Exploring Phytoremediation Potential: A Comprehensive Study of Flora Inventory and Soil Heavy Metal Contents in the Northeastern Mining Districts of Morocco
Abstract
1. Introduction
2. Results and Discussion
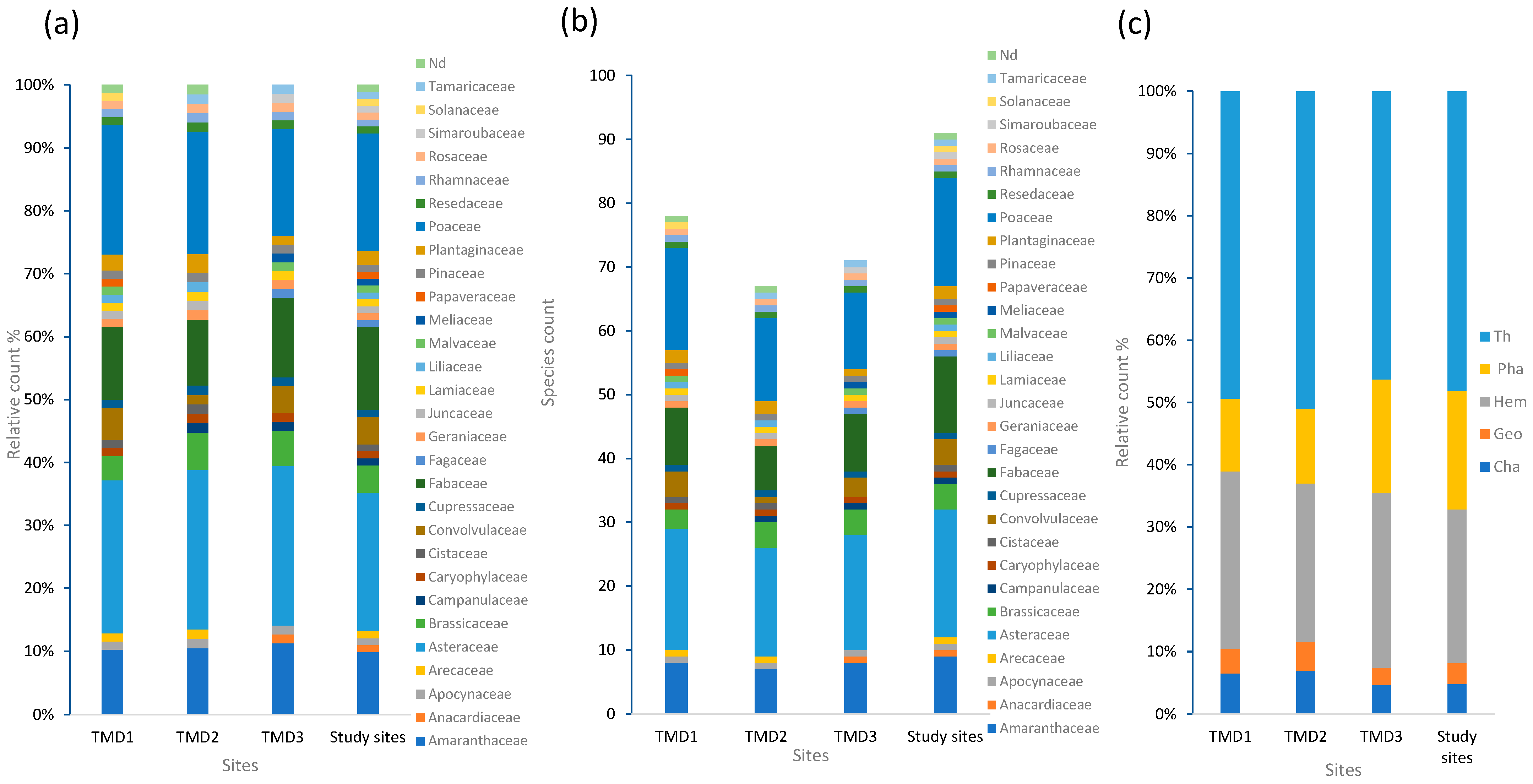
2.1. Flora Diversity and Composition
2.2. Analysis of Freqeuncy and Coverage of Species
- (S) Class IV (Figure 2) (between 60% and 80%) contained 3 species: Bromus mollis, Reseda lutea, and Aristida pungens.
- Class III (Figure 2) (between 40% and 60%) contained 14 species, including Atractylis caepistosa, Lamarckia aurea, Lomelosia stellata, Agatophora alopecoïdes, Carlina racemosa, Genista hirsuta, Phragmites australis, Astragatlus armatus, and Lotus maroccanus.
- Class II (between 20% and 40%) comprised 31 species, including Lotus corniculatus, Onopordum macracanthum schrub, Cardaria draba, Chenopodium murale, Genista trispucidata, and Juniperus oxycedrus.
- Class I (−20%) comprised 49 plant species, including Melia azedarach L. Pistacia lentiscus, Quercus ilex, and Retama monosperma.
2.2.1. Analyses of Species Composition in Different Sites
2.2.2. Local Plant Diversity: A Promising Avenue for Sustainable Mining Reclamation (AHC)
Ground Coverage and Frequency Analysis
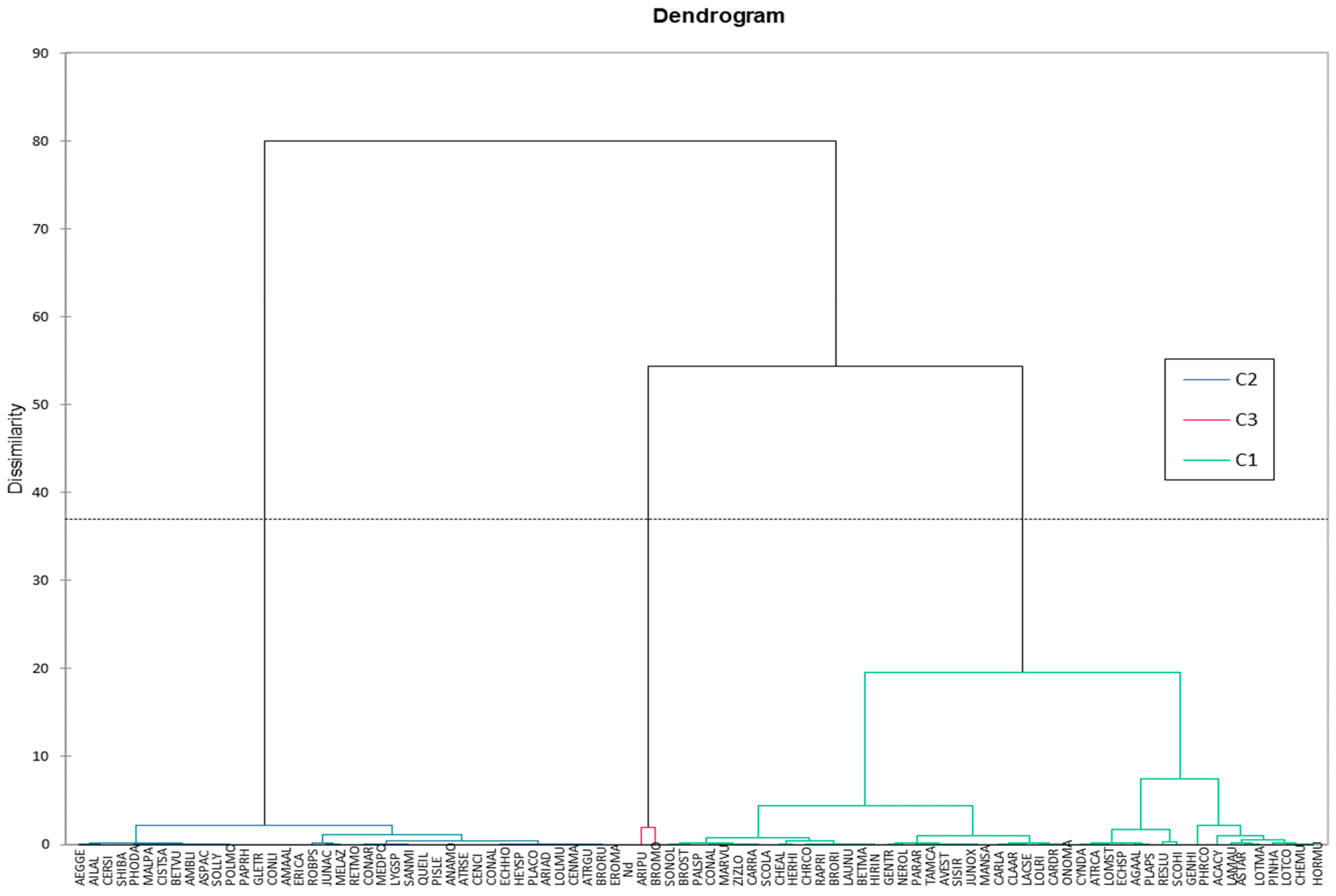
2.3. Physicochemical Properties of Soil
2.4. Heavy Metals in Rhizospheric Soils and Native Plants
2.5. Bioconcentration (BCF), Bioaccumulation Factor (BAC), and Translocation Factor (TF) of the Native Plants and Phytoremediation Potential
2.5.1. Phytoextraction
2.5.2. Phytostabilization (Metal Excluders)
2.6. Evaluation of Plants’ Heavy Metal Accumulation (PCA)
3. Materials and Methods
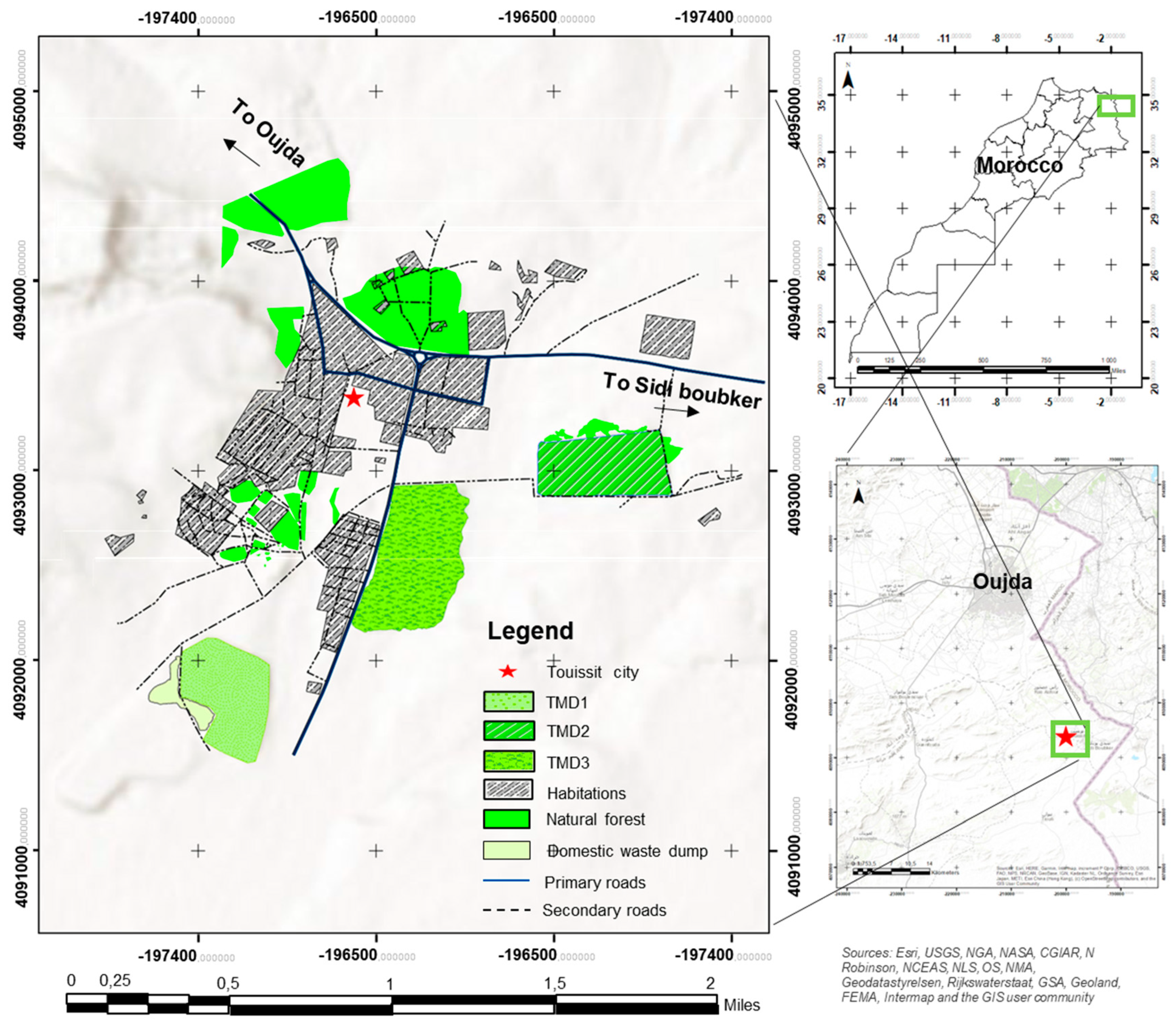
3.1. Description of the Study Site
3.2. Sampling
3.2.1. Flora Inventory
3.2.2. Soil and Plant Sample Collection and Pretreatment
3.3. Chemical Analyses of Soil and Plant Samples
3.4. Calculation of Phytoremediation Indices
3.5. Identification of Plant Species
3.6. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hooke, R.L.; Martín Duque, J.F.; de Pedraza Gilsanz, J. Land Transformation by Humans: A Review; Geological Society of America: Boulder, CO, USA, 2012. [Google Scholar]
- Le Roux, C. La réhabilitation des mines et carrières à ciel ouvert. Bois For. Trop. 2002, 272, 5–19. [Google Scholar]
- Zheng, J.; Noller, B.; Huynh, T.; Ng, J.; Taga, R.; Diacomanolis, V.; Harris, H. How the population in Mount Isa is living with lead exposure from mining activities. Extr. Ind. Soc. 2021, 8, 123–134. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. Int. Sch. Res. Not. 2011, 2011, e402647. [Google Scholar] [CrossRef]
- Li, T.; Wang, B. Effect and mechanism of nano-alumina on early hydration properties and heavy metals solidification/stabilization of alkali-activated MSWI fly ash solidified body. J. Hazard. Mater. 2023, 452, 131327. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Soil reclamation of abandoned mine land by revegetation: A review. Int. J. Soil Sediment Water 2010, 3, 13. [Google Scholar]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gu, H.; Van Le, Q.; Peng, W.; Lam, S.S.; Yang, Y.; Li, C.; Sonne, C. Perspectives on phytoremediation of zinc pollution in air, water and soil. Sustain. Chem. Pharm. 2021, 24, 100550. [Google Scholar] [CrossRef]
- Salas-Moreno, M.; Marrugo-Negrete, J. Phytoremediation potential of Cd and Pb-contaminated soils by Paspalum fasciculatum Willd. ex Flüggé. Int. J. Phytoremediation 2020, 22, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Ma, S.; Li, Y.; Zhao, C.; Tian, R. Pontederia cordata, an ornamental aquatic macrophyte with great potential in phytoremediation of heavy-metal-contaminated wetlands. Ecotoxicol. Environ. Saf. 2020, 203, 111024. [Google Scholar] [CrossRef]
- Emenike, C.U.; Jayanthi, B.; Agamuthu, P.; Fauziah, S.H. Biotransformation and removal of heavy metals: A review of phytoremediation and microbial remediation assessment on contaminated soil. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Jambhulkar, H.P. Phytoremediation of coal mine spoil dump through integrated biotechnological approach. Bioresour. Technol. 2008, 99, 4732–4741. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Goswami, P.; Mukherjee, A. Chapter 22—Stabilization of Iron Ore Mine Spoil Dump Sites with Vetiver System A2-Prasad, Majeti Narasimha Vara. In Bio-Geotechnologies for Mine Site Rehabilitation; Favas, P.J.d.C., Maiti, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 393–413. [Google Scholar] [CrossRef]
- Cetinkaya, G.; Sozen, N. Plant Species Potentially Useful in the Phytostabilization Process for the Abandoned CMC Mining Site in Northern Cyprus. Int. J. Phytoremediat. 2011, 13, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.S.; Ye, Z.H.; Zhang, Z.Q.; Lan, C.Y.; Wong, M.H. Natural colonization of plants on five lead/zinc mine tailings in Southern China. Restor. Ecol. 2005, 13, 49–60. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Y.; Hou, J.; Wu, L.; Christie, P.; Liu, W. Microbial community assembly of the hyperaccumulator plant Sedum plumbizincicola in two contrasting soil types with three levels of cadmium contamination. Sci. Total Environ. 2023, 863, 160917. [Google Scholar] [CrossRef]
- Steliga, T.; Kluk, D. Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2020, 194, 110409. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, G.; Santal, A.R.; Singh, N.P. Omics approaches in effective selection and generation of potential plants for phytoremediation of heavy metal from contaminated resources. J. Environ. Manag. 2023, 336, 117730. [Google Scholar] [CrossRef] [PubMed]
- Bharagava, R.N.; Saxena, G.; Mulla, S.I. Introduction to Industrial Wastes Containing Organic and Inorganic Pollutants and Bioremediation Approaches for Environmental Management. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Saxena, G., Bharagava, R.N., Eds.; Springer: Singapore, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of Heavy Metal-Contaminated Sites: Eco-environmental Concerns, Field Studies, Sustainability Issues, and Future Prospects. In Reviews of Environmental Contamination and Toxicology Volume 249; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 71–131. [Google Scholar] [CrossRef]
- Wadjinny, A. Le District a Plomb-zinc de Touissit: Presentation Gitologique et Synthese des Travaux Realises: Notes et Memoires du Service Geologique. R. Maroc 1997, 388, 151–164. [Google Scholar]
- Abdelaziz, S.; Ater, M.; Auguy, F.; Laplaze, L.; El Mzibri, M.; Fatiha, B.; Abdelkarim, F.-M.; Patrick, D. Evaluation de la contamination par les éléments-traces métalliques dans une zone minière du Maroc oriental. Cah. Agric. 2010, 19, 1–7. [Google Scholar] [CrossRef]
- Wu, B.; Peng, H.; Sheng, M.; Luo, H.; Wang, X.; Zhang, R.; Xu, F.; Xu, H. Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 2021, 220, 112368. [Google Scholar] [CrossRef]
- Ben Ghaya, A.; Hamrouni, L.; Mastouri, Y.; Hanana, M.; Charles, G. Impacts of toxic metals on vegetation of the Djebel Hallouf mine in the area of Sidi Bouaouane in BouSalem Northwestern Tunisia. Geo Eco Trop 2013, 37, 243–254. [Google Scholar]
- Taleb, A.; Maillet, J. Mauvaises herbes des céréales de la Chaouia (Maroc). I. Aspect floristique. Weed Res. 1994, 34, 345–352. [Google Scholar] [CrossRef]
- Tanji, A.; Boulet, C.; Hammoumi, M. Inventaire phytoécologique des adventices de la betterave sucrière dans le Gharb (Maroc). Weed Res. 1984, 24, 391–399. [Google Scholar] [CrossRef]
- Ater, M.; Hadi, A.; Meerts, P. Vegetation of the Mine Fields Touissite, Boubker and Oued El Heimer and Species with Potential in Phytoremediation. Available online: https://www.researchgate.net/publication/317329204_Vegetation_of_the_mine_fields_Touissite_Boubker_and_Oued_El_Heimer_and_species_with_potential_in_phytoremediation (accessed on 21 June 2024).
- Mohanty, M.; Patra, H.K. Phytoremediation Potential of Paragrass—An In Situ Approach for Chromium Contaminated Soil. Int. J. Phytoremediation 2012, 14, 796–805. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, H.; Guo, Q.; Song, B.; Zheng, G.; Zhang, Z.; Zhao, J.; Okoli, C.P. Heavy metal contents and enrichment characteristics of dominant plants in wasteland of the downstream of a lead-zinc mining area in Guangxi, Southwest China. Ecotoxicol. Environ. Saf. 2018, 151, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Karaca, O.; Cameselle, C.; Reddy, K.R. Mine tailing disposal sites: Contamination problems, remedial options and phytocaps for sustainable remediation. Rev. Environ. Sci. Biotechnol. 2018, 17, 205–228. [Google Scholar] [CrossRef]
- Yang, S.; Liang, S.; Yi, L.; Xu, B.; Cao, J.; Guo, Y.; Zhou, Y. Heavy metal accumulation and phytostabilization potential of dominant plant species growing on manganese mine tailings. Front. Environ. Sci. Eng. 2014, 8, 394–404. [Google Scholar] [CrossRef]
- Błońska, A.; Kompała-Bąba, A.; Sierka, E.; Bierza, W.; Magurno, F.; Besenyei, L.; Ryś, K.; Woźniak, G. Diversity of Vegetation Dominated by Selected Grass Species on Coal-Mine Spoil Heaps in Terms of Reclamation of Post-Industrial Areas. J. Ecol. Eng. 2019, 20, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hendrychová, M. Reclamation success in post-mining landscapes in the Czech Republic: A review of pedological and biological studies. J. Landsc. Stud. 2008, 1, 63–78. [Google Scholar]
- Lugo, A.E. The apparent paradox of reestablishing species richness on degraded lands with tree monocultures. For. Ecol. Manag. 1997, 99, 9–19. [Google Scholar] [CrossRef]
- Sarma, K.; Kushwaha, S.P.S.; Singh, K.J. Impact of coal mining on plant diversity and tree population structure in Jaintia Hills district of Meghalaya, North East India. N. Y. Sci. J. 2010, 3, 79–85. [Google Scholar]
- Holl, K.D. Long-term vegetation recovery on reclaimed coal surface mines in the eastern USA. J. Appl. Ecol. 2002, 39, 960–970. [Google Scholar] [CrossRef]
- Barralis, G. Méthode d’étude des groupements adventices des cultures annuelles; Application à la Côte d’Or. In Proceedings of the 5th International Colloquium on Weed Ecology and Biology, Dijon, France, 8–10 September 1976. [Google Scholar]
- Legendre, P. Legendre. Numerical Ecology with R, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Soufi, Z. Les principales mauvaises herbes des vergers dans la région maritime de Syrie. Weed Res. 1988, 28, 199–206. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; Volume 28. [Google Scholar]
- Ferhi, F.; Das, S.; Moussaoui, Y.; Elaloui, E.; Yanez, J.G. Paper from Stipagrostis pungens. Ind. Crops Prod. 2014, 59, 109–114. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- El Khazanti, F.; Rachid, A.; Harrou, A.; Nasri, H.; Et-tayea, Y.; El Ouahabi, M.; Gharibi, E.K. Assessment of a Mining-Waste Dump of Galena Mine in the East of Morocco for Possible Use in Civil Engineering. J. Ecol. Eng. 2022, 23, 336–349. [Google Scholar] [CrossRef]
- Hasnaoui, S.E.; Fahr, M.; Keller, C.; Levard, C.; Angeletti, B.; Chaurand, P.; Triqui, Z.E.A.; Guedira, A.; Rhazi, L.; Colin, F.; et al. Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation. Plants 2020, 9, 1458. [Google Scholar] [CrossRef]
- Pendias, H. Trace Elements in Soils and Plants; CRC press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Acosta, J.A.; Abbaspour, A.; Martínez, G.R.; Martínez-Martínez, S.; Zornoza, R.; Gabarrón, M.; Faz, A. Phytoremediation of mine tailings with Atriplex halimus and organic/inorganic amendments: A five-year field case study. Chemosphere 2018, 204, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, J.; Hagner, M.; Ruhanen, H.; Mäkitalo, K. Addition of recyclable biochar, compost and fibre clay to the growth medium layer for the cover system of mine tailings: A bioassay in a greenhouse. Environ. Earth Sci. 2020, 79, 422. [Google Scholar] [CrossRef]
- El Hachimi, M.L.; Fekhaoui, M.; Abidi, A.E.; Rhoujatti, A. Heavy metal contamination of soils from abandoned mines: The case of Aouli-Mibladen-Zeïda mines in Morocco. Cah. Agric. 2014, 23, 213–219. [Google Scholar] [CrossRef]
- Wu, H.; Yang, F.; Li, H.; Li, Q.; Zhang, F.; Ba, Y.; Cui, L.; Sun, L.; Lv, T.; Wang, N.; et al. Heavy metal pollution and health risk assessment of agricultural soil near a smelter in an industrial city in China. Int. J. Environ. Health Res. 2020, 30, 174–186. [Google Scholar] [CrossRef]
- Zhang, L.; Verweij, R.A.; Van Gestel, C.A.M. Effect of soil properties on Pb bioavailability and toxicity to the soil invertebrate Enchytraeus crypticus. Chemosphere 2019, 217, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Matanzas, N.; Afif, E.; Díaz, T.E.; Gallego, J.R. Phytoremediation Potential of Native Herbaceous Plant Species Growing on a Paradigmatic Brownfield Site. Water. Air. Soil Pollut. 2021, 232, 290. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, B.; Li, H.; Huang, J.; Jiang, L.; Zhang, X.; Tan, Z.; Wu, Z.; Qin, X.; Feng, C.; et al. Soil heavy metals and phytoremediation by Populus deltoides alter the structure and function of bacterial community in mine ecosystems. Appl. Soil Ecol. 2022, 172, 104359. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical Speciation, Plant Uptake, and Toxicity of Heavy Metals in Agricultural Soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Vibhandik, R.; Agrahari, R.; Daverey, A.; Rani, R. Role of root exudates on the soil microbial diversity and biogeochemistry of heavy metals. Appl. Biochem. Biotechnol. 2023, 196, 2673–2693. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, M.; Cania, B.; Thijs, S.; Vangronsveld, J.; Piotrowska-Seget, Z. Hydrocarbon degradation potential and plant growth-promoting activity of culturable endophytic bacteria of Lotus corniculatus and Oenothera biennis from a long-term polluted site. Environ. Sci. Pollut. Res. Int. 2017, 24, 19640–19652. [Google Scholar] [CrossRef] [PubMed]
- Malayeri, B.E.; Chehregani, A.; Mohsenzadeh, F.; Kazemeini, F.; Asgari, M. Plants growing in a mining area: Screening for metal accumulator plants possibly useful for bioremediation. Toxicol. Environ. Chem. 2013, 95, 434–444. [Google Scholar] [CrossRef]
- Kostić, O.; Gajić, G.; Jarić, S.; Vukov, T.; Matić, M.; Mitrović, M.; Pavlović, P. An Assessment of the Phytoremediation Potential of Planted and Spontaneously Colonized Woody Plant Species on Chronosequence Fly Ash Disposal Sites in Serbia—Case Study. Plants 2021, 11, 110. [Google Scholar] [CrossRef]
- Anawar, H.M.; Canha, N.; Santa-Regina, I.; Freitas, M.C. Adaptation, tolerance, and evolution of plant species in a pyrite mine in response to contamination level and properties of mine tailings: Sustainable rehabilitation. J. Soils Sediments 2013, 13, 730–741. [Google Scholar] [CrossRef]
- Mkadmi, Y.; Benabbi, O.; Fekhaoui, M.; Benakkam, R.; Bjijou, W.; Elazzouzi, M.; Kadourri, M.; Chetouani, A. Study of the impact of heavy metals and physico-chemical parameters on the quality of the wells and waters of the Holcim area (Oriental region of Morocco). J. Mater. Environ. Sci. 2018, 9, 672–679. [Google Scholar]
- Bacchetta, G.; Cappai, G.; Carucci, A.; Tamburini, E. Use of Native Plants for the Remediation of Abandoned Mine Sites in Mediterranean Semiarid Environments; Springer: Berlin/Heidelberg, Germany, 2013; Volume 94. [Google Scholar]
- Zine, H.; Hakkou, R.; Elmansour, A.; Elgadi, S.; Ouhammou, A.; Benzaazoua, M. Native plant diversity for ecological reclamation in Moroccan open-pit phosphate mines. Biodivers. Data J. 2023, 11, e104592. [Google Scholar] [CrossRef] [PubMed]
- Boularbah, A.; Schwartz, C.; Bitton, G.; Morel, J.L. Heavy metal contamination from mining sites in South Morocco: 1. Use of a biotest to assess metal toxicity of tailings and soils. Chemosphere 2006, 63, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, M.; Lorestani, B.; Khorasani, N.; Yousefi, N.; Karami, M. Findings on the phytoextraction and phytostabilization of soils contaminated with heavy metals. Biol. Trace Elem. Res. 2011, 144, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Nabil, B.; Najwa, A.; Aya, Z.; Nessma, A.; Rajab, E. Phytoremediation Potential of Malva Parviflora for Some Heavy Metals in Roadside Soil in Benghazi, Libya. 2022. Available online: https://repository.uob.edu.ly/bitstream/handle/123456789/1635/LCCA4%206.pdf?sequence=1&isAllowed=y (accessed on 30 May 2024).
- Barajas-Aceves, M.; Camarillo-Ravelo, D.; Rodríguez-Vázquez, R. Mobility and Translocation of Heavy Metals from Mine Tailings in Three Plant Species after Amendment with Compost and Biosurfactant. Soil Sediment Contam. Int. J. 2015, 24, 223–249. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Chaabani, S.; Abdelmalek-Babbou, C.; Ben Ahmed, H.; Chaabani, A.; Sebei, A. Phytoremediation assessment of native plants growing on Pb–Zn mine site in Northern Tunisia. Environ. Earth Sci. 2017, 76, 585. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Omeka, M.; Igwe, O. Heavy metals concentration in soils and crop plants within the vicinity of abandoned mine sites in Nigeria: An integrated indexical and chemometric approach. Int. J. Environ. Anal. Chem. 2021, 103, 4111–4129. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Phytoextraction of mine wastes—Options and impossibilities. Geochemistry 2005, 65, 29–42. [Google Scholar] [CrossRef]
- Nouri, J.; Lorestani, B.; Yousefi, N.; Khorasani, N.; Hasani, A.H.; Seif, F.; Cheraghi, M. Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ. Earth Sci. 2011, 62, 639–644. [Google Scholar] [CrossRef]
- Escaray, F.J.; Menendez, A.B.; Gárriz, A.; Pieckenstain, F.L.; Estrella, M.J.; Castagno, L.N.; Carrasco, P.; Sanjuán, J.; Ruiz, O.A. Ecological and agronomic importance of the plant genus Lotus. Its application in grassland sustainability and the amelioration of constrained and contaminated soils. Plant Sci. 2012, 182, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rajkumar, M.; Freitas, H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J. Hazard. Mater. 2009, 166, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Bouabdellah, M.; Boukirou, W.; Potra, A.; Melchiorre, E.; Bouzahzah, H.; Yans, J.; Zaid, K.; Idbaroud, M.; Poot, J.; Dekoninck, A.; et al. Origin of the Moroccan Touissit-Bou Beker and Jbel Bou Dahar Supergene Non-Sulfide Biomineralization and Its Relevance to Microbiological Activity, Late Miocene Uplift and Climate Changes. Minerals 2021, 11, 401. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Niedermann, S.; Velasco, F. The Touissit-Bou Beker Mississippi Valley-Type District of Northeastern Morocco: Relationships to the Messinian Salinity Crisis, Late Neogene-Quaternary Alkaline Magmatism, and Buoyancy-Driven Fluid Convection. Econ. Geol. 2015, 110, 1455–1484. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Bouamrane, A.; Hakkou, R. Valorisation des rejets miniers du district Pb-Zn de Touissit-Boubker (région orientale-Maroc). Environ. Ing. Dév. 2014, 66, 38–44. [Google Scholar] [CrossRef]
- Bell, D.T.; Rokich, D.P.; McChesney, C.J.; Plummer, J.A. Effects of temperature, light and gibberellic acid on the germination of seeds of 43 species native to Western Australia. J. Veg. Sci. 1995, 6, 797–806. [Google Scholar] [CrossRef]
- Lamin, H.; Alami, S.; Bouhnik, O.; ElFaik, S.; Abdelmoumen, H.; Bedmar, E.J.; Missbah-El Idrissi, M. Nodulation of Retama monosperma by Ensifer aridi in an Abandonned Lead Mine Soils in Eastern Morocco. Front. Microbiol. 2019, 10, 1456. [Google Scholar] [CrossRef]
- Maillet, J. Evolution de la Flore Adventice Dans le Montpellierais Sous la Pression des Techniques Culturales [Cereales, Vigne]. 1981. Available online: https://agris.fao.org/search/en/providers/123819/records/64735d822c1d629bc97cf2be (accessed on 12 January 2024).
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Le Bourgeois, T.; Merlier, H. Merlier, Adventrop. Les Adventices D’Afrique Soudano-Sahélienne. CIRAD-CA. 1995. Available online: https://agritrop.cirad.fr/326208/ (accessed on 12 January 2024).
- Mzabri, I.; Chafik, Z.; Berrichi, A. Weeds flora associated with Saffron (Crocus sativus L.) in Morocco. Mater. Today Proc. 2019, 13, 1108–1114. [Google Scholar] [CrossRef]
- Ka, S.L.; Gueye, M.; Mbaye, M.S.; Ngom, M.; Camara, A.A.; Cissokho, M.K.; Mballo, R.; Sidybe, M.; Diouf, N.; Diop, D.; et al. Taxonomic diversity and abundance of weed flora in upland rice fields of Southern Groundnut Basin, Senegal. J. Agric. Sci. Eng. 2020, 2, 48–56. [Google Scholar] [CrossRef]
- Wikum, D.A.; Shanholtzer, G.F. Application of the Braun-Blanquet cover-abundance scale for vegetation analysis in land development studies. Environ. Manag. 1978, 2, 323–329. [Google Scholar] [CrossRef]
- Pawłowski, B.; Pawłowski, B. Composition and structure of plant communities and methods of their research. In The Flora of Poland; PWN: Warszawa, Poland, 1972; pp. 237–269. [Google Scholar]
- Godron, Quelques Applications de la Notion de Fréquence en Ecologie Végétale: (Recouvrement, Information Mutuelle Entre Espèces et Facteurs Ecologiques, Echantillonnage)-Detail-Ermes, Volume III. In “Oecologia Plantarum”, Volume III. Gauthier-Villars. [Paris]. 1968. Available online: https://bibliotheques.mnhn.fr/medias/doc/EXPLOITATION/HORIZON/130159/quelques-applications-de-la-notion-de-frequence-en-ecologie-vegetale-recouvrement-information-mutuel (accessed on 31 January 2024).
- Midhat, L.; Ouazzani, N.; Hejjaj, A.; Ouhammou, A.; Mandi, L. Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Ecotoxicol. Environ. Saf. 2019, 169, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Qasim, B.; Motelica-Heino, M. Potentially toxic element fractionation in technosoils using two sequential extraction schemes. Environ. Sci. Pollut. Res. 2014, 21, 5054–5065. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Van Ranst, E.; Verloo, M.; Demeyer, A.; Pauwels, J.M. Manual for the Soil Chemistry and Fertility Laboratory-Analytical Methods for Soils and Plants, Equipment, and Management of Consumables; NUGI: Ghent, Belgium, 1999; Volume 243, p. 1999. ISBN 90-76603-01-4. Available online: http://hdl.handle.net/1854/LU-113771 (accessed on 4 May 2024).
- Sylvain, B.; Mikael, M.-H.; Florie, M.; Emmanuel, J.; Marilyne, S.; Sylvain, B.; Domenico, M. Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. Catena 2016, 136, 44–52. [Google Scholar] [CrossRef]
- Nandillon, R.; Lebrun, M.; Miard, F.; Gaillard, M.; Sabatier, S.; Villar, M.; Bourgerie, S.; Morabito, D. Capability of amendments (biochar, compost and garden soil) added to a mining technosol contaminated by Pb and As to allow poplar seed (Populus nigra L.) germination. Environ. Monit. Assess. 2019, 191, 465. [Google Scholar] [CrossRef] [PubMed]
- Bourlière, F.; Quezel, P. et Santa, S.—Nouvelle Flore de l’Algérie et de ses régions désertiques méridionales. Tome II. Paris, Editions du Centre National de la Recherche Scientifique, 1963. Rev. Décologie Terre Vie 1964, 18, 238. [Google Scholar]
- Faris, F.Z.; Oualidi, J.; Fennane, M.; Ibn Tattou, M.; Mathez, J.; Ouchbani, S.; Ouyahya, A.; Raymaud, C.; Salvo Tierra, Á.; Abidine, A.Z. Flore Pratique du Maroc; Fennane, M., Tattou, M., Mathez, J., Ouyahya, A., Oualidi, J., Eds.; Institut Scientifique, Université Mohammed V: Rabat, Morocco, 1999. [Google Scholar] [CrossRef]



| Family | Life-Forms | Code | Species | Frequency Class | Frequency % | Cover (D) |
|---|---|---|---|---|---|---|
| Amaranthaceae | Hem | AGAAL | Agatophora alopecoïdes (Delile) Bunge | III | 43.59 | 85.90 |
| Th | AMAAL | Amaranthus albus L. | I | 2.56 | 1.28 | |
| Pha | ATRSE | Atriplex semibaccatan R. Br. | I | 10.26 | 5.13 | |
| Hem | BETMA | Beta macrocarpa Guss. | II | 30.77 | 29.49 | |
| Hem | BETVU | Beta vulgaris L. | I | 5.13 | 2.56 | |
| Th | CHEAL | Chenopodium album L. | II | 23.08 | 50.00 | |
| Th | CHEMU | Chenopodium murale L. | II | 33.33 | 98.72 | |
| Anacardiaceae | Pha | PISLE | Pistacia lentiscus L. | I | 10.26 | 5.13 |
| Apocynaceae | Pha | NEROL | Nerium oleander L. | II | 28.21 | 78.21 |
| Arecaceae | Pha | PHODA | Phoenix dactylifera L. | I | 5.13 | 2.56 |
| Asteraceae | Th | AMBLI | Amberboa lipii L. | I | 5.13 | 2.56 |
| Th | ANAMO | Anacyclus monanthos L. | I | 10.26 | 5.13 | |
| Hem | ATRCA | Atractylis caepistosa Desf. | III | 48.72 | 62.82 | |
| Th | ATRGU | Atractylis gummifera L. | I | 12.82 | 6.41 | |
| Hem | CARLA | Carlina racemosa L. | III | 43.59 | 46.15 | |
| Th | CARRA | Carthamus lanatus L., 1753 | II | 20.51 | 8.97 | |
| Th | CENMA | Centaurea marocana Balt. | I | 12.82 | 6.41 | |
| Th | CHRCO | Chrysanthemum coronarium L. | II | 30.77 | 15.38 | |
| Th | ECHSP | Echinops spinosus L. | III | 51.28 | 88.46 | |
| Th | ECHHO | Echium horridum Batt. | I | 15.38 | 7.69 | |
| Th | ERICA | Eryngium campestre L., 1753 | I | 2.56 | 1.28 | |
| Th | LACSE | Lactuca serriola L. | II | 38.46 | 32.05 | |
| Hem | LAUNU | Launaea nudicaulis Hook.f. | II | 28.21 | 26.92 | |
| Th | LOMST | Lomelosia stellata (L.) Raf., 1838 | III | 46.15 | 60.26 | |
| Hem | MANSA | Mantisalca salmantica (L.) Briq. & Cavill. | II | 33.33 | 66.67 | |
| Hem | ONOMA | Onopordum macracanthum schrub sb, | II | 35.90 | 43.59 | |
| Hem | PALSP | Pallenis spinosa (L.) Cass., 1825 | II | 23.08 | 11.54 | |
| Hem | SCOHI | Scolymus hispanicus L., 1753 | III | 58.97 | 66.67 | |
| Th | SCOLA | Scorzonera laciniata L. | II | 20.51 | 8.97 | |
| Th | SONOL | Sonchus oleraceus L. | II | 25.64 | 12.82 | |
| Brassicaceae | Geo | CARDR | Cardaria draba L. | II | 33.33 | 41.03 |
| Th | HIRIN | Hirschfieldia incana (L.) W.D.J.Koch | II | 28.21 | 39.74 | |
| Th | RAPRI | Rapistrum rugosum (L.) All. | II | 28.21 | 14.10 | |
| Th | SISIR | Sisymbrium irio L. | II | 33.33 | 58.97 | |
| Campanulaceae | Th | HEYSP | Herniaria hirsuta L. | II | 15.38 | 7.69 |
| Caryophylaceae | Hem | PARAR | Paronychia argentea Lam., 1779 | II | 30.77 | 56.41 |
| Cistaceae | Pha | CISTSA | Cistus salviifolius L. | I | 5.13 | 2.56 |
| Convolvulaceae | Hem | CONAL | Convolvulus althaeoides L., 1753 | I | 15.38 | 6.41 |
| Hem | CONAL | Convolvulus arvensis L. | II | 20.51 | 23.08 | |
| Hem | CONAR | Convolvus lineatus L. | I | 7.69 | 3.85 | |
| Hem | CONLI | Foeniculum vulgare subsp. Vulgare | I | 2.56 | 1.28 | |
| Cupressaceae | Pha | JUNOX | Juniperus oxycedrus L. | II | 33.33 | 67.95 |
| Fabaceae | Pha | ACACY | Acacia cyanophylla Lindl. | II | 20.51 | 143.59 |
| Cha | ASTAR | Astragalus armatus Willd. | III | 41.03 | 97.44 | |
| Pha | CERSI | Cercis siliquastrum L., 1753 | I | 5.13 | 15.38 | |
| Cha | GENHI | Genista hirsuta Vahl. | III | 43.59 | 185.90 | |
| Cha | GENTR | Genista tricuspidata Desf. | II | 33.33 | 80.77 | |
| Pha | GLETR | Gledistia trianthos L. | I | 2.56 | 1.28 | |
| Th | HERHI | Hedysarum spinosissimum L. | II | 20.51 | 50.00 | |
| Hem | LOTCO | Lotus corniculatus L. | I | 35.90 | 112.82 | |
| Hem | LOTMA | Lotus maroccanus ball. | III | 41.03 | 110.26 | |
| Th | MEDPO | Medicago polymorpha L., 1753 | I | 7.69 | 3.85 | |
| Pha | RETMO | Retama monosperma (L.) Boiss., 1840 | I | 7.69 | 3.85 | |
| Pha | ROBPS | Robinia pseudoacacia L., 1753 | I | 12.82 | 75.64 | |
| Fagaceae | Pha | QUEIL | Quercus ilex L., 1753 | I | 10.26 | 5.13 |
| Georaniaceae | Th | EROMA | Erodium malacoïdes, Willd. | I | 12.82 | 5.13 |
| Juncaceae | Geo | JUNAC | Juncus acutus L. | I | 10.26 | 48.72 |
| Lamiaceae | Hem | MARVU | Marrubium vulgare L. | I | 17.95 | 21.79 |
| Liliaceae | Cha | ASPAC | Asparagus acutifolius L. | I | 5.13 | 2.56 |
| Malvaceae | Th | MALPA | Malva parviflora L. | I | 5.13 | 2.56 |
| Meliaceae | Pha | MELAZ | Melia azedarach L. | I | 10.26 | 30.77 |
| Papaveraceae | Th | PAPRH | Papaver rhoeas L. | I | 2.56 | 1.28 |
| Pinaceae | Pha | PINHA | Pinus halepensis Miller 1768. | II | 33.33 | 142.31 |
| Plantaginaceae | Th | PLACO | Plantago coronopus L. | I | 15.38 | 7.69 |
| Th | PLAPS | Plantago psyllium Moench 1794 | III | 43.59 | 71.79 | |
| Poaceae | Th | AEGGE | Aegilops Geniculata Roth | I | 7.69 | 16.67 |
| Hem | ARIAD | Arisitda adscensionis Desf. | I | 12.82 | 19.23 | |
| Hem | ARIPU | Arisitda pungens Desf. | IV | 66.67 | 462.82 | |
| Th | AVEST | Avena sterilis L. | I | 35.90 | 56.41 | |
| Th | BROMO | Bromus hordeaceus subsp. mollis (L.) Maire | IV | 71.79 | 330.77 | |
| Th | BRORI | Bromus rigidus Roth. | II | 25.64 | 25.64 | |
| Th | BRORU | Bromus rubens L. | I | 12.82 | 6.41 | |
| Th | BROST | Bromus sterilis (L.) Nevsk | II | 23.08 | 10.26 | |
| Th | CYNDA | Cynodon dactylon (L.) Pers. | III | 51.28 | 51.28 | |
| Th | HORMU | Hordeum murinum L. | II | 30.77 | 110.26 | |
| Th | LAMAU | Lamarckia aurea (L.) Moench | III | 46.15 | 130.77 | |
| Th | LOLMU | Lolium multiflorum Lam. | I | 12.82 | 19.23 | |
| Th | LOLRI | Lolium rigidum Gaudin | II | 35.90 | 16.67 | |
| Cha | LYGSP | Lygeum spartum L. | I | 10.26 | 3.85 | |
| Geo | PHRCO | Phragmites australis (Cav.) Trin. ex Steud. | III | 43.59 | 193.59 | |
| Th | POLMO | Polypogon monspeliensis (L.) Desf. | I | 2.56 | 1.28 | |
| Th | SHIBA | Schismus barbatus (L.) Thell. | I | 5.13 | 2.56 | |
| Resedaceae | Th | RESLU | Reseda lutea L. | IV | 71.79 | 60.26 |
| Rhamnaceae | Pha | ZIZLO | Zizyphus lotus (L.) Desf. | I | 17.95 | 8.97 |
| Rosaceae | Hem | SANMI | Sanguisorba minor L. 1753 | I | 10.26 | 5.13 |
| Simaroubaceae | Pha | AILAL | Ailanthus altissima (Mill.) Swingle, 1916 | I | 5.13 | 15.38 |
| Solanaceae | Th | SOLLY | Solanum lycopersicum L. | I | 2.56 | 1.28 |
| Tamaricaceae | Pha | TAMCA | Tamarix canariensis Willd. | II | 28.21 | 52.56 |
| Nd | - | Nd | Sp n.d | I | 12.82 | 5.13 |
| TMD1 | TMD2 | TMD3 | Study Sites | |
|---|---|---|---|---|
| Number of species (D > 100) | 11 | 17 | 7 | 10 |
| Number of species in Classes V and III | 11 | 28 | 10 | 17 |
| Species richness | 78 | 68 | 71 | 91 |
| Sum of coverage (D) | 3854 | 5050 | 3503 | 4130 |
| Sum of frequency % | 1823 | 2546.1 | 2076.86 | 2148.7 |
| Age | 24 | 22 | 16 | - |
| Site | TMD1 | TMD2 | TMD3 | |
|---|---|---|---|---|
| Age | 24 | 22 | 16 | |
| SOM% | Mean | 0.969 a | 1.024 a | 0.945 a |
| SD | 0.00 | 0.02 | 0.12 | |
| pH | Mean | 7.613 a | 7.490 a | 7.900 a |
| SD | 0.11 | 0.14 | 0.02 | |
| EC (µs·cm−3) | Mean | 129.267 b | 148.800 b | 254.200 a |
| SD | 28.05 | 15.36 | 8.43 | |
| Cu (mg·kg−1) | Mean | 818.616 b | 293.943 c | 933.866 a |
| SD | 14.21 | 8.41 | 35.08 | |
| Pb (mg·kg−1) | Mean | 13,426.182 a | 3417.775 c | 6527.435 b |
| SD | 126.03 | 50.72 | 344.37 | |
| Zn (mg·kg−1) | Mean | 7559.096 a | 1906.363 c | 3582.990 b |
| SD | 21.02 | 9.21 | 151.11 | |
| Bio-extractible Cu (mg·kg−1) | Mean | 1.422 ab | 1.709 a | 1.360 b |
| SD | 0.04 | 0.09 | 0.07 | |
| Bio-extractible Pb (mg·kg−1) | Mean | 3.663 a | 4.582 a | 5.129 a |
| SD | 0.21 | 0.03 | 0.66 | |
| Bio-extractible Zn (mg·kg−1) | Mean | 1.234 a | 1.073 a | 1.243 a |
| SD | 0.02 | 0.03 | 0.24 |
| Pb | Zn | Cu | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species Code | Shoots | SD | Roots | SD | Soil | SD | Shoots | SD | Roots | SD | Soil | SD | Shoots | SD | Roots | SD | Soil | SD |
| ARSPU | 416.870 c | 98.47 | 607.629 a | 79.18 | 3590.337 de | 126.22 | 832.202 abc | 208.41 | 1307.998 a | 278.25 | 14,070.731 b | 545.46 | 16.740 b | 3.09 | 38.123 b | 14.14 | 99.029 d | 4.39 |
| ASTAR | 501.871 c | 433.13 | 227.424 a | 62.20 | 5501.175 de | 1481.05 | 224.157 cd | 188.31 | 329.593 b | 65.83 | 1743.064 f | 70.60 | 42.600 b | 32.21 | 27.766 b | 4.95 | 209.078 cd | 7.46 |
| ATRCA | 572.682 bc | 254.65 | 568.098 a | 227.83 | 4908.724 de | 327.58 | 306.905 bcd | 133.10 | 176.512 b | 68.06 | 2408.230 f | 80.37 | 50.218 b | 25.22 | 42.964 b | 19.66 | 1077.393 a | 432.11 |
| CHEMU | 544.825 bc | 129.76 | 381.351 a | 110.75 | 4637.003 de | 98.06 | 236.134 bcd | 45.79 | 179.779 b | 41.33 | 3324.001 ef | 8.36 | 49.949 b | 5.05 | 41.891 b | 5.04 | 704.562 abcd | 9.99 |
| GENHI | 72.560 c | 19.75 | 152.993 a | 77.50 | 4072.320 de | 179.95 | 72.016 d | 18.41 | 71.157 b | 32.61 | 2064.299 f | 67.59 | 8.850 b | 0.97 | 18.257 b | 7.07 | 329.657 bcd | 7.14 |
| GENTR | 69.012 c | 2.76 | 451.040 a | 210.59 | 3417.775 de | 50.72 | 72.894 d | 6.07 | 242.356 b | 95.84 | 1906.363 f | 9.21 | 20.600 b | 3.33 | 50.065 ab | 21.98 | 293.943 cd | 8.41 |
| LOTCO | 2279.724 a | 284.91 | 264.210 a | 90.77 | 6307.600 cde | 233.68 | 846.271 ab | 90.56 | 116.482 b | 34.36 | 3333.050 ef | 82.34 | 180.113 a | 24.32 | 26.423 b | 5.11 | 592.092 abcd | 27.46 |
| LOTMA | 1484.512 ab | 342.93 | 864.085 a | 294.78 | 6527.435 cd | 344.37 | 1109.958 a | 307.16 | 575.610 b | 245.35 | 3582.990 ef | 151.11 | 221.332 a | 64.48 | 139.809 a | 48.11 | 933.866 ab | 35.08 |
| MELAZ | 248.873 c | 11.69 | nd | nd | 9844.292 bc | 345.08 | 267.758 bcd | 6.47 | nd | nd | 12,488.420 b | 919.13 | 21.493 b | 0.21 | nd | nd | 290.655 cd | 4.37 |
| PINHA | 76.555 c | 8.44 | 676.562 a | 64.35 | 10,038.321 bc | 921.77 | 88.972 d | 2.69 | 312.924 b | 27.83 | 6173.859 cd | 169.38 | 7.877 b | 0.40 | 31.630 b | 2.30 | 924.319 ab | 60.33 |
| PISLE | 61.991 c | 1.78 | nd | nd | 2396.654 e | 270.83 | 89.550 d | 3.38 | nd | nd | 14,065.316 b | 1063.89 | 11.710 b | 0.37 | nd | nd | 103.779 d | 17.01 |
| QUEIL | 151.694 c | 5.01 | nd | nd | 3633.154 de | 206.04 | 227.859 cd | 2.29 | nd | nd | 20,028.993 a | 543.59 | 8.797 b | 0.16 | nd | nd | 125.885 d | 9.41 |
| RESLU | 1609.817 a | 154.11 | 192.046 a | 35.47 | 5529.843 de | 186.33 | 1063.421 a | 89.08 | 183.274 b | 31.86 | 4889.819 de | 392.27 | 167.005 a | 16.46 | 30.879 b | 7.60 | 1124.250 a | 125.75 |
| RETMO | 93.629 c | 3.44 | nd | nd | 13,426.182 b | 126.03 | 112.726 d | 3.19 | nd | nd | 7559.096 c | 21.02 | 9.753 b | 0.45 | nd | nd | 818.616 abc | 14.21 |
| ROBPS | 49.165 c | 2.91 | nd | nd | 29,661.245 a | 2313.91 | 105.421 d | 3.90 | nd | nd | 19,042.828 a | 350.10 | 11.789 b | 0.46 | nd | nd | 556.457 abcd | 11.92 |
| Site * | Year of Soil Amendment | Year of Revegetation | Age | Area (ha) |
|---|---|---|---|---|
| TMD1 | 2000 | 2001 | 24 | 13.95 |
| TMD2 | 2002 | 2003 | 22 | 13.92 |
| TMD3 | 2008 | Not revegetated | 16 | 20.49 |
| Sampling Site | No. | Family Name | Scientific Name | Life-Form | Abbreviation | Replicates |
|---|---|---|---|---|---|---|
| Touissit mine dumps | 1 | Fabaceae | Astragalus armatus | Hc | ASTAR | 3 |
| 2 | Asteracea | Atractylis caespitosa | Hc | ATRCA | 3 | |
| 3 | Fabaceae | Genista hirsuta | Ph | GENHI | 3 | |
| 4 | Fabaceae | Genista tricuspidata | Ph | GENTR | 3 | |
| 5 | Fabaceae | Lotus corniculatus | Ph | LOTCO | 3 | |
| 6 | Fabaceae | Lotus maroccanus | Th | LOTMA | 3 | |
| 7 | Resedaceae | Reseda lutea | Th | RESLU | 3 | |
| 8 | Poaceae | Aristida pungens | Hc | ARSPU | 3 | |
| 9 | Chenopodiaceae | Chenopodium murale | Th | CHEMU | 3 | |
| 10 | Meliaceae | Mélia azedarach | Ph | MELAZ | 3 | |
| 11 | Anacardiaceae | Pistacia lentiscus | Ph | PISLE | 3 | |
| 12 | Fabaceae | Retama monosperma | Ph | RETMO | 3 | |
| 13 | Fagaceae | Quercus ilex | Ph | QUEIL | 3 | |
| 14 | Fabaceae | Robinia pseudoacacia | Ph | ROBPS | 3 | |
| 15 | Pinaceae | Pinus halpensis | Ph | PINHA | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oujdi, M.; Chafik, Y.; Boukroute, A.; Bourgerie, S.; Sena-Velez, M.; Morabito, D.; Addi, M. Exploring Phytoremediation Potential: A Comprehensive Study of Flora Inventory and Soil Heavy Metal Contents in the Northeastern Mining Districts of Morocco. Plants 2024, 13, 1811. https://doi.org/10.3390/plants13131811
Oujdi M, Chafik Y, Boukroute A, Bourgerie S, Sena-Velez M, Morabito D, Addi M. Exploring Phytoremediation Potential: A Comprehensive Study of Flora Inventory and Soil Heavy Metal Contents in the Northeastern Mining Districts of Morocco. Plants. 2024; 13(13):1811. https://doi.org/10.3390/plants13131811
Chicago/Turabian StyleOujdi, Mohammed, Yassine Chafik, Azzouz Boukroute, Sylvain Bourgerie, Marta Sena-Velez, Domenico Morabito, and Mohamed Addi. 2024. "Exploring Phytoremediation Potential: A Comprehensive Study of Flora Inventory and Soil Heavy Metal Contents in the Northeastern Mining Districts of Morocco" Plants 13, no. 13: 1811. https://doi.org/10.3390/plants13131811
APA StyleOujdi, M., Chafik, Y., Boukroute, A., Bourgerie, S., Sena-Velez, M., Morabito, D., & Addi, M. (2024). Exploring Phytoremediation Potential: A Comprehensive Study of Flora Inventory and Soil Heavy Metal Contents in the Northeastern Mining Districts of Morocco. Plants, 13(13), 1811. https://doi.org/10.3390/plants13131811










