Microwave Control of Reynoutria japonica Houtt., Including Ecotoxicological Aspects and the Resveratrol Content in Rhizomes
Abstract
1. Introduction
2. Results
2.1. Efficacy of Japanese Knotweed Control Using Microwaves
2.2. Soil Ecotoxicity after Microwave Radiation
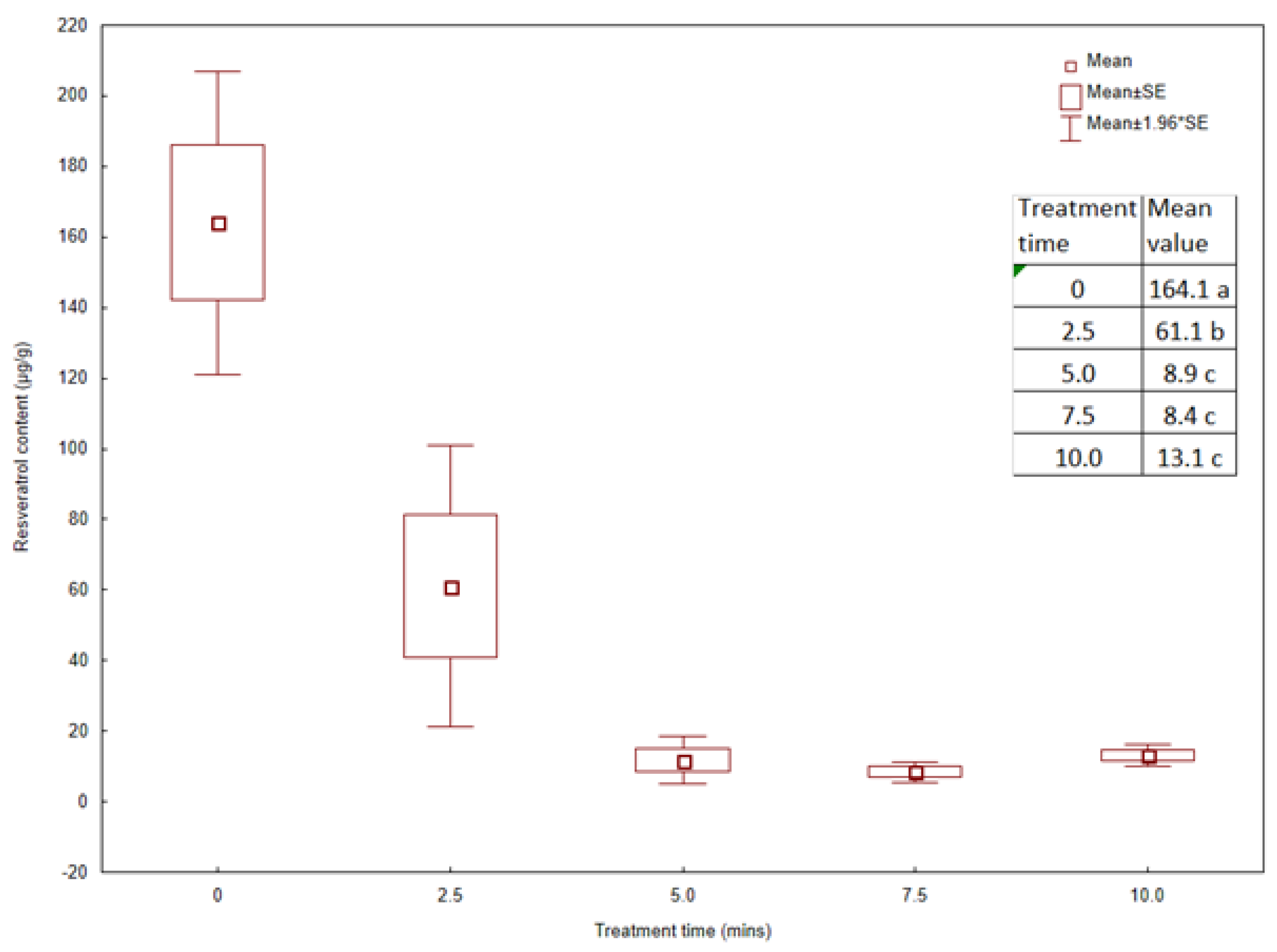
2.3. Content of Resveratrol in the Rhizomes of Japanese Knotweed Treated with Microwaves
3. Discussion
4. Materials and Methods
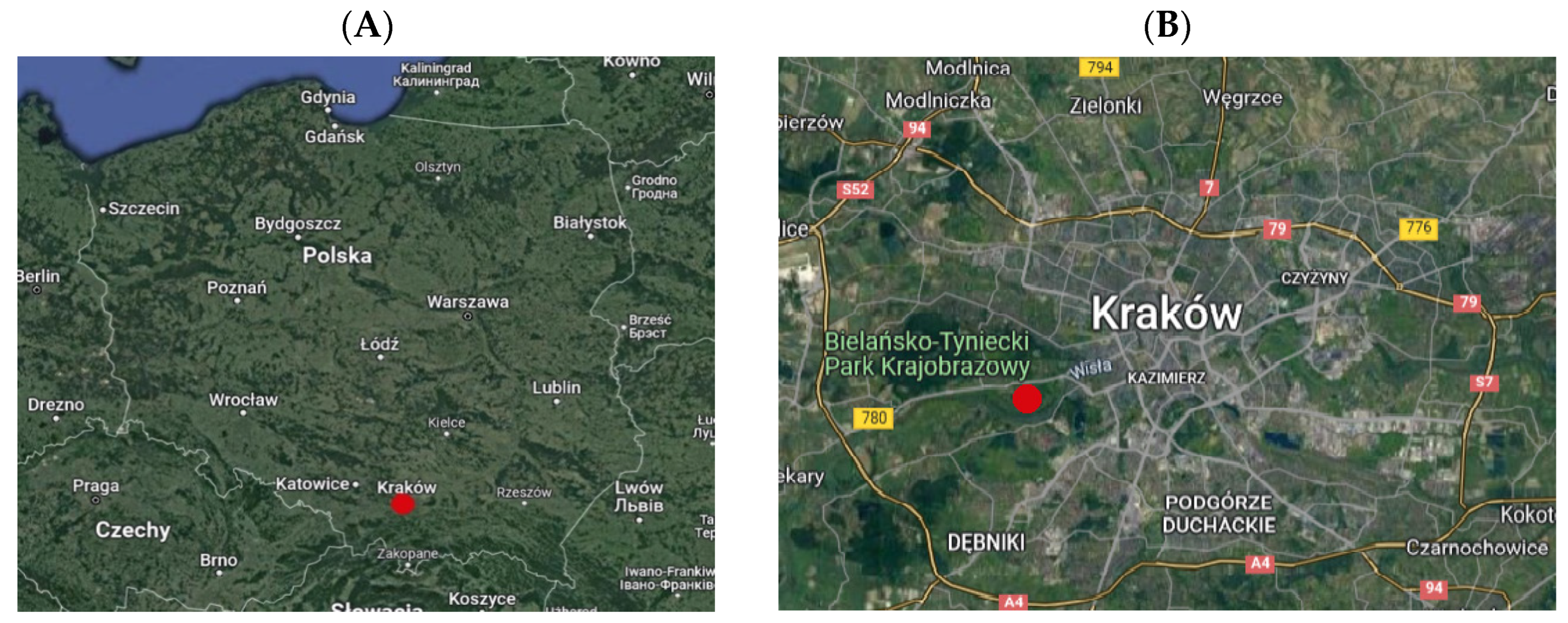
4.1. Characteristics of the Study Site and Japanese Knotweed Population
Chemical Characteristics of Soil and Weather Data from the Study Site
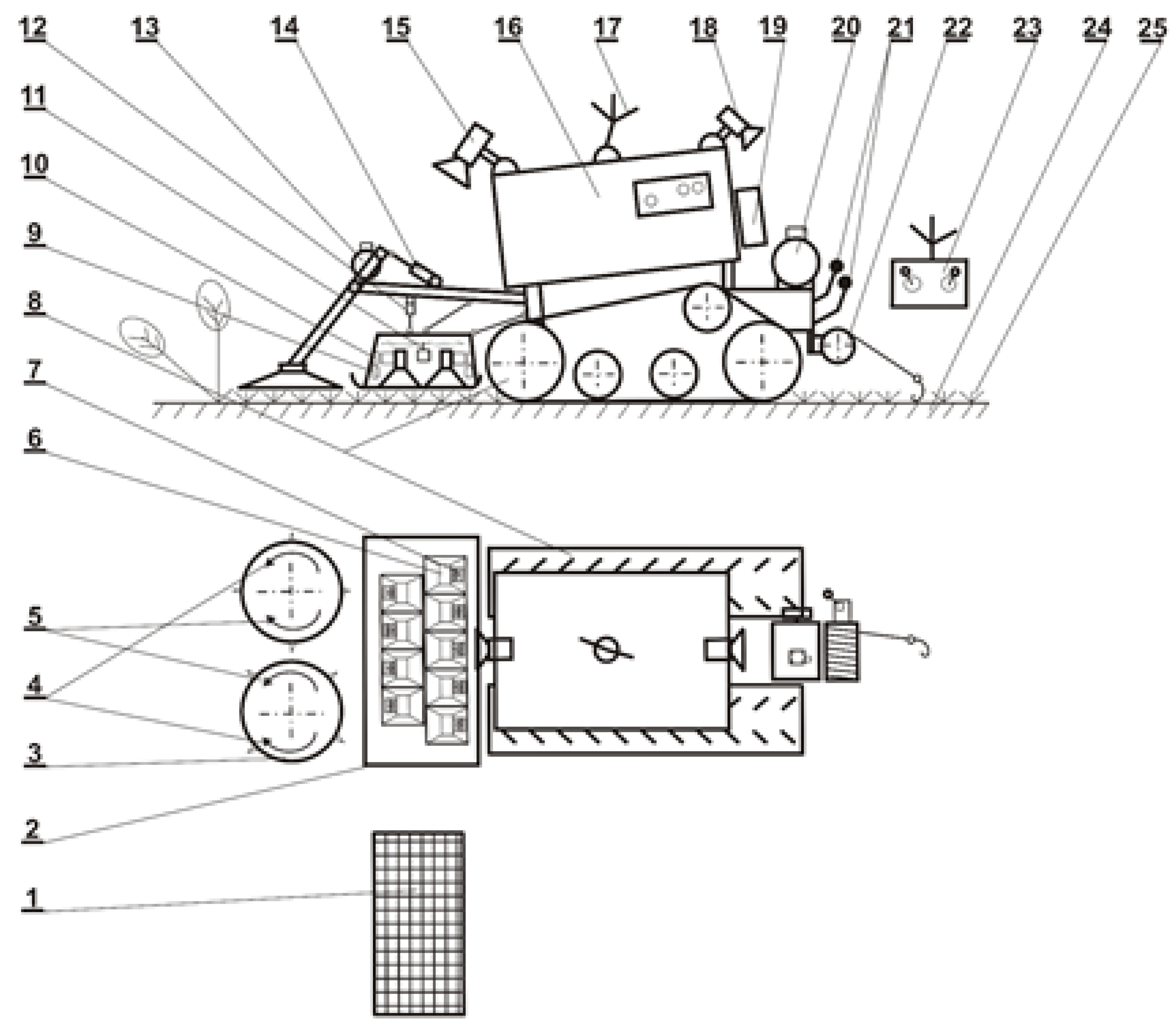
4.2. Control of Japanese Knotweed Using Microwaves
4.3. Ecotoxicological Analyses of Soil Treated with Microwaves
4.4. Analysis of Resveratrol Content in Rhizomes after Microwave Radiation
4.4.1. Reagents and Standard
4.4.2. Sample Preparation and Extraction Procedures
4.4.3. Method Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jug, U.; Naumoska, K.; Vovk, I. (-)-Epicatechin-An Important Contributor to the Antioxidant Activity of Japanese Knotweed. Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation. Antioxidants 2021, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Lindemann-Matthies, P. Beasts or beauties? Laypersons’ perception of invasive alien plant species in Switzerland and attitudes towards their management. NeoBiota 2016, 29, 15–33. [Google Scholar] [CrossRef]
- Rutkowski, L. Klucz Do Oznaczania Roślin Naczyniowych Polski Niżowej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2022. [Google Scholar]
- Chmura, D.; Tokarska-Guzik, B.; Nowak, T.; Woźniak, G.; Bzdęga, K.; Koszela, K.; Gancarek, M. The influence of invasive Fallopia taxa on resident plant species in two river valleys (southern Poland). Acta Soc. Bot. Pol. 2015, 84, 23–33. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B.; Bzdęga, K.; Knapik, D.; Jenczała, G. Changes in plant richness in some riparian plant communities as a result of their colonization by taxa of Reynoutria (Fallopia). Biodiv. Res. Conserv. 2006, 1–2, 123–130. [Google Scholar]
- GISD Global Invasive Species Database. Polygonum Cuspidatum (Sieb. & Zucc.). 2010. Available online: http://www.issg.org/database/species/ecology.asp?si=91&fr=1&sts=sss&lang=EN/ (accessed on 3 January 2023).
- Alberternst, B.; Böhmer, H.J. NOBANIS—Invasive Alien Species Fact Sheet—Fallopia japonica. Online Database of the North European and Baltic Network on Invasive Alien Species. 2011. Available online: www.nobanis.org/ (accessed on 3 January 2023).
- CABI Commonwealth Agricultural Bureau International. Datasheet: Fallopia japonica. 2014. Available online: https://www.cabdirect.org/cabdirect/abstract/20097401007/ (accessed on 2 January 2023).
- Kowalczyk, B. Rdestowiec japoński (Reynoutria japonica Houtt.)—Gatunek inwazyjny i leczniczy—Rozprzestrzenianie się w gminie Krzyżanowice (Kotlina Raciborska). Ann. Acad. Medicae Silesiensis 2009, 63, 48–53. (In Polish) [Google Scholar]
- Moravcová, L.; Pyšek, P.; Jarošík, V.; Zákravský, P. Potential phytotoxic and shading effects of invasive Fallopia (Polygonaceae) taxa on the germination of dominant native species. NeoBiota 2011, 9, 31–47. [Google Scholar] [CrossRef]
- Colleran, B.; Lacy, S.N.; Retamal, M.R. Invasive Japanese knotweed (Reynoutria japonica Houtt.) and related knotweeds as catalysts for streambank erosion. River Res. Applic. 2020, 36, 1962–1969. [Google Scholar] [CrossRef]
- Cucu, A.A.; Baci, G.M.; Dezsi, Ş.; Nap, M.E.; Beteg, F.I.; Bonta, V.; Bobiş, O.; Caprio, E.; Dezmirean, D.S. New approaches on Japanese knotweed (Fallopia japonica) bioactive compounds and their potential of pharmacological and beekeeping activities: Challenges and future directions. Plants 2021, 10, 2621. [Google Scholar] [CrossRef]
- Shimoda, M.; Yamasaki, N. Fallopia japonica (Japanese Knotweed) in Japan: Why is it not a pest for Japanese people? In Vegetation Structure and Function at Multiple Spatial, Temporal and Conceptual Scales; Pedrotti, F., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 447–473. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How much wine do you have to drink to stay healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J. Profile of bioactive compounds in the morphological parts of wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and their antioxidative activity. Molecules 2019, 24, 1436. [Google Scholar] [CrossRef] [PubMed]
- Vastano, B.C.; Chen, Y.; Zhu, N.; Ho, C.T.; Zhou, Z.; Rosen, R.T. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J. Agric. Food Chem. 2000, 48, 253–256. [Google Scholar] [CrossRef]
- Barney, J.N.; Tharayil, N.; DiTommaso, A.; Bhowmik, P.C. The biology of invasive alien plants in Canada. 5. Polygonum cuspidatum Sieb. & Zucc.[= Fallopia japonica (houtt.) Ronse Decr.]. Can. J. Plant Sci. 2006, 86, 887–906. [Google Scholar] [CrossRef]
- Jones, D.; Bruce, G.; Fowler, M.S.; Law-Cooper, R.; Graham, I.; Abel, A.; Street-Perrott, F.A.; Eastwood, D. Optimising physiochemical control of invasive Japanese knotweed. Biol. Invasions 2018, 20, 2091–2105. [Google Scholar] [CrossRef]
- Jones, D.; Eastwood, D. Sustainable Control of Japanese Knotweed. Outlooks Pest Manag. 2019, 30, 195–200. [Google Scholar] [CrossRef]
- Ito, M.; Ito, K. Japanese knotweed control with winter soil injection of chemicals targeting the rhizome system. Weed Biol. Manag. 2021, 21, 202–208. [Google Scholar] [CrossRef]
- Lawson, J.W.; Fennell, M.; Smith, M.W.; Bacon, K.L. Regeneration and growth in crowns and rhizome fragments of Japanese knotweed (Reynoutria japonica) and desiccation as a potential control strategy. PeerJ 2021, 9, e11783. [Google Scholar] [CrossRef]
- Francis, R.A.; Riley, K.A.; Hoggart, S.P.G. Vegetative regeneration of Fallopia japonica (Houtt.) Ronse Decraene (Japanese knotweed) at varying burial depths. Weed Biol. Manag. 2008, 8, 69–72. [Google Scholar] [CrossRef]
- Djeddour, D.H.; Shaw, R.H.; Evans, H.C.; Tanner, R.A.; Kurose, D.; Takahashi, N.; Seier, M. Could Fallopia japonica be the first target for classical weed biocontrol in Europe? In Proceedings of the XII International Symposium on Biological Control of Weeds, La Grande Motte, France, 22–27 April 2007; CABI: Wallingford, UK, 2008; pp. 463–469. [Google Scholar]
- Brodie, G.; Ryan, C.; Lancaster, C. Microwave Technologies as Part of an Integrated Weed Management Strategy: A Review. Int. J. Agron. 2012, 2012, 636905. [Google Scholar] [CrossRef]
- Kanwal, S.; Tariq, M.; Dawar, S. Effect of microwave radiation on plants infected with root rot pathogens. Pak. J. Bot. 2018, 50, 2389–2393. [Google Scholar]
- Maynaud, G.; Baudoin, E.; Bourillon, J.; Duponnois, R.; Cleyet-Marel, J.C.; Brune, B. Short-term effect of 915-MHz microwave treatments on soil physicochemical and biological properties. Eur. J. Soil Sci. 2019, 70, 443–453. [Google Scholar] [CrossRef]
- Słowiński, K.; Grygierzec, B.; Synowiec, A.; Tabor, S.; Araniti, F. Preliminary Study of Control and Biochemical Characteristics of Giant Hogweed (Heracleum sosnowskyi Manden.) Treated with Microwaves. Agronomy 2022, 12, 1335. [Google Scholar] [CrossRef]
- Cooper, A.; Brodie, G. The effect of microwave radiation and soil depth on soil pH, N, P, K, SO4 and bacterial colonies. Plant Prot. Q. 2009, 24, 67–70. [Google Scholar]
- Smith, J.M.D.; Ward, J.P.; Child, L.E.; Owen, M.R. A simulation model of rhizome networks for Fallopia japonica (Japanese knotweed) in the United Kingdom. Ecol. Model. 2007, 200, 421–432. [Google Scholar] [CrossRef]
- Halmagyi, A.; Surducan, E.; Surducan, V. The effect of low-and high-power microwave irradiation on in vitro grown Sequoia plants and their recovery after cryostorage. J. Biol. Phys. 2017, 43, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Skiles, J.W. Plant response to microwaves at 2.45 GHz. Acta Astronaut. 2006, 58, 258–263. [Google Scholar] [CrossRef]
- Senavirathna, M.D.H.J.; Asaeda, T. Microwaves affect Myriophyllum aquaticum plants differently depending on the wave polarization. Biol. Plant. 2017, 61, 378–384. [Google Scholar] [CrossRef]
- Surducan, V.; Surducan, E.; Neamtu, C.; Mot, A.C.; Ciorîta, A. Effects of Long-Term Exposure to Low-Power 915 MHz Unmodulated Radiation on Phaseolus vulgaris L. Bioelectromagnetics 2020, 41, 200–212. [Google Scholar] [CrossRef]
- Diprose, M.F.; Benson, F.A.; Willis, A.J. The effect of externally applied electrostatic fields, microwave radiation and electric currents on plants and other organisms, with special reference to weed control. Bot. Rev. 1984, 50, 171–223. [Google Scholar] [CrossRef]
- Heise, S.; Babut, M.; Casado, C.; Feiler, U.; Ferrari, B.J.D.; Marziali, L. Ecotoxicological testing of sediments and dredged material: An overlooked opportunity? J. Soils Sedim. 2020, 20, 4218–4228. [Google Scholar] [CrossRef]
- Baran, A.; Tack, F.M.G.; Delemazure, A.; Wieczorek, J.; Tarnawski, M.; Birch, G. Metal contamination in sediments of dam reservoirs: A multi-facetted generic risk assessment. Chemosphere 2023, 310, 136760. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, J.; Baran, A. Pollution indices and biotests as useful tools for the evaluation of the degree of soil contamination by trace elements. J. Soils Sedim. 2022, 22, 559–576. [Google Scholar] [CrossRef]
- Korablev, R.A.; Belocurov, V.P.; Busarin, E.N. Effect mechanisms of ultrahigh-frequency radiation on biological objects. IOP Conf. Ser. Earth Environ. Sci. 2021, 875, 012017. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Mujumdar, A.S.; Zhou, L.; Sun, J. Studies on hot air and microwave vacuum drying of wild cabbage. Drying Technol. 2004, 22, 2201–2209. [Google Scholar] [CrossRef]
- Yuan, J.F.; Wang, T.T.; Wang, D.H.; Zhou, G.H.; Zou, G.X.; Wang, Y.; Gong, M.G.; Zhang, B. Effect of Microwave on Changes of Gallic Acid and Resveratrol in a Model Extraction Solution. Food Bioprocess Technol. 2020, 13, 1246–1254. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Wu, X.; Wang, Y.; Lin, Y.; Wu, D.; Zhang, H.; Qin, J. Molecular Analysis of UV-C Induced Resveratrol Accumulation in Polygonum cuspidatum Leaves. Int. J. Mol. Sci. 2019, 20, 6185. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature. Policy Statement on Research Involving Species at Risk of Extinction. In Proceedings of the 27th Meeting of IUCN Council, Gland, Switzerland, 14 June 1989. [Google Scholar]
- Phytotoxkit, F.T.M. Seed germination and early growth microbiotest with higher plants. In Standard Operational Procedure; MicroBioTest Inc.: Nazareth, Belgium, 2004; p. 24. [Google Scholar]
- Ostracodtoxkit, F. Direct contact toxicity test for freshwater sediments. In Standard Operational Procedure; MicroBioTest Inc.: Nazareth, Belgium, 2001; p. 35. [Google Scholar]
- Microbics Corporation. Microtox Manual Toxicity Testing Handbook; Microbics Corporation: Carlsbad, CA, USA, 1992. [Google Scholar]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef]

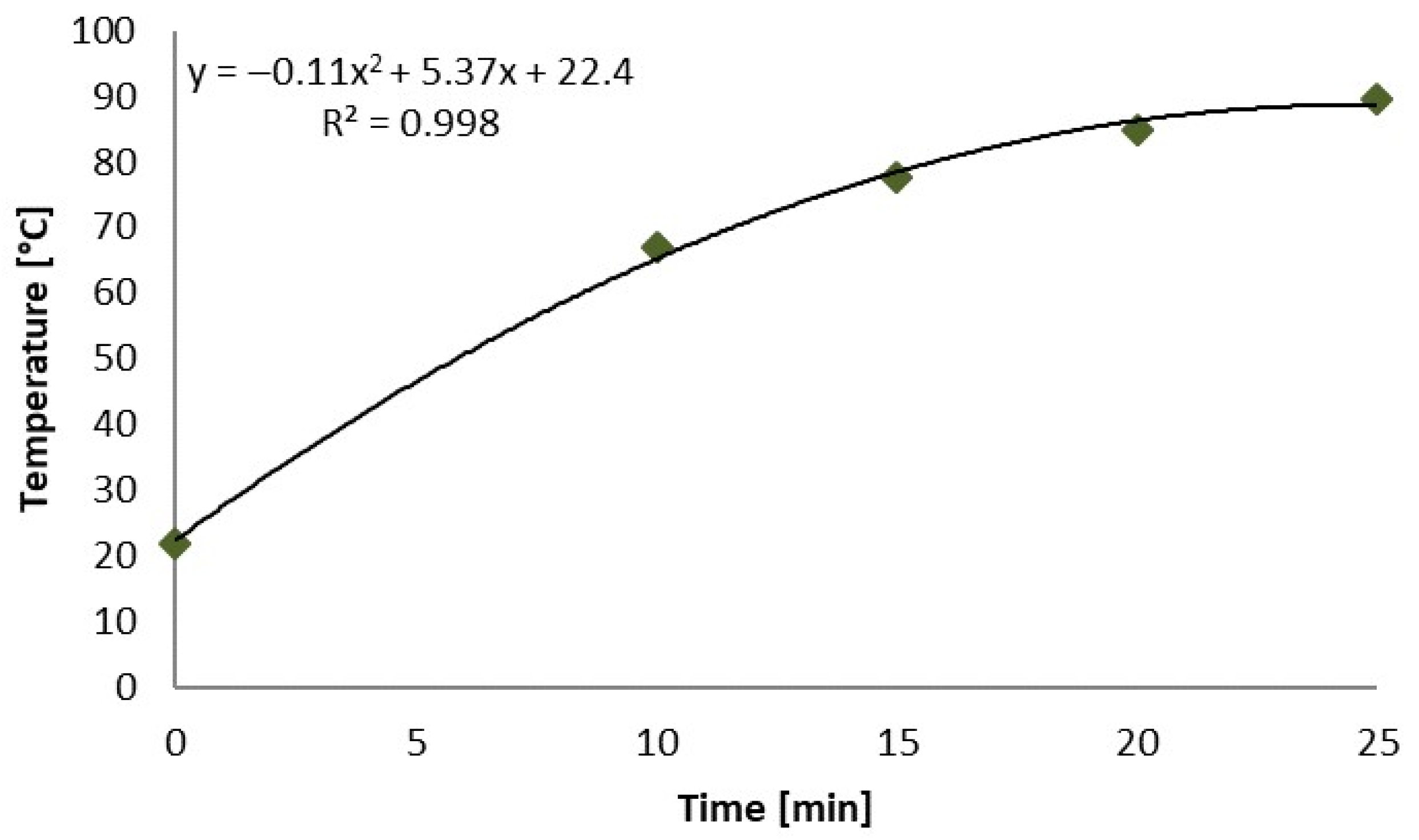


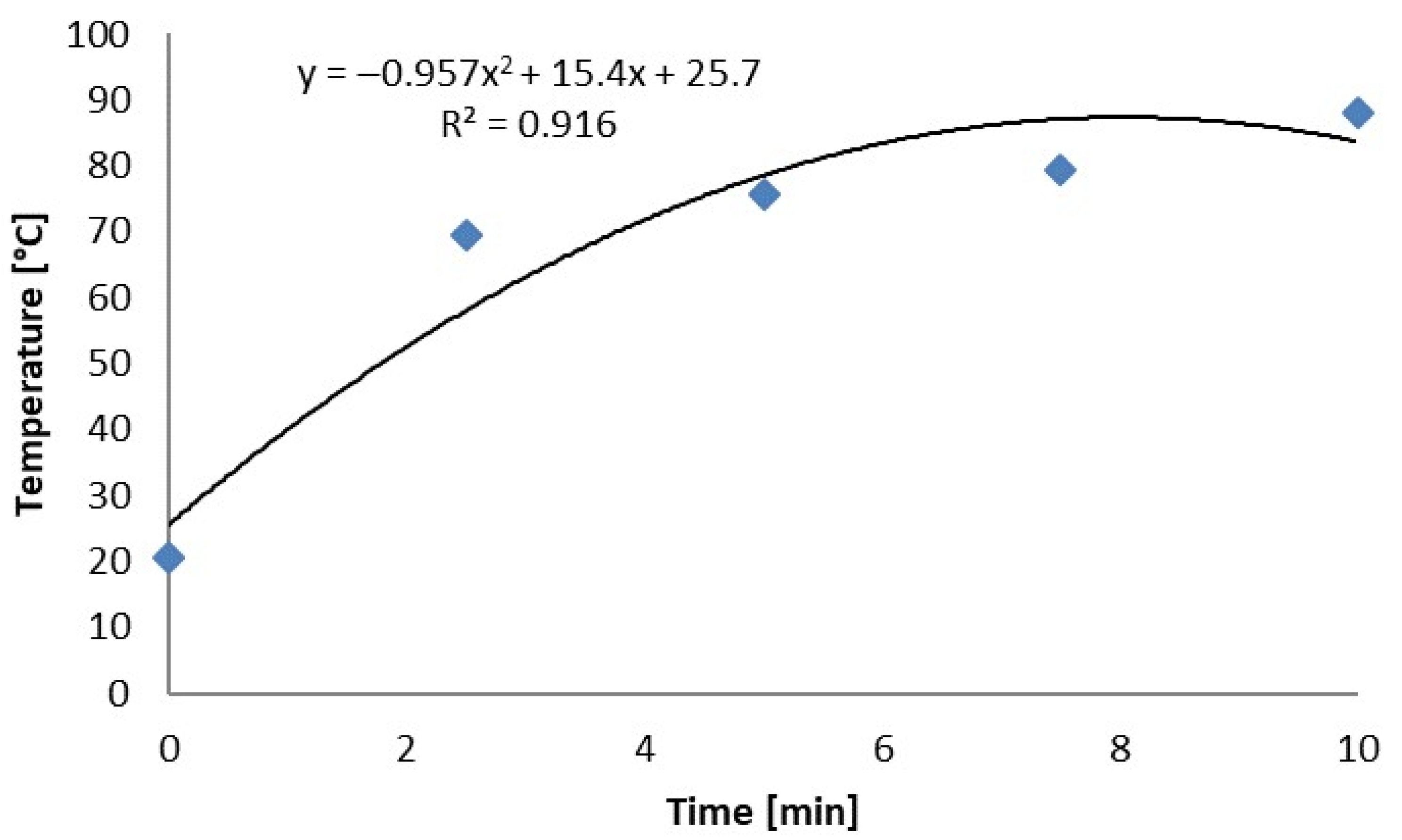
| Radiation Time (min) | 0.0 | 5.0 | 7.5 | 10.0 | 15.0 | 20.0 | 25.0 |
|---|---|---|---|---|---|---|---|
| Mean (°C) | 21.9 | 45.4 | 53.7 | 67.0 | 77.6 | 84.8 | 89.6 |
| Standard Deviation (°C) | 0.6 | 5.2 | 4.5 | 8.2 | 11.6 | 13.5 | 18.0 |
| Maximum (°C) | 24.3 | 56.1 | 64.0 | 95.9 | 112.3 | 137.7 | 150.2 |
| Minimum (°C) | 20.3 | 29.9 | 34.0 | 42.9 | 42.4 | 36.3 | 39.5 |
| Radiation | S. alba | S. saccharatum | H. incongruens | A. fischeri | Class Toxicity 6 | |||
|---|---|---|---|---|---|---|---|---|
| IG 1 | IR 2 | IG | IR | M 3 | GI 4 | IL 5 | ||
| Percentage Toxic Effect PE% | ||||||||
| 0 min (control) | 0 | 42 | 0 | 5 | 0 | 41 | 9 b 7 | II |
| 5 min | - | - | - | - | 0 | 13 | −9 a | I |
| 7.5 min | 21 | 25 | 8 | −14 | 0 | 35 | −4 a | II |
| 10 min | 7 | −10 | 0 | −25 | 0 | 37 | −3 a | II |
| 15 min | 14 | 21 | 0 | −5 | 0 | 35 | 20 c | II |
| 20 min | 21 | 43 | 0 | −7 | 0 | 37 | 22 c | II |
| 25 min | 14 | 37 | 0 | −14 | 0 | 48 | 26 c | II |
| Time of Radiation (min) | 0.0 | 2.5 | 5.0 | 7.5 | 10.0 |
|---|---|---|---|---|---|
| Mean (°C) | 20.3 | 69.4 | 75.8 | 79.5 | 87.9 |
| Std. Dev. (°C) | 0.1 | 13.0 | 7.4 | 10.2 | 10.6 |
| Maximum (°C) | 20.8 | 101.7 | 90.1 | 108.8 | 116.0 |
| Minimum (°C) | 19.9 | 40.2 | 49.1 | 42.8 | 45.8 |
| Sand % | Loam % | Clay % | pH - | C org. % | N g/kg | S g/kg | P g/kg | K g/kg | Ca g/kg |
| 70 | 26 | 4 | 6.06 | 2.55 | 1.95 | 0.38 | 0.68 | 2.47 | 6.21 |
| Mg g/kg | Na g/kg | Fe g/kg | Mn g/kg | Zn g/kg | Cu mg/kg | Ni mg/kg | Cd mg/kg | Pb mg/kg | Cr mg/kg |
| 3.20 | 0.19 | 13.23 | 0.68 | 153.55 | 17.35 | 34.44 | 0.66 | 32.20 | 32.60 |
| Test | Model Organism | Final Parameter | Time |
|---|---|---|---|
| Phytotoxkit | Sinapis alba, Sorghum saccharatum | Inhibition of germination, inhibition of root elongation | 72 h |
| Ostracodtoxkit | Heterocypris incongruens | Death, growth inhibition | 6 dni |
| Microtox | Aliivibrio fischeri | Luminescence inhibition | 15 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słowiński, K.; Grygierzec, B.; Baran, A.; Tabor, S.; Piatti, D.; Maggi, F.; Synowiec, A. Microwave Control of Reynoutria japonica Houtt., Including Ecotoxicological Aspects and the Resveratrol Content in Rhizomes. Plants 2024, 13, 152. https://doi.org/10.3390/plants13020152
Słowiński K, Grygierzec B, Baran A, Tabor S, Piatti D, Maggi F, Synowiec A. Microwave Control of Reynoutria japonica Houtt., Including Ecotoxicological Aspects and the Resveratrol Content in Rhizomes. Plants. 2024; 13(2):152. https://doi.org/10.3390/plants13020152
Chicago/Turabian StyleSłowiński, Krzysztof, Beata Grygierzec, Agnieszka Baran, Sylwester Tabor, Diletta Piatti, Filippo Maggi, and Agnieszka Synowiec. 2024. "Microwave Control of Reynoutria japonica Houtt., Including Ecotoxicological Aspects and the Resveratrol Content in Rhizomes" Plants 13, no. 2: 152. https://doi.org/10.3390/plants13020152
APA StyleSłowiński, K., Grygierzec, B., Baran, A., Tabor, S., Piatti, D., Maggi, F., & Synowiec, A. (2024). Microwave Control of Reynoutria japonica Houtt., Including Ecotoxicological Aspects and the Resveratrol Content in Rhizomes. Plants, 13(2), 152. https://doi.org/10.3390/plants13020152







