High Photosynthetic Photon Flux Density Differentially Improves Edible Biomass Space Use Efficacy in Edamame and Dwarf Tomato
Abstract
:1. Introduction
2. Results
2.1. EBSUE and SUE
2.2. Edible and Above-Ground Dry Weight and Dry Weight Fraction
2.3. Accumulated Cultivation Volume and Plant Height
2.4. Photosynthetic Capacity
3. Discussion
3.1. High PPFD Leads to High EBSUE by Increasing Both SUE and FE in Edamame
3.2. Effects of PPFD on FE, V, Plant Height, and Pmax in Dwarf Tomatoes
3.3. Effects of PPFD on FE, V, Plant Height, and Pmax in Edamame and Dwarf Tomatoes
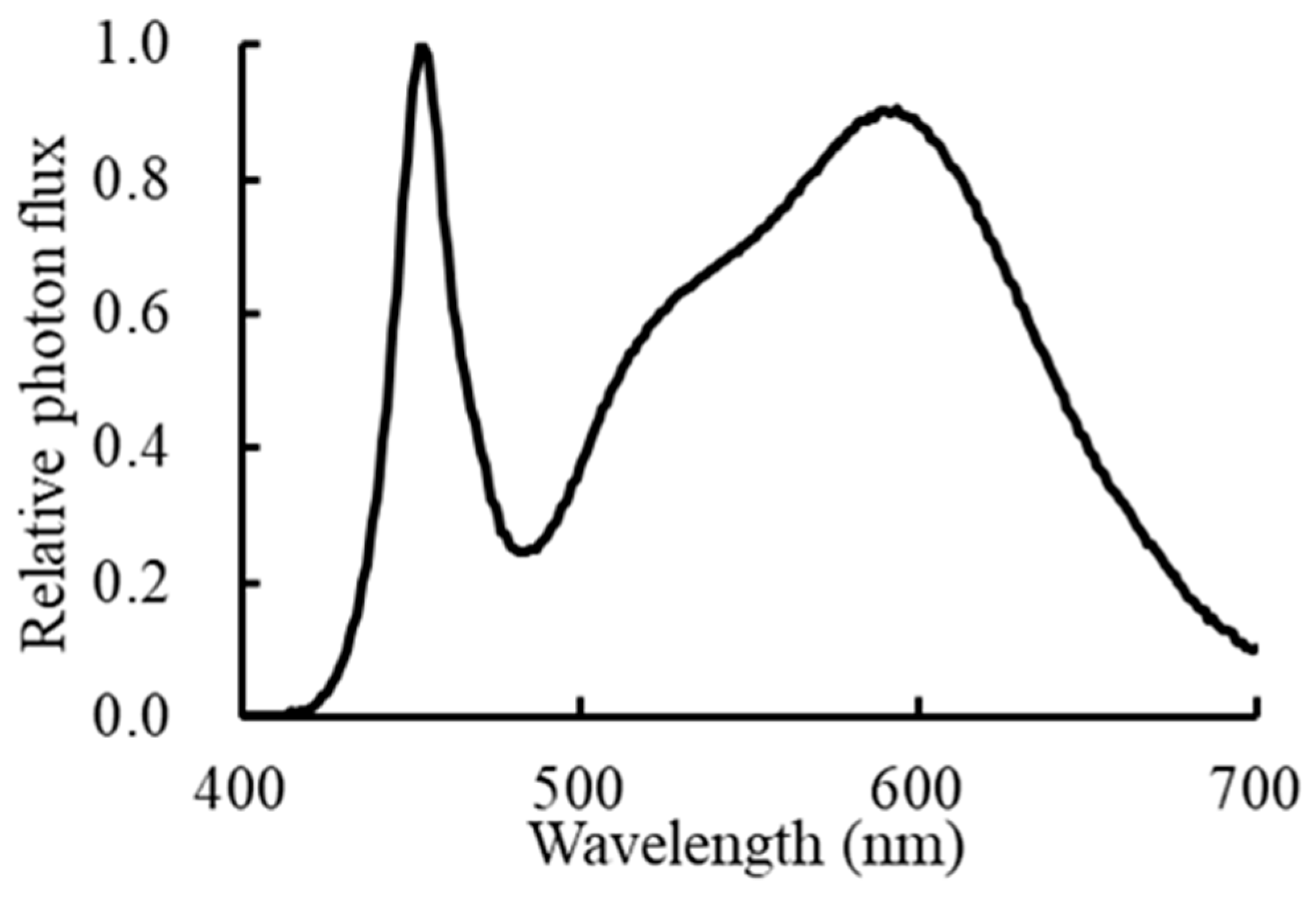
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Edible Biomass Space Use Efficacy and Photosynthetic Capacity
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozai, T.; Niu, G. Plant factory as a resource-efficient closed plant production system. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 69–90. [Google Scholar]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F. Vertical Farming: Moving from genetic to environmental modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Graamans, L.; Baeza, E.; Van Den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Konovsky, J.; Lumpkin, T.A.; MeClary, D. Edamame. The vegetable soybean. In Understanding the Japanese Food and Agrimarket, 1st ed.; A multifaceted Opportunity; Haworth Press: Boca Raton, FL, USA, 1994; pp. 173–181. [Google Scholar]
- Nair, R.M.; Boddepalli, V.N.; Yan, M.-R.; Kumar, V.; Gill, B.; Pan, R.S.; Wang, C.; Hartman, G.L.; Silva, E.S.R.; Somta, P. Global status of vegetable soybean. Plants 2023, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Yoshida, H.; Hikosaka, S.; Goto, E. Optimization of photosynthetic photon flux density and light quality for increasing radiation-use efficiency in dwarf tomato under LED light at the vegetative growth stage. Plants 2021, 11, 121. [Google Scholar] [CrossRef]
- Ribera, L.M.; Aires, E.S.; Neves, C.S.; Fernandes, G.D.C.; Bonfim, F.P.G.; Rockenbach, R.I.; Rodrigues, J.D.; Ono, E.O. Assessment of the physiological response and productive performance of vegetable vs. conventional soybean cultivars for edamame production. Agronomy 2022, 12, 1478. [Google Scholar] [CrossRef]
- Ke, X.; Yoshida, H.; Goto, E. Photosynthetic photon flux density affects fruit biomass radiation-use efficiency of dwarf tomatoes under LED light at the reproductive growth stage. Front. Plant Sci. 2023, 14, 1076423. [Google Scholar] [CrossRef] [PubMed]
- Saputra, H.; Mutaqin, Z. Growth response of edamame soybean intercropped with sweet corn at different planting date. J. Agrotropika 2021, 20, 42–48. [Google Scholar] [CrossRef]
- Utasi, L.; Kovács, V.; Gulyás, Z.; Marcek, T.; Janda, T.; Darko, E. Threshold or not: Spectral composition and light-intensity dependence of growth and metabolism in tomato seedlings. Sci. Hortic. 2023, 313, 111946. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, T.; Song, C.; Su, B.; Yang, F.; Yong, T.W.; Wu, Y.S.; Zhang, C.Y.; Yang, W.Y. Changes in light environment, morphology, growth and yield of soybean in maize-soybean intercropping systems. Field Crop. Res. 2017, 200, 38–46. [Google Scholar] [CrossRef]
- Mansoori, M.; Wu, B.S.; Addo, P.W.; MacPherson, S.; Lefsrud, M. Growth responses of tomato plants to different wavelength ratios of amber, red, and blue light. Sci. Hortic. 2023, 322, 112459. [Google Scholar] [CrossRef]
- Wu, Y.S.; Gong, W.Z.; Yang, F.; Wang, X.C.; Yong, T.W.; Yang, W.Y. Responses to shade and subsequent recovery of soybean in maize-soybean relay strip intercropping. Plant Prod. Sci. 2016, 19, 206–214. [Google Scholar] [CrossRef]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Bin Khalid, M.H.; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L.; et al. The influence of light intensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef]
- Hitz, T.; Graeff-Hönninger, S.; Munz, S. Modelling of Soybean (Glycine max (L.) Merr.) Response to blue light intensity in controlled environments. Plants 2020, 9, 1757. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Zhang, Y.; Cheng, R.; Zhang, Y.; Yang, Q.; Li, T. Effects of supplementary artificial light on growth of the tomato (Solanum lycopersicum) in a Chinese solar greenhouse. Hortic. J. 2018, 87, 516–523. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Emery, R.J.; Pharis, R.P.; Reid, D.M. The interaction of light quality and irradiance with gibberellins, cytokinins and auxin in regulating growth of Helianthus annuus hypocotyls. Plant Cell Environ. 2007, 30, 147–155. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Vriezen, W.H.; Smalle, J.; Laarhoven, L.J.; Harren, F.; Van Der Straeten, D. Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiol. 2003, 133, 517–527. [Google Scholar] [CrossRef]
- Hersch, M.; Lorrain, S.; de Wit, M.; Trevisan, M.; Ljung, K.; Bergmann, S.; Fankhauser, C. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 6515–6520. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, C.; Li, S.; Zhu, Q.; Zhang, H.; Wang, H.; Yu, C.; Steven, K.S.M.; Xie, F. Effect of shade on leaf photosynthetic capacity, light-intercepting, electron transfer and energy distribution of soybeans. Plant Growth Regul. 2017, 83, 409–416. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A. Shade effect on carbohydrates dynamics and stem strength of soybean genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Ookawa, T.; Takase, Y.; Ishihara, K.; Hirasawa, T. Differences in dry matter production and eco-physiological (characteristics between soybean cultivars, Enrei and Tachinagaha. Jpn. J. Crop Sci. 1999, 68, 105–111. [Google Scholar] [CrossRef]
- Gauhl, E. Photosynthetic response to varying light intensity in ecotypes of Solanum dulcamara L. from shaded and exposed habitats. Oecologia 1976, 22, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Ruimy, A.; Jarvis, P.G.; Baldocchi, D.D.; Saugier, B. CO2 fluxes over plant canopies and solar radiation: A review. Adv. Ecol. Res. 1995, 26, 1–68. [Google Scholar] [CrossRef]
- Schulze, E.D.; Caldwell, M.M. Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 133–143. [Google Scholar]
- Bowes, G.; Ogren, W.L.; Hageman, R.H. Light Saturation, photosynthesis rate, RuDP carboxylase activity, and specific leaf weight in soybeans grown under different light intensities 1. Crop Sci. 1972, 12, 77–79. [Google Scholar] [CrossRef]
- Bianchi, J.S.; Quijano, A.; Gosparini, C.O.; Morandi, E.N. Changes in leaflet shape and seeds per pod modify crop growth parameters, canopy light environment, and yield components in soybean. Crop J. 2020, 8, 351–364. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Jin, J.; Liu, J.D.; Zhang, Q.Y.; Liu, X.B. Effects of light enrichment and shade during reproductive stage on yield and yield components in soybean. Soy. Sci. 2008, 27, 764–772. [Google Scholar]
- Yang, F.; Liao, D.; Wu, X.; Gao, R.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; Liu, J. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crop. Res. 2017, 203, 16–23. [Google Scholar] [CrossRef]
- Kläring, H.P.; Krumbein, A. The effect of constraining the intensity of solar radiation on the photosynthesis, growth, yield and product quality of tomato. J. Agron. Crop Sci. 2013, 199, 351–359. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, J.; Lin, S.; Jin, C.; Shi, M.; Yang, B.; Yang, Y.; Jin, D.; Li, R.; Li, Y.; et al. Effects of different light intensity on the growth of tomato seedlings in a plant factory. PLoS ONE 2023, 18, e0294876. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, J.; Hu, J.T.; Jeong, B.R. Effect of supplementary light intensity on quality of grafted tomato seedlings and expression of two photosynthetic genes and proteins. Agronomy 2019, 9, 339. [Google Scholar] [CrossRef]
- Scott, J.W.; Harbaugh, B.K. Micro-Tom. A miniature dwarf tomato. Circ. Univ. Fla. Agric. Exp. Stn. 1989, 370, 6. [Google Scholar]
- Meissner, R.; Jacobson, Y.; Melamed, S.; Levyatuv, S.; Shalev, G.; Ashri, A.; Elkind, Y.; Levy, A. A new model system for tomato genetics. Plant J. 1997, 12, 1465–1472. [Google Scholar] [CrossRef]
- Pnueli, L.; Carmel-Goren, L.; Hareven, D.; Gutfinger, T.; Alvarez, J.; Ganal, M.; Zamir, D.; Lifschitz, E. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 1998, 125, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Q.; Xu, D.Q. Light intensity-dependent reversible down-regulation and irreversible damage of PSII in soybean leaves. Plant Sci. 2002, 163, 847–853. [Google Scholar] [CrossRef]
- Liu, Q.; Ke, X.; Yoshida, H.; Hikosaka, S.; Goto, E. Optimizing photosynthetic photon flux density and light quality for maximizing space use efficacy in edamame at the vegetative growth stage. Front. Sustain. Food Syst. 2024, 8, 1407359. [Google Scholar] [CrossRef]
- Kramer, P.J. Carbon dioxide concentration, photosynthesis, and dry matter production. BioScience 1981, 31, 29–33. [Google Scholar] [CrossRef]
- Niinemets, Ü. Research review. Components of leaf dry mass per area–thickness and density–alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999, 144, 35–47. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Thornley, J.H.M. Temperature and CO2 responses of leaf and canopy photosynthesis: A clarification using the non-rectangular hyperbola model of photosynthesis. Ann. Bot. 1998, 82, 883–892. [Google Scholar] [CrossRef]









| Environmental Element | Set Value |
|---|---|
| Light period (h d−1) | 12 (edamame) and 16 (tomato) |
| Air temperature (Light/Dark) (°C) | 25/20 |
| Relative humidity (%) | 60–70 |
| CO2 concentration (μmol mol−1) | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Ke, X.; Goto, E. High Photosynthetic Photon Flux Density Differentially Improves Edible Biomass Space Use Efficacy in Edamame and Dwarf Tomato. Plants 2024, 13, 1858. https://doi.org/10.3390/plants13131858
Liu Q, Ke X, Goto E. High Photosynthetic Photon Flux Density Differentially Improves Edible Biomass Space Use Efficacy in Edamame and Dwarf Tomato. Plants. 2024; 13(13):1858. https://doi.org/10.3390/plants13131858
Chicago/Turabian StyleLiu, Qingxin, Xinglin Ke, and Eiji Goto. 2024. "High Photosynthetic Photon Flux Density Differentially Improves Edible Biomass Space Use Efficacy in Edamame and Dwarf Tomato" Plants 13, no. 13: 1858. https://doi.org/10.3390/plants13131858
APA StyleLiu, Q., Ke, X., & Goto, E. (2024). High Photosynthetic Photon Flux Density Differentially Improves Edible Biomass Space Use Efficacy in Edamame and Dwarf Tomato. Plants, 13(13), 1858. https://doi.org/10.3390/plants13131858








