoirs? Environ. Sci. Pollut. Res. Int. 2022, 29, 52147–52161. [Google Scholar] [CrossRef] [PubMed]
- Diogo, B.S.; Rodrigues, S.; Lage, O.M.; Antunes, S.C. Are the Ecotoxicological Tools Viable to Evaluate the Effectiveness of Wastewater Treatment Plant Effluents? Int. J. Environ. Sci. Technol. 2023, 20, 11943–11962. [Google Scholar] [CrossRef]
- Fekete-Kertész, I.; Kunglné-Nagy, Z.; Gruiz, K.; Magyar, Á.; Farkas, É.; Molnár, M. Assessing Toxicity of Organic Aquatic Micropollutants Based on the Total Chlorophyll Content of Lemna minor as a Sensitive Endpoint. Period. Polytech. Chem. Eng. 2015, 59, 262–271. [Google Scholar] [CrossRef]
- Boros, B.-V.; Dascalu, D.; Ostafe, V.; Isvoran, A. Assessment of the Effects of Chitosan, Chitooligosaccharides and Their Derivatives on Lemna minor. Molecules 2022, 27, 6123. [Google Scholar] [CrossRef]
- Gavina, A.; Antunes, S.C.; Pinto, G.; Claro, M.T.; Santos, C.; Gonçalves, F.; Pereira, R. Can Physiological Endpoints Improve the Sensitivity of Assays with Plants in the Risk Assessment of Contaminated Soils? PLoS ONE 2013, 8, e59748. [Google Scholar] [CrossRef]
- Song, L.; Vijver, M.G.; Peijnenburg, W.J.G.M. Comparative Toxicity of Copper Nanoparticles across Three Lemnaceae Species. Sci. Total Environ. 2015, 518–519, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R.L.; Nussbaumer, C.; Pick, F.R.; Boutin, C. Comparing the Sensitivity of Geographically Distinct Lemna minor Populations to Atrazine. Ecotoxicol. Lond. Engl. 2013, 22, 718–730. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Lin, Y.-T.; Farhan, A.; Malhotra, N.; Luong, C.T.; Hung, C.-H.; Roldan, M.J.M.; Tsao, C.-C.; Cheng, T.-S.; Hsiao, C.-D. Deep Learning-Based Automatic Duckweed Counting Using StarDist and Its Application on Measuring Growth Inhibition Potential of Rare Earth Elements as Contaminants of Emerging Concerns. Toxics 2023, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Van Antro, M.; Prelovsek, S.; Ivanovic, S.; Gawehns, F.; Wagemaker, N.C.A.M.; Mysara, M.; Horemans, N.; Vergeer, P.; Verhoeven, K.J.F. DNA Methylation in Clonal Duckweed (Lemna minor L.) Lineages Reflects Current and Historical Environmental Exposures. Mol. Ecol. 2023, 32, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Roubeau Dumont, E.; Larue, C.; Lorber, S.; Gryta, H.; Billoir, E.; Gross, E.M.; Elger, A. Does Intraspecific Variability Matter in Ecological Risk Assessment? Investigation of Genotypic Variations in Three Macrophyte Species Exposed to Copper. Aquat. Toxicol. 2019, 211, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Fekete-Kertész, I.; Stirling, T.; Vaszita, E.; Berkl, Z.; Farkas, É.; Hedwig, S.; Remmen, K.; Lenz, M.; Molnár, M.; Feigl, V. Ecotoxicity Attenuation by Acid-Resistant Nanofiltration in Scandium Recovery from TiO2 Production Waste. Heliyon 2023, 9, e15512. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.; Nogueira, V.; Gavina, A.; Fernandes, S.; Rodrigues, J.L.; Pinto, V.V.; Ferreira, M.J.; Silva, A.M.; Pereira, C.M.; Pereira, R. Ecotoxicity to Freshwater Organisms and Cytotoxicity of Nanomaterials: Are We Generating Sufficient Data for Their Risk Assessment? Nanomaterials 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Iannilli, V.; Passatore, L.; Carloni, S.; Sciacca, G.; Cerasa, M.; Zacchini, M. Ecotoxicological and Genotoxic Effects of Dimethyl Phthalate (DMP) on Lemna Minor L. and Spirodela polyrhiza (L.) Schleid. Plants under a Short-Term Laboratory Assay. Sci. Total Environ. 2022, 806, 150972. [Google Scholar] [CrossRef] [PubMed]
- Groth, V.A.; Carvalho-Pereira, T.; da Silva, E.M.; Niemeyer, J.C. Ecotoxicological Assessment of Biosolids by Microcosms. Chemosphere 2016, 161, 342–348. [Google Scholar] [CrossRef]
- Sackey, L.N.A.; Mocová, K.A.; Kočí, V. Ecotoxicological Effect of Aged Wood Leachates to Aquatic Organisms. Water 2020, 12, 2091. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Ecotoxicological Evaluation of Propranolol Hydrochloride and Losartan Potassium to Lemna minor L. (1753) Individually and in Binary Mixtures. Ecotoxicology 2015, 24, 1112–1123. [Google Scholar] [CrossRef]
- Soares, C.; Fernandes, B.; Paiva, C.; Nogueira, V.; Cachada, A.; Fidalgo, F.; Pereira, R. Ecotoxicological Relevance of Glyphosate and Flazasulfuron to Soil Habitat and Retention Functions–Single vs Combined Exposures. J. Hazard. Mater. 2023, 442, 130128. [Google Scholar] [CrossRef]
- Ikebe Otomo, J.; Araujo de Jesus, T.; Gomes Coelho, L.H.; Rebelo Monteiro, L.; Hunter, C.; Helwig, K.; Roberts, J.; Pahl, O. Effect of Eight Common Brazilian Drugs on Lemna minor and Salvinia auriculata Growth. Environ. Sci. Pollut. Res. Int. 2021, 28, 43747–43762. [Google Scholar] [CrossRef]
- Hlavkova, D.; Caloudova, H.; Palikova, P.; Kopel, P.; Plhalova, L.; Beklova, M.; Havelkova, B. Effect of Gold Nanoparticles and Ions Exposure on the Aquatic Organisms. Bull. Environ. Contam. Toxicol. 2020, 105, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Alkimin, G.D.; Daniel, D.; Dionísio, R.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Effects of Diclofenac and Salicylic Acid Exposure on Lemna minor: Is Time a Factor? Environ. Res. 2019, 177, 108609. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.L.; Jonsson, C.M.; Silva, M.S.G.M.; Castanha, R.; Vallim, J.H.; da Silva, L.A.G.; de Oliveira, R.M.D.; Correa, D.S.; Ferreira, M.D. Estimates of AgNP Toxicity Thresholds in Support of Environmental Safety Policies. J. Nanoparticle Res. 2022, 24, 9. [Google Scholar] [CrossRef]
- Alkimin, G.D.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of Ketoprofen Toxicity in Two Freshwater Species: Effects on Biochemical, Physiological and Population Endpoints. Environ. Pollut. 2020, 265, 114993. [Google Scholar] [CrossRef]
- Alkimin, G.D.; Daniel, D.; Frankenbach, S.; Serôdio, J.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of Pharmaceutical Toxic Effects of Non-Standard Endpoints on the Macrophyte Species Lemna minor and Lemna gibba. Sci. Total Environ. 2019, 657, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.M.; Nogueira, V.; Lopes, I.; Rocha-Santos, T.; Pereira, R. Evaluation of the Potential Toxicity of Effluents from the Textile Industry before and after Treatment. Appl. Sci. 2019, 9, 3804. [Google Scholar] [CrossRef]
- Knežević, V.; Tunić, T.; Gajić, P.; Marjan, P.; Savić, D.; Tenji, D.; Teodorović, I. Getting More Ecologically Relevant Information from Laboratory Tests: Recovery of Lemna minor After Exposure to Herbicides and Their Mixtures. Arch. Environ. Contam. Toxicol. 2016, 71, 572–588. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Schott, J.; Hoenemann, L.; Feibicke, M. Glyceria maxima as New Test Species for the EU Risk Assessment for Herbicides: A Microcosm Study. Ecotoxicol. Lond. Engl. 2015, 24, 309–320. [Google Scholar] [CrossRef]
- Magahud, J.C.; Dalumpines, S.L.P. Growth of Duckweeds (Lemna minor L.) as Affected by Light Intensity, Nutrient Solution Concentration, and Light × Nutrient Interaction. Philipp. Sci. Lett. 2021, 14, 119–129. [Google Scholar]
- Burns, M.; Hanson, M.L.; Prosser, R.S.; Crossan, A.N.; Kennedy, I.R. Growth Recovery of Lemna gibba and Lemna minor Following a 7-Day Exposure to the Herbicide Diuron. Bull. Environ. Contam. Toxicol. 2015, 95, 150–156. [Google Scholar] [CrossRef]
- Havelkova, B.; Hlavkova, D.; Kovacova, V.; Beklova, M. Herbicides in the Cave Environment: Ecotoxicological Risks. Fresenius Environ. Bull. 2019, 28, 781–786. [Google Scholar]
- Xie, L.; Gomes, T.; Solhaug, K.A.; Song, Y.; Tollefsen, K.E. Linking Mode of Action of the Model Respiratory and Photosynthesis Uncoupler 3,5-Dichlorophenol to Adverse Outcomes in Lemna minor. Aquat. Toxicol. Amst. Neth. 2018, 197, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Rozman, U.; Jemec Kokalj, A.; Dolar, A.; Drobne, D.; Kalčíková, G. Long-Term Interactions between Microplastics and Floating Macrophyte Lemna minor: The Potential for Phytoremediation of Microplastics in the Aquatic Environment. Sci. Total Environ. 2022, 831, 154866. [Google Scholar] [CrossRef] [PubMed]
- Tofan, L.; Niță, V.; Nenciu, M.; Coatu, V.; Lazăr, L.; Damir, N.; Vasile, D.; Popoviciu, D.R.; Brotea, A.-G.; Curtean-Bănăduc, A.M.; et al. Multiple Assays on Non-Target Organisms to Determine the Risk of Acute Environmental Toxicity in Tebuconazole-Based Fungicides Widely Used in the Black Sea Coastal Area. Toxics 2023, 11, 597. [Google Scholar] [CrossRef]
- Varga, M.; Horvatić, J.; Barišić, L.; Lončarić, Z.; Dutour Sikirić, M.; Erceg, I.; Kočić, A.; Štolfa Čamagajevac, I. Physiological and Biochemical Effect of Silver on the Aquatic Plant Lemna gibba L.: Evaluation of Commercially Available Product Containing Colloidal Silver. Aquat. Toxicol. Amst. Neth. 2019, 207, 52–62. [Google Scholar] [CrossRef] [PubMed]
- de Alkimin, G.D.; Paisio, C.; Agostini, E.; Nunes, B. Phytoremediation Processes of Domestic and Textile Effluents: Evaluation of the Efficacy and Toxicological Effects in Lemna minor and Daphnia magna. Environ. Sci. Pollut. Res. Int. 2020, 27, 4423–4441. [Google Scholar] [CrossRef] [PubMed]
- Logeshwaran, P.; Sivaram, A.K.; Yadav, M.; Chadalavada, S.; Naidu, R.; Megharaj, M. Phytotoxicity of Class B Aqueous Firefighting Formulations, Tridol S 3 and 6% to Lemna minor. Environ. Technol. Innov. 2020, 18, 100688. [Google Scholar] [CrossRef]
- Pereira, S.P.P.; Jesus, F.; Aguiar, S.; de Oliveira, R.; Fernandes, M.; Ranville, J.; Nogueira, A.J.A. Phytotoxicity of Silver Nanoparticles to Lemna minor: Surface Coating and Exposure Period-Related Effects. Sci. Total Environ. 2018, 618, 1389–1399. [Google Scholar] [CrossRef]
- Mohiley, A.; Franzaring, J.; Calvo, O.C.; Fangmeier, A. Potential Toxic Effects of Aircraft De-Icers and Wastewater Samples Containing These Compounds. Environ. Sci. Pollut. Res. Int. 2015, 22, 13094–13101. [Google Scholar] [CrossRef]
- Di Baccio, D.; Pietrini, F.; Bertolotto, P.; Pérez, S.; Barcelò, D.; Zacchini, M.; Donati, E. Response of Lemna gibba L. to High and Environmentally Relevant Concentrations of Ibuprofen: Removal, Metabolism and Morpho-Physiological Traits for Biomonitoring of Emerging Contaminants. Sci. Total Environ. 2017, 584–585, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gopalapillai, Y.; Vigneault, B.; Hale, B.A. Root Length of Aquatic Plant, Lemna minor L., as an Optimal Toxicity Endpoint for Biomonitoring of Mining Effluents. Integr. Environ. Assess. Manag. 2014, 10, 493–497. [Google Scholar] [CrossRef]
- Daniel, D.; de Alkimin, G.D.; Nunes, B. Single and Combined Effects of the Drugs Salicylic Acid and Acetazolamide: Adverse Changes in Physiological Parameters of the Freshwater Macrophyte, Lemna gibba. Environ. Toxicol. Pharmacol. 2020, 79, 103431. [Google Scholar] [CrossRef]
- Gabriel, A.; Venâncio, C.; Sousa, J.P.; Leston, S.; Ramos, F.; Soares, A.M.V.M.; Lopes, I. Soil pH Matters in the Ecotoxicity of Basamid® to Freshwater Microalgae and Macrophytes. Sci. Total Environ. 2023, 859, 160165. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Patrolecco, L.; Rauseo, J.; Spataro, F.; Di Lenola, M.; Aimola, G.; Zacchini, M.; Pietrini, F.; Di Baccio, D.; Stanton, I.C.; et al. Sulfamethoxazole Persistence in a River Water Ecosystem and Its Effects on the Natural Microbial Community and Lemna minor Plant. Microchem. J. 2019, 149, 103999. [Google Scholar] [CrossRef]
- Sikorski, Ł.; Baciak, M.; Bęś, A.; Adomas, B. The Effects of Glyphosate-Based Herbicide Formulations on Lemna minor, a Non-Target Species. Aquat. Toxicol. Amst. Neth. 2019, 209, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Modlitbová, P.; Hlaváček, A.; Švestková, T.; Pořízka, P.; Šimoníková, L.; Novotný, K.; Kaiser, J. The Effects of Photon-Upconversion Nanoparticles on the Growth of Radish and Duckweed: Bioaccumulation, Imaging, and Spectroscopic Studies. Chemosphere 2019, 225, 723–734. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Novak, S.; Talaber, I.; Kononenko, V.; Mali, L.B.; Vodovnik, M.; Žegura, B.; Eleršek, T.; Kalčikova, G.; Gotvajn, A.Ž.; et al. The First Comprehensive Safety Study of Magnéli Phase Titanium Suboxides Reveals No Acute Environmental Hazard. Environ. Sci. Nano 2019, 6, 1131–1139. [Google Scholar] [CrossRef]
- Mihajlović, V.; Tomić, T.; Tubić, A.; Molnar Jazić, J.; Ivančev Tumbas, I.; Šunjka, D.; Lazić, S.; Teodorović, I. The Impact of Humic Acid on Toxicity of Individual Herbicides and Their Mixtures to Aquatic Macrophytes. Environ. Sci. Pollut. Res. Int. 2019, 26, 23571–23582. [Google Scholar] [CrossRef]
- Facin, F.; de Melo, J.V.S.; Lalau, C.M.; Nogueira, D.J.; Puerari, R.C.; Matias, W.G. Toxicological Effects of Leachate Extracts from Asphalt Mixtures Nanomodified under Daphnia magna and Landoltia punctata Test Organisms. Chemosphere 2021, 285, 131463. [Google Scholar] [CrossRef]
- Xie, L.; Song, Y.; Petersen, K.; Solhaug, K.A.; Lind, O.C.; Brede, D.A.; Salbu, B.; Tollefsen, K.E. Ultraviolet B Modulates Gamma Radiation-Induced Stress Responses in Lemna minor at Multiple Levels of Biological Organisation. Sci. Total Environ. 2022, 846, 157457. [Google Scholar] [CrossRef]
- Roubeau Dumont, E.; Gao, X.; Zheng, J.; Macairan, J.; Hernandez, L.M.; Baesu, A.; Bayen, S.; Robinson, S.A.; Ghoshal, S.; Tufenkji, N. Unraveling the Toxicity of Tire Wear Contamination in Three Freshwater Species: From Chemical Mixture to Nanoparticles. J. Hazard. Mater. 2023, 453, 131402. [Google Scholar] [CrossRef]
- Van Hoeck, A.; Horemans, N.; Van Hees, M.; Nauts, R.; Knapen, D.; Vandenhove, H.; Blust, R. β-Radiation Stress Responses on Growth and Antioxidative Defense System in Plants: A Study with Strontium-90 in Lemna minor. Int. J. Mol. Sci. 2015, 16, 15309–15327. [Google Scholar] [CrossRef]


| Median Effective Concentration (RGRfrond) | ||||
|---|---|---|---|---|
| Current Study (L. gibba) | Naumann et al. [47] (L. minor) | W | p | |
| EC20 | 2.27 | 0.086 | 2 | 0.023 |
| EC50 | 3.21 | 0.683 | 3 | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, M.; Mészáros, I.; Szabó, S.; Oláh, V. Comparative Phytotoxicity of Metallic Elements on Duckweed Lemna gibba L. Using Growth- and Chlorophyll Fluorescence Induction-Based Endpoints. Plants 2024, 13, 215. https://doi.org/10.3390/plants13020215
Irfan M, Mészáros I, Szabó S, Oláh V. Comparative Phytotoxicity of Metallic Elements on Duckweed Lemna gibba L. Using Growth- and Chlorophyll Fluorescence Induction-Based Endpoints. Plants. 2024; 13(2):215. https://doi.org/10.3390/plants13020215
Chicago/Turabian StyleIrfan, Muhammad, Ilona Mészáros, Sándor Szabó, and Viktor Oláh. 2024. "Comparative Phytotoxicity of Metallic Elements on Duckweed Lemna gibba L. Using Growth- and Chlorophyll Fluorescence Induction-Based Endpoints" Plants 13, no. 2: 215. https://doi.org/10.3390/plants13020215
APA StyleIrfan, M., Mészáros, I., Szabó, S., & Oláh, V. (2024). Comparative Phytotoxicity of Metallic Elements on Duckweed Lemna gibba L. Using Growth- and Chlorophyll Fluorescence Induction-Based Endpoints. Plants, 13(2), 215. https://doi.org/10.3390/plants13020215








