Beneficial Effects of Phosphite in Arabidopsis thaliana Mediated by Activation of ABA, SA, and JA Biosynthesis and Signaling Pathways
Abstract
1. Introduction
2. Results
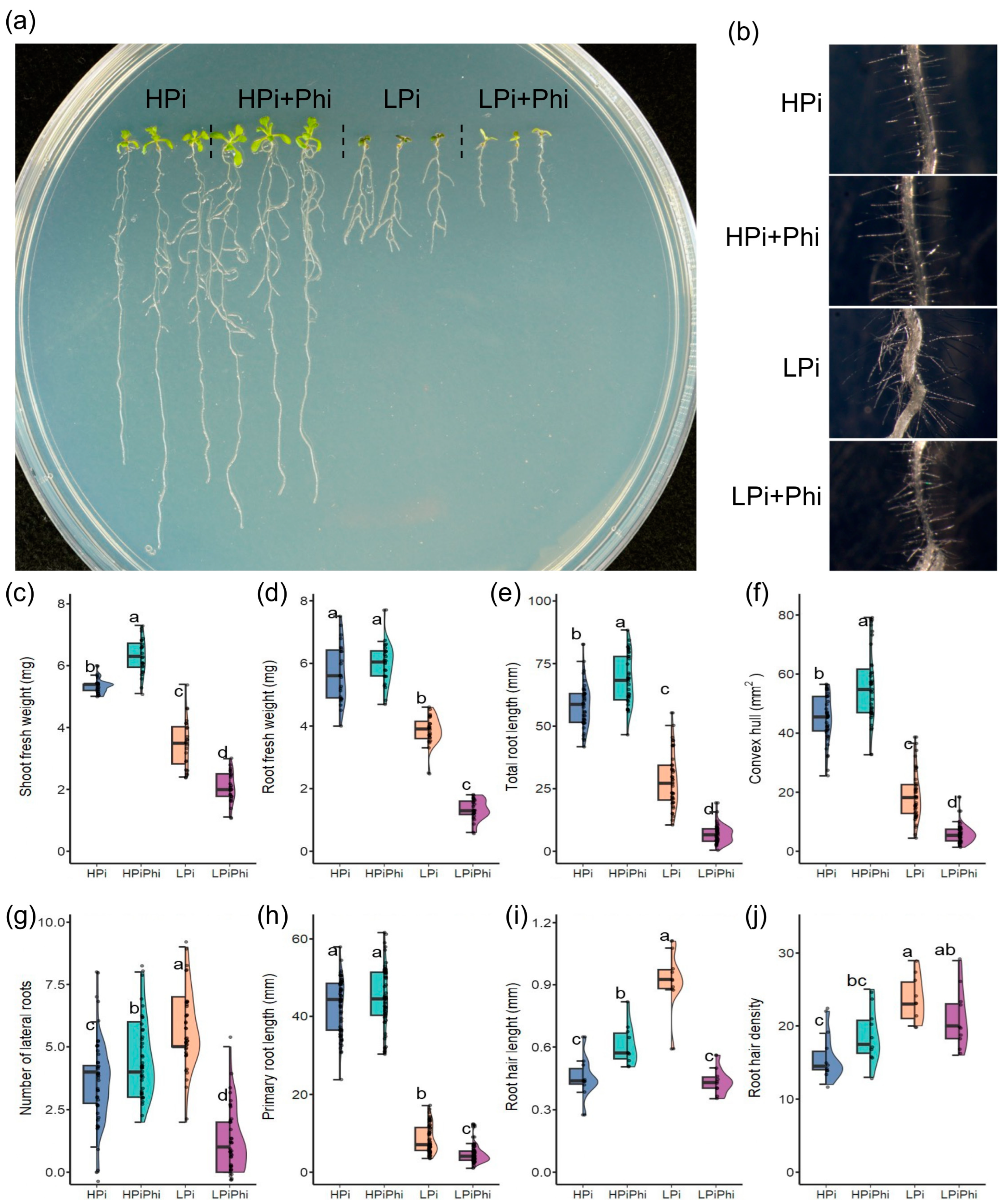
2.1. Phi Suppresses Pi Starvation Responses and Positively Affects Arabidopsis Growth under Optimal Pi Conditions
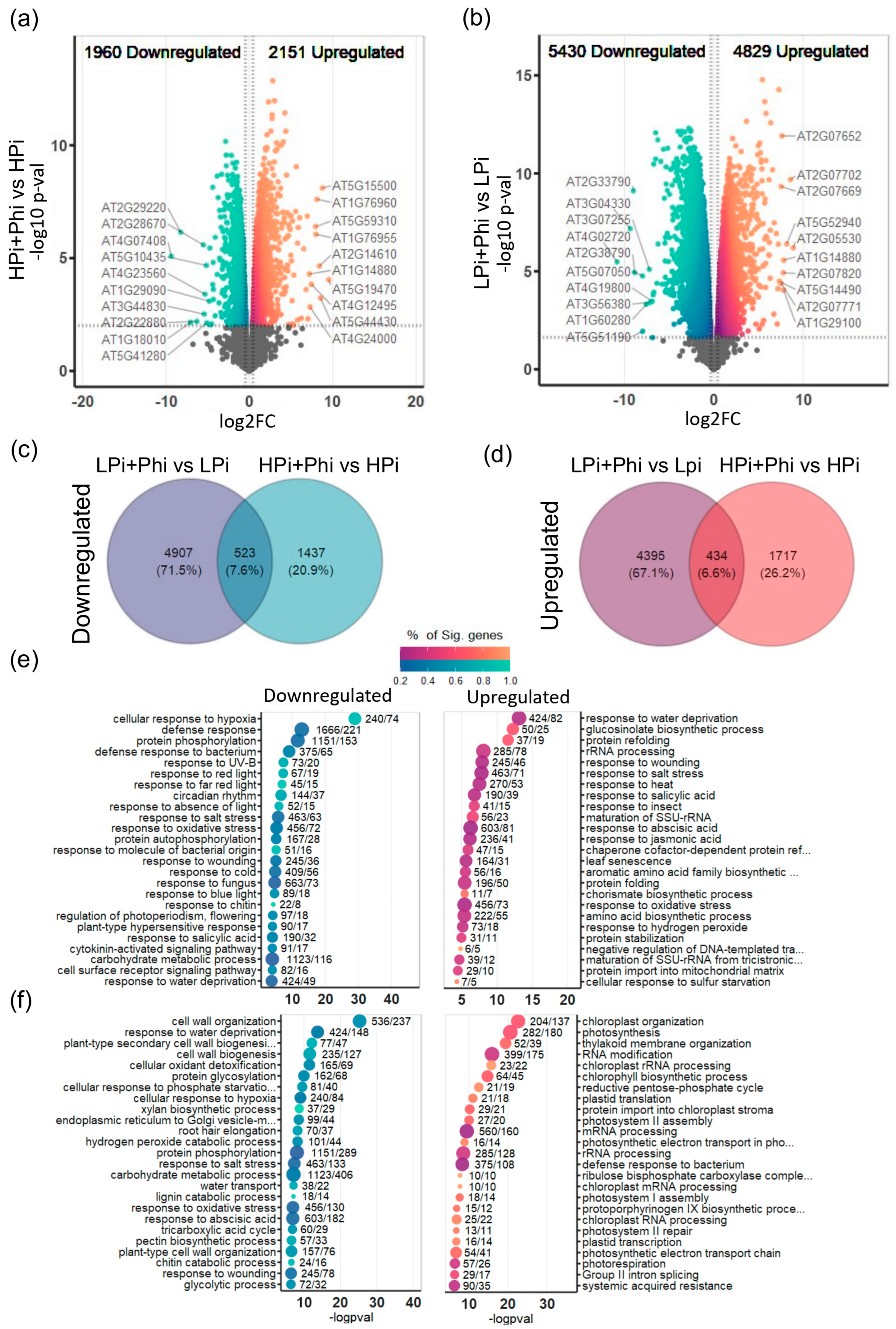
2.2. Phi induces Transcriptional Changes in Arabidopsis under Both Low and Optimal Pi Levels
2.3. Phi Activates Plant Defense Responses under Pi Sufficiency
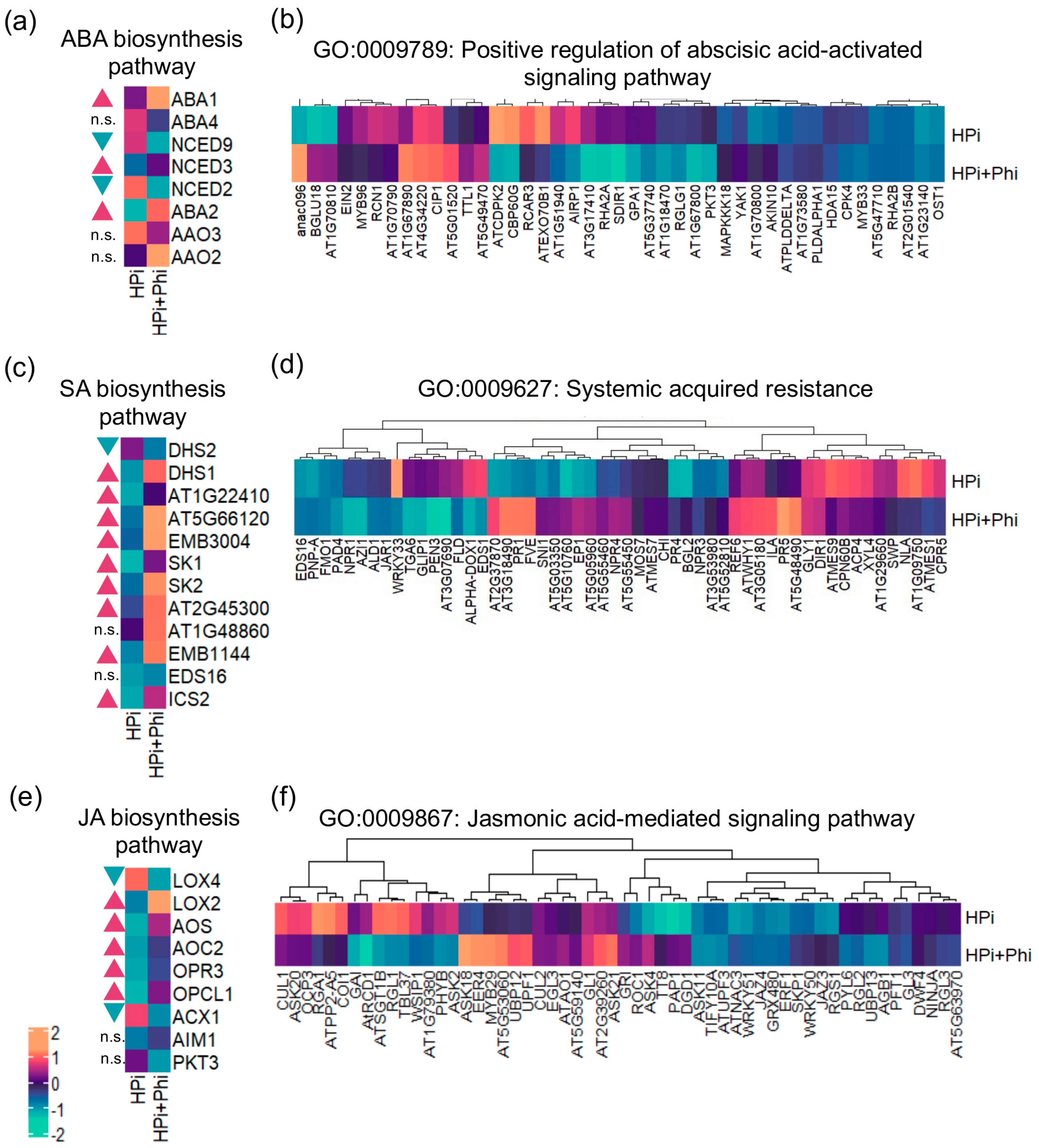
2.4. Phi Enhances the Biosynthesis of ABA, JA, and SA, and Shows a Priming Effect on the Associated Signaling Pathways
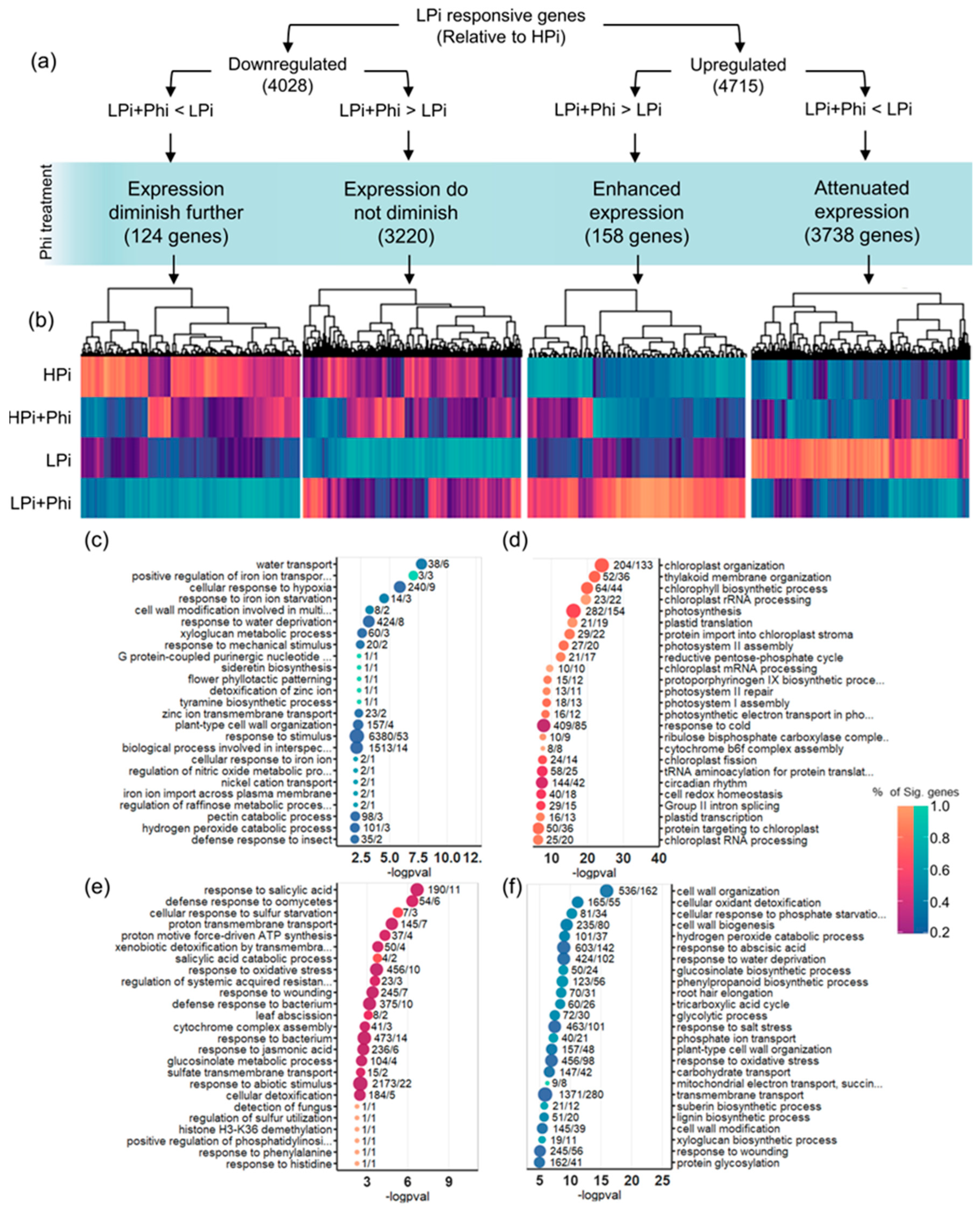
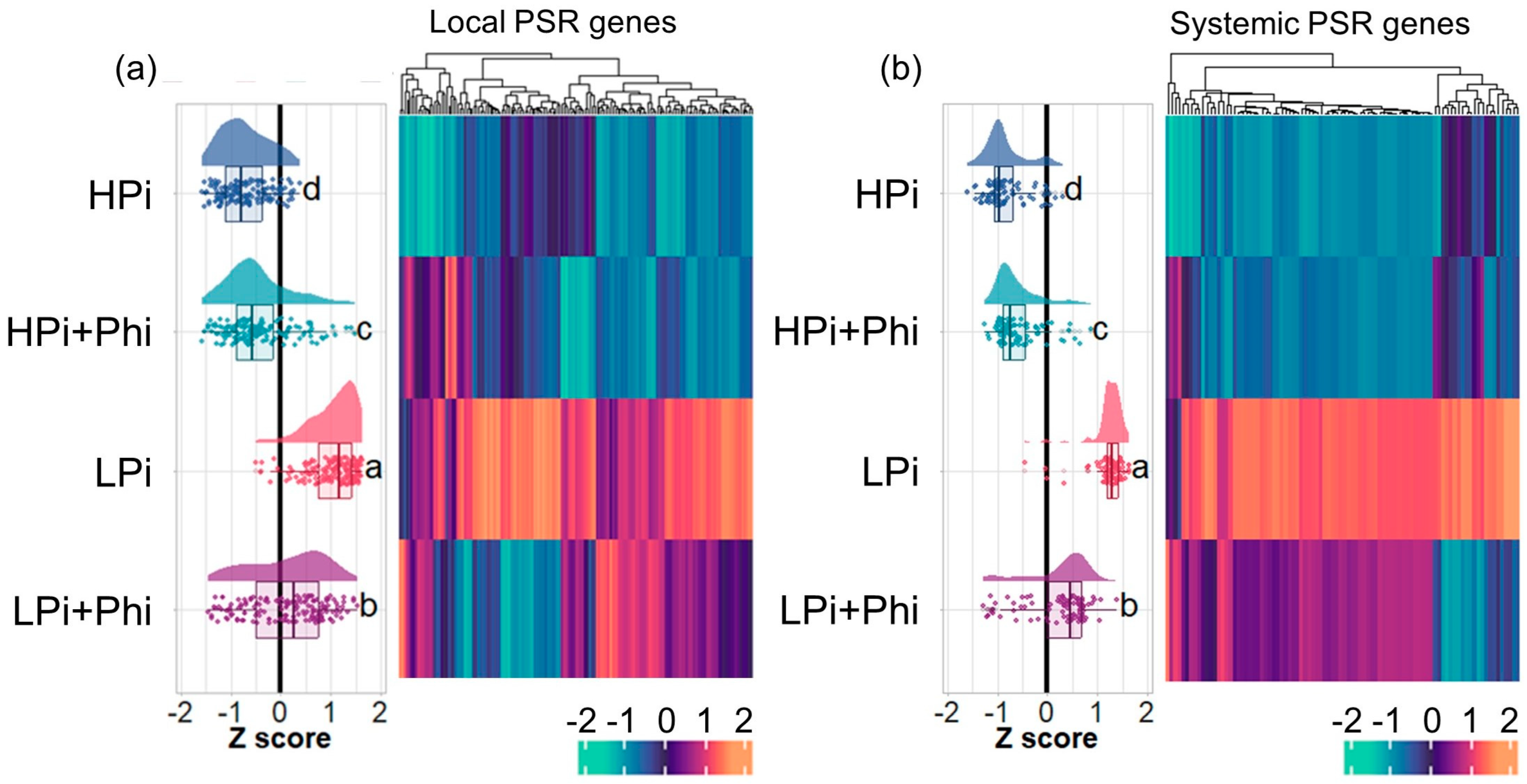
2.5. Phi Suppresses Local and Systemic Responses to Pi Starvation
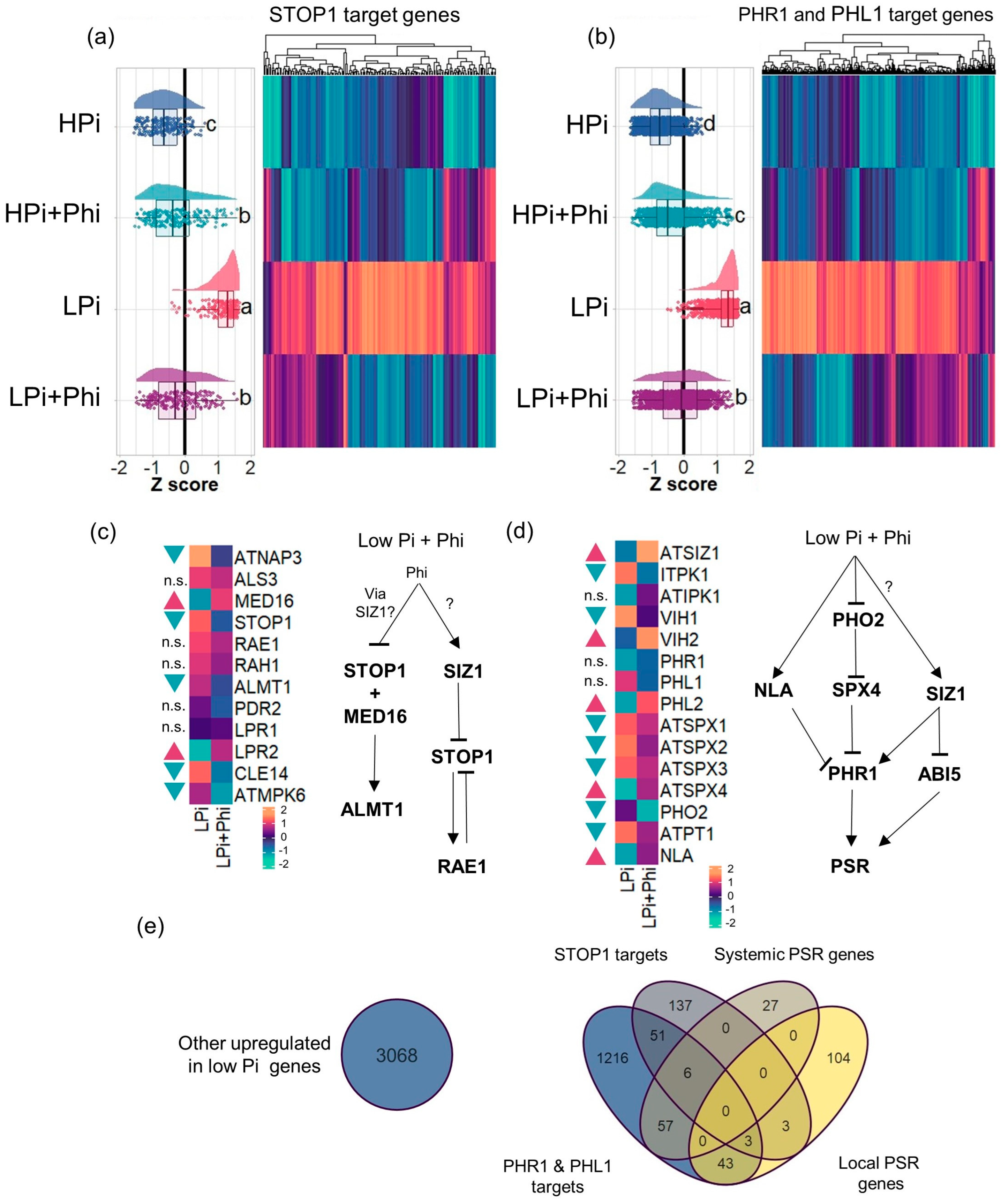
2.6. Phi-Dependent Shutdown of STOP1 and PHR1 Signaling Pathways
2.7. Phi-Dependent Shutdown of STOP1 and PHR1 Signaling Pathways
3. Discussion
3.1. Phi Has a Positive Effect on Plant Growth
3.2. Phi Activates Defense Responses against Multiple Biotic and Abiotic Stresses Using ABA, SA, and JA Signaling Pathways
3.3. Phi Effects on Low Pi Conditions Are Mainly Caused by Suppressing the Activation of the PSRs
3.4. Phi Modulates the Local and Systemic Responses to Low Pi
3.5. Practical Implications under Field Conditions
4. Conclusions
5. Materials and Methods
5.1. Biological Material and Plant Growth
5.2. Evaluation of Phi Effect on Arabidopsis Shoot and Root Growth
5.3. Experiments for RNA-seq Studies
5.4. Data Checks and RNA-seq Data Analysis
5.5. Histochemical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bogaard, A.; Fraser, R.; Heaton, T.H.E.; Wallace, M.; Vaiglova, P.; Charles, M.; Jones, G.; Evershed, R.P.; Styring, A.K.; Andersen, N.H.; et al. Crop Manuring and Intensive Land Management by Europe’s First Farmers. Proc. Natl. Acad. Sci. USA 2013, 110, 12589–12594. [Google Scholar] [CrossRef] [PubMed]
- Russel, D.A.; Williams, G.G. History of Chemical Fertilizer Development. Soil Sci. Soc. Am. J. 1977, 41, 260–265. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Merino, F.C.; Gómez-Trejo, L.F.; Ruvalcaba-Ramírez, R.; Trejo-Téllez, L.I. Application of Phosphite as a Biostimulant in Agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering: Sustainable Agriculture: Revisiting Green Chemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 135–153. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 135–189. [Google Scholar] [CrossRef]
- Hoang, T.; Bich, T.; Yamakawa, T.; Thi, H.; Thao, B. Phosphite (Phosphorous Acid): Fungicide, Fertilizer or Bio-Stimulator? Soil Sci. Plant Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- Guest, D.; Grant, B. The Complex Action of Phosphonates as Antifungal Agents. Biol. Rev. 1991, 66, 159–187. [Google Scholar] [CrossRef]
- Yáñez-Juárez, M.G.; López-Orona, C.A.; Ayala-Tafoya, F.; Partida-Ruvalcaba, L.; Velázquez-Alcaraz, T.d.J.; Medina-López, R. Phosphites as Alternative for the Management of Phytopathological Problems. Rev. Mex. Fitopatol. 2018, 36, 79–94. [Google Scholar] [CrossRef]
- Graham, J.H. Phosphite for Control of Phytophthora Diseases in Citrus: Model for Management of Phytophthora Species on Forest Trees? N. Z. J. For. Sci. 2011, 41, 49–56. [Google Scholar]
- Cerioni, L.; Rapisarda, V.A.; Doctor, J.; Fikker, S.; Ruiz, T.; Fassel, R.; Smilanick, J.L. Use of Phosphite Salts in Laboratory and Semicommercial Tests to Control Citrus Postharvest Decay. Plant Dis. 2013, 97, 201–212. [Google Scholar] [CrossRef]
- Farooq, Q.U.A.; McComb, J.; Hardy, G.E.S.J.; Burgess, T. Soil Amendments and Suppression of Phytophthora Root Rot in Avocado (Persea americana). Australas. Plant Pathol. 2023, 52, 1–11. [Google Scholar] [CrossRef]
- Pegg, K.G.; Whiley, A.W.; Saranah, J.B.; Glass, R.J. Control of Phytophthora Root Rot of Avocado with Phosphorus Acid. Australas. Plant Pathol. 1985, 14, 25–29. [Google Scholar] [CrossRef]
- Da Silva, S.R.; Cantuarias-Avilés, T.; Bremer Neto, H.; Mourão Filho, F.D.A.A.; Medina, R.B. Management of Root Rot in Avocado Trees. Rev. Bras. Frutic. 2016, 38, e-175. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Ram, B.; Manna, M.; Datta, D.; Bhatt, A.; Reddy, M.K.; Agrawal, P.K. Phosphite: A Novel P Fertilizer for Weed Management and Pathogen Control. Plant Biotechnol. J. 2017, 15, 1493–1508. [Google Scholar] [CrossRef]
- Havlin, J.L.; Schlegel, A.J.; Havlin, J.L.; Schlegel, A.J. Review of Phosphite as a Plant Nutrient and Fungicide. Soil Syst. 2021, 5, 52. [Google Scholar] [CrossRef]
- Market Reports World Global Potassium Phosphite Market—Market Reports World. Available online: https://www.marketreportsworld.com/global-potassium-phosphite-market-26318022 (accessed on 18 February 2024).
- Bertsch, F.; Ramírez, F.; Henríquez, C. Agronomía Costarricense. Agron. Costarric. 2009, 33, 249–265. [Google Scholar]
- Mohammed, U.; Davis, J.; Rossall, S.; Swarup, K.; Czyzewicz, N.; Bhosale, R.; Foulkes, J.; Murchie, E.H.; Swarup, R. Phosphite Treatment Can Improve Root Biomass and Nutrition Use Efficiency in Wheat. Front. Plant Sci. 2022, 13, 1017048. [Google Scholar] [CrossRef]
- Rickard, D.A. Review of Phosphorus Acid and Its Salts as Fertilizer Materials. J. Plant Nutr. 2000, 23, 161–180. [Google Scholar] [CrossRef]
- Estrada-Ortiz, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Núñez-Escobar, R.; Sandoval-Villa, M. The Effects of Phosphite on Strawberry Yield and Fruit Quality. J. Soil Sci. Plant Nutr. 2013, 13, 612–620. [Google Scholar] [CrossRef]
- Batista, P.F.; da Costa, A.C.; da Silva, A.A.; Almeida, G.M.; Rodrigues, M.F.M.; Santos, E.C.D.; Rodrigues, A.A.; Müller, C. Potassium Phosphite Induces Tolerance to Water Deficit Combined with High Irradiance in Soybean Plants. Agronomy 2023, 13, 382. [Google Scholar] [CrossRef]
- Liu, P.; Li, B.; Lin, M.; Chen, G.; Ding, X.; Weng, Q.; Chen, Q. Phosphite-Induced Reactive Oxygen Species Production and Ethylene and ABA Biosynthesis, Mediate the Control of Phytophthora capsici in Pepper (Capsicum annuum). Funct. Plant Biol. 2016, 43, 563–574. [Google Scholar] [CrossRef]
- Lim, S.; Borza, T.; Peters, R.D.; Coffin, R.H.; Al-Mughrabi, K.I.; Pinto, D.M.; Wang-Pruski, G. Proteomics Analysis Suggests Broad Functional Changes in Potato Leaves Triggered by Phosphites and a Complex Indirect Mode of Action against Phytophthora infestans. J. Proteom. 2013, 93, 207–223. [Google Scholar] [CrossRef]
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an Analog of Phosphate, Suppresses the Coordinated Expression of Genes under Phosphate Starvation. Plant Physiol. 2002, 129, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.E.; Grant, B.R.; Plaxton, W.C. Phosphite (Phosphorous Acid): Its Relevance in the Environment and Agriculture and Influence on Plant Phosphate Starvation Response. J. Plant Nutr. 2001, 24, 1505–1519. [Google Scholar] [CrossRef]
- Lee, T.M.; Tsai, P.F.; Shyu, Y.T.; Sheu, F. The Effects of Phosphite on Phosphate Starvation Responses of Ulva lactuca (Ulvales, Chlorophyta). J. Phycol. 2005, 41, 975–982. [Google Scholar] [CrossRef]
- Jost, R.; Pharmawati, M.; Lapis-Gaza, H.R.; Rossig, C.; Berkowitz, O.; Lambers, H.; Finnegan, P.M. Differentiating Phosphate-Dependent and Phosphate-Independent Systemic Phosphate-Starvation Response Networks in Arabidopsis thaliana through the Application of Phosphite. J. Exp. Bot. 2015, 66, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.A.; Delatorre, C.A.; Abel, S. Attenuation of Phosphate Starvation Responses by Phosphite in Arabidopsis. Plant Physiol. 2001, 127, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rivera, J.O.; Alejo-Jacuinde, G.; Nájera-González, H.R.; López-Arredondo, D. Prospects of Genetics and Breeding for Low-Phosphate Tolerance: An Integrated Approach from Soil to Cell. Theor. Appl. Genet. 2022, 135, 4125–4150. [Google Scholar] [CrossRef]
- Madison, I.; Gillan, L.; Peace, J.; Gabrieli, F.; Van den Broeck, L.; Jones, J.L.; Sozzani, R. Phosphate Starvation: Response Mechanisms and Solutions. J. Exp. Bot. 2023, 74, 6417–6430. [Google Scholar] [CrossRef]
- Mccarthy, D.J.; Smyth, G.K. Testing Significance Relative to a Fold-Change Threshold Is a TREAT. Bioinformatics 2009, 25, 765. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A.D. Elucidation of the Indirect Pathway of Abscisic Acid Biosynthesis by Mutants, Genes, and Enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Xiong, L.; Lee, H.; Ishitani, M.; Zhu, J.K. Regulation of Osmotic Stress-Responsive Gene Expression by the LOS6/ABA1 Locus in Arabidopsis. J. Biol. Chem. 2002, 277, 8588–8596. [Google Scholar] [CrossRef]
- Thibaud, M.C.; Arrighi, J.F.; Bayle, V.; Chiarenza, S.; Creff, A.; Bustos, R.; Paz-Ares, J.; Poirier, Y.; Nussaume, L. Dissection of Local and Systemic Transcriptional Responses to Phosphate Starvation in Arabidopsis. Plant J. 2010, 64, 775–789. [Google Scholar] [CrossRef]
- O’Malley, R.C.; Huang, S.S.C.; Song, L.; Lewsey, M.G.; Bartlett, A.; Nery, J.R.; Galli, M.; Gallavotti, A.; Ecker, J.R. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell 2016, 165, 1280–1292. [Google Scholar] [CrossRef]
- Castrillo, G.; Teixeira, P.J.P.L.; Paredes, S.H.; Law, T.F.; De Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root Microbiota Drive Direct Integration of Phosphate Stress and Immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.T.; Wang, L.F.; Guo, J.X.; Lu, K.K.; Song, R.F.; Zuo, J.X.; Chen, H.H.; Liu, W.C. Abscisic Acid Facilitates Phosphate Acquisition through the Transcription Factor ABA INSENSITIVE5 in Arabidopsis. Plant J. 2022, 111, 269–281. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Du, J.; Han, X.; Li, H.; Kui, M.; Zhang, J.; Huang, Z.; Fu, Q.; Jiang, Y.; Hu, Y. PHOSPHATE STARVATION RESPONSE1 (PHR1) Interacts with JASMONATE ZIM-DOMAIN (JAZ) and MYC2 to Modulate Phosphate Deficiency-Induced Jasmonate Signaling in Arabidopsis. Plant Cell 2023, 35, 2132–2156. [Google Scholar] [CrossRef]
- Song, L.; Liu, D. Ethylene and Plant Responses to Phosphate Deficiency. Front. Plant Sci. 2015, 6, 796. [Google Scholar] [CrossRef] [PubMed]
- Glinicki, R.; Sas-Paszt, L.; Jadczuk-Tobjasz, E. The Effect of Plant Stimulant/Fertilizer “Resistim” on Growth and Development of Strawberry Plants. J. Fruit Ornam. Plant Res. 2010, 18, 111–124. [Google Scholar]
- William Ávila, F.; Faquin, V.; Ramos, S.J.; Pinheiro, G.L.; Marques, D.J.; Klynger, A.; Lobato, S.; Ferreira De Oliveira Neto, C.; Ávila, P.A. Effects of Phosphite and Phosphate Supply in a Weathered Tropical Soil on Biomass Yield, Phosphorus Status and Nutrient Concentrations in Common Bean. J. Food Agric. Environ. 2012, 10, 312–317. [Google Scholar]
- Bachiega Zambrosi, F.C. Phosphite and Phosphate Have Contrasting Effects on Nutrient Status of Plants. J. Crop Improv. 2016, 30, 421–432. [Google Scholar] [CrossRef]
- Thao, H.T.B.; Yamakawa, T.; Shibata, K. Effect of Phosphite-Phosphate Interaction on Growth and Quality of Hydroponic Lettuce (Lactuca sativa). J. Plant Nutr. Soil Sci. 2009, 172, 378–384. [Google Scholar] [CrossRef]
- Ali, M.S.; Sutradhar, A.; Edano, M.L.; Edwards, J.T.; Girma, K. Response of Winter Wheat Grain Yield and Phosphorus Uptake to Foliar Phosphite Fertilization. Int. J. Agron. 2014, 2014, 801626. [Google Scholar] [CrossRef]
- Omar, M.M.; Taha, A.A.; Shokir, S.A. Effect of Applying Potassium Phosphite with Potassium Fulvate on Plant Growth. J. Soil Sci. Agric. Eng. 2020, 11, 255–263. [Google Scholar] [CrossRef]
- Whan, A.P.; Verbyla, A.P.; Mieog, J.C.; Howitt, C.A.; Ral, J.-P. Transferring a Biomass Enhancement Biotechnology from Glasshouse to Field: A Case Study on Wheat GWD RNAi. Agronomy 2017, 7, 82. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic Adjustment and Plant Adaptation to Drought Stress. In Drought Stress Tolerance in Plants; Physiology and Biochemistry; Springer: Cham, Switzerland, 2016; Volume 1, pp. 105–143. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, K.S.; Paz, V.P.d.S.; Silva, F.d.L.; Hongyu, K.; de Almeida, W.F. Potassium Phosphite and Water Deficit: Physiological Response of Eucalyptus Using Multivariate Analysis. J. Agric. Sci. 2019, 11, 565. [Google Scholar] [CrossRef]
- Rey-Burusco, M.F.; Daleo, G.R.; Feldman, M.L. Identification of Potassium Phosphite Responsive MiRNAs and Their Targets in Potato. PLoS ONE 2019, 14, e0222346. [Google Scholar] [CrossRef] [PubMed]
- Merlot, S.; Gosti, F.; Guerrier, D.; Vavasseur, A.; Giraudat, J. The ABI1 and ABI2 Protein Phosphatases 2C Act in a Negative Feedback Regulatory Loop of the Abscisic Acid Signalling Pathway. Plant J. 2001, 25, 295–303. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, H.; Yuan, B.; Wang, S.; Su, C.; Yao, B.; Zhao, H.; Li, X. ABA Signalling Is Fine-Tuned by Antagonistic HAB1 Variants. Nat. Commun. 2015, 6, 8138. [Google Scholar] [CrossRef]
- Kumar, M.; Kesawat, M.S.; Ali, A.; Lee, S.C.; Gill, S.S.; Kim, H.U. Integration of Abscisic Acid Signaling with Other Signaling Pathways in Plant Stress Responses and Development. Plants 2019, 8, 592. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate Action in Plant Growth and Development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways. Front. Plant Sci 2017, 8, 263702. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Dursun, A. Effect of Foliar Salicylic Acid Applications on Plant Growth and Yield of Tomato under Greenhouse Conditions. Acta Hortic. 2009, 807, 395–400. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Mohamed, G.F.; Rady, M.M. Combined Effect of Foliar-Applied Salicylic Acid and Deficit Irrigation on Physiological–Anatomical Responses, and Yield of Squash Plants under Saline Soil. S. Afr. J. Bot. 2016, 106, 8–16. [Google Scholar] [CrossRef]
- Mady, E.; Abd El-Wahed, A.H.M.; Awad, A.H.; Asar, T.O.; Al-Farga, A.; Abd El-Raouf, H.S.; Randhir, R.; Alnuzaili, E.S.; El-Taher, A.M.; Randhir, T.O.; et al. Evaluation of Salicylic Acid Effects on Growth, Biochemical, Yield, and Anatomical Characteristics of Eggplant (Solanum melongena L.) Plants under Salt Stress Conditions. Agronomy 2023, 13, 2213. [Google Scholar] [CrossRef]
- Vinas, M.; Mendez, J.C.; Jiménez, V.M. Effect of Foliar Applications of Phosphites on Growth, Nutritional Status and Defense Responses in Tomato Plants. Sci. Hortic. 2020, 265, 109200. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; de Oliveira, T.S.; Simmi, F.Z.; Farias, R.S.; Peres, L.E.P.; Pascholati, S.F.; Camargo, L.E.A. The Contributions of Potassium Phosphite and Hormonal Pathways to the Control of Phytophthora infestans in Tomato Cv. Micro-Tom. Plant Pathol. 2023, 72, 468–475. [Google Scholar] [CrossRef]
- Machinandiarena, M.F.; Lobato, M.C.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B. Potassium Phosphite Primes Defense Responses in Potato against Phytophthora infestans. J. Plant Physiol. 2012, 169, 1417–1424. [Google Scholar] [CrossRef]
- Lobato, M.C.; Olivieri, F.P.; Altamiranda, E.A.G.; Wolski, E.A.; Daleo, G.R.; Caldiz, D.O.; Andreu, A.B. Phosphite Compounds Reduce Disease Severity in Potato Seed Tubers and Foliage. Eur. J. Plant Pathol. 2008, 122, 349–358. [Google Scholar] [CrossRef]
- Wadlington, W.H.; Sandoya-Miranda, G.V.; Miller, C.F.; Villegas, J.; Raid, R.N. Stemphylium Leaf Spot in Spinach: Chemical and Breeding Solutions for This Threatening Disease in Florida. Proc. Fla. State Hort. Soc. 2018, 131, 151–158. [Google Scholar]
- Picanço, B.B.M.; Silva, B.N.; Rodrigues, F.A. Potentiation of Soybean Resistance against Phakopsora pachyrhizi Infection Using Phosphite Combined with Free Amino Acids. Plant Pathol. 2022, 71, 1496–1510. [Google Scholar] [CrossRef]
- Guo, M.; Li, B.; Xiang, Q.; Wang, R.; Liu, P.; Chen, Q. Phosphite Translocation in Soybean and Mechanisms of Phytophthora sojae Inhibition. Pestic. Biochem. Physiol. 2021, 172, 104757. [Google Scholar] [CrossRef] [PubMed]
- Giudice, G.; Moffa, L.; Niero, M.; Duso, C.; Sandrini, M.; Vazzoler, L.F.; Luison, M.; Pasini, E.; Chitarra, W.; Nerva, L. Novel Sustainable Strategies to Control Plasmopara viticola in Grapevine Unveil New Insights on Priming Responses and Arthropods Ecology. Pest Manag. Sci. 2022, 78, 2342–2356. [Google Scholar] [CrossRef]
- Groves, E.; Howard, K.; Hardy, G.; Burgess, T. Role of Salicylic Acid in Phosphite-Induced Protection against Oomycetes; a Phytophthora cinnamomi–Lupinus augustifolius Model System. Eur. J. Plant Pathol. 2015, 141, 559–569. [Google Scholar] [CrossRef]
- William Ávila, F.; Faquin, V.; Lopes Araújo, J.; José Marques, D.; Martins Ribeiro Júnior, P.; Klynger da Silva Lobato, A.; Junio Ramos, S.; Pereira Baliza, D. Phosphite Supply Affects Phosphorus Nutrition and Biochemical Responses in Maize Plants. Aust. J. Crop Sci. 2011, 5, 646–653. [Google Scholar]
- Fang, Q.; Zhang, J.; Yang, D.L.; Huang, C.F. The SUMO E3 Ligase SIZ1 Partially Regulates STOP1 SUMOylation and Stability in Arabidopsis thaliana. Plant Signal. Behav. 2021, 16, 1899487. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, J.; Liu, J.; Wang, J.; Ding, Z.; Tian, H. SIZ1 Negatively Regulates Aluminum Resistance by Mediating the STOP1–ALMT1 Pathway in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 1147–1160. [Google Scholar] [CrossRef]
- Raya-González, J.; Ojeda-Rivera, J.O.; Mora-Macias, J.; Oropeza-Aburto, A.; Ruiz-Herrera, L.F.; López-Bucio, J.; Herrera-Estrella, L. MEDIATOR16 Orchestrates Local and Systemic Responses to Phosphate Scarcity in Arabidopsis Roots. New Phytol. 2021, 229, 1278–1288. [Google Scholar] [CrossRef]
- Manna, M.; Islam, T.; Kaul, T.; Reddy, C.S.; Fartyal, D.; James, D.; Reddy, M.K. A Comparative Study of Effects of Increasing Concentrations of Phosphate and Phosphite on Rice Seedlings. Acta Physiol. Plant 2015, 37, 258. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Hu, J.; Yang, G.; Wang, Z.; Sun, J. Dissection of the Response Mechanism of Alfalfa under Phosphite Stress Based on Metabolomic and Transcriptomic Data. Plant Physiol. Biochem. 2022, 192, 35–49. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Herrera-Estrella, L. Engineering Phosphorus Metabolism in Plants to Produce a Dual Fertilization and Weed Control System. Nat. Biotechnol. 2012, 30, 889–893. [Google Scholar] [CrossRef]
- Dormatey, R.; Sun, C.; Ali, K.; Fiaz, S.; Xu, D.; Calderón-Urrea, A.; Bi, Z.; Zhang, J.; Bai, J. PtxD/Phi as Alternative Selectable Marker System for Genetic Transformation for Bio-Safety Concerns: A Review. PeerJ 2021, 9, e11809. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechtold, N.; Weisbeek, P.; et al. An Auxin-Dependent Distal Organizer of Pattern and Polarity in the Arabidopsis Root. Cell 1999, 99, 463–472. [Google Scholar] [CrossRef]
- Colón-Carmona, A.; You, R.; Haimovitch-Gal, T.; Doerner, P. Spatio-Temporal Analysis of Mitotic Activity with a Labile Cyclin–GUS Fusion Protein. Plant J. 1999, 20, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Pound, M.P.; French, A.P.; Atkinson, J.A.; Wells, D.M.; Bennett, M.J.; Pridmore, T. RootNav: Navigating Images of Complex Root Architectures. Plant Physiol. 2013, 162, 1802–1814. [Google Scholar] [CrossRef]
- Krueger, F. Trim Galore! 2012. Available online: https://github.com/FelixKrueger/TrimGalore (accessed on 11 September 2023).
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics: Cambridge, UK, 2010. [Google Scholar]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-Optimal Probabilistic RNA-Seq Quantification. Nat Biotechnol 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Krishnakumar, V.; Chan, A.P.; Thibaud-Nissen, F.; Schobel, S.; Town, C.D. Araport11: A Complete Reannotation of the Arabidopsis thaliana Reference Genome. Plant J. 2017, 89, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential Analyses for RNA-Seq: Transcript-Level Estimates Improve Gene-Level Inferences. F1000Res 2016, 4, 1521. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential Expression Analysis of Multifactor RNA-Seq Experiments with Respect to Biological Variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Zavala, F.G.; Atriztán-Hernández, K.; Martínez-Irastorza, P.; Oropeza-Aburto, A.; López-Arredondo, D.; Herrera-Estrella, L. Titanium Nanoparticles Activate a Transcriptional Response in Arabidopsis That Enhances Tolerance to Low Phosphate, Osmotic Stress and Pathogen Infection. Front. Plant Sci. 2022, 13, 994523. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.P.; Nettleton, D.; McCarthy, D.J.; Smyth, G.K. Detecting Differential Expression in RNA-Sequence Data Using Quasi-Likelihood with Shrunken Dispersion Estimates. Stat. Appl. Genet. Mol. Biol. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Lun, A.T.L.; Chen, Y.; Smyth, G.K. It’s DE-Licious: A Recipe for Differential Expression Analyses of RNA-Seq Experiments Using Quasi-Likelihood Methods in EdgeR. Methods Mol. Biol. 2016, 1418, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Alexa, A.; Rahnenfuhrer, J.; Lengauer, T. Improved Scoring of Functional Groups from Gene Expression Data by Decorrelating GO Graph Structure. Bioinformatics 2006, 22, 1600–1607. [Google Scholar] [CrossRef]
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. 2021. Available online: https://dplyr.tidyverse.org/ (accessed on 11 September 2023).
- Yan, L. ggvenn: Draw Venn Diagram by “Ggplot2”. 2021. Available online: https://cran.r-project.org/web/packages/ggvenn/index.html (accessed on 11 September 2023).
- Wickham, H. Ggplot2 Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2009; ISBN 9780387981406. [Google Scholar]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS Fusions: Beta-glucuronidase as a Sensitive and Versatile Gene Fusion Marker in Higher Plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Malamy, J.E.; Benfey, P.N. Down and out in Arabidopsis: The Formation of Lateral Roots. Trends Plant Sci. 1997, 2, 390–396. [Google Scholar] [CrossRef]
- Hawkins, C.; Ginzburg, D.; Zhao, K.; Dwyer, W.; Xue, B.; Xu, A.; Rice, S.; Cole, B.; Paley, S.; Karp, P.; et al. Plant Metabolic Network 15: A Resource of Genome-Wide Metabolism Databases for 126 Plants and Algae. J. Integr. Plant Biol. 2021, 63, 1888–1905. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Zavala, F.G.; Ojeda-Rivera, J.O.; Herrera-Estrella, L.; López-Arredondo, D. Beneficial Effects of Phosphite in Arabidopsis thaliana Mediated by Activation of ABA, SA, and JA Biosynthesis and Signaling Pathways. Plants 2024, 13, 1873. https://doi.org/10.3390/plants13131873
Pérez-Zavala FG, Ojeda-Rivera JO, Herrera-Estrella L, López-Arredondo D. Beneficial Effects of Phosphite in Arabidopsis thaliana Mediated by Activation of ABA, SA, and JA Biosynthesis and Signaling Pathways. Plants. 2024; 13(13):1873. https://doi.org/10.3390/plants13131873
Chicago/Turabian StylePérez-Zavala, Francisco Gabriel, Jonathan Odilón Ojeda-Rivera, Luis Herrera-Estrella, and Damar López-Arredondo. 2024. "Beneficial Effects of Phosphite in Arabidopsis thaliana Mediated by Activation of ABA, SA, and JA Biosynthesis and Signaling Pathways" Plants 13, no. 13: 1873. https://doi.org/10.3390/plants13131873
APA StylePérez-Zavala, F. G., Ojeda-Rivera, J. O., Herrera-Estrella, L., & López-Arredondo, D. (2024). Beneficial Effects of Phosphite in Arabidopsis thaliana Mediated by Activation of ABA, SA, and JA Biosynthesis and Signaling Pathways. Plants, 13(13), 1873. https://doi.org/10.3390/plants13131873






