Nano-Integrated Plant Tissue Culture to Increase the Rate of Callus Induction, Growth, and Curcuminoid Production in Curcuma longa
Abstract
1. Introduction
2. Materials and Methods
2.1. Green Synthesis of Fe3O4 Nanoparticles
2.2. Characterization of Synthesized Nanoparticles
2.2.1. Ultraviolet Visible (UV–Vis) Spectroscopy
2.2.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.3. X-ray Diffraction (XRD) Analysis
2.2.4. Scanning Electron Microscopy (SEM)
2.3. Tissue Culture of Turmeric Plant
2.4. Preparation of Turmeric Extract
2.5. Liquid Chromatography–Mass Spectroscopy (LC-MS)
2.6. Statistical Analysis
3. Results
3.1. Effect of Fe3O4 NPs on the Regeneration of Turmeric Plant through Tissue Culture
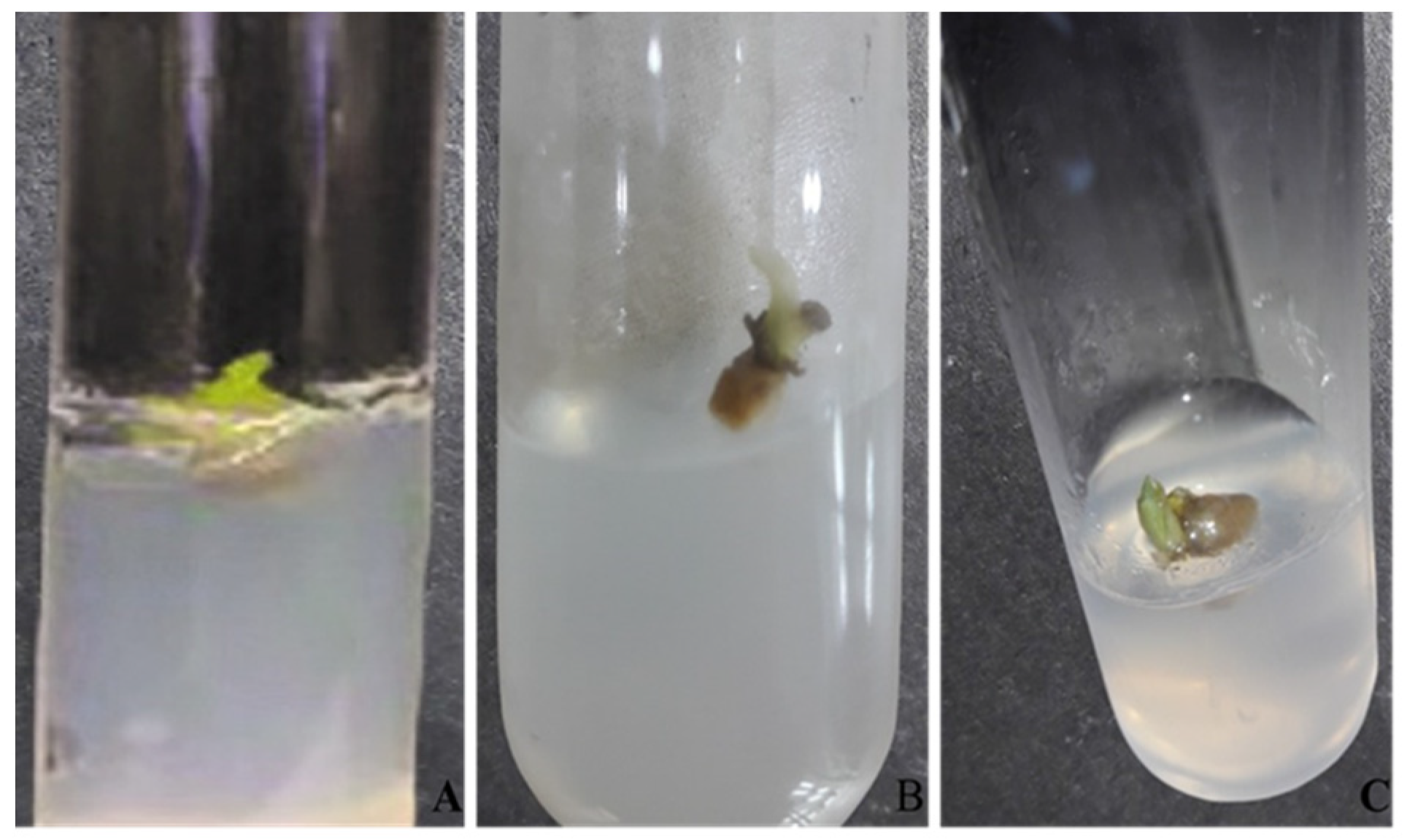
3.1.1. Effect of Fe3O4 NPs on Callus Induction
3.1.2. Effect of Fe3O4 NPs on In Vitro Shoot Growth of Turmeric
3.1.3. Effect of Fe3O4 NPs on In Vitro Root Induction
3.2. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis of Turmeric
3.3. Characterization of Green Synthesized Fe3O4 NPs
3.3.1. UV–Vis Spectroscopy
3.3.2. Fourier Transformed Infrared Spectroscopy (FTIR)
3.3.3. X-ray Diffraction (XRD)
3.3.4. Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brijesh, H.; Ajjappala, B. Micropropagation strategies in medicinally important turmeric (Curcuma sp.): Current research and future challenges. J. Appl. Biol. Biotechnol. 2023, 11, 1–8. Available online: http://www.jabonline.in (accessed on 26 June 2024). [CrossRef]
- Kumar, A.; Singh, A.K.; Kaushik, M.S.; Mishra, S.K.; Raj, P.; Singh, P.K.; Panday, K.D. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: A review. 3 Biotech 2017, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Yixuan, L.; Quaria, M.A.; Sivasamy, S.; Jianzohng, S.; Daochen, Z. Curcumin production and bioavailability: A comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind. Crops Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Sinchana, N.S.; Kattimani, K.N.; Prabhuling, G.; Sudesh, K.; Jagadeesha, N. Standardization of tissue culture protocol for turmeric (Curcuma longa L.) Cv. Salem. Int. J. Chem. Stud. 2020, 8, 2721–2726. [Google Scholar] [CrossRef]
- Chitra, R. Comparative studies on growth and Yield of Conventional and Tissue culture plants of Turmeric (Curcuma longa) var. CO2. J. Hortic. Sci. 2019, 14, 162–165. [Google Scholar] [CrossRef]
- Ramkumar Kollana, Y.; Raj, S.D.; Suman, P.; Vani, P.R.; Sreeramulu, S.H. Studies on nutritional evaluation and secondary metabolites of some selected curcuma species collected from Eastern Ghats of Udayagiri Hills, Gajapathi District, Odisha. Eur. J. Biomed. Pharm. Sci. 2020, 7, 276–281. [Google Scholar]
- Panda, D.; Behera, A.K.; Padhan, B.; Nayak, J.K. Chemical profiling of selected plants of Zingiberaceae used in ethnomedicine of Koraput, India. J. Stress Physiol. Biochem. 2020, 16, 50–60. [Google Scholar]
- Ibanez, M.D.; Blazquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Marchent, M.J.; Molina, P.; Montecinos, M.; Guzman, L.; Balada, C.; Fassio, C.; Castro, M. In Vitro Propagation of Easter Island Curcuma longa from Rhizome Explants Using Temporary Immersion System. Agronomy 2021, 11, 2121. [Google Scholar] [CrossRef]
- Shashikant Rajak, K.K.; Koshore, C.; Parasad, B.C.; Trivedi, M.P. Callus induction in rhizome of Curcuma caesia: A medicinal plant. J. Pharmacogn. Phytochem. 2019, 8, 1989–1993. [Google Scholar]
- Flores, S.; Retana-Cordero, M.; Fisher, P.R.; Freyer, R.; Gomez, C. Effect of Photoperiod, Propagative Material, and Production Period on Greenhouse-grown Ginger and Turmeric Plants. Hortiscience 2021, 56, 1476–1485. [Google Scholar] [CrossRef]
- Upendri HF, L.; Seran, T.H. In vitro propagation of turmeric (Curcuma longa L.) through direct somatic embryogenesis with reference to types of explants and plant growth regulators: A review. Cienc. Agron. 2021, 38. [Google Scholar]
- Singh, Y.; Kumar, U.; Panigrahi, S.; Balyan, P.; Mehla, S.; Sihag, P.; Sagwal, V.; Singh, K.P.; White, J.C.; Dhankher, O.P. Nanoparticles as novel elicitors in plant tissue culture applications: Current status and future outlook. Plant Physiol. Biochem. 2023, 203, 108004. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Zahra, S.A.; Shahbaz, A.; Kanwal, S.; Rabbani, A.; Mahmood, T. Bioinspired synthesis and activity characterization of iron oxide nanoparticles made using Rhamnus Triquetra leaf extract. Mater. Res. Express 2020, 6, 1250e7. [Google Scholar] [CrossRef]
- Pacheco, I.; Buzea, C. Nanoparticle uptake by plants: Beneficial or detrimental? Phytotoxicity Nanoparticles 2018, 1–61. [Google Scholar] [CrossRef]
- Asoufi, H.M.; Al-Antary, T.M.; Awwad, A.M. Green route for synthesis hematite (α-Fe2O3) nanoparticles: Toxicity effect on the green peach aphid, Myzus persicae (Sulzer). Environ. Nanotechnol. Monit. Manag. 2018, 9, 107–111. [Google Scholar]
- Abusalem, M.; Awwad, A.; Ayad, J.; Abu Rayyan, A. Green synthesis of α-Fe2O3 nanoparticles using pistachio leaf extract influenced seed germination and seedling growth of tomatos. JJEES 2019, 10, 161–166. [Google Scholar]
- Niluxsshun, M.C.D.; Masilamani, K.; Mathiventhan, U. Green Synthesis of Silver Nanoparticles from the Extracts of Fruit Peel of Citrus tangerina, Citrus sinensis, and Citrus limon for Antibacterial Activities. Bioinorg. Chem. Appl. 2021, 2021, 6695734. [Google Scholar] [CrossRef]
- Priya Ashique, S.; Afzal, O.; Khalid, M.; Ahmad, M.F.; Upadhyay, A.; Kumar, S.; Garg, A.; Ramzan, M.; Hussain, A.; Altamimi, M.A.; et al. Biogenic nanoparticles from waste fruit peels: Synthesis, applications, challenges and future perspectives. Int. J. Pharm. 2023, 643, 123223. [Google Scholar] [CrossRef]
- Al-Amri, N.; Tombuloglu, H.; Slimani, Y.; Akhtar, S.; Barghouthi, M.; Almessiere, M.; Alshammari, T.; Baykal, A.; Sabit, H.; Ercan, I.; et al. Size effect of iron (III) oxide nanomaterials on the growth, and their uptake and translocation in common wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2020, 194, 110377. [Google Scholar] [CrossRef]
- Shirsat, S.; Suthindhiran, K. Iron oxide nanoparticles as iron micronutrient fertilizer—Opportunities and limitations. J. Plant Nutr. Soil Sci. 2023. [Google Scholar] [CrossRef]
- Abou Seeda, M.A.; El-Sayed, A.A.; Yassen, A.A.; Abou El-Nour EA, A.; Zaghloul, S.M.; Mervat, G.M. Nickel, iron and their diverse role in plants: A review, approaches and future prospective. Middle East J. Appl. Sci. 2020, 10, 196–219. [Google Scholar]
- Maswada, H.; Djanaguiraman, M.; Prasad, P. Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agron. Crop Sci. 2018, 204, 577–587. [Google Scholar] [CrossRef]
- Irum, S.; Jabeen, N.; Ahmad, K.S.; Shafique, S.; Khan, T.F.; Gul, H.; Anwaar, S.; Shah, N.I.; Mehmood, A.; Hussain, S.Z. Biogenic iron oxide nanoparticles enhance callogenesis and regeneration pattern of recalcitrant Cicer arietinum L. PLoS ONE 2020, 15, e0242829. [Google Scholar] [CrossRef] [PubMed]
- Harmansah, C.; Kutman, M.K.; Muftuler, F.B.Z. Preparation of iron oxide nanoparticles by banana peel extract and its usage in NDT. Measurement 2022, 204, 112081. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakherdiev, S.I.; Shabala, S. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xi, H.; Wang, P.; Yin, H. Nanopartcles in Plants: Uptake, Transpoty and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Ali, I.; Khan, A.; Ali, A.; Ullah, Z.; Dai, D.Q.; Khan, N.; Khan, A.; Al-Tawaha, A.R.; Sher, H. Iron and zinc micronutrients and soil inoculation of Trichoderma harzianum enhance wheat grain quality and yield. Front. Plant Sci. 2022, 13, 960948. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Almessiere, M.; Baykal, A. Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.). Chemosphere 2019, 226, 110–122. [Google Scholar] [CrossRef]
- Kokina, I.; Mickeviča, I.; Jahundoviča, I.; Ogurcovs, A.; Krasovska, M.; Jermaļonoka, M.; Mihailova, I.; Tamanis, E.; Gerbreders, V. Plant Explants Grown on Medium Supplemented with Fe3O4 Nanoparticles Have a Significant Increase in Embryogenesis. J. Nanomater. 2017, 2017, 4587147. [Google Scholar] [CrossRef]
- Gebre, S.H. Bio-inspired synthesis of metal and metal oxide nanoparticles: The key role of phytochemicals. J. Clust. Sci. 2022, 33, 1–40. [Google Scholar] [CrossRef]
- Nhut, D.T.; Duc, H.H.; Hoang, N.H.; Ngan, H.T.M.; Diem, L.T.; Tung, H.T.; Khai, H.D.; Mai, N.T.N.; Cuong, D.M.; Luan, V.Q.; et al. Efficient transgenic plantlet regeneration from hairy roots via somatic embryogenesis and hardening plantlets of Panax vietnamensis by iron nanoparticles-supplied culture. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 151, 335–345. [Google Scholar] [CrossRef]
- Hernández-Hernández, A.A.; Aguirre-Álvarez, G.; Cariño-Cortés, R.; Mendoza-Huizar, L.H.; Jiménez-Alvarado, R. Iron oxide nanoparticles: Synthesis, functionalization, and applications in diagnosis and treatment of cancer. Chem. Pap. 2020, 74, 3809–3824. [Google Scholar] [CrossRef]
- Marcus, N.; Lee, J.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.R.; Hsiao, C.D. Potential cytotoxicity of Iron Oxide Nanaoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Ezealigo, U.S.; Ezealigo, B.N.; Aisida, S.O.; Ezema, F.I. Iron Oxide Nanaoparticles in Biological systems: Antbacterial and toxicology Perspective. JCIS Open 2021, 4, 100027. [Google Scholar] [CrossRef]
- Madubuonu, N.; Aisida, S.O.; Ali, A.; Ahmad, I.; ZTing-Kai Botha, S.; Mazza, M.; Ezema, F.I. Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study. J. Photochem. Photobiol. B Biol. 2019, 199, 111601. [Google Scholar] [CrossRef]
- Begum, S.; Sahoo, T.; Swain, S.; Nayak, A.; Das, S.P.S.; Rath, S.K.; Rath, C.C. Fabrication of iron nanoparticles using different bioactive precursors, their characterization and bioactivity evaluation. Sustain. Chem. Environ. 2024, 6, 100100. [Google Scholar] [CrossRef]
- Jamzad, M.; Kamari, B.M. Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis L. leaves and evaluation of the antimicrobial activity. J. Nanostruct. Chem. 2020, 10, 193–201. [Google Scholar] [CrossRef]
- Madubuonu, N.; Aisida, S.O.; Ahmad, I.; Botha, S.; Zhao, T.K.; Maaza, M. Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A Mater. Sci. Process. 2020, 126, 72. [Google Scholar] [CrossRef]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Jadczak, P.; Kulpa, D.; Bihun, M.; Przewodowski, W. Positive effect of AgNPs and AuNPs in in vitro cultures of Lavandula angustifolia Mill. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 139, 191–197. [Google Scholar] [CrossRef]
- Desai, A.S.; Singh, A.; Edis, Z.; Bloukh, S.H.; Shah, P.; Panday, B.; Agrawal, N.; Bhagat, N. An In Vitro and In Vivo Study of the Efficacy and Toxicity of Plant-Extract-Derived Silver Nanoparticles. J. Funct. Biomater. 2022, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagam, M.; Pavan, G.; Vasudevan, V. A comprehensive study of the hormetic influence of biosynthesized AgNPs on regenerating rice calli of indica cv. IR64. Sci. Rep. 2019, 9, 8821. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.R.; Bhosale, R.R.; Kamble, G.S.; Shukla, S.S.; Gadale, S.R.; Dhavale, R.P.; Anbhule, P.V. Enriching the antimicrobial efficacy of iron oxide with bioderived mesoporous carbon. Org. Chem. Commun. 2024, 161, 112111. [Google Scholar] [CrossRef]
- Eldeeb, B.A.; El-Raheem WM, A.; Elbeltagi, S. Green synthesis of biocompatible Fe3O4 magnetic nanoparticles using Citrus Sinensis peels extract for their biological activities and magnetic-hyperthermia applications. Sci. Rep. 2023, 13, 19000. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.I.; Magro, M.Y.; Ming, L.C.; da Silva, M.B.; Rodrigues, L.F.O.S.; Prado, D.Z.; Bonaiuto, E.; Baratella, D.; Roger, J.D.A.; Lima, G.P.P.; et al. Sustainable production of high purity curcuminoids from Curcuma longa by magnetic nanoparticles: A case study in Brazil. J. Clean. Prod. 2017, 154, 233–241. [Google Scholar] [CrossRef]
- Ajanaku, C.O.; Ademosun, O.T.; Atohengbe, P.O.; Ajayi, S.O.; Obafemi, Y.D.; Owolabi, O.D.; Akinduti, P.A.; Ajanaku, P.A. Functional bioactive compounds in ginger, turmeric, and garlic. Front. Nutr. 2022, 9, 1012023. [Google Scholar] [CrossRef]
- Khattab, S.; Alkuwayti, M.A.; Yap, Y.; Meligy, A.M.A.; Ismail, M.B.; Sherif, F.E. Foliar Spraying of ZnO Nanoparticals on Curcuma longa Had Increased Growth, Yield, Expression of Curcuminoid Synthesis Genes, and Curcuminoid Accumulation. Horticulturae 2023, 9, 355. [Google Scholar] [CrossRef]
- Aldayel, M.F. Enhancement of the Bioactive Compound Content and Antibacterial Activities in Curcuma longa Using Zinc Oxide Nanoparticles. Molecules 2023, 28, 4935. [Google Scholar] [CrossRef]






| Treatment | Callus Initiation (Day) | Callus Induction (%) | Proliferation Rate |
|---|---|---|---|
| No Fe3O4 NPs (Control) | 42 | 50 | ++ |
| Fe3O4 NPs (1 mg/L) | 42 | 52 | ++ |
| Fe3O4 NPs (5 mg/L) | 39 | 55 | ++ |
| Fe3O4 NPs (10 mg/L) | 33 | 70 | +++ |
| Fe3O4 NPs (15 mg/L) | 28 | 80 | ++++ |
| Fe3O4 NPs (20 mg/L) | 41 | 40 | + |
| Treatments | Percent Response (%) | Shoot Height (cm) | No. of Leaves per Plantlet | Shoot Fresh Weight (mg) |
|---|---|---|---|---|
| No Fe3O4 NPs (Control) | 42.3 ± 0.8 b | 3.4 ± 0.4 b | 1.3 ± 0.3 b | 51.3 ± 0.6 b |
| Fe3O4 NPs (1 mg/L) | 44.0 ± 1.0 b | 3.7 ± 0.2 b | 1.3 ± 0.3 b | 52.6 ± 0.7 b |
| Fe3O4 NPs (5 mg/L) | 46.0 ± 0.5 b | 4.5 ± 0.5 bc | 2.0 ± 0.5 b | 57.1 ± 0.8 c |
| Fe3O4 NPs (10 mg/L) | 60.0 ± 1.1 c | 6.2 ± 0.7 c | 3.0 ± 1.0 c | 74.1 ± 1.0 d |
| Fe3O4 NPs (15 mg/L) | 70.0 ± 0.5 d | 9.0 ± 0.7 d | 5.6 ± 0.8 d | 100.3 ± 0.1 e |
| Fe3O4 NPs (20 mg/L) | 33.2 ± 0.5 a | 2.1 ± 0.4 a | 1.1 ± 0.4 a | 40.2 ± 0.7 a |
| Treatments | Percent Rooting (%) | No of Roots | Root Fresh Weight (mg) |
|---|---|---|---|
| No Fe3O4 NPs (Control) | 45.3 ± 1.8 a | 1.3 ± 0.3 a | 27.0 ± 1.7 a |
| Fe3O4 NPs (1 mg/L) | 47.6 ± 0.3 a | 1.6 ± 0.3 a | 29.2 ± 0.5 a |
| Fe3O4 NPs (5 mg/L) | 49.3 ± 0.8 a | 2.3 ± 0.6 ab | 30.2 ± 0.6 a |
| Fe3O4 NPs (10 mg/L) | 62.0 ± 2.1 b | 4.0 ± 0.5 b | 38.2 ± 1.9 b |
| Fe3O4 NPs (15 mg/L) | 75.0 ± 2.1 c | 7.0 ± 0.5 c | 51.1 ± 2.2 c |
| Fe3O4 NPs (20 mg/L) | 43.3 ± 0.9 a | 1.1 ± 0.2 a | 25.0 ± 2.1 a |
| Compound | MW | RT | Formula | m/z | Concentration mg/g | |
|---|---|---|---|---|---|---|
| With NPs | Without NPs | |||||
| Curcumin | 368 | 17.91 | C12H20O6 | 369, 285, 177 | 13.73 | 10.35 |
| Demethoxycurcumin | 338 | 14.10 | C20H18O5 | 339, 177, 147 | 8.05 | 6.49 |
| Bisdemethoxycurcumin | 308 | 10.75 | C19H16O4 | 309, 225, 147 | 2.16 | 1.13 |
| Dihydrocurcumin | 370 | 10.50 | C12H22O6 | 371, 137, 177 | 0.02 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.; Aftab, Z.-e.-H.; Anjum, T.; Rizwana, H.; Akram, W.; Aftab, A.; Sajid, Z.A.; Li, G. Nano-Integrated Plant Tissue Culture to Increase the Rate of Callus Induction, Growth, and Curcuminoid Production in Curcuma longa. Plants 2024, 13, 1819. https://doi.org/10.3390/plants13131819
Iqbal M, Aftab Z-e-H, Anjum T, Rizwana H, Akram W, Aftab A, Sajid ZA, Li G. Nano-Integrated Plant Tissue Culture to Increase the Rate of Callus Induction, Growth, and Curcuminoid Production in Curcuma longa. Plants. 2024; 13(13):1819. https://doi.org/10.3390/plants13131819
Chicago/Turabian StyleIqbal, Muhammad, Zill-e-Huma Aftab, Tehmina Anjum, Humaira Rizwana, Waheed Akram, Arusa Aftab, Zahoor Ahmad Sajid, and Guihua Li. 2024. "Nano-Integrated Plant Tissue Culture to Increase the Rate of Callus Induction, Growth, and Curcuminoid Production in Curcuma longa" Plants 13, no. 13: 1819. https://doi.org/10.3390/plants13131819
APA StyleIqbal, M., Aftab, Z.-e.-H., Anjum, T., Rizwana, H., Akram, W., Aftab, A., Sajid, Z. A., & Li, G. (2024). Nano-Integrated Plant Tissue Culture to Increase the Rate of Callus Induction, Growth, and Curcuminoid Production in Curcuma longa. Plants, 13(13), 1819. https://doi.org/10.3390/plants13131819






