Integrating Proteomics and Metabolomics Approaches to Elucidate the Mechanism of Responses to Combined Stress in the Bell Pepper (Capsicum annuum)
Abstract
1. Introduction
2. Results
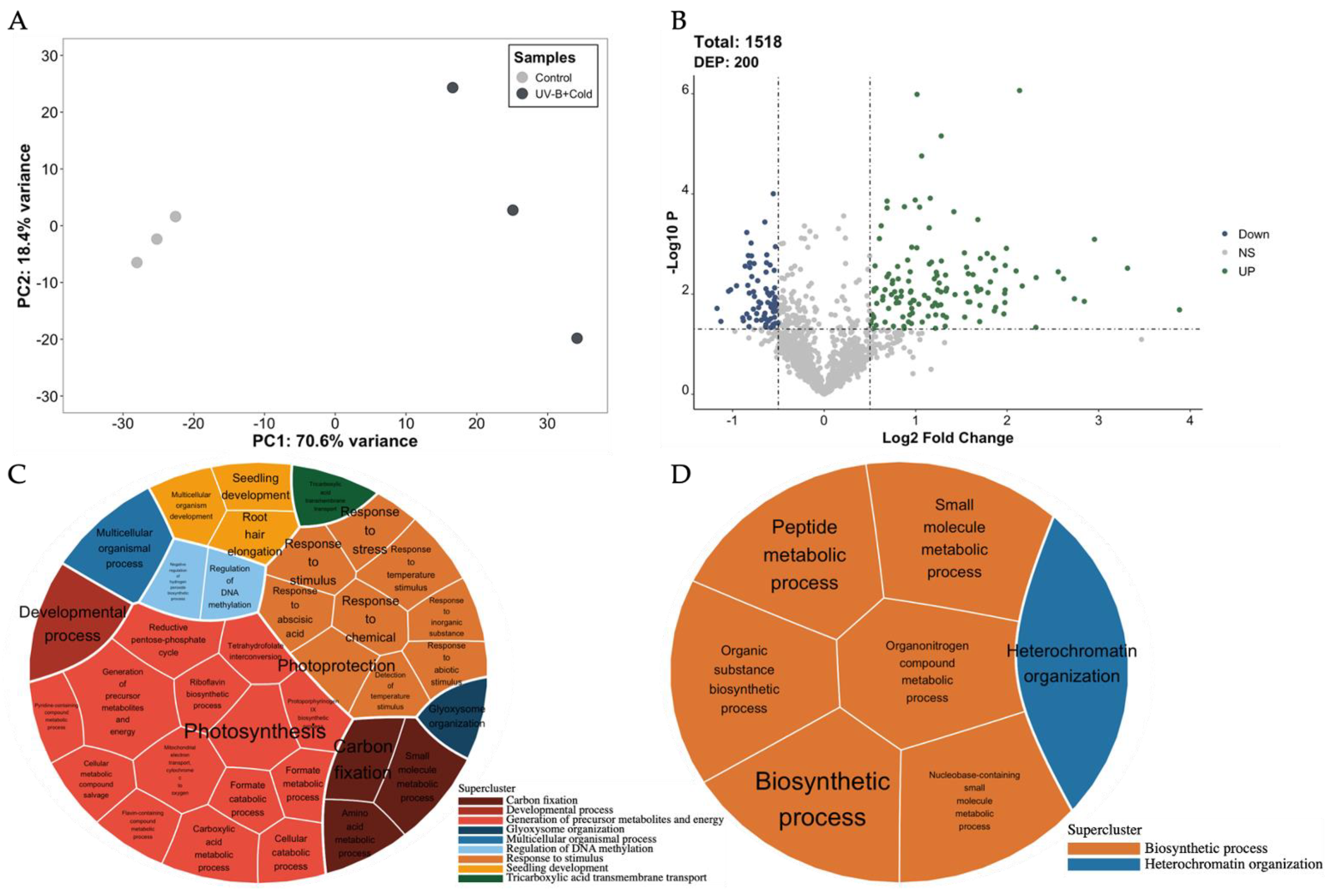
2.1. Differentially Expressed Proteins (DEPs) Identification
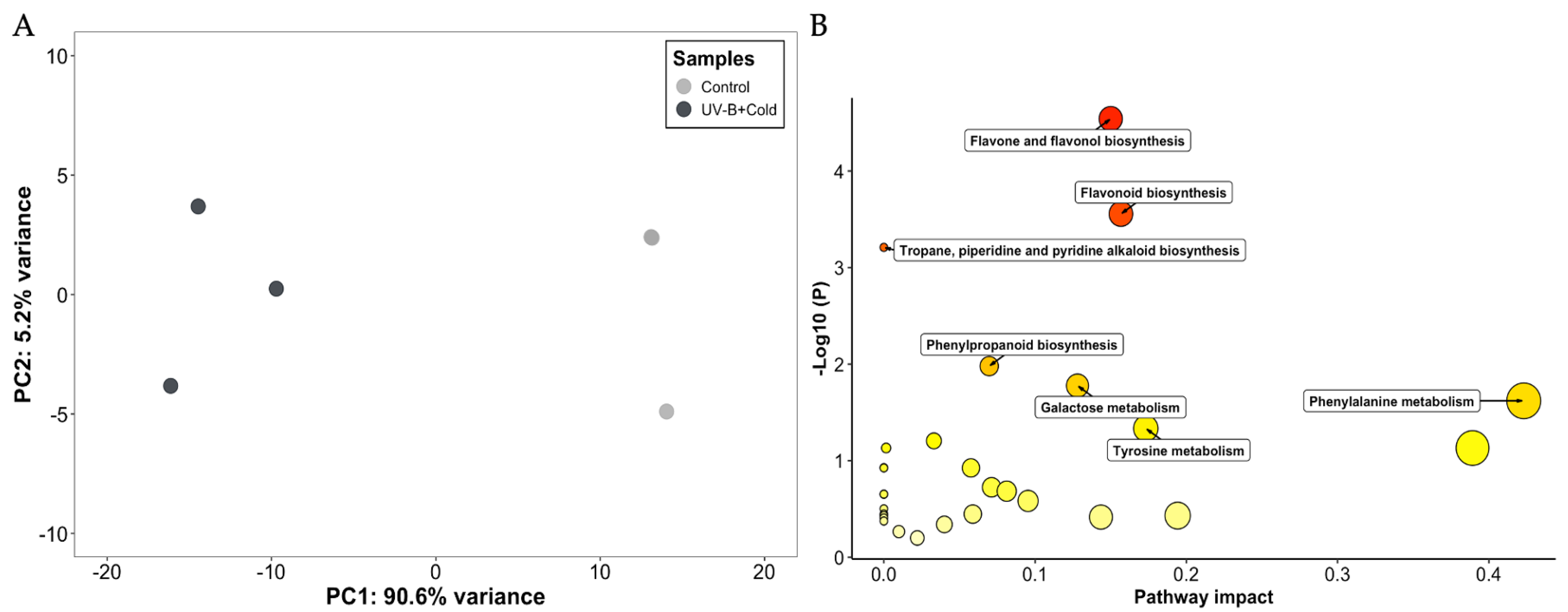
2.2. Untargeted Metabolomic Analyses
2.3. Targeted Metabolomic Analyses
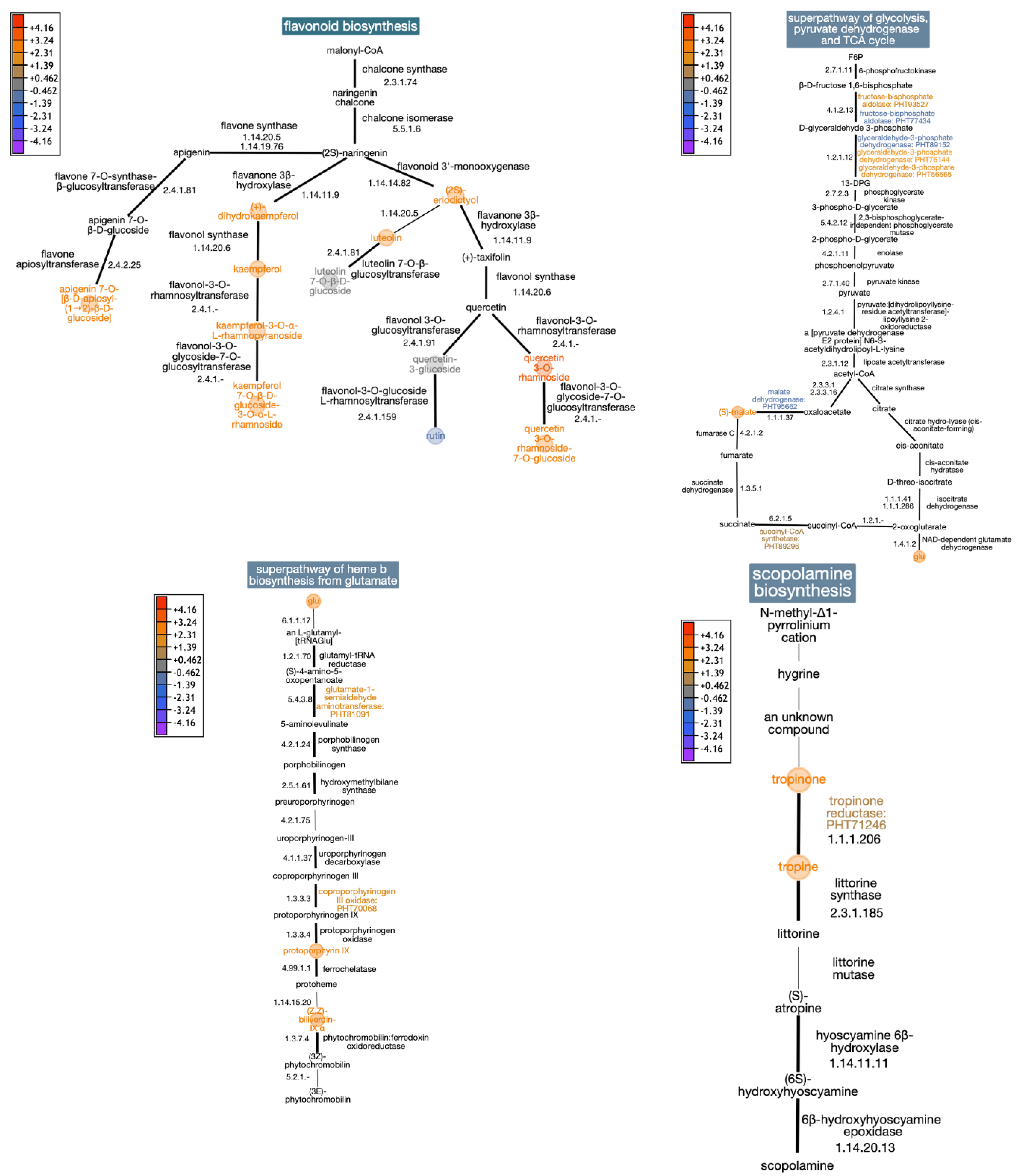
2.4. Data Integration
3. Discussion
3.1. Combined UV-B and Cold Stress Induces Changes in Flavonoid Biosynthesis
3.2. Effects of Combined UV-B and Cold Stress on Respiration and Photosynthesis Metabolism
3.3. Combined UV-B and Cold Stress Induces Changes in Tropine Biosynthesis
4. Materials and Methods
4.1. The Plant Materials
4.2. Proteomic Analysis
4.3. Metabolomic Analyses
4.4. Integration Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. The Plant Materials
| DAS * | Product Applied | Composition |
|---|---|---|
| 15 | Ultra P | P (33.53%), K (2.65%) |
| 18 | Ultra NPK | N (10%), P (10%), K (10%) |
| 21 | Ultra NPK | N (10%), P (10%), K (10%) |
| 24 | HBK | N (10%), P (5%), K (10%) |
| 27 | Ultra Ca | N (17%), Ca (13%), K (3.5%) |
| 30 | Ultra S | K (30%), S (30%) |
Appendix A.2. Proteomic Analysis
Appendix A.2.1. Protein Extraction
Appendix A.2.2. Protein Digestion, Prefractionation, Desalting, and Labeling
Appendix A.2.3. Nano LC-MS/MS
Appendix A.2.4. Synchronous Precursor Selection (SPS)-MS3
Appendix A.2.5. Data Analyses and Interpretation
Appendix A.2.6. Annotation Methods
Appendix A.3. Metabolomic Analyses
Appendix A.3.1. Metabolites Extraction
Appendix A.3.2. Untargeted Metabolomic Analyses
Appendix A.3.3. Targeted Metabolomic Analyses
References
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Sheth, B.P.; Thaker, V.S. Plant systems biology: Insights, advances and challenges. Planta 2014, 240, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Pandey, P.; Pandey, A.K. Proteomics in Relation to Abiotic Stress Tolerance in Plants. In Plant Life under Changing Environment: Responses and Management; Academic Press: Cambridge, MA, USA, 2020; pp. 513–541. ISBN 9780128182048. [Google Scholar]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T.; Renaut, J. Plant abiotic stress proteomics: The major factors determining alterations in cellular proteome. Front. Plant Sci. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Jorge, T.F.; Rodrigues, J.A.; Caldana, C.; Schmidt, R.; Van Dongen, J.T.; Thomas-Oates, J.; Antonio, C. Mass spectrometry-based plant metabolomics: Metabolite responses to abiotic stress. Mass Spectrom. Rev. 2016, 35, 620–649. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Chao, H.; Zhang, S.; Hu, Y.; Ni, Q.; Xin, S.; Zhao, L.; Ivanisenko, V.A.; Orlov, Y.L.; Chen, M. Integrating omics databases for enhanced crop breeding. J. Integr. Bioinform. 2023, 20, 20230012. [Google Scholar] [CrossRef]
- Jamil, I.N.; Remali, J.; Azizan, K.A.; Nor Muhammad, N.A.; Arita, M.; Goh, H.H.; Aizat, W.M. Systematic Multi-Omics Integration (MOI) Approach in Plant Systems Biology. Front. Plant Sci. 2020, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Palevitch, D.; Craker, L.E. Nutritional and Medical Importance of Red Pepper (Capsicum spp.). J. Herbs. Spices Med. Plants 1996, 3, 55–83. [Google Scholar] [CrossRef]
- Saleh, B.K.; Omer, A.; Teweldemedhin, B. Medicinal uses and health benefits of chili pepper (Capsicum spp.): A review. MOJ Food Process. Technol. 2018, 6, 325–328. [Google Scholar] [CrossRef]
- Chhapekar, S.S.; Jaiswal, V.; Ahmad, I.; Gaur, R.; Ramchiary, N. Progress and Prospects in Capsicum Breeding for Biotic and Abiotic Stresses; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9789811090295. [Google Scholar]
- Momo, J.; Kumar, A.; Islam, K.; Ahmad, I.; Rawoof, A.; Ramchiary, N. A comprehensive update on Capsicum proteomics: Advances and future prospects. J. Proteomics 2022, 261, 104578. [Google Scholar] [CrossRef] [PubMed]
- Rawoof, A.; Ahmad, I.; Islam, K.; Momo, J.; Kumar, A.; Jaiswal, V.; Ramchiary, N. Integrated omics analysis identified genes and their splice variants involved in fruit development and metabolites production in Capsicum species. Funct. Integr. Genom. 2022, 22, 1189–1209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Yi, C.; Chen, F.; Liu, Y.; Liao, Y.; Zhang, Z.; Liu, W.; Lv, J. Integrative analysis of non-targeted metabolome and transcriptome reveals the mechanism of volatile formation in pepper fruit. Front. Genet. 2023, 14, 1290492. [Google Scholar] [CrossRef]
- Heng, Z.; Xu, X.; Xu, X.; Li, Y.; Wang, H.; Huang, W.; Yan, S.; Li, T. Integrated transcriptomic and metabolomic analysis of chili pepper fruits provides new insight into the regulation of the branched chain esters and capsaicin biosynthesis. Food Res. Int. 2023, 169, 112856. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, H.; Fan, Y.; Yan, L. Anthocyanins accumulation analysis of correlated genes by metabolome and transcriptome in green and purple peppers (Capsicum annuum). BMC Plant Biol. 2022, 22, 358. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Liu, Z.; Liu, Y.; Song, J.; Ma, Y.; Ou, L.; Zhang, X.; Liang, C.; Wang, F.; et al. Integration of transcriptomics and metabolomics for pepper (Capsicum annuum L.) in response to heat stress. Int. J. Mol. Sci. 2019, 20, 5042. [Google Scholar] [CrossRef]
- Liu, C.; Luo, S.; Zhao, Y.; Miao, Y.; Wang, Q.; Ye, L.; Gao, L.; Ahammed, G.J.; Cheng, Y. Multiomics analyses reveal high temperature-induced molecular regulation of ascorbic acid and capsaicin biosynthesis in pepper fruits. Environ. Exp. Bot. 2022, 201, 104941. [Google Scholar] [CrossRef]
- Reimer, J.J.; Shaaban, B.; Drummen, N.; Ambady, S.S.; Genzel, F.; Poschet, G.; Wiese-Klinkenberg, A.; Usadel, B.; Wormit, A. Capsicum Leaves under Stress: Using Multi-Omics Analysis to Detect Abiotic Stress Network of Secondary Metabolism in Two Species. Antioxidants 2022, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, L.; Xie, Y.; Zhao, Z.; Su, L.; Tang, Y.; Sun, B.; Lai, Y.; Li, H. Transcriptome and Metabolome Analyses Reveal Molecular Responses of Two Pepper (Capsicum annuum L.) Cultivars to Cold Stress. Front. Plant Sci. 2022, 13, 819630. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, L.; Yang, B.; Cui, Q.; Dai, Y.; Li, X.; Chen, Y.; Dai, X.; Zou, X.; Ou, L.; et al. A multi-omics approach identifies bHLH71-like as a positive regulator of yellowing leaf pepper mutants exposed to high-intensity light. Hortic. Res. 2023, 10, uhad098. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yuan, S.; Chen, B.; Shi, J.; Sui, Y.; Gao, L.; Geng, S.; Zuo, J.; Wang, Q. A comparative proteomic and metabolomic analysis of the low-temperature response of a chilling-injury sensitive and a chilling-injury tolerant cultivar of green bell pepper. Sci. Hortic. 2023, 318, 112092. [Google Scholar] [CrossRef]
- Brand, D.; Wijewardana, C.; Gao, W.; Reddy, K.R. Interactive effects of carbon dioxide, low temperature, and ultraviolet-B radiation on cotton seedling root and shoot morphology and growth. Front. Earth Sci. 2016, 10, 607–620. [Google Scholar] [CrossRef]
- Coffey, A.; Jansen, M.A.K. Effects of natural solar UV-B radiation on three Arabidopsis accessions are strongly affected by seasonal weather conditions. Plant Physiol. Biochem. 2019, 134, 64–72. [Google Scholar] [CrossRef] [PubMed]
- León-Chan, R.G.; López-Meyer, M.; Osuna-Enciso, T.; Sañudo-Barajas, J.A.; Heredia, J.B.; León-Félix, J. Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environ. Exp. Bot. 2017, 139, 143–151. [Google Scholar] [CrossRef]
- Nichelmann, L.; Pescheck, F. Solar UV-B effects on composition and UV screening efficiency of foliar phenolics in Arabidopsis thaliana are augmented by temperature. Physiol. Plant. 2021, 173, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Tohge, T.; Winkler, J.B.; Albert, A.; Schaffner, A.R.; Fernie, A.R.; Zuther, E.; Hincha, D.K. Natural variation among arabidopsis accessions in the regulation of flavonoid metabolism and stress gene expression by combined uv radiation and cold. Plant Cell Physiol. 2021, 62, 502–514. [Google Scholar] [CrossRef]
- León-Chan, R.G.; Lightbourn-Rojas, L.A.; López-Meyer, M.; Amarillas, L.; Heredia, J.B.; Martínez-Bastidas, T.F.; Villicaña, C.; León-Félix, J. Differential gene expression of anthocyanin biosynthetic genes under low temperature and ultraviolet-B radiation in bell pepper (Capsicum annuum). Int. J. Agric. Biol. 2020, 23, 501–508. [Google Scholar]
- Morales-Merida, B.E.; Villicaña, C.; Perales-Torres, A.L.; Martínez-Montoya, H.; Castillo-Ruiz, O.; León-Chan, R.; Lighbourn-Rojas, L.A.; Heredia, J.B.; León-Félix, J. Transcriptomic Analysis in Response to Combined Stress by UV-B Radiation and Cold in Belle Pepper (Capsicum annuum). Int. J. Agric. Biol. 2021, 25, 969–980. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. Genetic Engineering for Modern Agriculture: Challenges and Perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef]
- Balfagón, D.; Sengupta, S.; Gómez-Cadenas, A.; Fritschi, F.B.; Azad, R.K.; Mittler, R.; Zandalinasc, S.I. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Sengupta, S.; Fritschi, F.B.; Azad, R.K.; Nechushtai, R.; Mittler, R. The impact of multifactorial stress combination on plant growth and survival. New Phytol. 2021, 230, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Shaar-Moshe, L.; Blumwald, E.; Peleg, Z. Unique physiological and transcriptional shifts under combinations of salinity, drought, and heat. Plant Physiol. 2017, 174, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Del Socorro Sánchez Correa, M.; el Rocío Reyero Saavedra, M.; Antonio Estrella Parra, E.; Nolasco Ontiveros, E.; del Carmen Benítez Flores, J.; Gerardo Ortiz Montiel, J.; Eduardo Campos Contreras, J.; López Urrutia, E.; Guillermo Ávila Acevedo, J.; Edith Jiménez Nopala, G.; et al. Ultraviolet Radiation and Its Effects on Plants. In Abiotic Stress in Plants—Adaptations to Climate Change; IntechOpen: London, UK, 2023; pp. 1–21. [Google Scholar]
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar] [CrossRef]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Fang, J.; Lin, M.; Hu, C.; Qi, X.; Chen, J.; Zhong, Y.; Muhammad, A.; Li, Z.; Li, Y. Comparative Metabolomic and Transcriptomic Studies Reveal Key Metabolism Pathways Contributing to Freezing Tolerance Under Cold Stress in Kiwifruit. Front. Plant Sci. 2021, 12, 628969. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wang, S.; Zhang, H.; Liu, Y.; Yang, M. Integrative transcriptomic and metabolomic analyses reveal the flavonoid biosynthesis of Pyrus hopeiensis flowers under cold stress. Hortic. Plant J. 2023, 9, 395–413. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, J.; Gao, T.; Zhang, N.; Jing, T.; Wang, J.; Ban, Q.; Schwab, W.; Song, C. Glucosyltransferase CsUGT78A14 Regulates Flavonols Accumulation and Reactive Oxygen Species Scavenging in Response to Cold Stress in Camellia sinensis. Front. Plant Sci. 2019, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Kim, J.H.; Kim, J.; Lee, C.; Ahn, J.H. Accumulation of flavonols in response to ultraviolet-B irradiation in soybean is related to induction of flavanone 3-β-hydroxylase and flavonol synthase. Mol. Cells 2008, 25, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Bumke-Vogt, C. Flavonoid glycosides in brassica species respond to uv-b depending on exposure time and adaptation time. Molecules 2021, 26, 494. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Gu, Y.; Liu, S.; Jiang, L.; Han, M.; Geng, D. Flavonoids metabolism and physiological response to ultraviolet treatments in Tetrastigma hemsleyanum Diels et Gilg. Front. Plant Sci. 2022, 13, 926197. [Google Scholar] [CrossRef] [PubMed]
- Genzel, F.; Dicke, M.D.; Junker-Frohn, L.V.; Neuwohner, A.; Thiele, B.; Putz, A.; Usadel, B.; Wormit, A.; Wiese-Klinkenberg, A. Impact of Moderate Cold and Salt Stress on the Accumulation of Antioxidant Flavonoids in the Leaves of Two Capsicum Cultivars. J. Agric. Food Chem. 2021, 69, 6431–6443. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Kleinschmidt, K.; Weissenböck, G.; Klein, M. Flavonoid biosynthesis in barley primary leaves requires the presence of the vacuole and controls the activity of vacuolar flavonoid transport. Plant Physiol. 2007, 144, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Zhou, C.; Li, J.; Zhang, Y. Molecular characterization of the C-glucosylation for puerarin biosynthesis in Pueraria lobata. Plant J. 2017, 90, 535–546. [Google Scholar] [CrossRef]
- Liu, Z.; Song, J.; Miao, W.; Yang, B.; Zhang, Z.; Chen, W.; Tan, F.; Suo, H.; Dai, X.; Zou, X.; et al. Comprehensive Proteome and Lysine Acetylome Analysis Reveals the Widespread Involvement of Acetylation in Cold Resistance of Pepper (Capsicum annuum L.). Front. Plant Sci. 2021, 12, 730489. [Google Scholar] [CrossRef]
- Muñoz-Bertomeu, J.; Cascales-Miñana, B.; Mulet, J.M.; Baroja-Fernández, E.; Pozueta-Romero, J.; Kuhn, J.M.; Segura, J.; Ros, R. Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiol. 2009, 151, 541–558. [Google Scholar] [CrossRef]
- Muñoz-Bertomeu, J.; Bermúdez, M.A.; Segura, J.; Ros, R. Arabidopsis plants deficient in plastidial glyceraldehyde-3-phosphate dehydrogenase show alterations in abscisic acid (ABA) signal transduction: Interaction between ABA and primary metabolism. J. Exp. Bot. 2011, 62, 1229–1239. [Google Scholar] [CrossRef]
- Petersen, J.; Brinkmann, H.; Cerff, R. Origin, evolution, and metabolic role of a novel glycolytic GAPDH enzyme recruited by land plant plastids. J. Mol. Evol. 2003, 57, 16–26. [Google Scholar] [CrossRef]
- Li, X.; Wei, W.; Li, F.; Zhang, L.; Deng, X.; Liu, Y.; Yang, S. The plastidial glyceraldehyde-3-phosphate dehydrogenase is critical for abiotic stress response in wheat. Int. J. Mol. Sci. 2019, 20, 1104. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, R.; Guo, Z.; Yang, S.; Deng, X. Genome-wide identification and characterization of Glyceraldehyde-3-phosphate dehydrogenase genes family in wheat (Triticum aestivum). BMC Genom. 2016, 17, 1104. [Google Scholar] [CrossRef] [PubMed]
- Carrera, D.Á.; George, G.M.; Fischer-Stettler, M.; Galbier, F.; Eicke, S.; Truernit, E.; Streb, S.; Zeeman, S.C. Distinct plastid fructose bisphosphate aldolases function in photosynthetic and non-photosynthetic metabolism in Arabidopsis. J. Exp. Bot. 2021, 72, 3739–3755. [Google Scholar] [CrossRef]
- Koßmann, J.; Sonnewald, U.; Willmitzer, L. Reduction of the chloroplastic fructose-1,6-bisphosphatase in transgenic potato plants impairs photosynthesis and plant growth. Plant J. 1994, 6, 637–650. [Google Scholar] [CrossRef]
- Li, T.; Hou, X.; Sun, Z.; Ma, B.; Wu, X.; Feng, T.; Ai, H.; Huang, X.; Li, R. Characterization of FBA genes in potato (Solanum tuberosum L.) and expression patterns in response to light spectrum and abiotic stress. Front. Genet. 2024, 15, 1364944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiao, F.; Tang, H.; Xu, H.; Zhang, L.; Wu, H. Genome-wide characterization, evolution, and expression profiling of FBA gene family in response to light treatments and abiotic stress in Nicotiana tabacum. Plant Signal. Behav. 2021, 16, 1938442. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Li, Q.; Xu, Y.; Yang, L.; Bi, H.; Ai, X. Genome-wide analysis of the fructose 1,6-bisphosphate aldolase (FBA) gene family and functional characterization of FBA7 in tomato. Plant Physiol. Biochem. 2016, 108, 251–265. [Google Scholar] [CrossRef]
- Cai, B.; Li, Q.; Liu, F.; Bi, H.; Ai, X. Decreasing fructose-1,6-bisphosphate aldolase activity reduces plant growth and tolerance to chilling stress in tomato seedlings. Physiol. Plant. 2018, 163, 247–258. [Google Scholar] [CrossRef]
- Tomaz, T.; Bagard, M.; Pracharoenwattana, I.; Lindén, P.; Lee, C.P.; Carroll, A.J.; Ströher, E.; Smith, S.M.; Gardeström, P.; Millar, A.H. Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Physiol. 2010, 154, 1143–1157. [Google Scholar] [CrossRef]
- Pracharoenwattana, I.; Cornah, J.E.; Smith, S.M. Arabidopsis peroxisomal malate dehydrogenase functions in β-oxidation but not in the glyoxylate cycle. Plant J. 2007, 50, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Cousins, A.B.; Pracharoenwattana, I.; Zhou, W.; Smith, S.M.; Badger, M.R. Peroxisomal malate dehydrogenase is not essential for photorespiration in Arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release. Plant Physiol. 2008, 148, 786–795. [Google Scholar] [CrossRef]
- Beeler, S.; Liu, H.C.; Stadler, M.; Schreier, T.; Eicke, S.; Lue, W.L.; Truernit, E.; Zeeman, S.C.; Chen, J.; Kötting, O. Plastidial NAD-dependent malate dehydrogenase is critical for embryo development and heterotrophic metabolism in Arabidopsis. Plant Physiol. 2014, 164, 1175–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.F.; Zhao, Y.; Yi, Q.; Li, K.Z.; Yu, Y.X.; Chen, L.M. Overexpression of malate dehydrogenase in transgenic tobacco leaves: Enhanced malate synthesis and augmented Al-resistance. Acta Physiol. Plant. 2010, 32, 1209–1220. [Google Scholar] [CrossRef]
- Imran, M.; Munir, M.Z.; Ialhi, S.; Abbas, F.; Younus, M.; Ahmad, S.; Naeem, M.K.; Waseem, M.; Iqbal, A.; Gul, S.; et al. Identification and Characterization of Malate Dehydrogenases in Tomato (Solanum lycopersicum L.). Int. J. Mol. Sci. 2022, 23, 28. [Google Scholar] [CrossRef]
- Li, Z.; Shi, L.; Lin, X.; Tang, B.; Xing, M.; Zhu, H. Genome-Wide Identification and Expression Analysis of Malate Dehydrogenase Gene Family in Sweet Potato and Its Two Diploid Relatives. Int. J. Mol. Sci. 2023, 24, 6549. [Google Scholar] [CrossRef]
- Larkin, R.M. Tetrapyrrole signaling in plants. Front. Plant Sci. 2016, 7, 1586. [Google Scholar] [CrossRef]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef]
- Salama, H.M.H.; Al Watban, A.A.; Al-Fughom, A.T. Effect of ultraviolet radiation on chlorophyll, carotenoid, protein and proline contents of some annual desert plants. Saudi J. Biol. Sci. 2011, 18, 79–86. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Jin, L.Q.; Li, Y.T.; Tikkanen, M.; Li, Q.M.; Ai, X.Z.; Gao, H.Y. Ultraviolet-B radiation (UV-B) relieves chilling-light-induced PSI photoinhibition and accelerates the recovery of CO2 assimilation in cucumber (Cucumis sativus L.) Leaves. Sci. Rep. 2016, 6, srep34455. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Wang, X.; Essemine, J.; Jin, J.; Qu, M.; Xiang, Y.; Chen, W. Cold-induced inhibition of photosynthesis-related genes integrated by a TOP6 complex in rice mesophyll cells. Nucleic Acids Res. 2023, 51, 1823–1842. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, H.; Wang, L.; Zhao, Q.; Wang, D.; Zhang, T. Cold acclimation alleviates cold stress-induced PSII inhibition and oxidative damage in tobacco leaves. Plant Signal. Behav. 2022, 17, 2013638. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, S.; Sun, L.; Song, F.; Liu, F.; Li, X. Cold tolerance of photosynthetic electron transport system is enhanced in wheat plants grown under elevated CO2. Front. Plant Sci. 2018, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrapyrrole Metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0145. [Google Scholar] [CrossRef] [PubMed]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant metabolomics in biotic and abiotic stress: A critical overview. Phytochem. Rev. 2022, 21, 503–524. [Google Scholar] [CrossRef]
- Mabuchi, T.; Miura, Y.; Ohtsuka, T. Herbicidal activity and characteristics of pyraflufen-ethyl for controlling broad-leaved weeds in cereals. J. Pestic. Sci. 2002, 27, 39–46. [Google Scholar] [CrossRef]
- Murata, S.; Kimura, Y.; Mabuchi, T.; Miura, Y. Accumulation of protoporphyrinogen IX prior to protoporphyrin IX in intact plants treated with protoporphyrinogen IX oxidase-inhibiting herbicides. J. Pestic. Sci. 2004, 29, 195–199. [Google Scholar] [CrossRef]
- Grossmann, K.; Niggeweg, R.; Christiansen, N.; Looser, R.; Ehrhardt, T. The Herbicide Saflufenacil (KixorTM) is a New Inhibitor of Protoporphyrinogen IX Oxidase Activity. Weed Sci. 2010, 58, 1–9. [Google Scholar] [CrossRef]
- Phung, T.H.; Jung, S. Alterations in the porphyrin biosynthesis and antioxidant responses to chilling and heat stresses in Oryza sativa. Biol. Plant. 2015, 59, 341–349. [Google Scholar] [CrossRef]
- Phung, T.H.; Jung, H.; Park, J.H.; Kim, J.G.; Back, K.; Jung, S. Porphyrin biosynthesis control under water stress: Sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol. 2011, 157, 1746–1764. [Google Scholar] [CrossRef]
- Tran, L.H. Expression of the Arabidopsis Mg-chelatase H subunit alleviates iron deficiency-induced stress in transgenic rice. Front. Plant Sci. 2023, 14, 1098808. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Tran, L.H.; Jung, S. Salt Stress-Induced Modulation of Porphyrin Biosynthesis, Photoprotection, and Antioxidant Properties in Rice Plants (Oryza sativa). Antioxidants 2023, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Kohchi, T.; Mukougawa, K.; Frankenberg, N.; Masuda, M.; Yokota, A.; Lagarias, J.C. The Arabidopsis HY2 gene encodes phytochromobilin synthase, a ferredoxin-dependent biliverdin reductase. Plant Cell 2001, 13, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane alkaloids: Chemistry, pharmacology, biosynthesis and production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef] [PubMed]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiu, F.; Zeng, J.; Xu, Z.; Tang, Y.; Zhao, T.; Gou, Y.; Su, F.; Wang, S.; Sun, X.; et al. Revealing evolution of tropane alkaloid biosynthesis by analyzing two genomes in the Solanaceae family. Nat. Commun. 2023, 14, 1446. [Google Scholar] [CrossRef] [PubMed]
- Monribot-Villanueva, J.L.; Elizalde-Contreras, J.M.; Aluja, M.; Segura-Cabrera, A.; Birke, A.; Guerrero-Analco, J.A.; Ruiz-May, E. Endorsing and extending the repertory of nutraceutical and antioxidant sources in mangoes during postharvest shelf life. Food Chem. 2019, 285, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Monribot-Villanueva, J.L.; Altúzar-Molina, A.; Aluja, M.; Zamora-Briseño, J.A.; Elizalde-Contreras, J.M.; Bautista-Valle, M.V.; Arellano de los Santos, J.; Sánchez-Martínez, D.E.; Rivera-Reséndiz, F.J.; Vázquez-Rosas-Landa, M.; et al. Integrating proteomics and metabolomics approaches to elucidate the ripening process in white Psidium guajava. Food Chem. 2022, 367, 130565. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant Graphics for Data Analysis. J. Stat. Softw. 2009, 35, 160–167. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Jiménez-Fernández, V.M.; Suárez-Montaño, R.; Aguilar-Colorado, Á.S.; Guerrero-Analco, J.A.; Jiménez, M. Phytochemical characterization of Izote (Yucca elephantipes) flowers. J. Appl. Bot. Food Qual. 2018, 91, 202–210. [Google Scholar] [CrossRef]
- Zhang, P.; Dreher, K.; Karthikeyan, A.; Chi, A.; Pujar, A.; Caspi, R.; Karp, P.; Kirkup, V.; Latendresse, M.; Lee, C.; et al. Creation of a genome-wide metabolic pathway database for populus trichocarpa using a new approach for reconstruction and curation of metabolic pathways for plants. Plant Physiol. 2010, 153, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Dorfer, V.; Pichler, P.; Stranzl, T.; Stadlmann, J.; Taus, T.; Winkler, S.; Mechtler, K. MS Amanda, a universal identification algorithm optimized for high accuracy tandem mass spectra. J. Proteome Res. 2014, 13, 3679–3684. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef]
| Compound | Samples | |
|---|---|---|
| Control | UV-B+Cold | |
| Phenylalanine 1 | 101.75 ± 2.39 a | 91.78 ± 1.30 b |
| Protocatechuic acid 1 | 0.31 ± 0.01 * a | 0.29 ± 0.03* a |
| 4-hydroxybenzoic acid 2 | 1 ± 0.05 a | 0.69 ± 0.00 a |
| Vanillic acid 2 | 1.04 ± 0.01 a | 0.55 ± 0.03 a |
| Chlorogenic acid 1 | 22.89 ± 0.20 a | 57.17 ± 0.03 b |
| 4-Coumaric acid 2 | 0.26 ± 0.01 * a | 0.11 ± 0.01* a |
| 3-Coumaric acid 1 | 3.61 ± 0.03 a | 3.04 ± 0.06 b |
| Ferulic acid 3 | 0.05 ± 0.01 * | --- |
| Salicylic acid 1 | 2.31 ± 0.14 a | 0.30 ± 0.06* b |
| Vanillin 2 | 1.44 ± 0.06 a | 0.92 ± 0.02 a |
| Luteolin 1 | 0.66 ± 0.01 * a | 0.72 ± 0.04* a |
| Luteolin-7-O-glucoside 1 | 82.86 ± 2.17 a | 63.99 ± 3.38 b |
| Quercetin-3-glucoside 1 | 4.97 ± 0.12 a | 4.93 ± 0.05 a |
| Quercitrin 1 | 3.97 ± 0.05 a | 4.34 ± 0.05 b |
| Rutin 1 | 9.17 ± 0.33 a | 6.31 ± 0.19 b |
| Penta-O-galloyl-B-D-glucose 1 | 1.47 ± 0.42 * a | 0.32 ± 0.05* b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Merida, B.E.; Grimaldi-Olivas, J.C.; Cruz-Mendívil, A.; Villicaña, C.; Valdez-Torres, J.B.; Heredia, J.B.; León-Chan, R.G.; Lightbourn-Rojas, L.A.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; et al. Integrating Proteomics and Metabolomics Approaches to Elucidate the Mechanism of Responses to Combined Stress in the Bell Pepper (Capsicum annuum). Plants 2024, 13, 1861. https://doi.org/10.3390/plants13131861
Morales-Merida BE, Grimaldi-Olivas JC, Cruz-Mendívil A, Villicaña C, Valdez-Torres JB, Heredia JB, León-Chan RG, Lightbourn-Rojas LA, Monribot-Villanueva JL, Guerrero-Analco JA, et al. Integrating Proteomics and Metabolomics Approaches to Elucidate the Mechanism of Responses to Combined Stress in the Bell Pepper (Capsicum annuum). Plants. 2024; 13(13):1861. https://doi.org/10.3390/plants13131861
Chicago/Turabian StyleMorales-Merida, Brandon Estefano, Jesús Christian Grimaldi-Olivas, Abraham Cruz-Mendívil, Claudia Villicaña, José Benigno Valdez-Torres, J. Basilio Heredia, Rubén Gerardo León-Chan, Luis Alberto Lightbourn-Rojas, Juan L. Monribot-Villanueva, José A. Guerrero-Analco, and et al. 2024. "Integrating Proteomics and Metabolomics Approaches to Elucidate the Mechanism of Responses to Combined Stress in the Bell Pepper (Capsicum annuum)" Plants 13, no. 13: 1861. https://doi.org/10.3390/plants13131861
APA StyleMorales-Merida, B. E., Grimaldi-Olivas, J. C., Cruz-Mendívil, A., Villicaña, C., Valdez-Torres, J. B., Heredia, J. B., León-Chan, R. G., Lightbourn-Rojas, L. A., Monribot-Villanueva, J. L., Guerrero-Analco, J. A., Ruiz-May, E., & León-Félix, J. (2024). Integrating Proteomics and Metabolomics Approaches to Elucidate the Mechanism of Responses to Combined Stress in the Bell Pepper (Capsicum annuum). Plants, 13(13), 1861. https://doi.org/10.3390/plants13131861











