Pharmacognostic Evaluation, Chemical Characterization, and Antibacterial Activity of Bassia indica (Wight) A.J. Scott
Abstract
1. Introduction
2. Results
2.1. Extract Preparation and Fractionation
2.2. Pharmacognostic Evaluation
2.2.1. Macroscopic Analysis
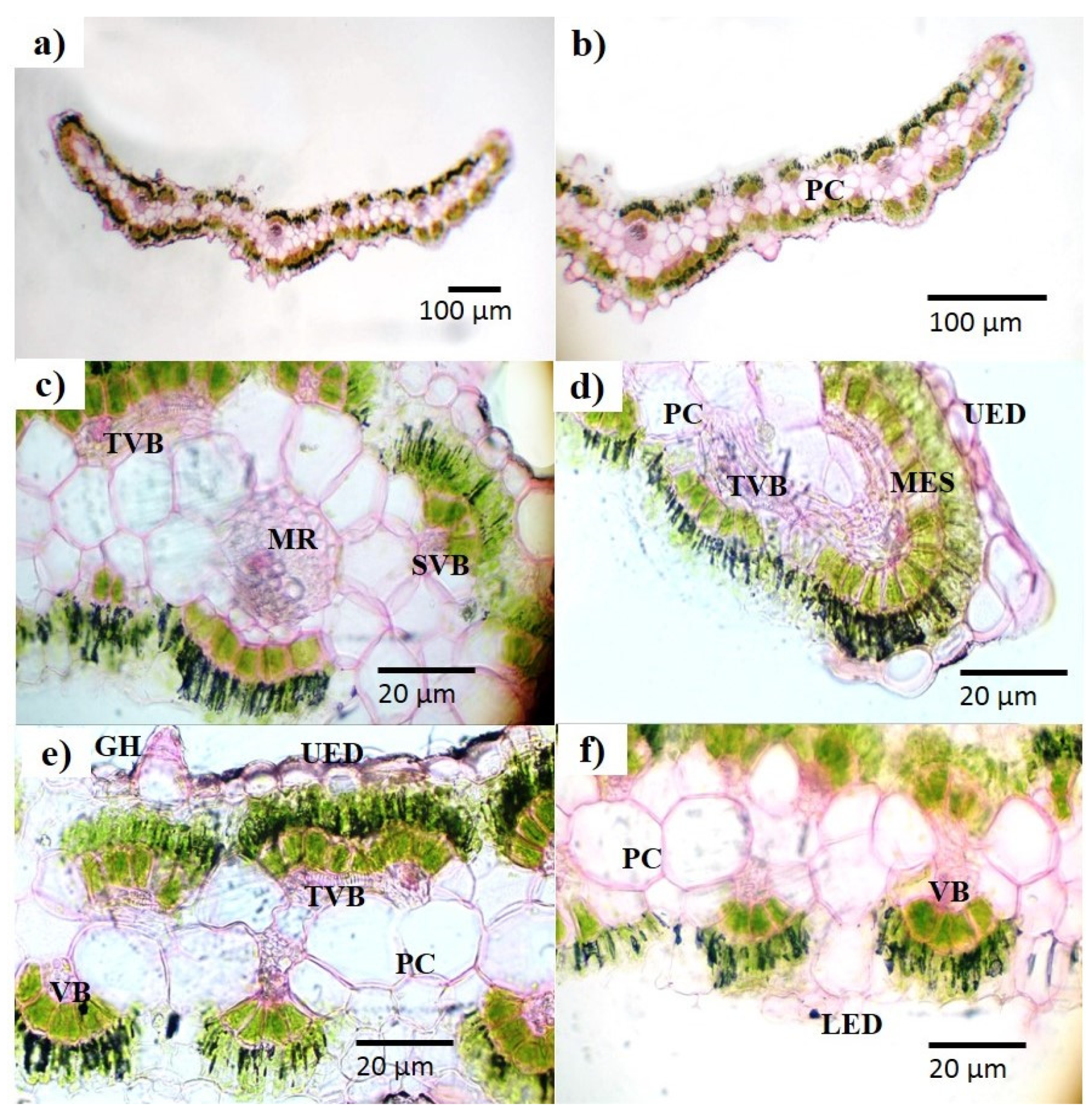
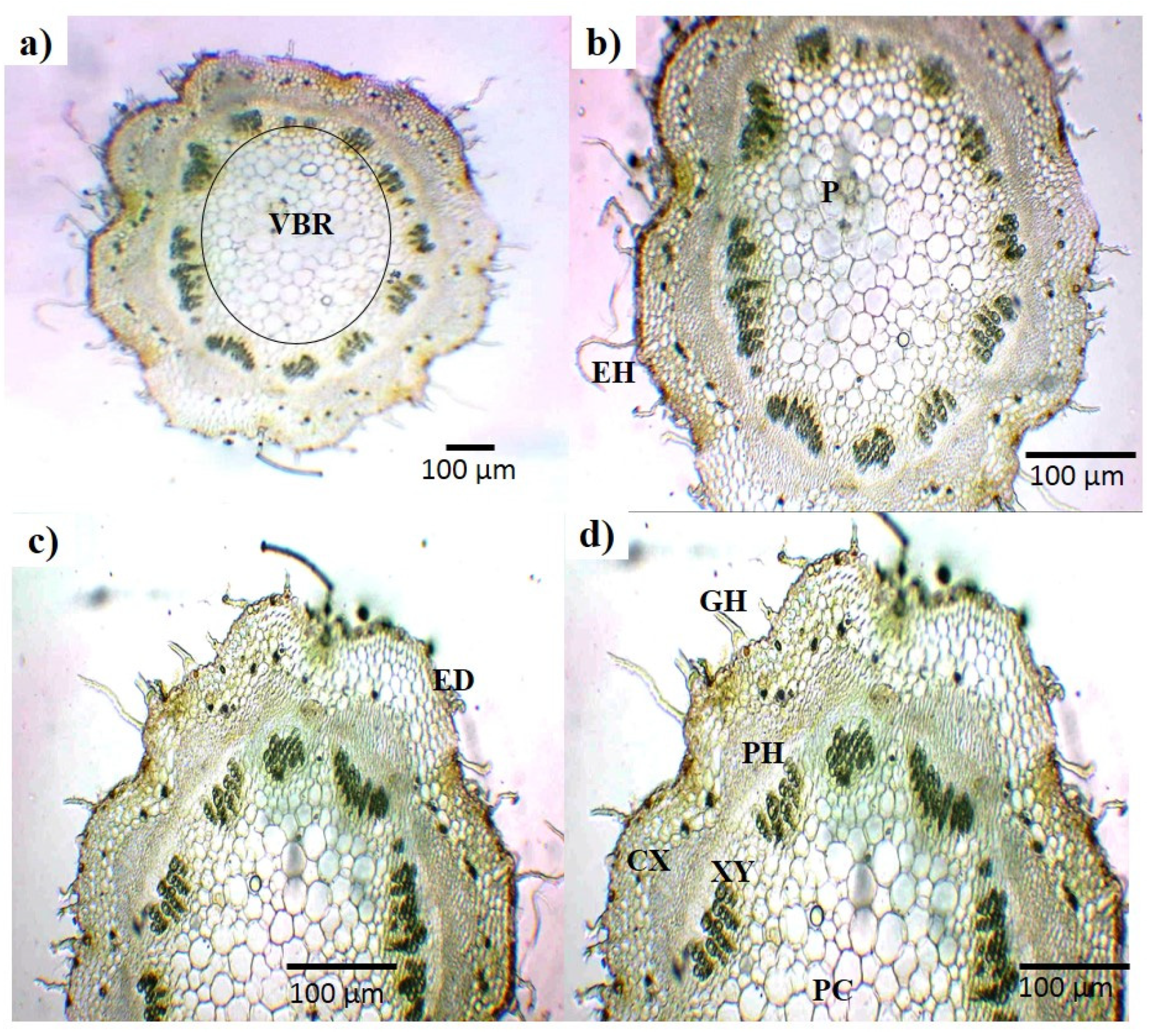
2.2.2. Microscopic Analysis
2.2.3. Scanning Electron Microscopy
2.2.4. Extractive Value and Swelling Index
2.2.5. Nutritional and Elemental Analysis
2.2.6. Fluorescence Analysis
2.2.7. Thin Layer Chromatography
2.3. Phytochemical Analysis
2.3.1. Qualitative Analysis
2.3.2. Quantitative Analysis of Total Phenols, Tannins, Flavonoids, and Saponins
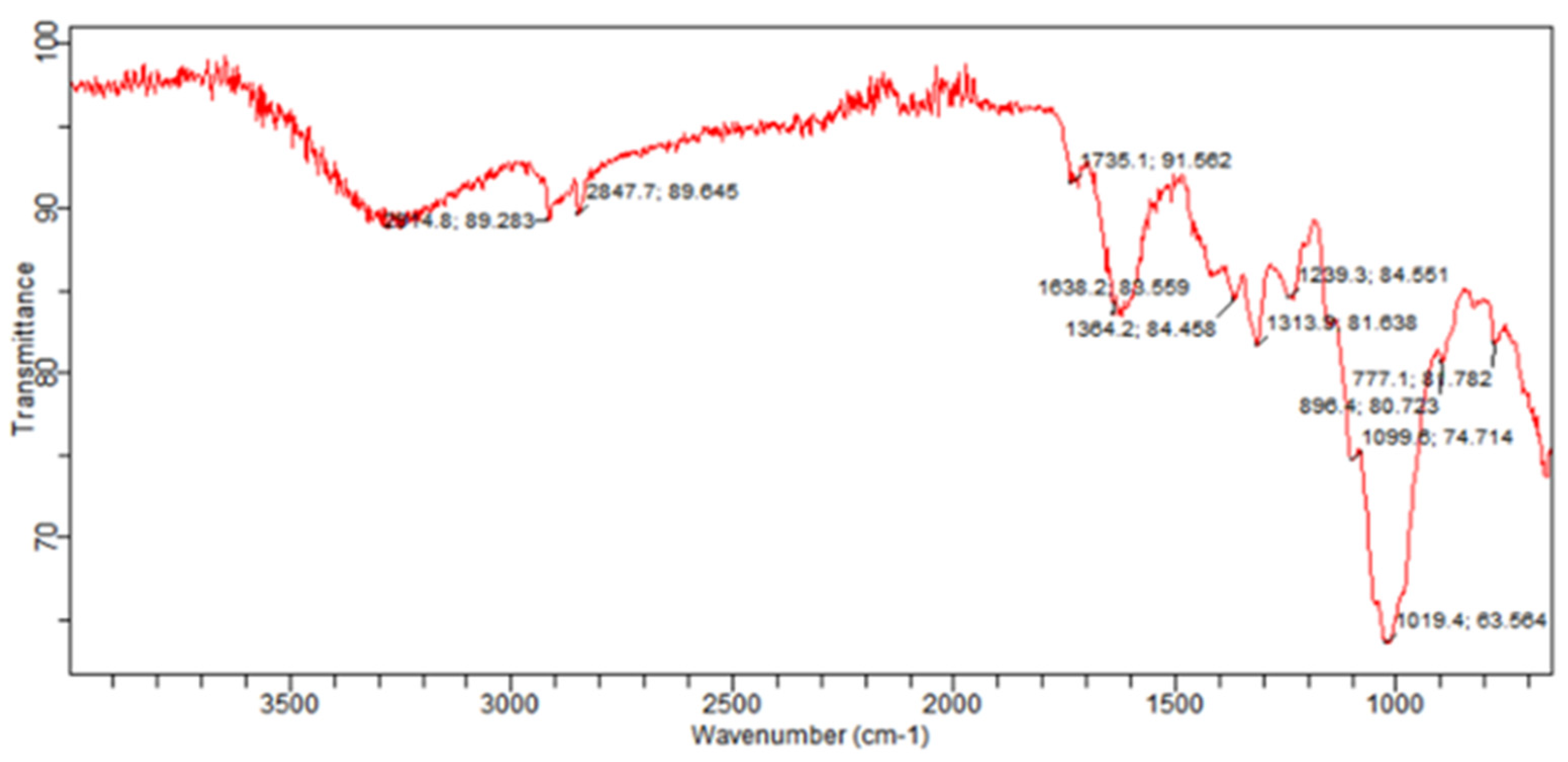
2.4. FTIR Spectroscopy
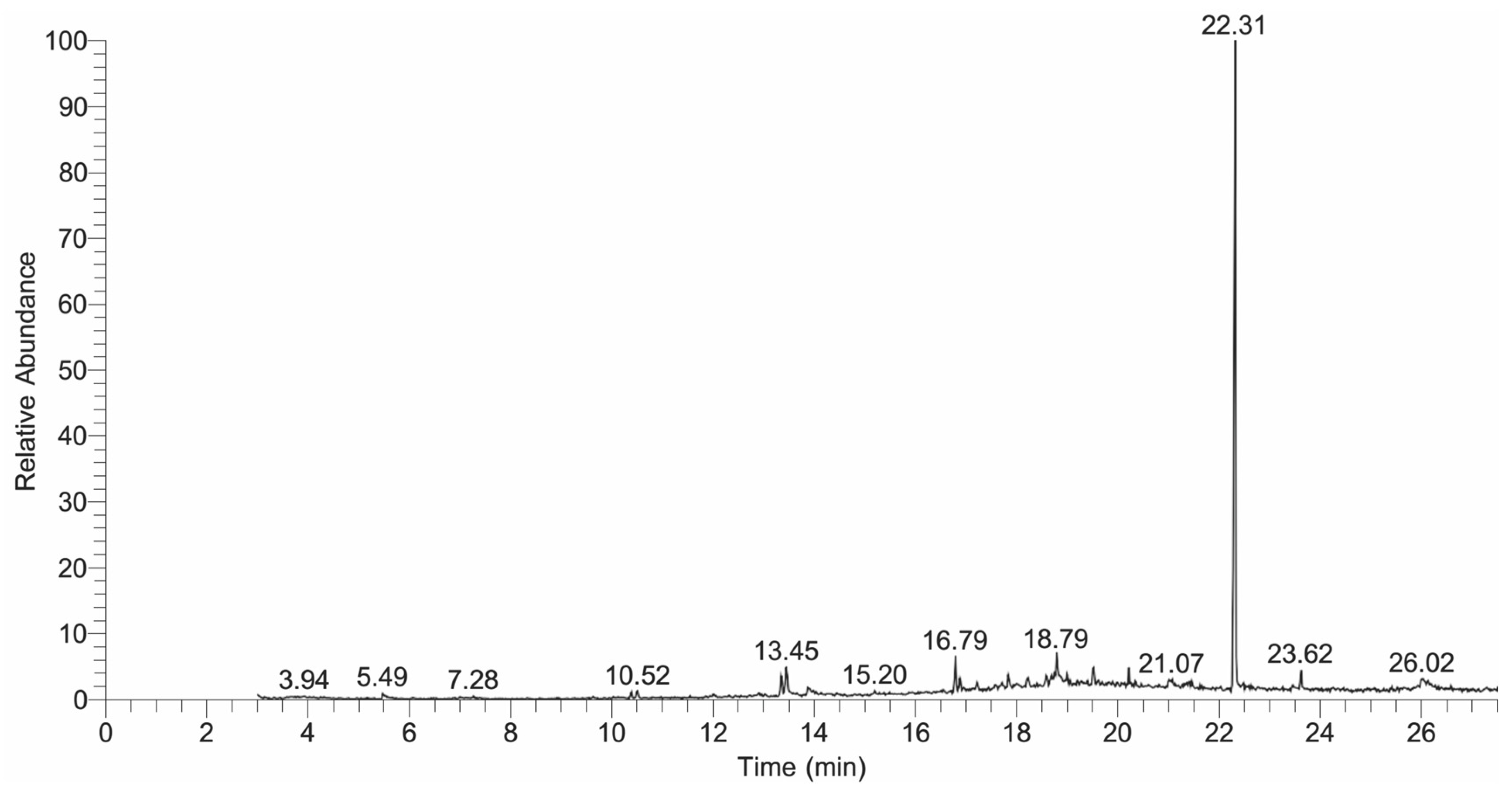
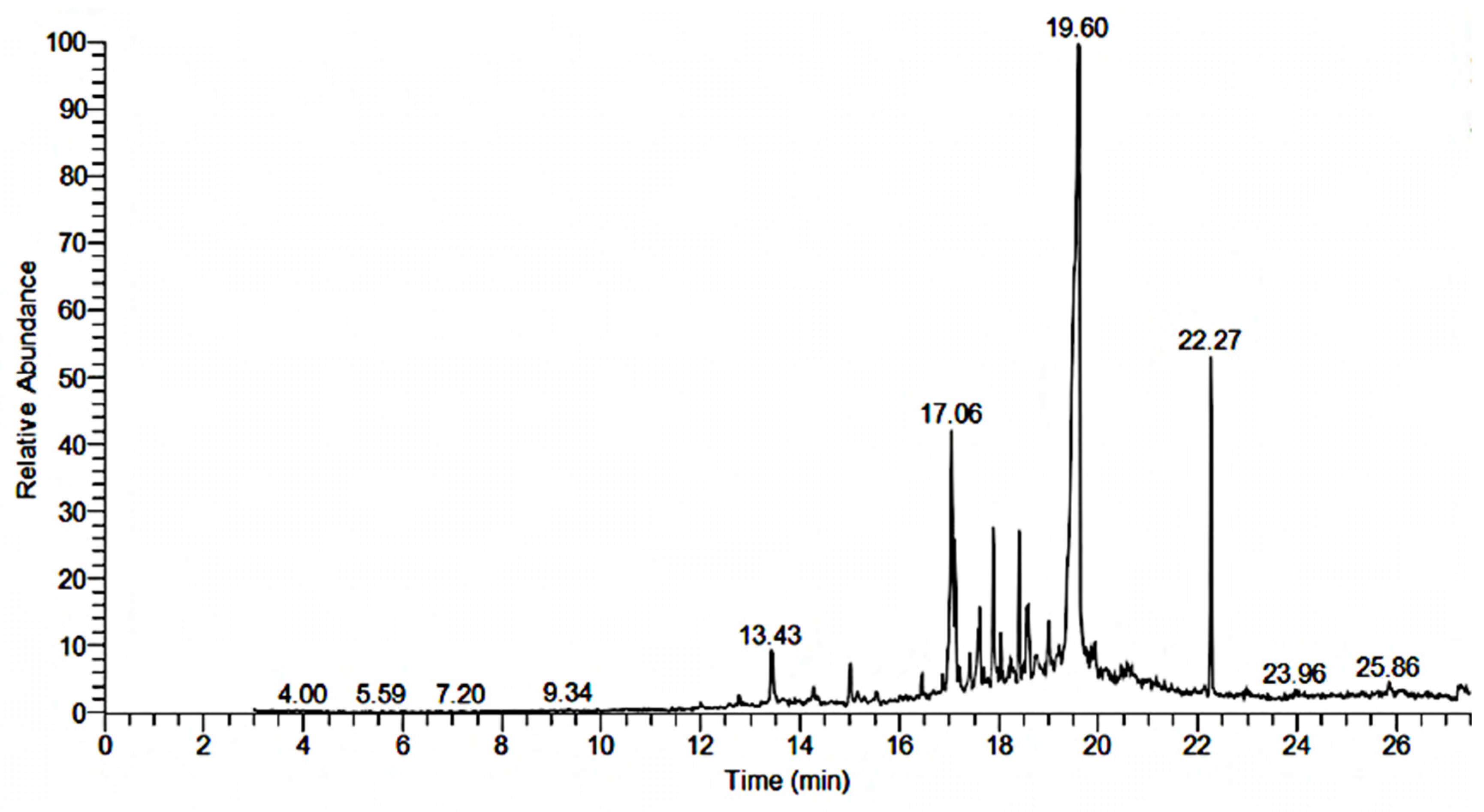
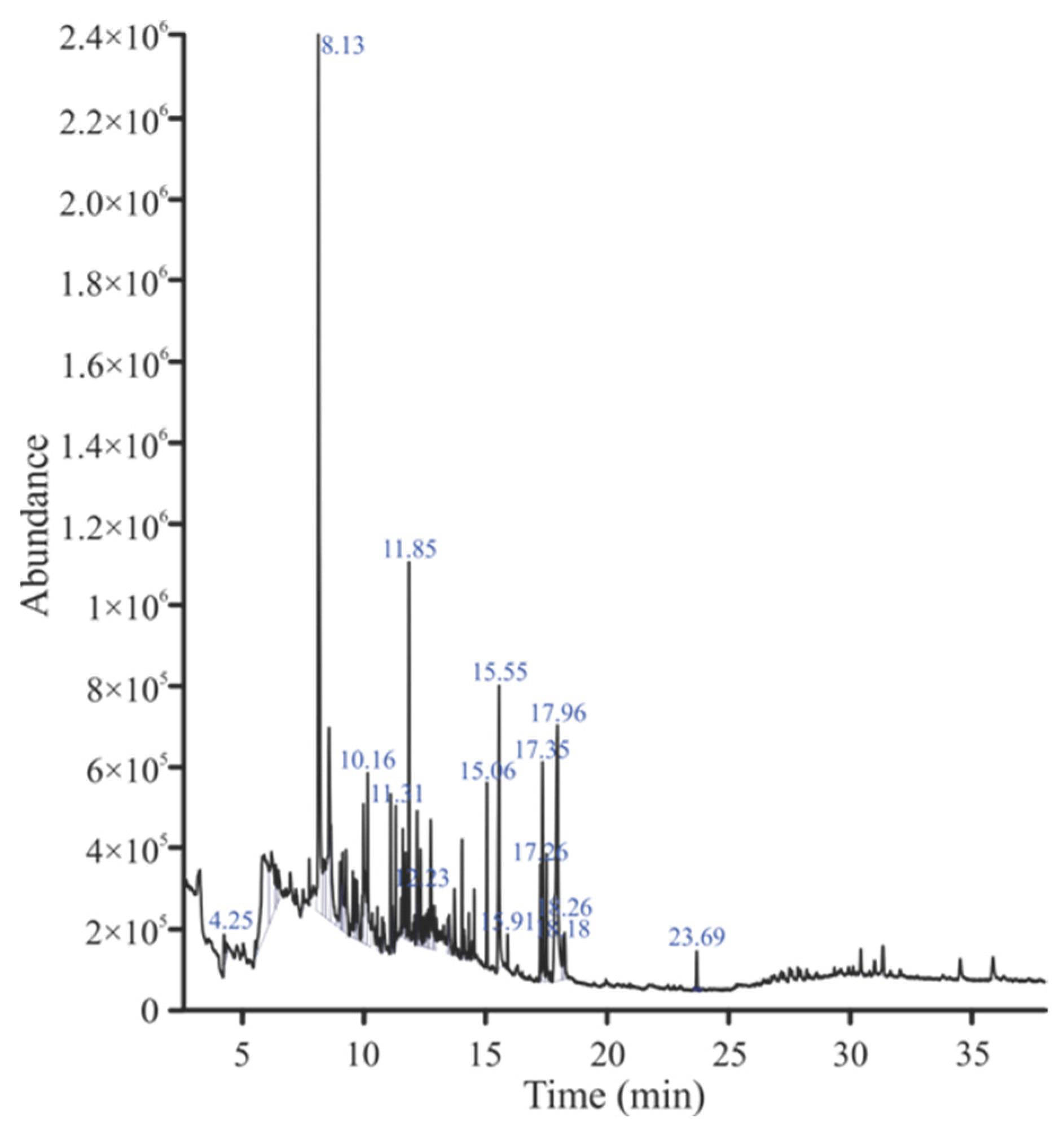
2.5. GC-MS Analysis
2.6. Acute Toxicity and Cytotoxicity
2.7. Antimicrobial Activity
2.7.1. Disc Diffusion Method
2.7.2. Minimum Inhibitory Concentration
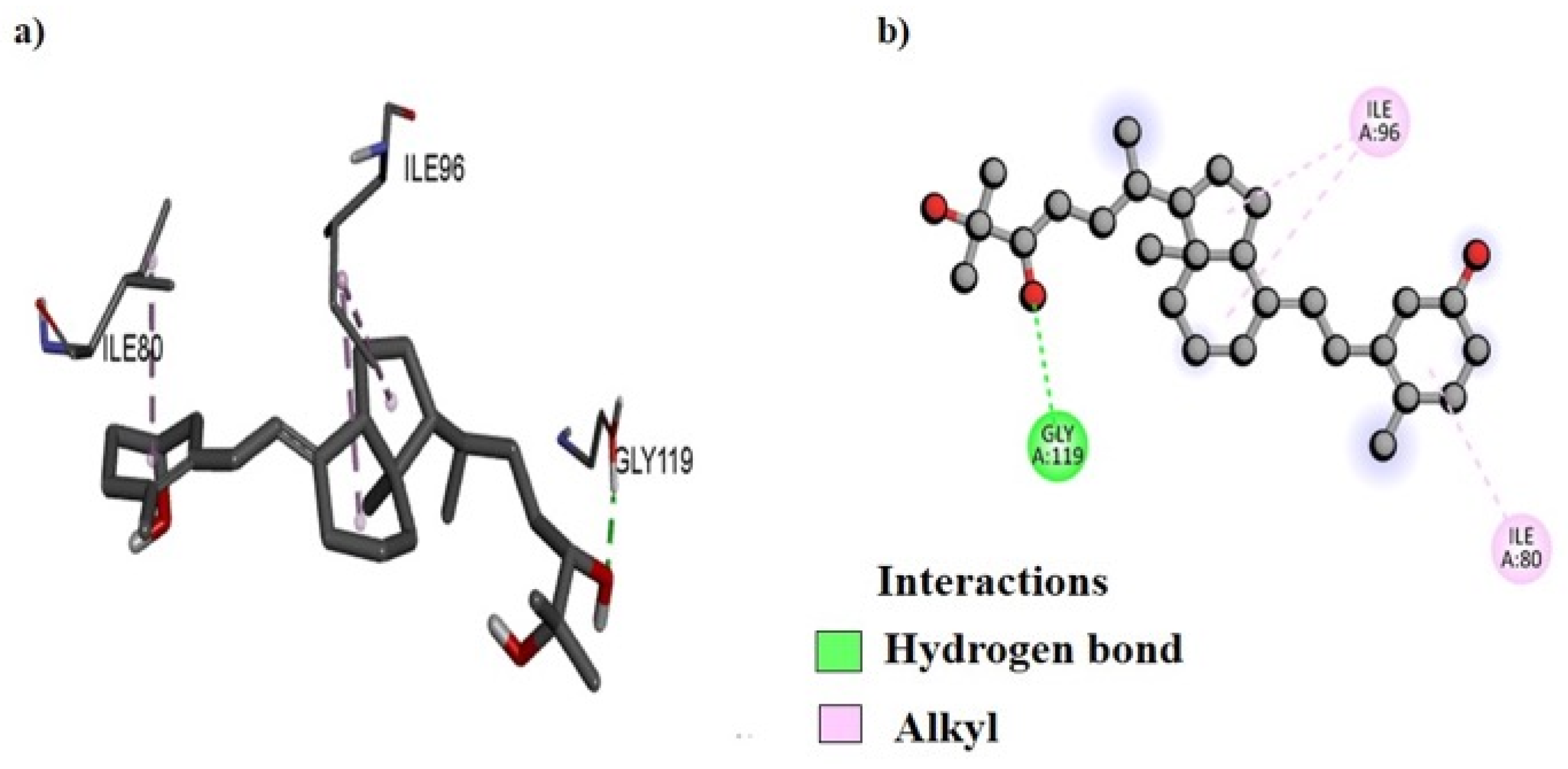
2.8. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Extract Preparation and Fractionation
4.3. Experimental Animals
4.4. Pharmacognostic Evaluation
4.4.1. Macroscopic Studies
4.4.2. Microscopic Studies
4.4.3. Scanning Electron Microscopy
4.4.4. Swelling Index
4.4.5. Extractive Value Determination
4.4.6. Elemental and Nutritional Analysis
4.4.7. Fluorescence Analysis
4.4.8. Thin Layer Chromatography
4.5. Phytochemical Analysis
4.5.1. Qualitative Analysis
4.5.2. Quantitative Analysis of Total Phenols, Tannins, Flavonoids, and Saponins
4.6. FTIR Spectroscopy
4.7. GC-MS Analysis
4.8. Cytotoxicity Study
4.9. Acute Toxicity Study
4.10. Antibacterial Activity
4.10.1. Zone of Inhibition by Disc Diffusion Method
4.10.2. Minimum Inhibitory Concentration
4.11. Molecular Docking
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Guidelines on the Conservation of Medicinal Plants; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 1993. [Google Scholar]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Puneshwar, K. Controversy, adulteration and substitution: Burning problems in ayurveda practices. In Natural Medicinal Plants; Hany, A.E.-S., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Khan, S.A.; Barkatullah; Ibrar, M.; Ullah, S. Microscopic investigations and pharmacognostic techniques used for the standardization of leaf of Rhus succedanea var. Himalaica JD Hook. Microsc. Res. Tech. 2019, 82, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Ichim, M.C. The DNA-based authentication of commercial herbal products reveals their globally widespread adulteration. Front. Pharmacol. 2019, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.W.A.; Sharif, H.; Gilani, S.M.U.; Ali, S.T.; Ahmed, S.; Siddiqui, M.U.A.; Hasan, M.M. Pharmacognostic and phytochemical study of the flowers of Cordia sebestena L. Pak. J. Pharm. Sci 2022, 35, 069–076. [Google Scholar] [CrossRef]
- Uza, N.U.; Dastagir, G. Microscopic and pharmacognostic standardization of Astragalus scorpiurus Bunge. Microsc. Res. Tech. 2022, 85, 324–338. [Google Scholar] [CrossRef]
- WFO. Bassia indica (Wight) A.J. Scott. 2023. Available online: https://wfoplantlist.org/taxon/wfo-0000561151-2024-06?page=1 (accessed on 23 April 2024).
- Grabowska, K.; Buzdygan, W.; Galanty, A.; Wróbel-Biedrawa, D.; Sobolewska, D.; Podolak, I. Current knowledge on genus Bassia All: A comprehensive review on traditional use, phytochemistry, pharmacological activity, and nonmedical applications. Phytochem. Rev. 2023, 22, 1197–1246. [Google Scholar] [CrossRef]
- Youssef, S. Medicinal and non-medicinal uses of some plants found in the middle region of Saudi Arabia. J. Med. Plant Res. 2013, 7, 2501–2517. [Google Scholar] [CrossRef]
- El-Gendy, Z.A.; Taher, R.F.; Elgamal, A.M.; Serag, A.; Hassan, A.; Jaleel, G.A.A.; Farag, M.A.; Elshamy, A.I. Metabolites profiling and bioassays reveal Bassia indica ethanol extract protective effect against stomach ulcers development via HMGB1/TLR-4/NF-κB pathway. Antioxidants 2023, 12, 1263. [Google Scholar] [CrossRef]
- Qureshi, M.Z.; Javed, S.; Javaid, A.; Al-Taie, A.H. Identification of antimicrobial compounds from n-hexane stem extract of Kochia indica by GC-MS analysis. Mycopath 2020, 16, 51–55. [Google Scholar]
- Mohammed, M.; Heikal, E.A.; Ellessy, F.M.; Aboushousha, T.; Ghareeb, M.A. Comprehensive chemical profiling of Bassia indica Wight. aerial parts extract using UPLC-ESI–MS/MS, and its antiparasitic activity in Trichinella spiralis infected mice: In silico supported in vivo study. BMC Complement. Med. Ther. 2023, 23, 1–22. [Google Scholar] [CrossRef]
- Othman, A.; Amen, Y.; Inoue, Y.; Shimizu, K. Phytochemical analysis, anti-inflammatory, and anticancer activities of the halophyte herb Bassia indica. Nat. Prod. Commun. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Bouaziz, M.; Dhouib, A.; Loukil, S.; Boukhris, M.; Sayadi, S. Polyphenols content, antioxidant and antimicrobial activities of extracts of some wild plants collected from the south of Tunisia. Afr. J. Biotechnol. 2009, 8, 7017–7027. [Google Scholar]
- Bibi, H.; Hussain, M.; Jan, G.; Shah, G.; Khan, S.; Ullah, I. Phytochemical analysis and antimicrobial activities of Kochia indica (Wight), plant growing in District Karak Khyber Puhktunkhuwa. Pure Appl. Biol. 2021, 10, 789–796. [Google Scholar] [CrossRef]
- Javed, S.; Javaid, A.; Qureshi, M.Z. Antifungal phytocomponents in n-butanol fraction of leaf extract of Kochia indica Wight. Int. J. Biol. Biotechnol. 2018, 15, 661–666. [Google Scholar]
- Othman, A.; Sayed, A.M.; Amen, Y.; Shimizu, K. Possible neuroprotective effects of amide alkaloids from Bassia indica and Agathophora alopecuroides: In vitro and in silico investigations. RSC Adv. 2022, 12, 18746–18758. [Google Scholar] [CrossRef] [PubMed]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evid. Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef]
- Kim, O.K.; Barrett, J.F.; Ohemeng, K. Advances in DNA gyrase inhibitors. Expert Opin. Investig. Drug 2001, 10, 199–212. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Zhang, R.; Chen, Y.; Zhang, C.; Heidi, H.; Li, M. Comparison of the guidelines on good agricultural and collection practices in herbal medicine of the European Union, China, the WHO, and the United States of America. Pharmacol. Res. 2021, 167, 105533. [Google Scholar] [CrossRef]
- WHO. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- El Shereef, A.A. Kochia plant as potential forage for ruminants under desert conditions. Annu. Res. Rev. Biol. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Turki, Z.; El-Shayeb, F.; Shehata, F. Taxonomic studies in the camphorosmeae (chenopodiaceae) in Egypt. Flora Mediterr. 2006, 16, 275–294. [Google Scholar] [CrossRef]
- Zaman, S.; Barkatulllah; Zahoor, M.; Wadood Ali Shah, S.; Ullah, Z.; Ullah, R.; Alotaibi, A. Pharmacognostic evaluation of Artemisia maritima L. a highly medicinal specie of genus Artemisia. Saudi J. Biol. Sci. 2022, 29, 103419. [Google Scholar] [CrossRef]
- Adu, O.T.; Naidoo, Y.; Adu, T.S.; Sivaram, V.; Dewir, Y.H. Micromorphology and histology of the secretory apparatus of Diospyros villosa (L.) de winter leaves and stem bark. Plants 2022, 11, 2498. [Google Scholar] [CrossRef]
- Hameed, A.; Ghani, N.; Mughal, T.A.; Abbas, M.; Abrar, A.; Javed, H. Pharmacognostical evaluation and physiochemical analysis of Salsola Kali as medicinal plant. Microsc. Res. Tech. 2023, 26, 1322–1332. [Google Scholar] [CrossRef]
- Yusufoglu, H.S. Pharmacognostic and wound healing studies of the leaves of Bassia eriophora (family: Chenopodiaceae) on albino rats. Annu. Res. Rev. Biol. 2015, 5, 400–408. [Google Scholar] [CrossRef]
- Ukwubile, C.A.; Ikpefan, E.O.; Dibal, M.Y.; Umeano, V.A.; Menkiti, D.N.; Kaosi, C.C.; Paul, S.; Famurewa, A.C.; Nettey, H.; Yerima, T.S. Pharmacognostic profiles, evaluation of analgesic, anti-inflammatory and anticonvulsant activities of Newbouldia laevis (P. Beauv.) Seem. ex Bureau leaf and root extracts in Wistar rats. J. Ethnopharmacol. 2023, 314, 116632. [Google Scholar] [CrossRef]
- Sree, P.R.; Thoppil, J. Comparative seed morphology, pharmacognostic, phytochemical, and antioxidant potential of Memecylon l. Fruits. Turk. J. Pharm. Sci. 2021, 18, 213. [Google Scholar] [CrossRef] [PubMed]
- Dastagir, G.; Hussain, F.; Uza, N.U. Anatomical and histochemical characterization of some highly medicinal plants as a tool for quality control. Microsc. Res. Tech. 2023, 86, 966–990. [Google Scholar] [CrossRef]
- Jones, P.W.; Chin, Y.-W.; Kinghorn, D.A. The role of pharmacognosy in modern medicine and pharmacy. Curr. Drug Targets 2006, 7, 247–264. [Google Scholar] [CrossRef]
- Pirintsos, S.; Panagiotopoulos, A.; Bariotakis, M.; Daskalakis, V.; Lionis, C.; Sourvinos, G.; Karakasiliotis, I.; Kampa, M.; Castanas, E. From traditional ethnopharmacology to modern natural drug discovery: A methodology discussion and specific examples. Molecules 2022, 27, 4060. [Google Scholar] [CrossRef]
- Balami, S.; Sharma, A.; Karn, R. Significance of nutritional value of fish for human health. Malays. J. Halal Res. J. 2019, 2, 32–34. [Google Scholar] [CrossRef]
- Radha; Kumar, M. Evaluation of nutritional, phytochemical, and mineral composition of selected medicinal plants for therapeutic uses from cold desert of Western Himalaya. Plants 2021, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Alhadrami, H.A.A.; Bhandari, A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrum Linn fruits. Asian Pac. J. Trop. Biomed. 2015, 5, 101–107. [Google Scholar] [CrossRef]
- Nafea, E. Nutritive values of some wetland plants in the Deltaic Mediterranean coast of Egypt. Egypt. J. Bot. 2017, 57, 1–10. [Google Scholar] [CrossRef][Green Version]
- Mensah, E.; Kyei-Baffour, N.; Ofori, E.; Obeng, G. Influence of Human Activities and Land Use on Heavy Metal Concentrations in Irrigated Vegetables in Ghana and Their Health Implications; Yanful, E.K., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 9–14. [Google Scholar]
- Mirzaei, F. Minerals profile of forages for grazing ruminants in Pakistan. Open J. Anim. Sci. 2012, 2, 9. [Google Scholar] [CrossRef]
- El Shaer, H.M. Halophytes and salt-tolerant plants as potential forage for ruminants in the Near East region. Small Rumin. Res. 2010, 91, 3–12. [Google Scholar] [CrossRef]
- Younus, M.; Hasan, M.M.; Rehman, M.S.; Abbas, K.; Sarwar, G. Report: Pharmacognostic and physicochemical screening of Euphorbia nivulia Buch.-Ham. Pak. J. Pharm. Sci 2019, 32, 1111–1119. [Google Scholar]
- Sajid-Ur-Rehman, M.; Ishtiaq, S.; Khalil-Ur-Rehman, M.; Bauer, R. Pharmacognostic screening, physico-chemical and cytotoxic potential of Sesuvium sesuvioides (Fenzyl) Verdc. Pak. J. Pharm. Sci 2021, 34, 1585–1595. [Google Scholar] [CrossRef]
- Ahmed, F.A.; Baraka, D.M.; Donia, A.E.R.M.; Mostafa, R.M.; Morsy, Z.M. Phytochemical investigation, HPLC analysis and antimicrobial activity of some plants from chenopodiaceae family. Egypt. Acad. J. Biol. 2022, 13, 13–24. [Google Scholar] [CrossRef]
- Vasavilbazo-Saucedo, A.; Almaraz-Abarca, N.; González-Ocampo, H.A.; Ávila-Reyes, J.A.; González-Valdez, L.S.; Luna-González, A.; Delgado-Alvarado, E.A.; Torres-Ricario, R. Phytochemical characterization and antioxidant properties of the wild edible acerola Malpighia umbellata Rose. CyTA J. Food 2018, 16, 698–706. [Google Scholar] [CrossRef]
- González-Ocampo, H.A.; Martínez-Álvarez, I.G.; Jaramillo-Flores, M.E.; Luna-González, A. Comparison of phenolic and flavonoid content and antioxidant and chelating activities of Rhizophora mangle in different anthropogenically-polluted coastal lagoons. Front. Mar. Sci. 2022, 9, 791748. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Lombardo, G.E.; Cirmi, S.; Süntar, I.; Barreca, D. Pharmacology and toxicology of tannins. Arch. Toxicol. 2022, 96, 1257–1277. [Google Scholar] [CrossRef] [PubMed]
- Elekofehinti, O.O.; Iwaloye, O. Saponins in cancer treatment: Current progress and future prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Maulidya, V.; Hasanah, A.N. Quality control and authentication of black betel leaf extract (Piper acre Blume) from East Kalimantan as an antimicrobial agent using a combination of high-performance liquid chromatography and chemometric fourier transform infrared. Molecules 2023, 28, 5666. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Javaid, A. Antifungal, antibacterial and antioxidant components of ethyl acetate extract of quinoa stem. Plant Prot. 2019, 3, 125–130. [Google Scholar] [CrossRef]
- Selvakumar, J.N.; Chandrasekaran, S.D.; Doss, G.P.C.; Kumar, T.D. Inhibition of the ATPase domain of human topoisomerase IIa on HepG2 Cells by 1, 2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester: Molecular docking and dynamics simulations. Curr. Cancer Drug Targets 2019, 19, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Fadipe, L.A.; Haruna, A.; Mohammed, I. Antibacterial activity of 1, 2-benzenedicarboxylic acid, dioctyl ester isolated from the ethyl acetate soluble sub-portion of the unripe fruits of Nauclea latifolia. Int. J. Pure Appl. Biosci. 2014, 2, 223–230. [Google Scholar]
- Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef]
- Henry, H.L.; Norman, A.W.; Taylor, A.N.; Hartenbower, D.L.; Coburn, J.W. Biological activity of 24,25-dihydroxycholecalciferol in chicks and rats. J. Nutr. 1976, 106, 724–734. [Google Scholar] [CrossRef]
- Yang, S.; Chou, G.; Li, Q. Cardioprotective role of azafrin in against myocardial injury in rats via activation of the Nrf2-ARE pathway. Phytomedicine 2018, 47, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Brintha, S.; Rajesh, S.; Renuka, R.; Santhanakrishnan, V.; Gnanam, R. Phytochemical analysis and bioactivity prediction of compounds in methanolic extracts of Curculigo orchioides Gaertn. J. Pharmacogn. Phytochem. 2017, 6, 192–197. [Google Scholar]
- Anifalaje, E.O.; Ibok, M.G. Chemical compositions and antioxidant activities of Albizia lebbeck L. essential oils. J. Essent. Oil-Bear. Plants 2020, 23, 810–820. [Google Scholar] [CrossRef]
- Shokrollahi, N.; Ho, C.-L.; Mohd Zainudin, N.A.I.; Abdul Wahab, M.A.w.B.; Wong, M.-Y. Plant defense inducers and antioxidant metabolites produced during oil palm-Ganoderma boninense interaction in vitro. Chem. Afr. 2023, 6, 499–511. [Google Scholar] [CrossRef]
- Gardner, D.R.; Panter, K.E.; Stegelmeier, B.L. Implication of agathic acid from Utah juniper bark as an abortifacient compound in cattle. J. Appl. Toxicol. 2010, 30, 115–119. [Google Scholar] [CrossRef]
- Hashimoto, T.; Rathore, H.; Satoh, D.; Griffin, J.F.; From, A.H.; Ahmed, K.; Fullerton, D.S.; Hong, G. Cardiac glycosides. 6. Gitoxigenin C16 acetates, formates, methoxycarbonates, and digitoxosides. Synthesis and Na+, K+-ATPase inhibitory activities. J. Med. Chem. 1986, 29, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Arora, S.; Singh, R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci. Rep. 2022, 12, 9370. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Asami, E.; Kitami, M.; Ida, T.; Kobayashi, T.; Saeki, M. Anti-inflammatory activity of 2-methoxy-4-vinylphenol involves inhibition of lipopolysaccharide-induced inducible nitric oxidase synthase by heme oxygenase-1. Immunopharmacol. Immunotoxicol. 2023, 45, 589–596. [Google Scholar] [CrossRef]
- Yang, S.; Liu, J.; Jiao, J.; Jiao, L. Ar-turmerone exerts anti-proliferative and anti-inflammatory activities in HaCaT keratinocytes by inactivating hedgehog pathway. Inflammation 2020, 43, 478–486. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef]
- Mohammad Shafiqur, R.; Jaripa, B.; JU, C.; MN, A. Antibacterial activity of 1, 2-benzenedicarboxylic acid, diisooctyl ester isolated from the root of Pumbago zeylanica linn. Hamdard Med. 2007, 50, 23–28. [Google Scholar]
- Shahid, A.; Khan, D.A.; Aati, H.Y.; Sherif, A.E.; Ovatlarnporn, C.; Hussain, M.; Rao, H.; Khan, M.I.; Younus, M.; Basit, A.; et al. Chemical profiling and biological activities of Dipterygium glaucum Decne.: An in-vivo, in-vitro and in-silico evaluation. S. Afr. J. Bot. 2023, 160, 715–730. [Google Scholar] [CrossRef]
- Aati, H.Y.; Anwar, M.; Al-Qahtani, J.; Al-Taweel, A.; Khan, K.-u.-R.; Aati, S.; Usman, F.; Ghalloo, B.A.; Asif, H.M.; Shirazi, J.H. Phytochemical profiling, in vitro biological activities, and in-silico studies of Ficus vasta Forssk.: An unexplored plant. Antibiotics 2022, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.M.; Mushtaq, A.; Jabeen, Q. Antimicrobial, antioxidant and calcium channel blocking activities of Amberboa divaricata. Bangladesh J. Pharmacol. 2014, 9, 29–36. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Nafees, M.; Barkatullah; Ullah, S.; Ikram, N. Phytochemical and pharmacognostic studies of Buddleja asiatica leaves. Microsc. Res. Tech. 2022, 85, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Barkatullah; Khan, B. Anatomy, micromorphology, and physiochemical analysis of Rhus succedanea var. himalaica root. Microsc. Res. Tech. 2020, 83, 424–435. [Google Scholar] [CrossRef]
- Farooq, M.; Shoaib, M.H.; Yousuf, R.I.; Qazi, F.; Hanif, M. Development of extended release loxoprofen sodium multiparticulates using different hydrophobic polymers. Polym. Bull. 2019, 76, 2537–2558. [Google Scholar] [CrossRef]
- WHO. Quality Control Methods for Herbal Materials; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Volume 15. [Google Scholar]
- Altemimi, A.; Watson, D.G.; Kinsel, M.; Lightfoot, D.A. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem. Cent. J. 2015, 9, 39. [Google Scholar] [CrossRef]
- Masood, N.; Jamil, Q.; Aslam, M.I.; Masood, M.I.; Shirazi, J.H.; Jamil, Q.A.; Jan, M.S.; Alsuwayt, B.; Ahmad, A.; Alnasser, S.M.A. Antioxidant, carbonic anhydrase inhibition and diuretic activity of Leptadenia pyrotechnica Forssk. Decne. Heliyon 2023, 9, e22485. [Google Scholar] [CrossRef]
- Ayaz, A.; Jamil, Q.; Hussain, M.; Anjum, F.; Sarfraz, A.; Alqahtani, T.; Hussain, N.; Gahtani, R.M.; Dera, A.A.; Alharbi, H.M. Antioxidant and gastroprotective activity of Suaeda fruticosa Forssk. Ex JF Gmel. Molecules 2022, 27, 4368. [Google Scholar] [CrossRef]
- Aslam, M.I.; Touqeer, S.; Jamil, Q.; Masood, M.I.; Sarfraz, A.; Khan, S.Y.; Jan, M.S.; Alnasser, S.M.A.; Ahmad, A.; Aslam, F.; et al. Cenchrus ciliaris L. ameliorates cigarette-smoke induced acute lung injury by reducing inflammation and oxidative stress. S. Afr. J. Bot. 2024, 171, 216–227. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Nawaz, I.; Tahir, A.; Iqbal, S.M.; Anjum, F.; Naseem, M.; Aslam, M.I.; Hussain, M.; Jamil, Q.A.; Shirazi, J.H.; Jamil, Q. Anti-inflammatory, anti-nociceptive and anti-pyretic activities of Cenchrus ciliaris L. J. Ethnopharmacol. 2023, 309, 116332. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Saleem, U.; Amin, S.; Ahmad, B.; Azeem, H.; Anwar, F.; Mary, S. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicol. Rep. 2017, 4, 580–585. [Google Scholar] [CrossRef]
- Iqbal, S.M.; Jamil, Q.; Jamil, N.; Kashif, M.; Mustafa, R.; Jabeen, Q. Antioxidant, antibacterial and gut modulating activities of Kalanchoe laciniata. Acta. Pol. Pharm. 2016, 73, 1221–1227. [Google Scholar]
- Konstantinovitch, K.k.Y.; Arsene, M.M.J.; Aliya, M.V.; Viktorovna, P.I.; Elena, V.G.; Azova, M.M.; Amira, A.A. Assessment of antimicrobial activity of ethanolic and aqueous extracts of Aesculus hippocastanum L. (horse chestnut) bark against bacteria isolated from urine of patients diagnosed positive to urinary tract infections. Front. Biosci. (Schol. Ed.) 2022, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Bank, P.D. Protein data bank. Nat. New Biol. 1971, 233, 223. [Google Scholar]
- Goodsell, D.S.; Sanner, M.F.; Olson, A.J.; Forli, S. The AutoDock suite at 30. Protein Sci. 2021, 30, 31–43. [Google Scholar] [CrossRef]
- Brown, T. ChemDraw. Sci. Teach. 2014, 81, 67. [Google Scholar]
- Ejaz, S.A.; Aziz, M.; Zafar, Z.; Akhtar, N.; Ogaly, H.A. Revisiting the inhibitory potential of protein kinase inhibitors against NEK7 protein via comprehensive computational investigations. Sci. Rep. 2023, 13, 4304. [Google Scholar] [CrossRef]
- Ejaz, S.A.; Aziz, M.; Fayyaz, A.; Wani, T.A.; Zargar, S. Computer-aided approach for the identification of lead molecules as the inhibitors of cholinesterase’s and monoamine oxidases: Novel target for the treatment of Alzheimer’s disease. J. Serb. Chem. Soc. 2023, 89, 441–442. [Google Scholar] [CrossRef]
- Aziz, M.; Ejaz, S.A.; Tamam, N.; Siddique, F.; Riaz, N.; Qais, F.A.; Chtita, S.; Iqbal, J. Identification of potent inhibitors of NEK7 protein using a comprehensive computational approach. Sci. Rep. 2022, 12, 6404. [Google Scholar] [CrossRef]





| Solvent | Value (%) |
|---|---|
| Distilled water | 10.5 |
| n-Butanol | 3 |
| Ethyl acetate | 2 |
| Dichloromethane | 1.5 |
| n-Hexane | 1 |
| Parameter | Value |
|---|---|
| Nutritional analysis | (%) |
| Crude protein | 12.39 |
| Crude fat | 1.10 |
| Crude fiber | 24.21 |
| Carbohydrate | 16.61 |
| Ash value | 14.52 |
| Moisture content | 31.17 |
| Caloric value | 111.43 (kcal/100 g) |
| Elemental content | (mg/kg) |
| Calcium | 470 |
| Cadmium | 0.1 |
| Chromium | 0.05 |
| Copper | 34.5 |
| Iron | 161 |
| Magnesium | 55.6 |
| Manganese | 5.5 |
| Reagents | Daylight | UV 254 nm |
|---|---|---|
| Powder as such | Greenish brown | Brown |
| Methanol | Brown | Dark brown |
| Ethyl acetate | Light green | Dark green |
| Dichloromethane | Dark green | Blue |
| Chloroform | Brown | Dark brown |
| n-Butanol | Dark brown | Blue |
| n-Hexane | Dark green | Blackish green |
| 50% HNO3 | Green | Pink |
| Picric acids | Yellowish | Brown |
| 50% HCl | Green | Light pink |
| Conc. H2SO4 | Black | Dark brown |
| 1% NaOH | Green | Dark green |
| Iodine | Light brown | Blue |
| 5% FeCl3 | Yellow | Dark brown |
| Extract | Solvent | Ratio | Rf Value |
|---|---|---|---|
| n-Hexane | H:E.A | 1:1 | 0.34, 0.55, 0.67, 0.85 |
| H:E.A | 3:1 | 0.15 | |
| Dichloromethane | CH:M | 4:1 | 0.50, 0.62, 0.70, 0.90 |
| H:E.A | 1:1 | 0.28, 0.52 | |
| Ethyl acetate | H:E.A | 1:1 | 0.544, 0.68, 0.85 |
| CH:M | 4:1 | 0.11, 0.26, 0.34 | |
| n-Butanol | CH:M | 4:1 | 0.10, 0.16, 0.51, 0.63, 0.85 |
| H:E.A | 1:1 | 0.23, 0.50, 0.59, 0.66 | |
| Aqueous | CH:M | 4:1 | 0.88 |
| Crude | E.A:B:AA:W | 16:2:1:1 | 0.08, 0.94 |
| Fraction | TPC (mg TAEg−1) | TTC (mg TAEg−1) | TFC (mg QEg−1) |
|---|---|---|---|
| n-Hexane | 36.00 ± 2.9 | 3.99 ± 0.48 | 24.089 ± 3.952 |
| Dichloromethane | 303.48 ± 25.28 | 190.95 ± 37.26 | 106.223 ± 3.437 |
| Ethyl acetate | 419.10 ± 11.76 | 249.60 ± 10.50 | 161.790 ± 1.375 |
| n-Butanol | 296.07 ± 10.14 | 156.97 ± 29.47 | 109.658 ± 9.107 |
| Aqueous | 169.86 ± 12.08 | 104.17 ± 26.93 | 47.973 ± 0.344 |
| Crude | 214.27 ± 14.98 | 107.50 ± 30.27 | 86.943 ± 5.670 |
| Wave Number (cm−1) | Intensity of Estimation | Group or Functional Group Class |
|---|---|---|
| 2914.8 | S | O-H stretching (alcohol) |
| M | C-H stretching (alkane) | |
| 2847.7 | M | C-H stretching (alkane) |
| 1735.1 | W | C-H bending (aromatic compound) |
| 1638.2 | M | C=C stretching (conjugated alkene) |
| 1364.2 | M | O-H bending (alcohol) |
| 1313.9 | S | S=O stretching (sulfone) |
| M | O-H bending (phenol) | |
| 1239.3 | S | C-O stretching (alkyl aryl ether) |
| M | C-N stretching (amine) | |
| 1099.6 | S | C-O stretching (secondary alcohol) |
| 1019.4 | S | C=C bending (alkene) |
| 896.4 | M | C=C bend (alkenes like vanylidine) |
| 777.1 | S | C-Cl stretching (halo compound) |
| Sr. NO | RT | Area | Compound | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|
| NB1 | 3.92 | 1.10 | 6-Methoxy-2-phenyl-hexahydropyrano[2,3-b][1,3]dioxine-7,8 diol | C14H18O6 | 282.29 |
| NB2 | 4.28 | 0.17 | 2,5-Dihydro-5-methoxy-2-furanone | C5H6O3 | 114.10 |
| NB3 | 7.28 | 0.9 | 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl)methyl ester, cis | C28H44O4 | 444 |
| NB4 | 9.62 | 0.15 | Acetamide, N-methyl-N-[4-[2-acetoxymethyl-1-pyrrolidyl]-2-butynyl]- | C14H22N2O3 | 266.33 |
| NB5 | 10.50 | 1.28 | Tetradecane | C14H30 | 198.38 |
| NB6 | 12.01 | 0.49 | Dodecane, 5,8-diethyl- | C16H34 | 226.44 |
| NB7 | 12.31 | 0.20 | 2H-indeno[1,2-b]furan-2-one, 3,3a,4,5,6,7,8,8b-octahydro-8,8-dimethyl | C13H18O2 | 206.28 |
| NB8 | 12.92 | 0.74 | Pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester | C16H30O4 | 286.40 |
| NB9 | 13.43 | 5.20 | Hexadecane | C16H34 | 226.44 |
| NB10 | 13.92 | 3.16 | N-Acetyl-4-phenylbutylamine | C12H17NO | 191 |
| NB11 | 15.20 | 0.77 | Myristyl monoethoxylate | C16H34O2 | 258.44 |
| NB12 | 15.92 | 0.15 | 2(3H)-Furanone, dihydro-5-tetradecyl | C18H34O2 | 282.46 |
| NB13 | 16.18 | 0.16 | trans-1,2-diaminocyclohexane-N,N,N,N-tetraacetic acid | C14H22N2O8 | 346 |
| NB14 | 16.51 | 0.14 | 17-Pentatriacontene | C35H70 | 490.93 |
| NB15 | 16.81 | 3.39 | Bacteriochlorophyll-c-stearyl | C52H72MgN4O4 | 841.5 |
| NB16 | 17.20 | 0.77 | 2- Nonadecanone 2,4- dinitrophenylhydrazine | C25H42N4O4 | 462 |
| NB17 | 17.83 | 2.38 | 1-Docosanol | C22H46O | 326.6 |
| NB18 | 18.22 | 0.68 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | C17H24O3 | 276.37 |
| NB19 | 18.79 | 8.08 | Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | C35H68O5 | 568.91 |
| NB20 | 20.56 | 0.11 | 3-Pyridinecarboxylic acid, 2,7,10-tris(acetyloxy)-1,1a,2,3,4,6,7,10, 11,11a-decahydro-1,1,3,6,9-pentamethyl-4-oxo-4a,7a-epoxy-5H-cyclopenta[a]cyclo propa [f]cycloundecen-11-yl ester, [1aR(1aR*,2R*,3S*,4aR*,6S*,7S*,7aS*,8E,10R*,11R*,11aS*)]- | C32H39NO10 | 597.25 |
| NB21 | 21.05 | 1.77 | Dodecanecarboxamide, N-[2-(3-indolyl)ethyl]- | C22H34N2O | 342 |
| NB22 | 21.40 | 1.86 | Erucic acid | C22H42O2 | 338.57 |
| NB23 | 21.99 | 0.18 | 18,19-Secoyohimban-19-oic acid, 16,17,20,21-tetradehydro-16-(hydroxy methyl)-, methyl ester, (15α,16E)- | C21H24N2O3 | 352 |
| NB24 | 22.31 | 48.18 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | C24H38O4 | 390 |
| NB25 | 23.62 | 2.08 | Squalene | C30H50 | 410 |
| NB26 | 23.92 | 0.22 | 24,25-Dihydroxycholecalciferol | C27H44O3 | 416 |
| NB27 | 24.29 | 0.76 | Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) | C22H28N2O3 | 368 |
| NB28 | 25.0 | 0.54 | 9,12,15-Octadecatrienoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester, (Z,Z,Z)- | C27H52O4Si2 | 496 |
| NB29 | 26.04 | 5.91 | Azafrin | C27H38O4 | 426 |
| NB30 | 27.8 | 0.13 | Butanoic acid,1a,2,5,5a,6,9,10,10a-octahydro-5,5a-di hydroxy-4-(hydroxymethyl)-1,1,7,9-tetra methyl-11-oxo-1H-2,8a-methanocyclop enta[a]cyclopropa[e]cyclodecen-6-yl ester | C24H34O6 | 418 |
| Sr. No | RT | Area | Compound | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|
| EA1 | 12.0 | 0.15 | Octadecane, 3-ethyl-5-(2-ethylbutyl)- | C26H54 | 366.70 |
| EA2 | 12.80 | 0.59 | Illudol | C15H24O3 | 252.35 |
| EA3 | 13.45 | 3.16 | Hexadecane | C16H34 | 226.44 |
| EA4 | 14.29 | 0.95 | 2-Naphthalenemethanol, decahydro-α,α,4a-trimethyl-8-methylene-, [2R-(2α,4aα,8aα)]- | C15H26O | 222.36 |
| EA5 | 15.02 | 1.58 | Isoaromadendrene epoxide | C15H24O | 220.35 |
| EA6 | 15.53 | 0.40 | Alpha-Santanol acetate | C17H26O2 | 262.38 |
| EA7 | 16.10 | 0.12 | 17-Pentatriacontene | C35H70 | 490.93 |
| EA8 | 16.44 | 0.46 | Acetate, [6-(acetyloxy)-5,5,8a-trimethyl-2-methyleneperhydro-1-naphthalenyl]methyl ester | C19H30O4 | 322.00 |
| EA9 | 17.06 | 12.11 | Bicyclo[2.2.2]octa-2,5-diene, 1,2,3,6-tetramethyl- | C12H18 | 162.27 |
| EA10 | 17.61 | 2.97 | 5-Hydroxymethyl-1,1,4a-trimethyl-6-methylenedecahydronaphthalen-2-ol | C15H26O2 | 238.37 |
| EA11 | 17.89 | 3.36 | Benzene, hexamethyl- | C12H18 | 162.27 |
| EA12 | 19.01 | 1.05 | Gitoxigenin | C23H34O5 | 390.50 |
| EA13 | 19.56 | 55.80 | 4,4-Dimethyladamantan-2-ol | C12H20O | 180.29 |
| EA14 | 20.17 | 0.11 | Androst-4-en-3-one, 9-fluoro-11,17-dihydroxy-17-methyl-, (11α,17α)- | C20H29FO3 | 336.40 |
| EA15 | 20.62 | 1.65 | Methyl 3-(acetyloxy)-7,12-dioxocholan-24-oate | C27H40O6 | 460.00 |
| EA16 | 21.01 | 0.73 | Agathic acid | C20H30O4 | 334.40 |
| EA17 | 22.27 | 5.58 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | C24H38O4 | 390.00 |
| EA18 | 22.98 | 0.48 | 2,7-Diphenyl-1,6-dioxopyridazino[4,5:2′,3′]pyrrolo [4′,5′-d]pyridazine | C20H13N5O2 | 355.30 |
| EA19 | 23.98 | 0.67 | 9-Octadecene, 1-[2-(octadecyloxy)ethoxy]- | C38H76O2 | 565.00 |
| EA20 | 24.43 | 0.17 | 5aH-3a,12-methano-1H-cyclopropa[5′,6′]cyclodeca [1′,2′:1,5]cyclopenta[1,2-d][1,3]dioxol-13-one, 1a,2,3,9,12,12a-hexahydro-9-hydroxy-10 -(hydroxymethyl)-1,1,3,5,7,7-hexamethyl-, [1aR-(1aα,3α,3aα,5aα,8aR*,9α,12α,12aα)]- | C23H32O5 | 388.50 |
| EA21 | 24.43 | 0.17 | Betulin | C30H50O2 | 442.70 |
| EA22 | 24.72 | 0.13 | 24,25-Dihydroxycholecalciferol | C27H44O3 | 416.00 |
| EA23 | 25.86 | 2.47 | Stigmasterol | C29H48O | 412.70 |
| EA24 | 26.63 | 0.63 | 2,2,3,5,5-Pentachloro-7,7-bis(chloromethyl)-1 (dichloromethyl)bicyclo[2.2.1]heptane | C10H9Cl9 | 448.20 |
| EA25 | 27.30 | 0.68 | 9,12,15-Octadecatrienoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester, (Z,Z,Z)- | C27H52O4Si2 | 496.90 |
| Sr. No | RT | Area | Compound | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|
| NH1 | 4.25 | 0.54 | 5-Methylfurfural | C6H6O2 | 110.11 |
| NH2 | 8.13 | 16.94 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.17 |
| NH3 | 10.16 | 2.59 | 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | C13H18O | 190.28 |
| NH4 | 11.31 | 2.99 | Megastigmatrienone | C13H18O | 190.28 |
| NH5 | 12.23 | 0.65 | Ar-turmerone | C15H20O | 216.31 |
| NH6 | 15.06 | 1.58 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.45 |
| NH7 | 15.25 | 4.41 | n-Hexadeconic acid | C16H32O2 | 256.42 |
| NH8 | 15.91 | 0.28 | Hexadeconic acid, ethyl ester | C18H36O2 | 284.47 |
| NH9 | 17.26 | 1.09 | 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | 294.47 |
| NH10 | 17.35 | 2.91 | 9,12,15-Octadecatrienoic acid | C18H30O2 | 278.40 |
| NH11 | 17.96 | 8.58 | 9,12,15-Octadecatrien-1-ol, (Z,Z,Z)- | C18H32O | 264.44 |
| NH12 | 18.18 | 0.59 | Linoleic acid ethyl ester | C20H36O2 | 308.49 |
| NH13 | 18.26 | 0.89 | Ethyl Oleate | C20H38O2 | 310.51 |
| NH14 | 23.69 | 0.49 | 1,2-Benzenedicarboxylic acid, monopentyl ester | C13H16O4 | 236.26 |
| Fractions | Conc. | B. subtilis | S. aureus | P. aeruginosa | S. typhi | E. coli |
|---|---|---|---|---|---|---|
| (mg/mL) | Mm | |||||
| n-Hexane | 10 | 6 | 10.5 | 8.5 | 6 | 10.5 |
| 30 | 12 | 9 | 9 | 8.5 | 15 | |
| 100 | 11.5 | 14 | 9.5 | 9 | 12.5 | |
| Dichloromethane | 10 | 9 | 7.5 | 7.5 | 4.5 | 4 |
| 30 | 9.5 | 11 | 8 | 9 | 13 | |
| 100 | 13 | 12 | 11.5 | 10.5 | 13 | |
| Ethyl acetate | 10 | 10 | 9.5 | 8.5 | 8 | 5 |
| 30 | 15.5 | 11.5 | 9 | 9.5 | 8.5 | |
| 100 | 13 | 14.5 | 10.5 | 9.5 | 27 | |
| n-Butanol | 10 | 9 | 8 | 5 | 9 | 9.5 |
| 30 | 10.5 | 9 | 7.5 | 4.5 | 11.5 | |
| 100 | 13 | 10.5 | 11.5 | 11 | 14 | |
| Aqueous | 10 | 7.5 | 9.5 | 8.5 | 9.5 | 9.5 |
| 30 | 8 | 13.5 | 7 | 7 | 5.5 | |
| 100 | nil | 9.5 | 7.5 | 6.5 | 9 | |
| Crude | 10 | 11 | 8.5 | 7 | 5 | 8.5 |
| 30 | 11 | 9 | 4.5 | 8 | 10.5 | |
| 100 | 14.5 | 11 | 9 | 8 | 12.5 | |
| Ceftriaxone | 1 | 15 | 16 | 7.5 | 11 | 15.5 |
| Fractions | B. subtilis | S. aureus | P. aeruginosa | S. typhi | E. coli |
|---|---|---|---|---|---|
| mg/mL | |||||
| n-Hexane | >100 | >100 | >100 | >100 | Nil |
| Dichloromethane | 3.91 | 7.81 | 3.91 | 62.5 | 31.25 |
| Ethyl acetate | 1.95 | 3.91 | 3.91 | 7.81 | 3.91 |
| n-Butanol | 3.91 | 31.25 | 3.91 | 31.25 | 3.91 |
| Aqueous | 31.25 | 62.5 | 31.25 | 62.5 | 62.5 |
| Crude | 31.25 | 31.25 | 31.25 | 62.5 | 62.5 |
| Ceftriaxone | 1.95 | 3.91 | 0.98 | 0.98 | 7.81 |
| Compounds | B. subtilis | E. coli | P. aeruginosa | S. aureus | S. typhi |
|---|---|---|---|---|---|
| kcal/mol | |||||
| NB1 | −6.6 | −7.3 | −8.0 | −7.5 | −7.5 |
| NB7 | −5.7 | −7.4 | −7.4 | −7.0 | −6.4 |
| NB26 | −7.2 | −6.6 | −8.4 | −7.8 | −7.3 |
| NB27 | −6.3 | −6.5 | −7.8 | −7.3 | −7.5 |
| EA10 | −5.7 | −5.4 | −6.3 | −7.2 | −6.2 |
| EA12 | −7.5 | −7.5 | −8.4 | −7.8 | −8.6 |
| EA15 | −6.6 | −7.1 | −8.8 | −8.0 | −8.3 |
| EA16 | −6.6 | −7.1 | −7.6 | −7.3 | −7.5 |
| EA17 | −4.2 | −4.4 | −5.3 | −6.4 | −5.6 |
| EA18 | −8.2 | −8.5 | −9.1 | −9.2 | −9.4 |
| EA20 | −7.5 | −7.4 | −8.7 | −8.1 | −9.3 |
| EA21 | −7.4 | −6.6 | −7.5 | −7.7 | −8.6 |
| EA22 | −6.8 | −6.0 | −7.3 | −7.4 | −7.0 |
| EA23 | −7.5 | −7.1 | −8.3 | −7.8 | −8.1 |
| NH1 | −4.2 | −4.5 | −4.8 | −4.6 | −4.2 |
| NH2 | −4.8 | −5.5 | −5.9 | −5.5 | −5.0 |
| NH3 | −5.5 | −5.9 | −6.4 | −6.4 | −5.5 |
| NH4 | −5.8 | −6.1 | −6.8 | −6.7 | −5.8 |
| NH14 | −5.0 | −6.2 | −6.4 | −5.8 | −5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjum, F.; Touqeer, S.; Khan, M.Y.; Jamil, Q.; Rida, A.; Shirazi, J.H.; Ejaz, S.A.; Attaullah, H.M.; Sarwar, G.; Khan, Z.H.; et al. Pharmacognostic Evaluation, Chemical Characterization, and Antibacterial Activity of Bassia indica (Wight) A.J. Scott. Plants 2024, 13, 1753. https://doi.org/10.3390/plants13131753
Anjum F, Touqeer S, Khan MY, Jamil Q, Rida A, Shirazi JH, Ejaz SA, Attaullah HM, Sarwar G, Khan ZH, et al. Pharmacognostic Evaluation, Chemical Characterization, and Antibacterial Activity of Bassia indica (Wight) A.J. Scott. Plants. 2024; 13(13):1753. https://doi.org/10.3390/plants13131753
Chicago/Turabian StyleAnjum, Fayyaz, Saad Touqeer, Muhammad Younus Khan, QurratUlAin Jamil, Ayesha Rida, Jafir Hussain Shirazi, Syeda Abida Ejaz, Hafiz Muhammad Attaullah, Ghulam Sarwar, Zaeem Hayat Khan, and et al. 2024. "Pharmacognostic Evaluation, Chemical Characterization, and Antibacterial Activity of Bassia indica (Wight) A.J. Scott" Plants 13, no. 13: 1753. https://doi.org/10.3390/plants13131753
APA StyleAnjum, F., Touqeer, S., Khan, M. Y., Jamil, Q., Rida, A., Shirazi, J. H., Ejaz, S. A., Attaullah, H. M., Sarwar, G., Khan, Z. H., Wazir, M. A., Malik, B., Aufy, M., & Iqbal, S. M. (2024). Pharmacognostic Evaluation, Chemical Characterization, and Antibacterial Activity of Bassia indica (Wight) A.J. Scott. Plants, 13(13), 1753. https://doi.org/10.3390/plants13131753







