Seasonal Variation and Mean Degree of Polymerization of Proanthocyanidin in Leaves and Branches of Rabbiteye Blueberry (Vaccinium virgatum Aiton)
Abstract
1. Introduction
2. Results
2.1. Seasonal Changes in Leaves and Branches
2.2. Total Polyphenol Content
2.3. Polyphenol Component
2.4. Total PAC Content
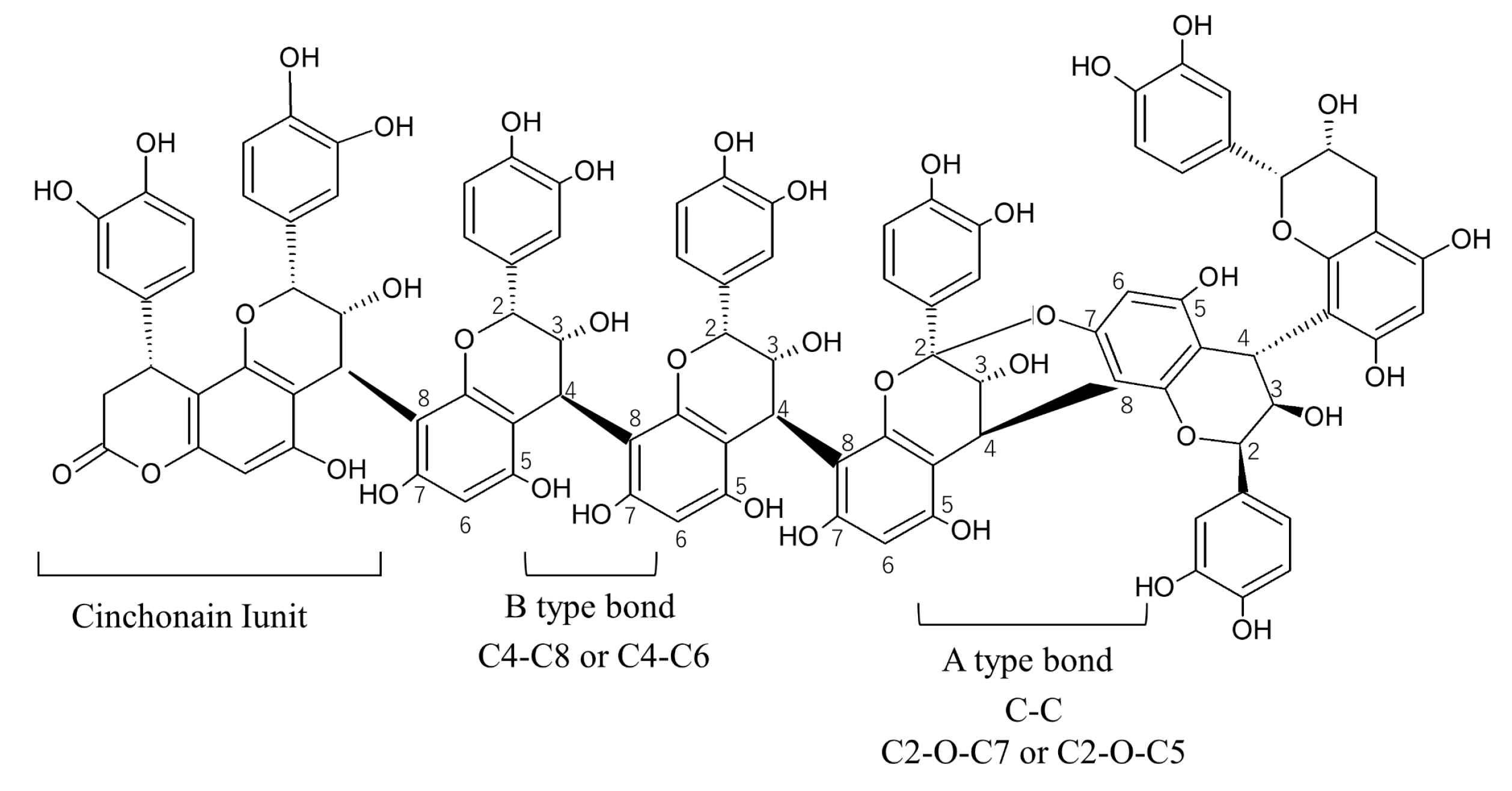
2.5. Mean Degree of Polymerization (mDP) of PAC
2.6. Antioxidant Activity Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Polyphenol Analysis
4.2.1. Total Polyphenol Content
4.2.2. Polyphenol Composition Analysis
4.3. Total Proanthocyanidin (PAC) Analysis
4.3.1. p-Dimethylaminocinnamaldehyde (DMACA) Method
4.3.2. Butanol/HCl Method
4.4. Mean Degrees of Polymerization (mDP) of PACs Polymerization
4.5. Antioxidant Activity
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krishna, P.; Pandey, G.; Thomas, R.; Parks, S. Improving blueberry fruit nutritional quality through physiological and genetic interventions: A review of current research and future directions. Antioxidants 2023, 12, 810. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.; Joseph Kamal, S.W.; Bajrang Chole, P.; Dayal, D.; Chaubey, K.K.; Pal, A.K.; Manjunath, B.T.; Bachheti, R.K. Functional Foods: Exploring the Health Benefits of Bioactive Compounds from Plant and Animal Sources. J. Food Qual. 2023, 2023, 5546753. [Google Scholar] [CrossRef]
- Matsuura, Y.; Sakakibara, H.; Kawaguchi, M.; Murayama, E.; Yokoyama, D.; Yukizaki, C.; Kunitake, H.; Sakono, M. Effects of blueberry leaf and stem extracts on hepatic lipid levels in rats consuming a high-sucrose diet. FFHD 2018, 8, 447–461. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Nemes, S.A.; Teleky, B.E.; Călinoiu, L.F.; Mitrea, L.; Martău, G.A.; Szabo, K.; Mihai, M.; Vodnar, V.C.; Crișan, G. Microencapsulation and Bioaccessibility of phenolic compounds of vaccinium leaf extracts. Antioxidants 2022, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Toyama, Y.; Fujita, Y.; Toshima, S.; Hirano, T.; Yamasaki, M.; Kunitake, H. Comparison of proanthocyanidin content in rabbiteye blueberry (Vaccinium virgatum Aiton) leaves and the promotion of apoptosis against hl-60 promyelocytic leukemia cells using ‘Kunisato 35 Gou’leaf extract. Plants 2023, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Nagao, K.; Higa, K.; Shirouchi, B.; Nomura, S.; Inoue, N.; Inafuku, M.; Yanagita, T. Effect of Vaccinium ashei reade leaves on lipid metabolism in Otsuka Long-Evans Tokushima Fatty rats. Biosci. Biotechnol. Biochem. 2008, 72, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Sakaida, H.; Nagao, K.; Higa, K.; Shirouchi, B.; Inoue, N.; Hidaka, F.; Kai, T.; Yanagita, T. Effect of Vaccinium ashei reade leaves on angiotensin converting enzyme activity in vitro and on systolic blood pressure of spontaneously hypertensive rats in vivo. Biosci. Biotechnol. Biochem. 2007, 71, 2335–2337. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hamada, K.; Fujii, K.; Nishiyama, K.; Yamasaki, Y.; Tari, H.; Araki, K.; Arakawa, T. Vaccinium ashei leaves extract alleviates insulin resistance via AMPK independent pathway in C2C12 myotube model. Biochem. Biophys. Rep. 2018, 14, 182–187. [Google Scholar] [CrossRef]
- Matsuo, Y.; Fujita, Y.; Ohnishi, S.; Tanaka, T.; Hirabaru, H.; Kai, T.; Sakaida, H.; Nisizono, S.; Kouno, I. Chemical constituents of the leaves of rabbiteye blueberry (Vaccinium ashei) and characterisation of polymeric proanthocyanidins containing phenylpropanoid units and A-type linkages. Food Chem. 2010, 121, 1073–1079. [Google Scholar] [CrossRef]
- Takeshita, M.; Ishida, Y.I.; Akamatsu, E.; Ohmori, Y.; Sudoh, M.; Uto, H.; Tsubouchi, H.; Kataoka, H. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J. Biol. Chem. 2009, 284, 21165–21176. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Kiue, Y.; Fujii, K.; Sushida, M.; Yamasaki, Y.; Sugamoto, K.; Suzuku, Y.; Koga, Y.; Kunitake, H.; Kai, H.; et al. Vaccinium virgatum Aiton Leaves Extract Suppressed Lipid Accumulation and Uric Acid Production in 3T3-L1 Adipocytes. Plants 2021, 10, 2638. [Google Scholar] [CrossRef] [PubMed]
- Sugamoto, K.; Tanaka, Y.L.; Saito, A.; Goto, Y.; Nakayama, T.; Okabayashi, T.; Kunitake, H.; Morishita, K. Highly polymerized proanthocyanidins (PAC) components from blueberry leaf and stem significantly inhibit SARS-CoV-2 infection via inhibition of ACE2 and viral 3CLpro enzymes. Biochem. Biophys. Res. Commun. 2022, 615, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Sharma, S.; Khatkar, S.K. Antioxidative, structural and thermal characterisation of simulated fermented matrix of quinoa, chia and teff with caseinate. Int. J. Food Sci. Technol. 2022, 57, 5663–5672. [Google Scholar] [CrossRef]
- Ogawa, K.; Urata, K.; Maeda, S.; Ohno, Y.; Satoh, K.; Yamada, Y.; Suzuki, Y.; Koga, Y.; Sugamoto, K.; Kawaguti, M.; et al. Blueberry leaf extract prevents lacrimal hyposecretion in Sjögren’s syndrome-like model of non-obese diabetic mice. In Vivo 2023, 37, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Okada, Y.; Goto, Y.; Nakayama, T.; Sugamoto, K.; Ogawa, K.; Yamasaki, M.; Morishita, K.; Matsuno, K.; Kunitake, H. Prediction of the adult T-cell leukemia inhibitory activity of blueberry leaves/stems using direct-injection electron ionization-mass spectrometry metabolomics. Plants 2022, 11, 1343. [Google Scholar] [CrossRef]
- Botha, J.J.; Ferreira, D.; Roux, D.G. Condensed tannins: Direct synthesis, structure, and absolute configuration of four biflavonoids from black wattle bark (‘mimosa’) extract. J. Chem. Soc. Chem. Commun. 1978, 16, 700–702. [Google Scholar] [CrossRef]
- Ariga, T. Antioxidative Functions, Preventive Action toward Disease and Utilization of Proanthocyanidins. Jpn. Oil Chem. Soc. 1999, 48, 1087–1096+1200. (In Japanese) [Google Scholar] [CrossRef]
- Steigerwalt, R.; Belcaro, G.; Cesarone, M.R.; Di Renzo, A.; Grossi, M.G.; Ricci, A.; Dugall, M.; Cacchio, M.; Schönlau, F. Pycnogenol® improves microcirculation, retinal edema, and visual acuity in early diabetic retinopathy. J. Ocul. Pharmacol. Ther. 2009, 25, 537–540. [Google Scholar] [CrossRef]
- Li, Y.G.; Tanner, G.; Larkin, P. The DMACA–HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- Rutkowska, M.; Balcerczak, E.; Świechowski, R.; Dubicka, M.; Olszewska, M.A. Seasonal variation in phenylpropanoid biosynthesis and in vitro antioxidant activity of Sorbus domestica leaves: Harvesting time optimisation for medicinal application. Ind. Crops Prod. 2020, 156, 112858. [Google Scholar] [CrossRef]
- Chen, H.; Wu, H.; Yan, B.; Zhao, H.; Liu, F.; Zhang, H.; Sheng, Q.; Miao, F.; Liang, Z. Core microbiome of medicinal plant Salvia miltiorrhiza seed: A rich reservoir of beneficial microbes for secondary metabolism? Int. J. Mol. Sci. 2018, 19, 672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Tam, N.F.Y.; Lin, Y.M.; Ding, Z.H.; Chai, W.M.; Wei, S.D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant Ceriops tagal. PLoS ONE 2014, 9, e107606. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Liu, L.; Cui, J.; Zhao, Y.; Zhang, D.; Zhou, D.; Wu, J.; Li, B.; Wang, T.; Li, M.; et al. Oligomeric proanthocyanidins: An updated review of their natural sources, synthesis, and potentials. Antioxidants 2023, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Dixon, R.A.; Duan, C. A role for ascorbate conjugates of (+)-catechin in proanthocyanidin polymerization. Nat. Commun. 2022, 13, 3425. [Google Scholar] [CrossRef]
- Izumi, H.; Ito, T.; Yoshida, Y. Seasonal changes in ascorbic acid, sugar and chlorophyll contents in Sun and shade leaves of Satsuma mandarin and their interrelationships. J. Jpn. Soc. Hort. Sci. 1990, 59, 389–397. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Makkar, H.P.; Gamble, G.; Becker, K. Limitation of the butanol–hydrochloric acid–iron assay for bound condensed tannins. Food Chem. 1999, 66, 129–133. [Google Scholar] [CrossRef]
- Suda, E. Antioxidant function, measurement of DPPH radical scavenging ability with spectrophotometer. Food Funct. Res. Method 2000, 8, 219–220. (In Japanese) [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koga, Y.; Setoguchi, Y.; Sugamoto, K.; Goto, Y.; Hirano, T.; Kunitake, H. Seasonal Variation and Mean Degree of Polymerization of Proanthocyanidin in Leaves and Branches of Rabbiteye Blueberry (Vaccinium virgatum Aiton). Plants 2024, 13, 1864. https://doi.org/10.3390/plants13131864
Koga Y, Setoguchi Y, Sugamoto K, Goto Y, Hirano T, Kunitake H. Seasonal Variation and Mean Degree of Polymerization of Proanthocyanidin in Leaves and Branches of Rabbiteye Blueberry (Vaccinium virgatum Aiton). Plants. 2024; 13(13):1864. https://doi.org/10.3390/plants13131864
Chicago/Turabian StyleKoga, Yasuko, Yuno Setoguchi, Kazuhiro Sugamoto, Yo Goto, Tomonari Hirano, and Hisato Kunitake. 2024. "Seasonal Variation and Mean Degree of Polymerization of Proanthocyanidin in Leaves and Branches of Rabbiteye Blueberry (Vaccinium virgatum Aiton)" Plants 13, no. 13: 1864. https://doi.org/10.3390/plants13131864
APA StyleKoga, Y., Setoguchi, Y., Sugamoto, K., Goto, Y., Hirano, T., & Kunitake, H. (2024). Seasonal Variation and Mean Degree of Polymerization of Proanthocyanidin in Leaves and Branches of Rabbiteye Blueberry (Vaccinium virgatum Aiton). Plants, 13(13), 1864. https://doi.org/10.3390/plants13131864







