Orchestrated Movement Sequences and Shape-Memory-like Effects in Pine Cones
Abstract
:1. Introduction
2. Methods
2.1. Kinematic Analyses of Cones
2.1.1. General Procedures and Settings for Video Analyses of Whole Cones
2.1.2. Experiment A: Unmanipulated Cone Opening
2.1.3. Experiment B: Manipulation of Cone Surfaces with Vaseline
| Cone | Treatment |
|---|---|
| c1 | Distal 2/3 region of cone covered with Vaseline |
| c2 | Cone completely covered with Vaseline |
| c3 | Basal 2/3 region covered with Vaseline |
| c4 | Basal 1/3 region covered with Vaseline |
| c5 | One half of the cone (longitudinal) covered with Vaseline |
| c6 | Cone completely covered with Vaseline, except for four connected central scales |
| c7 | Cone completely covered with Vaseline, except for a helical row of scales |
| c8 | Cone completely covered with Vaseline, except for a central horizontal row of scales |
2.2. Kinematic Analyses of Scales
2.2.1. General Procedures and Settings for Video Analyses of Scales
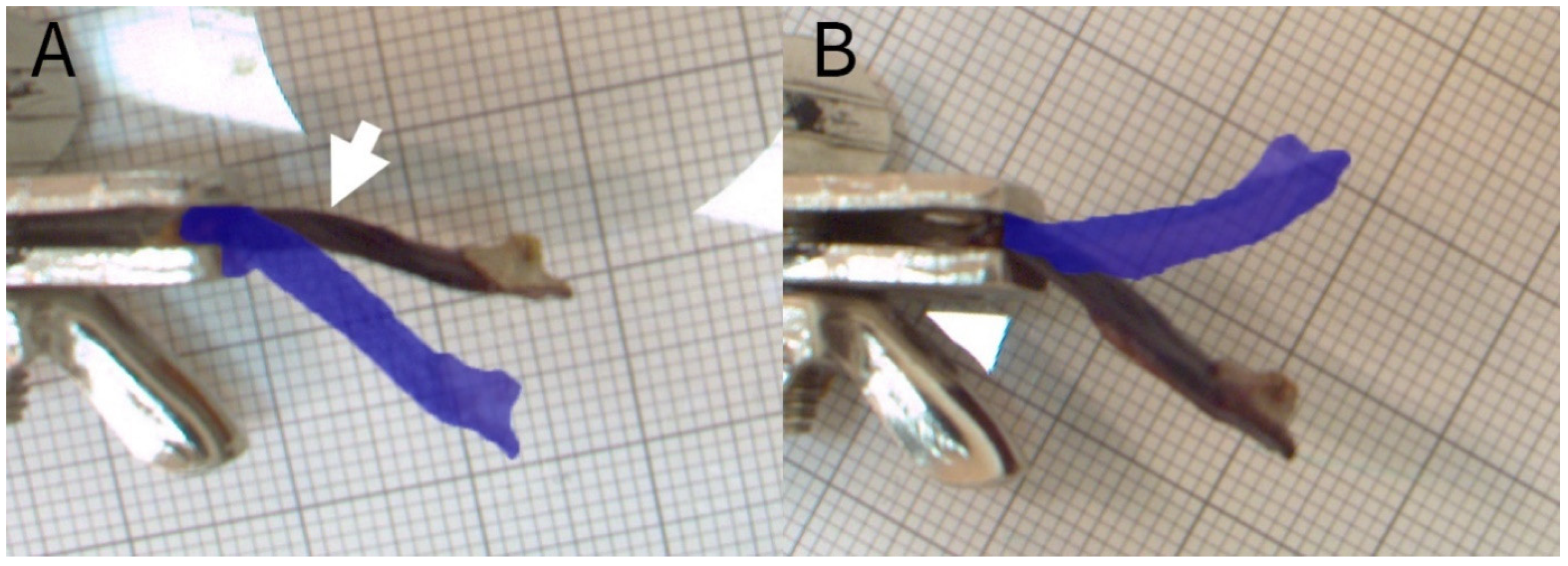
2.2.2. Experiment C: Shape-Memory-like Effects in Scales
2.2.3. Experiment D: Manipulation of Scale Surfaces with Vaseline
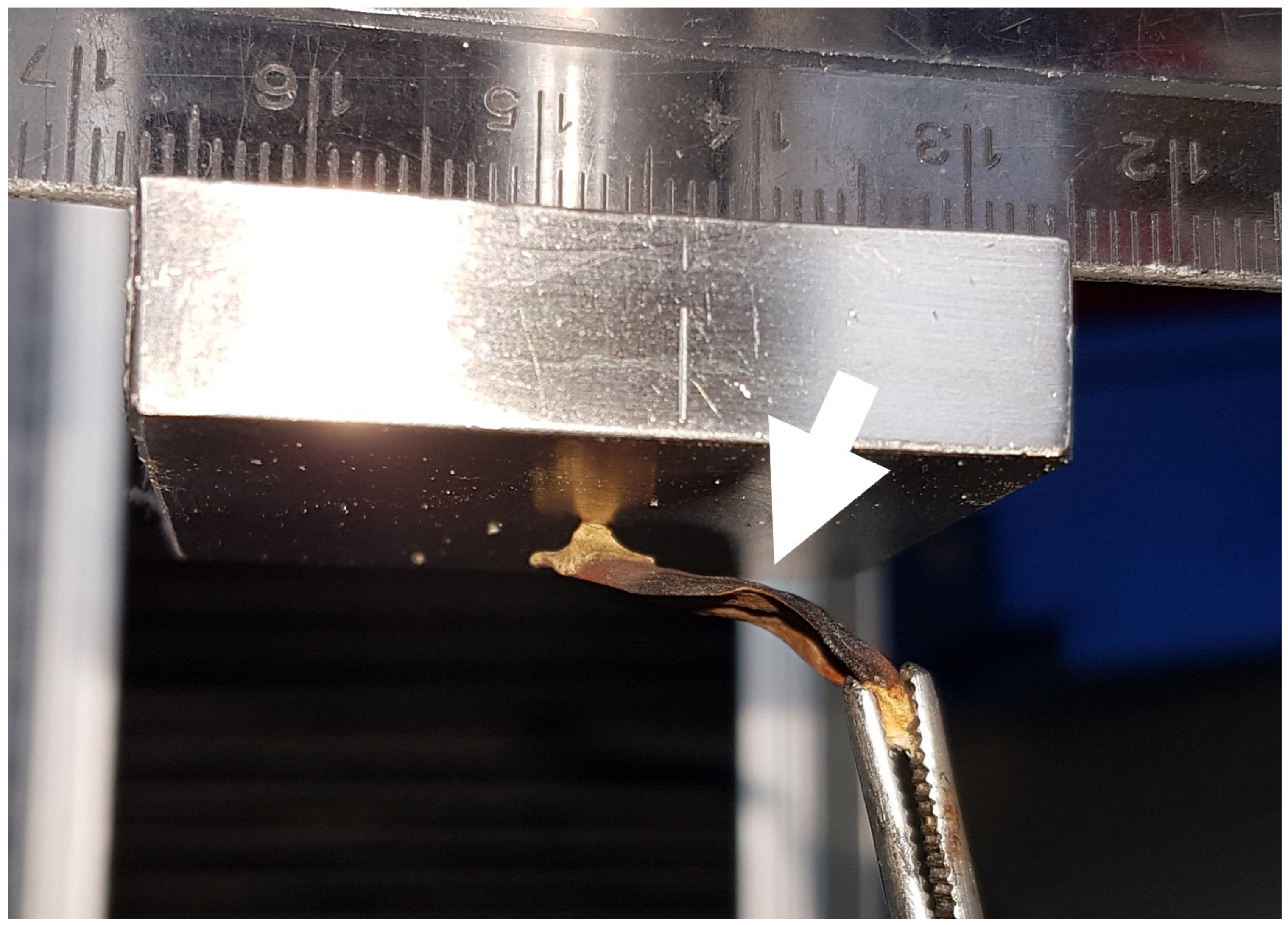
2.3. Force Measurements
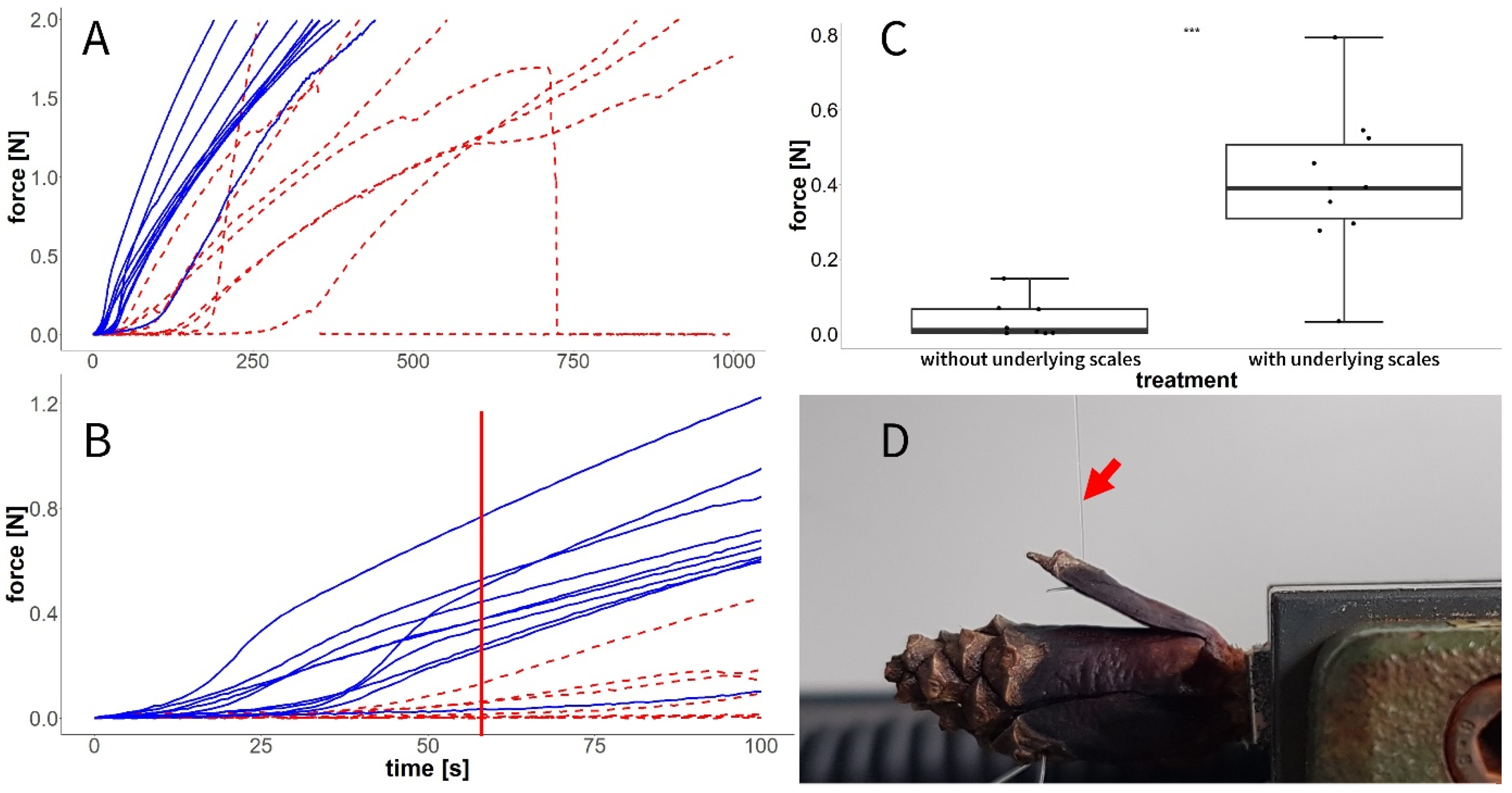
2.3.1. Experiment E/F: Repeated Blocking Force Measurements
2.3.2. Experiment G: Forces in the Closed Cone
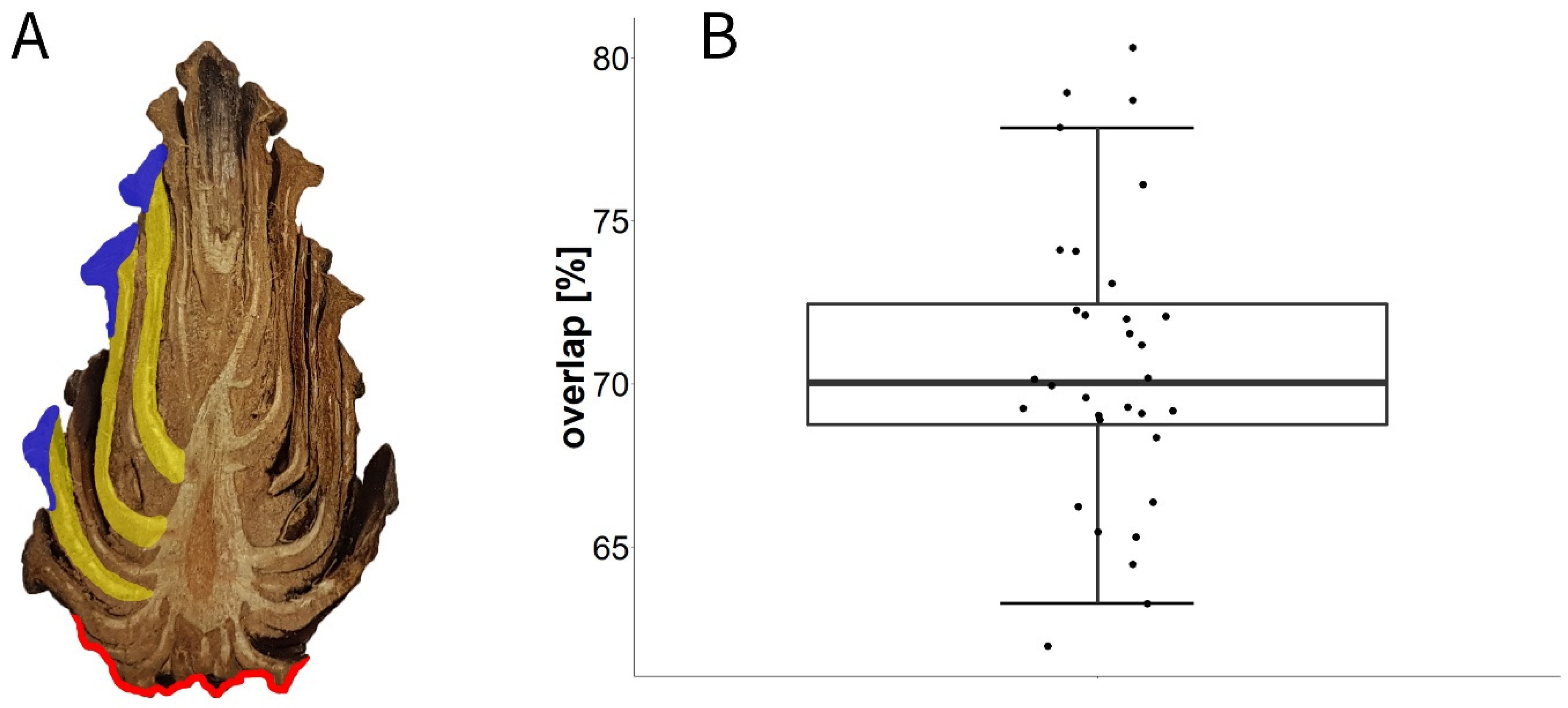
2.4. Experiment H: Morphological Overlap Measurements
2.5. Statistics
3. Results
3.1. Experiment A: Repetitive Cone Opening and Scale Movement Orchestration
3.2. Experiment B: Manipulated Repetitive Cone Opening
3.3. Manipulated Repetitive Scale Movement
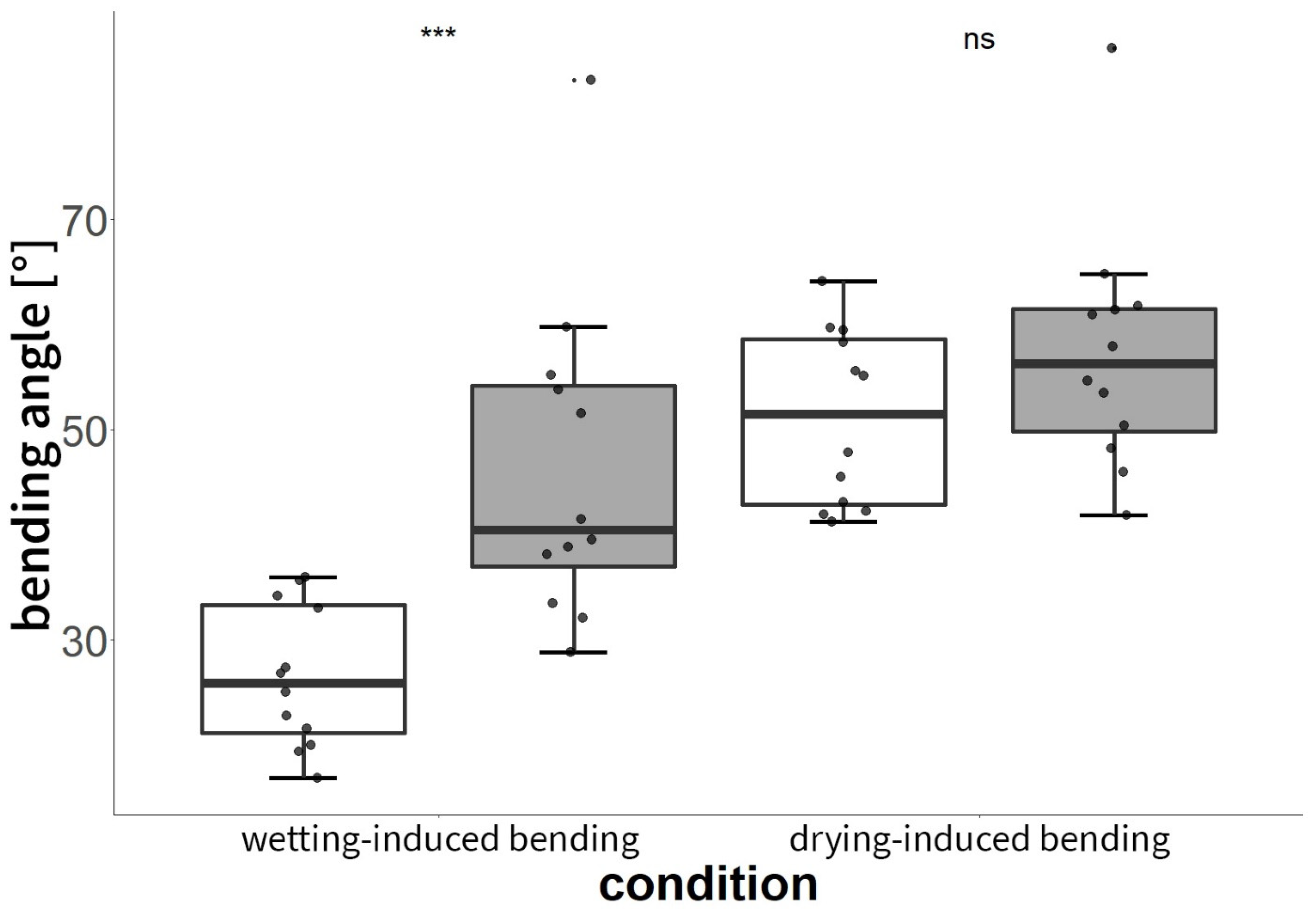
3.3.1. Experiment C: Shape-Memory-like Effects of Scales
3.3.2. Experiment D: Manipulation of Isolated Scales with Vaseline
3.4. Blocking Force Measurements
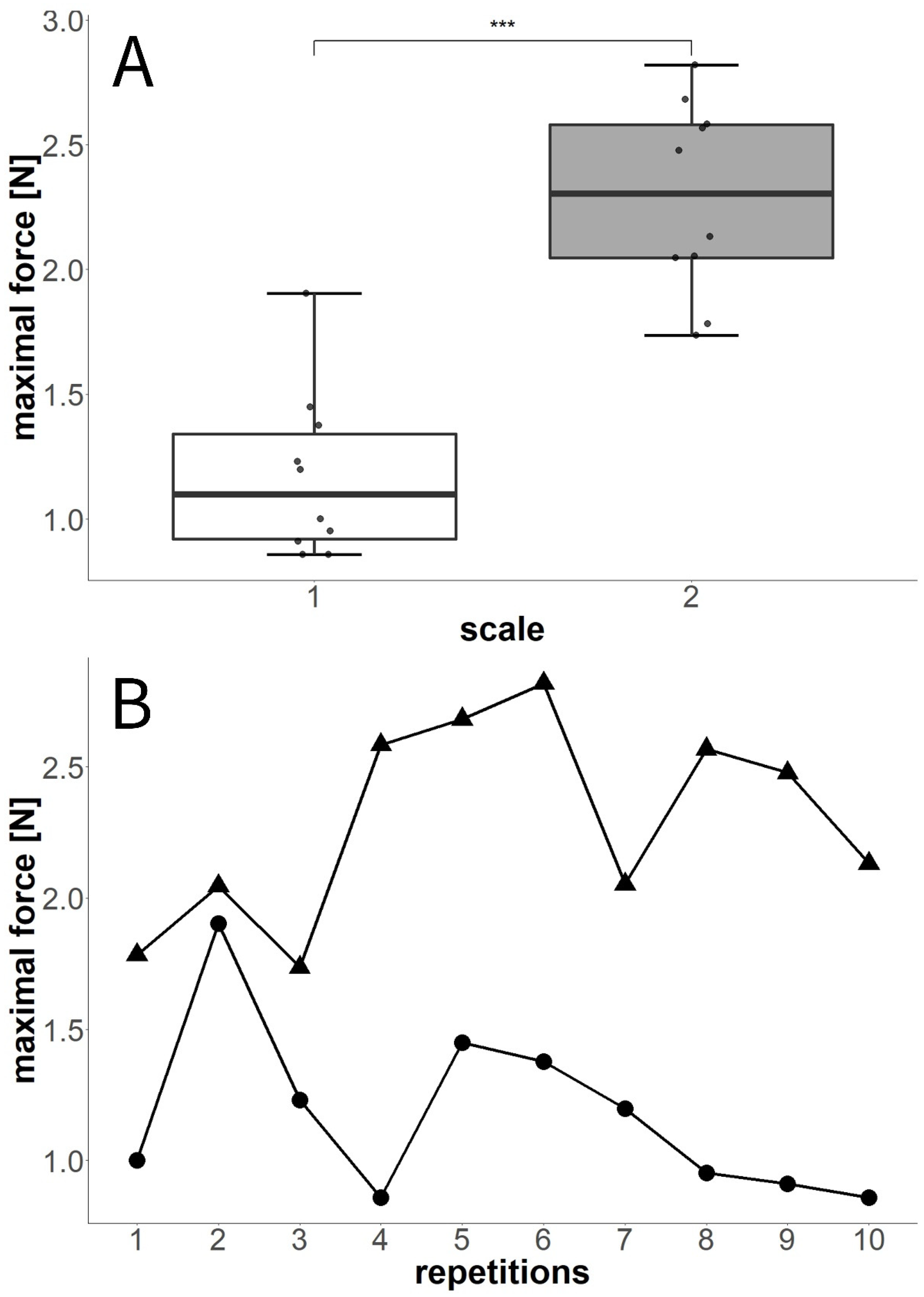
3.4.1. Experiment E: Twice-Repeated Blocking Force Measurements
3.4.2. Experiment F: Tenfold-Repeated Blocking Force Measurements
3.5. Experiment G: Forces in the Closed State
3.6. Experiment H: Overlap Measurements
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaw, G.R. The Genus Pinus; Publications of the Arnold Arboretum No. 5; The Riverside Press: Rolling Meadows, IL, USA; Cambridge, MA, USA, 1914. [Google Scholar] [CrossRef]
- Allen, R.; Wardrop, A.B. The opening and shedding mechanism of the female cones of Pinus radiata. Aust. J. Bot. 1964, 12, 125–134. [Google Scholar] [CrossRef]
- Harlow, W.M.; Côté, W.A.; Day, A.C. The opening mechanism of pine cone scales. J. For. 1964, 62, 538–540. [Google Scholar] [CrossRef]
- Dawson, C.; Vincent, J.F.V.; Rocca, A.-M. How pine cones open. Nature 1997, 390, 668. [Google Scholar] [CrossRef]
- Quan, H.; Pirosa, A.; Yang, W.; Ritchie, R.O.; Meyers, M.A. Hydration-induced reversible deformation of the pine cone. Acta Biomater. 2021, 128, 370–383. [Google Scholar] [CrossRef]
- Song, K.; Chang, S.S.; Lee, S.J. How the pine seeds attach to/detach from the pine cone scale? Front. Life Sci. 2017, 10, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Yeom, E.; Seo, S.J.; Kim, K.; Kim, H.; Lim, J.H.; Lee, S.J. Journey of water in pine cones. Sci. Rep. 2015, 5, 9963. [Google Scholar] [CrossRef]
- Losada, J.M.; Blanco-Moure, N.; Leslie, A.B. Not all ‘pine cones’ flex: Functional trade-offs and the evolution of seed release mechanisms. New Phytol. 2019, 222, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Eger, C.J.; Horstmann, M.; Poppinga, S.; Sachse, R.; Thierer, R.; Nestle, N.; Bruchmann, B.; Speck, T.; Bischoff, M.; Rühe, J. The structural and mechanical basis for passive-hydraulic pine cone actuation. Adv. Sci. 2022, 9, 2200458. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, M.; Xu, X.; Liu, X.; Liu, H.; Jiang, L.; Wang, S. Unperceivable motion mimicking hygroscopic geometric reshaping of pine cones. Nat. Mater. 2022, 21, 1357–1365. [Google Scholar] [CrossRef]
- Correa, D.; Poppinga, S.; Mylo, M.D.; Westermeier, A.S.; Bruchmann, B.; Menges, A.; Speck, T. 4D pine scale: Biomimetic 4D printed autonomous scale and flap structures capable of multi-phase movement. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190445. [Google Scholar] [CrossRef]
- Ulrich, K.; Genter, L.; Schäfer, S.; Masselter, T.; Speck, T. Investigation of the resilience of cyclically actuated pine cone scales of Pinus jeffreyi. Bioinspir. Biomim. 2024, 19, 046009. [Google Scholar] [CrossRef] [PubMed]
- McVean, D.N. Ecology of Scots Pine in the Scottish Highlands. J. Ecol. 1963, 51, 671–686. [Google Scholar] [CrossRef]
- Nathan, R.; Safriel, U.N.; Noy-Meir, I.; Schiller, G. Seed release without fire in Pinus halepensis, a Mediterranean serotinous wind-dispersed tree. J. Ecol. 1999, 87, 659–669. [Google Scholar] [CrossRef]
- Despain, D.G. Dispersal ecology of lodgepole pine (Pinus contorta Dougl.) in its native environment as related to Swedish forestry. For. Ecol. Manag. 2001, 141, 59–68. [Google Scholar] [CrossRef]
- Wyse, S.V.; Brown, J.E.; Hulme, P.E. Seed release by a serotinous pine in the absence of fire: Implications for invasion into temperate regions. AoB PLANTS 2019, 11, plz077. [Google Scholar] [CrossRef] [PubMed]
- Wyse, S.V.; Hulme, P.E.; Holland, E.P. Partitioning intraspecific variation in seed dispersal potential using a low-cost method for rapid estimation of samara terminal velocity. Methods Ecol. Evol. 2019, 10, 1298–1307. [Google Scholar] [CrossRef]
- Horstmann, M.; Buchheit, H.; Speck, T.; Poppinga, S. The cracking of Scots pine (Pinus sylvestris) cones. Front. Plant Sci. 2022, 13, 982756. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Xie, Y.M.; Li, Q.; Huang, X.; Zhou, S. On the shape transformation of cone scales. Soft Matter 2016, 12, 9797–9802. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, W.L. On growing pine cones and other Fibonacci fruits—McCulloch’s localized algorithm. Math. Biosci. 1971, 11, 53–57. [Google Scholar] [CrossRef]
- Le Duigou, A.; Castro, M. Evaluation of force generation mechanisms in natural, passive hydraulic actuators. Sci. Rep. 2016, 6, 18105. [Google Scholar] [CrossRef]
- Skotheim, J.M.; Mahadevan, L. Physical limits and design principles for plant and fungal movements. Science 2005, 308, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B. The Crossbill. In Forestry Commission Leaflets, 36; Her Majesty’s Stationery Office: London, UK, 1955. [Google Scholar]
- Carlisle, A.; Brown, A.H.F. Pinus sylvestris L. J. Ecol. 1968, 56, 269–307. [Google Scholar] [CrossRef]
- Coffey, K.; Benkman, C.W.; Milligan, B.G. The adaptive significance of spines on pine cones. Ecology 1999, 80, 1221–1229. [Google Scholar] [CrossRef]
- Leslie, A.B. Predation and protection in the macroevolutionary history of conifer cones. Proc. R. Soc. B Biol. Sci. 2011, 278, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Reyssat, E.; Mahadevan, L. Hygromorphs: From pine cones to biomimetic bilayers. J. R. Soc. Interface 2009, 6, 951–957. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bae, H.; Kim, J. Functional principles of morphological and anatomical structures in pinecones. Plants 2020, 9, 1343. [Google Scholar] [CrossRef]
- Kossuth, S.V.; Biggs, R.H. Role of apophysis and outer scale tissue in pine cone opening. For. Sci. 1981, 27, 828–836. [Google Scholar] [CrossRef]
- Hellum, A.K.; Barker, N.A. Cone moisture content influences seed release in lodgepole pine. Can. J. For. Res. 1980, 10, 239–244. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Young, R.A. Characterization of conifer seed cone polysaccharides and lignin. Holzforschung 1996, 50, 401–407. [Google Scholar] [CrossRef]
- Summers, R.W.; Proctor, R. Tree and cone selection by crossbills Loxia sp. and red squirrels Sciurus vulgaris at Abernethy forest, Strathspey. For. Ecol. Manag. 1999, 118, 173–182. [Google Scholar] [CrossRef]
- Keeley, J.E. Ecology and evolution of pine life histories. Ann. For. Sci. 2012, 69, 445–453. [Google Scholar] [CrossRef]
- Przybylski, P.; Konatowska, M.; Jastrzębowski, S.; Tereba, A.; Mohytych, V.; Tyburski, Ł.; Rutkowski, P. The possibility of regenerating a pine stand through natural regeneration. Forests 2021, 12, 1055. [Google Scholar] [CrossRef]
- Perry, D.A.; Lotan, J.E. Opening Temperatures in Serotinous Cones of Lodgepole Pine; Research Note INT-228; USDA Forest Service: Ogden, UT, USA, 1977. [Google Scholar]
- Johnson, M.; Vander Wall, S.B.; Borchert, M. A comparative analysis of seed and cone characteristics and seed-dispersal strategies of three pines in the subsection Sabinianae. Plant Ecol. 2003, 168, 69–84. [Google Scholar] [CrossRef]
- Johnson, E.A.; Gutsell, S.L. Heat budget and fire behaviour associated with the opening of serotinous cones in two Pinus species. J. Veg. Sci. 1993, 4, 745–750. [Google Scholar] [CrossRef]
- Moya, D.; Saracino, A.; Salvatore, R.; Lovreglio, R.; De Las Heras, J.; Leone, V. Anatomic basis and insulation of serotinous cones in Pinus halepensis Mill. Trees—Struct. Funct. 2008, 22, 511–519. [Google Scholar] [CrossRef]
- Hernández-Serrano, A.; Verdú, M.; González-Martínez, S.C.; Pausas, J.G. Fire structures pine Serotiny at different scales. Am. J. Bot. 2013, 100, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Lamont, B.B.; Pausas, J.G.; He, T.; Witkowski, E.T.F.; Hanley, M.E. Fire as a selective agent for both serotiny and nonserotiny over space and time. CRC Crit. Rev. Plant Sci. 2020, 39, 140–172. [Google Scholar] [CrossRef]
- Poppinga, S.; Nestle, N.; Šandor, A.; Reible, B.; Masselter, T.; Bruchmann, B.; Speck, T. Hygroscopic motions of fossil conifer cones. Sci. Rep. 2017, 7, 40302. [Google Scholar] [CrossRef] [PubMed]
- Lienhard, J.; Schleicher, S.; Poppinga, S.; Masselter, T.; Milwich, M.; Speck, T.; Knippers, J. Flectofin: A hingeless flapping mechanism inspired by nature. Bioinspir. Biomim. 2011, 6, 045001. [Google Scholar] [CrossRef]
- Keckes, J.; Burgert, I.; Frühmann, K.; Müller, M.; Kölln, K.; Hamilton, M.; Burghammer, M.; Roth, S.V.; Stanzl-Tschegg, S.; Fratzl, P. Cell-wall recovery after irreversible deformation of wood. Nat. Mater. 2003, 2, 810–814. [Google Scholar] [CrossRef]
- Mylo, M.D.; Speck, O. Longevity of System Functions in Biology and Biomimetics: A Matter of Robustness and Resilience. Biomimetics 2023, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Climent, J.; Nanos, N.; Mutke, S.; Ortiz, I.; Schiller, G. Cone morphology variation in Pinus canariensis Sm. Plant Syst. Evol. 2002, 235, 35–51. [Google Scholar] [CrossRef]
- Poppinga, S.; Masselter, T.; Lienhard, J.; Schleicher, S.; Knippers, J.; Speck, T. Plant movements as concept generators for deployable systems in architecture. In Design & Nature V: Comparing Design in Nature with Science and Engineering; Brebbia, C.A., Ed.; WIT Press: Southampton, UK; Boston, MA, USA, 2010; pp. 403–409. [Google Scholar] [CrossRef]
- Tahouni, Y.; Krüger, F.; Poppinga, S.; Wood, D.; Pfaff, M.; Rühe, J.; Speck, T.; Menges, A. Programming sequential motion steps in 4D-printed hygromorphs by architected mesostructure and differential hygro-responsiveness. Bioinspir. Biomim. 2021, 16, 055002. [Google Scholar] [CrossRef]
- Wood, D.; Cheng, T.; Tahouni, Y.; Menges, A. Material programming for bio-inspired and bio-based hygromorphic building envelopes. In Advanced Materials in Smart Building Skins for Sustainability; Springer International Publishing: Cham, Switzerland, 2023; pp. 99–112. [Google Scholar] [CrossRef]
- Van Opdenbosch, D.; Fritz-Popovski, G.; Wagermaier, W.; Paris, O.; Zollfrank, C. Moisture-driven ceramic bilayer actuators from a biotemplating approach. Adv. Mater. 2016, 28, 5235–5240. [Google Scholar] [CrossRef] [PubMed]






| Scale Set | Treatment |
|---|---|
| s1 | Whole abaxial surface covered |
| s2 | Abaxial surface covered except for the apophysis |
| s3 | Whole adaxial surface covered |
| s4 | Scale completely covered |
| s5 | Scale completely covered except for the apophysis |
| s6 | Only apophysis covered |
| Treatment with Vaseline | n | Unmanipulated | Manipulated | Difference Δ between Manipulated and Unmanipulated Scales | |||
|---|---|---|---|---|---|---|---|
| Wetting-Induced Bending (min) | Drying-Induced Bending (min) | Wetting-Induced Bending (min) | Drying-Induced Bending (min) | Wetting-Induced Bending [min] | Drying-Induced Bending [min] | ||
| No manipulation | 12 | 118 ± 25 | 903 ± 259 | - | - | - | - |
| Adaxial scale surface covered | 4 | 110 ± 22 | 981 ± 321 | 331 ± 66 | 1238 ± 43 | 221 | 256 |
| Surface of apophysis covered | 3 | 130 ± 28 | 758 ± 29 | 375 ± 25 | 1250 ± 43 | 245 | 492 |
| Abaxial scale surface covered, but not apophysis | 4 | 141 ± 13 | 650 ± 29 | 588 ± 75 | 2006 ± 783 | 446 | 1356 |
| Abaxial scale surface covered | 4 | 86 ± 18 | 1075 ± 140 | 750 ± 65 | 1800 ± 767 | 664 | 725 |
| Scale surface covered, but not apophysis | 4 | 126 ± 6 | 738 ± 48 | 1375 ± 261 | 3150 ± 0 | 1249 | 2413 |
| Scale surface entirely covered | 3 | 113 ± 24 | 1275 ± 152 | 2150 ± 520 | 7125 ± 130 | 2037 | 5850 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horstmann, M.; Speck, T.; Poppinga, S. Orchestrated Movement Sequences and Shape-Memory-like Effects in Pine Cones. Plants 2024, 13, 2078. https://doi.org/10.3390/plants13152078
Horstmann M, Speck T, Poppinga S. Orchestrated Movement Sequences and Shape-Memory-like Effects in Pine Cones. Plants. 2024; 13(15):2078. https://doi.org/10.3390/plants13152078
Chicago/Turabian StyleHorstmann, Martin, Thomas Speck, and Simon Poppinga. 2024. "Orchestrated Movement Sequences and Shape-Memory-like Effects in Pine Cones" Plants 13, no. 15: 2078. https://doi.org/10.3390/plants13152078
APA StyleHorstmann, M., Speck, T., & Poppinga, S. (2024). Orchestrated Movement Sequences and Shape-Memory-like Effects in Pine Cones. Plants, 13(15), 2078. https://doi.org/10.3390/plants13152078








