Abstract
Fungi as heterotrophs are key participants in the decomposition of organic materials and the transformation of nutrients in agroecosystems. Ditch-buried straw return as a novel conservation management strategy can improve soil fertility and alter hydrothermal processes. However, how ditch-buried straw return strategies affect the soil fungal community is still unclear. Herein, a 7-year field trial was conducted to test the influences of burial depth (0, 10, 20, 30, and 40 cm) and the amount of ditch-buried straw (half, full, double) on the diversity, composition, and predicted functions of a soil fungal community, as well as the activities of carbon-degraded enzymes. Under the full amount of straw burial, the abundance of phylum Ascomycota was 7.5% higher as compared to other burial amount treatments. This further increased the activity of cellobiohydrolase by 32%, as revealed by the positive correlation between Ascomycota and cellobiohydrolase. With deeper straw burial, however, the abundance of Ascomycota and β-D-glucopyranoside activity decreased. Moreover, genus Alternaria and Fusarium increased while Mortierella decreased with straw burial amount and depth. FUNgild prediction showed that plant fungal pathogens were 1- to 2-fold higher, whilst arbuscular mycorrhizal fungi were 64% lower under straw buried with double the amount and at a depth of 40 cm. Collectively, these findings suggest that ditch-buried straw return with a full amount and buried at a depth less than 30 cm could improve soil nutrient cycles and health and may be beneficial to subsequent crop production.
1. Introduction
Straw return is an important practice for increasing soil organic matter in agroecosystems [1,2]. This can improve soil structure and diversify nutrient supply [3,4]. Soil microorganisms play important roles in the degradation of straw through releasing cellulase and ligninase [5,6]. Simultaneously, the returned straw supplies plentiful carbon and energy for soil microorganisms, thus promoting their growth and activity [7,8,9]. Since soil microbial community structure has been widely used as an indicator of soil quality [10,11], it deserves more efforts to decipher how straw incorporation influences soil microbial communities and functioning.
As one of the largest contributors to global biomass, soil-inhabiting fungi perform crucial roles in sustaining soil health, nutrient cycling, and crop productivity in terrestrial ecosystems [12,13]. For instance, symbiotic fungi could stimulate nutrient transfer, e.g., nitrogen and phosphorus to crops from the soils beyond the rhizosphere [14,15]. Fungal decomposers could recycle soil organic matter and mineral nutrients [16]. However, pathogenic fungi would passively influence plant growth and subsequently productivity [17]. Given that soil fungi are sensitive to anthropogenic disturbances such as tillage and straw incorporation [18,19], variations in soil fungal community structure may therefore influence their functioning, such as soil organic matter decomposition and nutrient utilization, as well as the acquisition by crops, which can directly affect soil health and crop productivity [20,21].
Soil fungi can excrete a comprehensive set of enzymes to degrade organic materials and thus play a major role in the recycling and reutilization of straw residues [22,23]. Yet, there are few studies focusing on fungal ecology and functioning in response to straw return practices. For example, Huang et al. [24] reported that soil fungal diversity was increased by straw returning, and a larger enhancement was observed at a higher rate of returned straw. Soil fungal community changed after rice straw incorporation, particularly for cellulose-decomposing fungi, which became dominant [25,26]. Although straw carries plant-parasitic fungi [27], ditch-buried straw return increased the abundance of symbiotic fungi, whilst decreasing the pathotrophic fungal abundance [28]. Despite these findings, it is not well known how fungal community composition and functional guilds change with different straw return strategies.
Rice–wheat rotation is the major farming system in Eastern China, with an annual wheat and rice yield of ~4500 kg ha−1 and ~9000 kg ha−1, respectively [29]. The effective management of straw residues is therefore quite important for the development of sustainable agriculture. Ditch-buried straw return (DB-SR) is a novel conservation management strategy that can combine deep ploughing (10% ditching) and no till (90% without ditching) into one rotational tillage regime in the same cropping season [30]. Our previous findings demonstrated that DB-SR can enhance soil carbon accumulation and nutrient availability, alter hydrothermal processes, and improve crop growth and yield performance [30]. However, how different DB-SR strategies, e.g., burial amount and depth, affect soil fungal community structure and functioning is still unclear. We therefore conducted two 7-year field experiments with different burial amounts and depths in a rice–wheat double cropping system under DB-SR practice in the eastern China. We hypothesized that (1) with a greater amount of straw returned to the soils, fungal community structure would be altered and then stimulate the production of C-degraded enzymes; (2) with deeper buried straw, more pathogenic fungi would be stimulated. We analyzed the diversity, composition, functional guilds, and C-degrading enzymatic activity of soil fungal communities.
2. Materials and Methods
2.1. Site Description
A field experiment was performed at the Nantong Academy of Agricultural Sciences, Jiangsu Province (32°13′ N, 120°63′ E). The region belongs to a subtropical monsoonal climate, with an average annual temperature and precipitation of 14.4 °C and 1057 mm, respectively. Before starting the experiment, soil properties were as follows: bulk density = 1.4 g cm−3, pH = 6.7, soil organic C (SOC) = 11.86 g kg−1, total nitrogen (TN) = 1.62 g kg−1, available phosphorus (AP) = 12.76 mg kg−1.
2.2. Experimental Design
2.2.1. Experiment 1: Straw Burial Depth
A field experiment was started in November 2008 with a random block design, including five treatments: straw removal (Control) and ditch-buried straw return to depths of 10 cm, 20 cm, 30 cm, and 40 cm. Each treatment had 3 replications, yielding 15 plots in total with a size of 18 m2 (3 × 6 m). For ditch-buried treatments, straw ditches were first manually created to corresponding depths (10 cm, 20 cm, 30 cm, and 40 cm) with a width of 20 cm and interval of 2 m between adjacent ditches. The intact straws were then manually returned to the bottom of the ditches and covered with soil from ditching. The amount of straw returned to rice and wheat was 9000 kg ha−1 and 4500 kg ha−1, respectively.
2.2.2. Experiment 2: Straw Burial Amount
A field experiment was started in November 2008 with random block design which contained three treatments: straw removal (Control) and DB-SR with half straw amount (H), full straw amount (F), and double straw amount (D). Each treatment was replicated 3 times, yielding 12 plots in total with a size of 18 m2 (3 × 6 m). All treatments were conducted at a burial depth of 20 cm. Half straw amounts were 4500 kg ha−1 for rice and 2250 kg ha−1 for wheat. Full straw amounts were 9000 and 4500 kg ha−1 for rice and wheat, respectively, which represented the whole amount of straw residues produced at the current farming level. Double straw amounts were 18,000 kg ha−1 for rice and 9000 kg ha−1 for wheat, which were designed for the future high or superhigh yielding level. The agronomic managements were similar between the two field experiments except for straw practices. The rice variety was Nangeng 5055, and the wheat variety was Yangmai 13. The applied amounts of N fertilizer were 300 and 180 kg ha−1 for rice and wheat seasons, respectively. The P fertilizer (P2O5) was applied at rates of 180 and 80 kg ha−1 for rice and wheat seasons, and the K fertilizer (K2O) was applied at rates of 250 and 150 kg ha−1 for rice and wheat seasons, respectively.
2.3. Soil Sample and Measurements
Soil was collected from top 10 cm depth in May 2015. The location of the straw ditch was first labeled. Soil cores were sampled at the middle position of each straw ditch, and then mixed thoroughly as one composite sample. Soils were passed through a 2 mm sieve to remove undecomposed straws, root debris, and small stones and then separated into three parts. One sub-sample was stored at −20 °C for the determination of DNA sequencing; the second part was stored at 4 °C for NO3− and NH4+ measurement; and the other sub-sample was air-dried to determine pH, AP, and AK. The contents of soil NO3− and NH4+ were analyzed using a San++ Continuous Flow Analyzer (Skalar, Breda, The Netherlands). The concentration of soil AP was determined by the molybdenum blue colorimetric assay, and the concentration of AK was determined by flame photometry.
2.4. Enzyme Activity Analysis
The activities of β-D-Glucosidase, cellobiohydrolase, and peroxidase were determined according to the methods described by Tiemann and Grandy [31]. Briefly, one gram of fresh soil was mixed with buffer (pH = 6.5), and labile substrates were added to the buffer to incubate at 25 °C for 18 h before analyzing fluorescence for β-D-Glucosidase and cellobiohydrolase, as well as determining absorbance for peroxidase on a Synergy HT plate reader (BioTek, Winooski, VT, USA).
2.5. DNA Extraction
The DNA was extracted from 0.5 g of each soil using a Fast DNA SPIN Kit for Soil (Illumina, San Diego, CA, USA). DNA quality was tested by 1.5% agarose gel electrophoresis in 1× TAE buffer, and the concentration of DNA was determined using an ND-1000 spectrophotometer (Nanodrop Technology, Wilmington, NC, USA). The extracted DNA was stored at −80 °C before PCR amplification. The fungal ITS rDNA gene was amplified with the primer pair ITS1F (5′-barcode-CTTGGTCATTTAGAGG AAGTAA-3′) and 2043R (5′-GCTGCGTTCT TCATCGATGC-3′) [32]. For detailed determination, please see the Supplementary Information. Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1, http://drive5.com/uparse/ (accessed on 20 January 2024)), and chimeric sequences were identified and removed using UCHIME v.4.2.40 [33].
2.6. Statistical Analysis
After confirming the normal distribution and homogeneity of variances, the analysis of variance (ANOVA) was performed to compare the means of variables among treatments using SPSS v.19.1 software (SPSS Inc., Chicago, IL, USA). Principal coordinate analysis (PCoA) was used to illustrate the dissimilarities among fungal communities using Bray–Curtis distance constructed on the OTU table, which was performed using the VEGAN package in R (version 4.1.3). To evaluate the relationships between fungal community structure and soil physicochemical properties, redundancy analysis (RDA) was conducted in Canoco v5.0. The ecological function categories of fungi were predicted with the FUNGuild database (https://github.com/UMNFuN/FUNGuild (accessed on 20 January 2024)) based on relative abundance at the OTU level. This step was performed on the FUNGuild website. According to the results of the confidence assessment of the FUNGuild database, only the confidence levels “highly probable” and “probable” were used for subsequent analysis.
3. Results
3.1. Soil Fungal Community Diversity and Composition
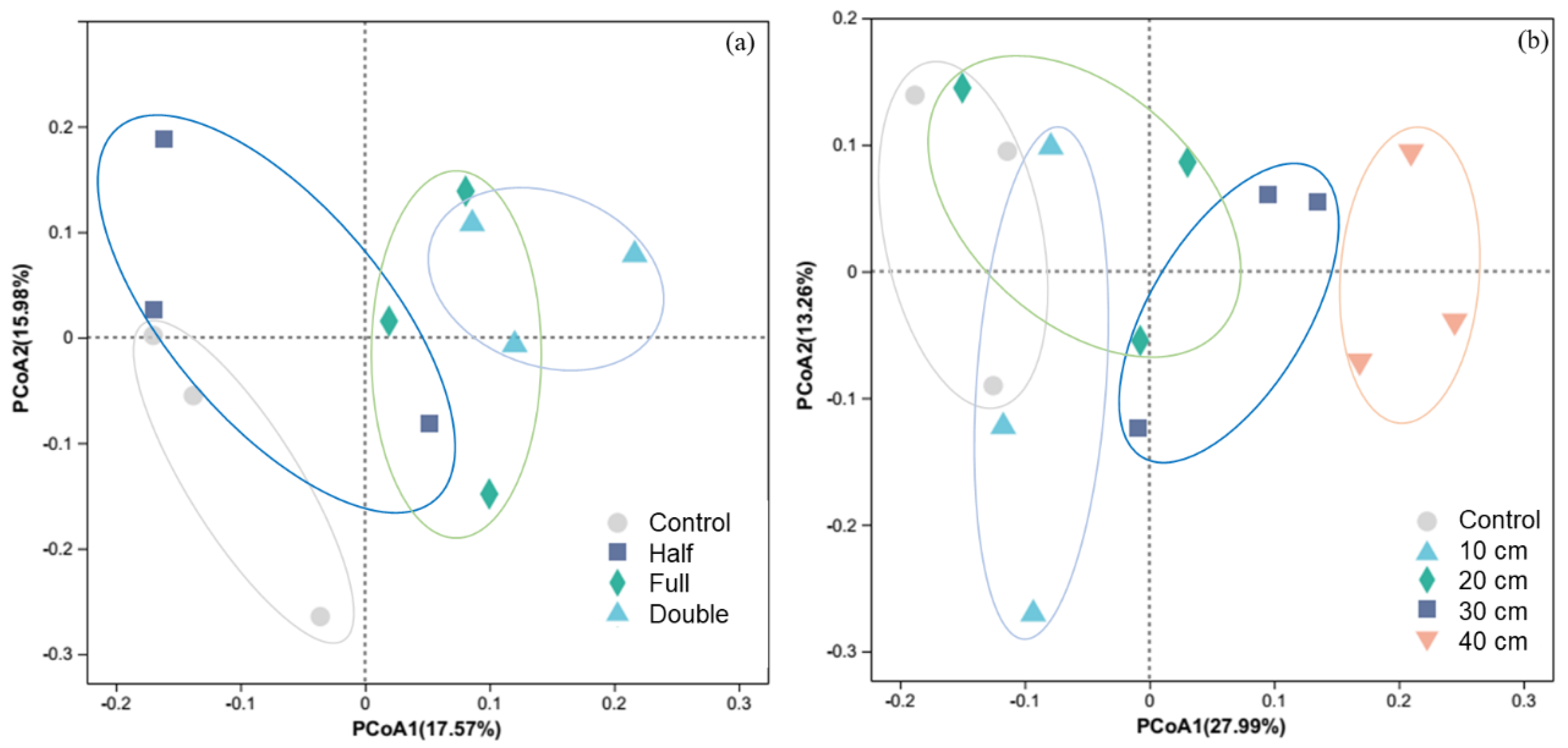
Regardless of DB-SR amount or depth, soil fungal diversity was not affected (p > 0.05, Figure S1). However, PCoA results showed that soil fungal community composition under DB-SR with variable amounts differed from control (p < 0.05, Figure 1a). Similarly, straw burial depth at 30 and 40 cm altered soil fungal community composition in relative to burial depth at 10 and 20 cm (p < 0.05, Figure 1b).
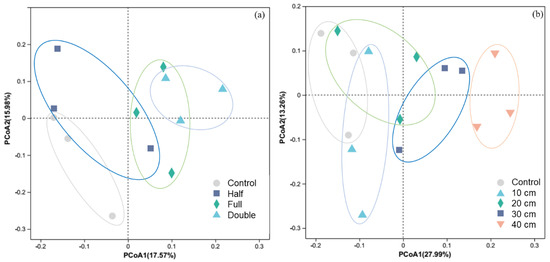
Figure 1.
Principal coordinate analysis (PCoA) of soil fungi community structure in response to straw return amount (a) and buried depth (b). Control, no tillage and no straw return; 10 cm, straw return with full amount return at a depth of 10 cm; 20 cm, straw return with full amount return at a depth of 20 cm; 30 cm, straw return with full amount return at a depth of 30 cm; 40 cm, straw return with full amount return at a depth of 40 cm; Half, straw return with half amount; Full, straw return with full amount; Double, straw return with double amount.
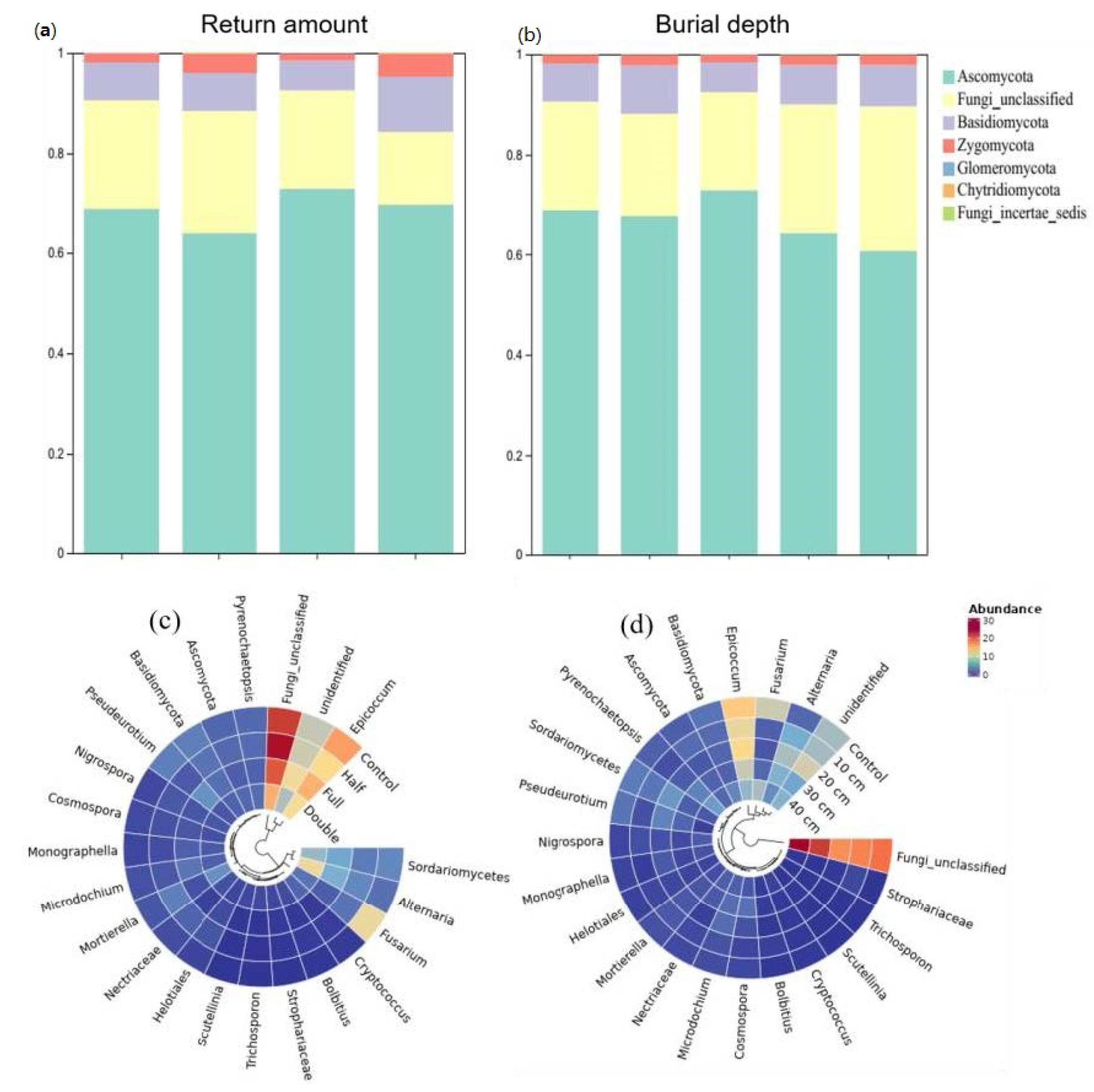
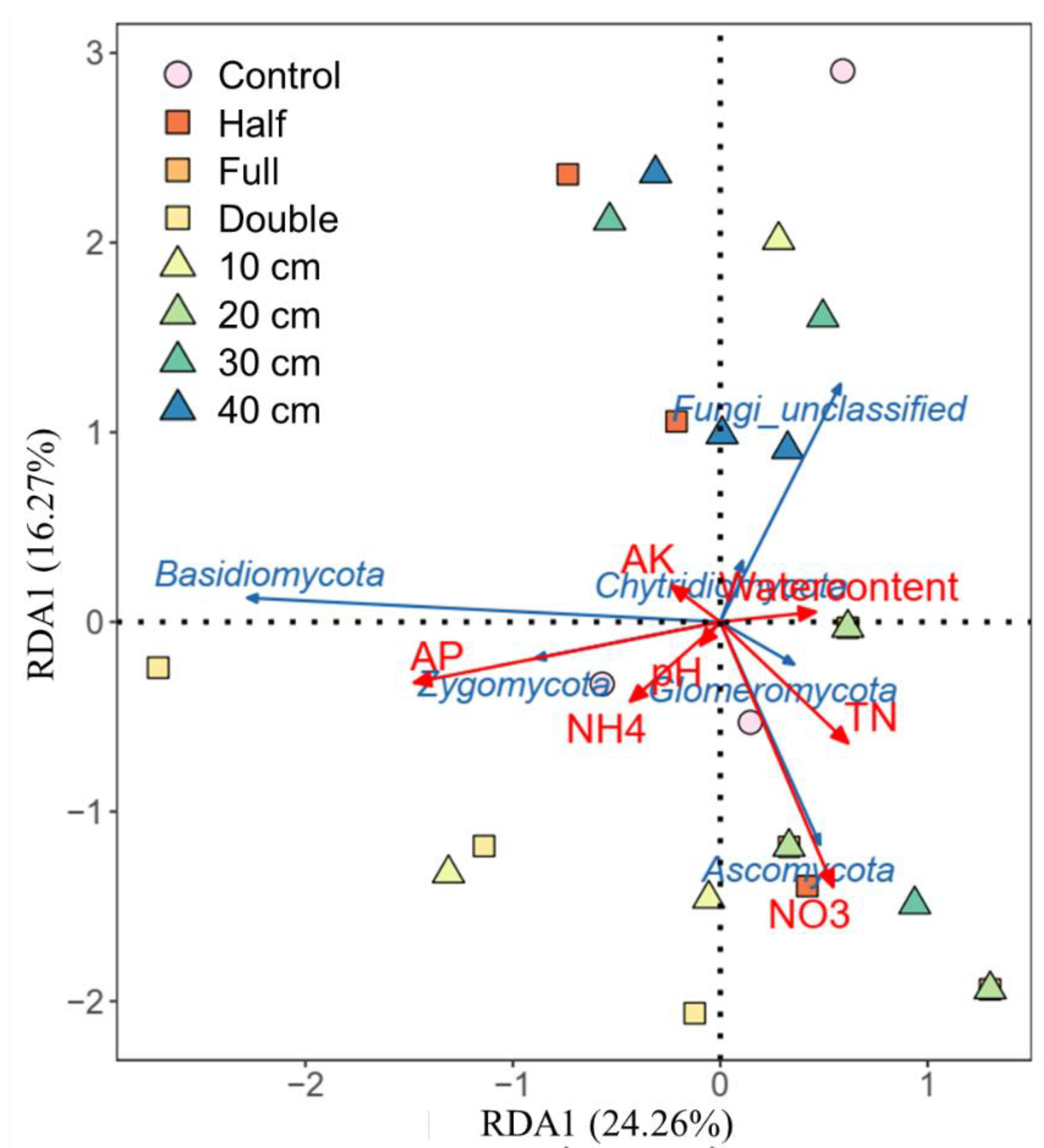
Compared to control, straw buried at 20 cm increased the relative abundance of Ascomycota by 8.2% and Basidiomycota by 62% (p < 0.05, Figure 2a). The relative abundance of Zygomycota was 25%, 16%, and 18% higher under straw buried at 10, 30, and 40 cm than that under control, respectively. Also, straw buried at 20 cm decreased the abundance of Basidiomycota and Zygomycota but increased the abundance of Ascomycota relative to other straw burial depth treatments. In addition, straw buried at 10, 20, 30, and 40 cm increased the abundance of genus Microdochium and Nigrospora, while it decreased the relative abundance of Epicoccum, Fusarium, and Humicola (Figure 2c). Relative to control, straw with a half amount increased the abundance of Ascomycota by 7.5% but decreased Zygomycota by 133% (p < 0.05, Figure 2b). On the contrary, straw with double amount increased the abundance of Basidiomycota but decreased Zygomycota. Compared to half and double straw return treatments, DB-SR-F increased the abundance of Ascomycota while it decreased Basidiomycota and Chytridiomycota (p < 0.05). Moreover, NO3- and TN were positively correlated with Ascomycota and Glomeromycota, and AP was positively correlated to Basidiomycota and Zygomycota (p < 0.05, Figure 3).
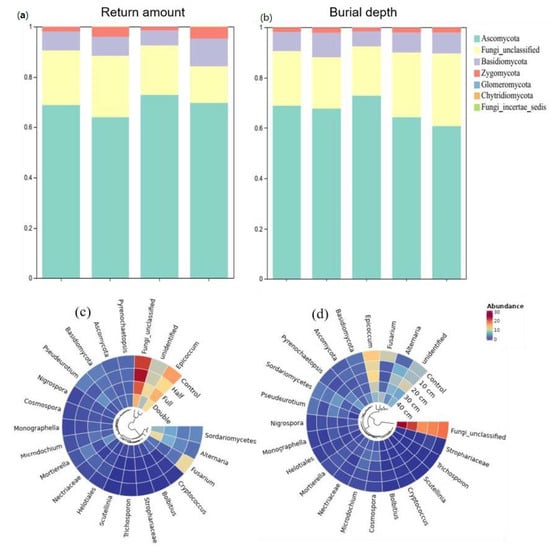
Figure 2.
Relative abundances of soil fungi community composition under different straw returned amounts and burial depth managements at the phylum (a,c) and genus levels (b,d). Control, no tillage and no straw return; 10 cm, straw return with full amount return at a depth of 10 cm; 20 cm, straw return with full amount return at a depth of 20 cm; 30 cm, straw return with full amount return at a depth of 30 cm; 40 cm, straw return with full amount return at a depth of 40 cm; Half, straw return with half amount; Full, straw return with full amount; Double, straw return with double amount.
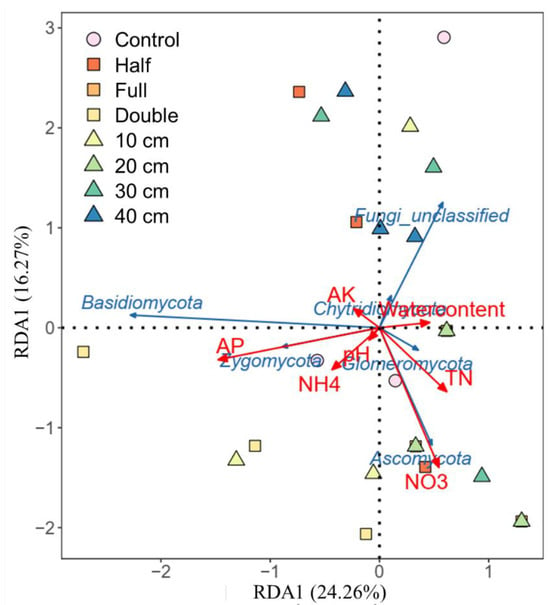
Figure 3.
Effects of soil chemical properties on soil fungi community composition based on distance-based redundancy analysis (RDA) in response to straw management practices. TN, total N; NH4, Ammonia N; NO3, nitrate N; AP, available phosphorus; AK, available potassium.
3.2. Soil Fungal Function
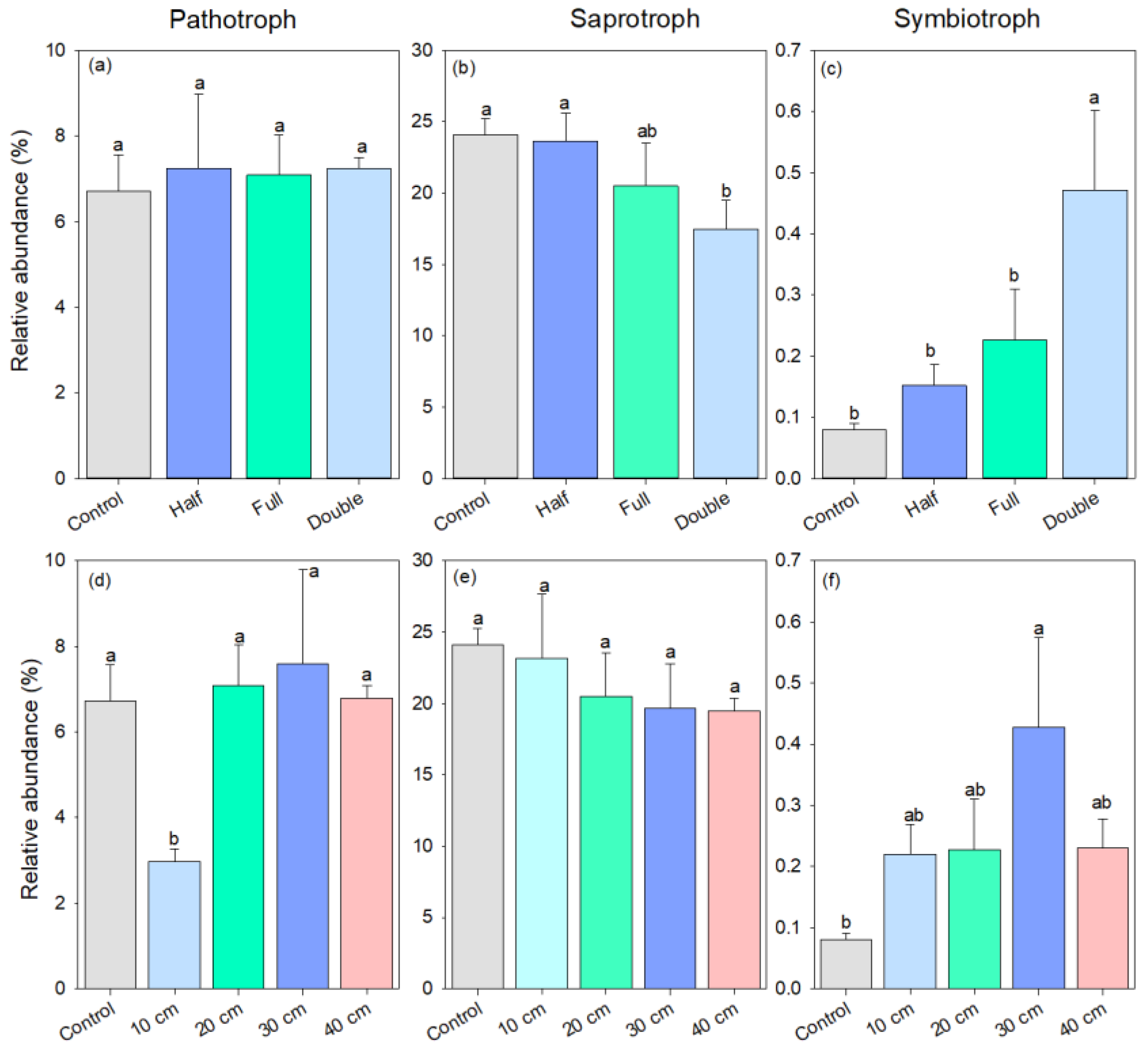
As for fungal trophic mode, the relative abundance of pathotrophic fungi was similar among half and full DB-SR amount treatments and control (p > 0.05, Figure 4a). However, DB-SR with a double amount increased the abundance of plant fungal pathogens by 2-fold and decreased the abundance of fungal saprotrophs by 27% relative to DB-SR with full amount return (p < 0.05, Figure 4b). The abundance of symbiotrophic fungi was 90–489% higher under DB-SR amount treatments than control, but it was 52% lower under DB-SR with full amount relative to double amount (p < 0.05, Figure 4c). Regarding DB-SR depth treatments, The abundance of fungal saprotroph was 15–22% lower under straw buried at 20, 30 cm, and 40 cm than under control (p < 0.05, Figure 4e). By contrast, the abundance of symbiotrophic fungi was 1.7 to 4.3 times higher under DB-SR depth treatments as compared to control (Figure 4f).
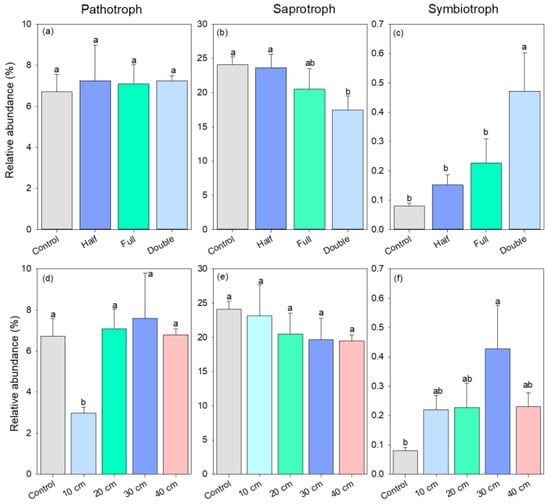
Figure 4.
Relative abundance of soil fungal functional groups under different straw management practices at trophic mode level under FUNGuild: (a,d) pathotroph; (b,e) saprotroph; (c,f) symbiotroph. Control, no tillage and no straw return; 10 cm, straw return with full amount return at a depth of 10 cm; 20 cm, straw return with full amount return at a depth of 20 cm; 30 cm, straw return with full amount return at a depth of 30 cm; 40 cm, straw return with full amount return at a depth of 40 cm; Half, straw return with half amount; Full, straw return with full amount; Double, straw return with double amount. Different letters indicate significant differences between treatments (p < 0.05).
As for fungal guild, DB-SR with double amount increased the abundance of plant fungal pathogens by 2-fold and decreased the abundance of arbuscular mycorrhizal fungi by 45% relative to DB-SR with full amount return (p < 0.05, Figure S3a,b). Additionally, straw buried at 40 cm increased the abundance of plant fungal pathogens by 52–97% whilst it decreased the abundance of arbuscular mycorrhizal by 64% compared with straw buried at 30 cm (Figure S3c).
3.3. C-Degrading Enzymes
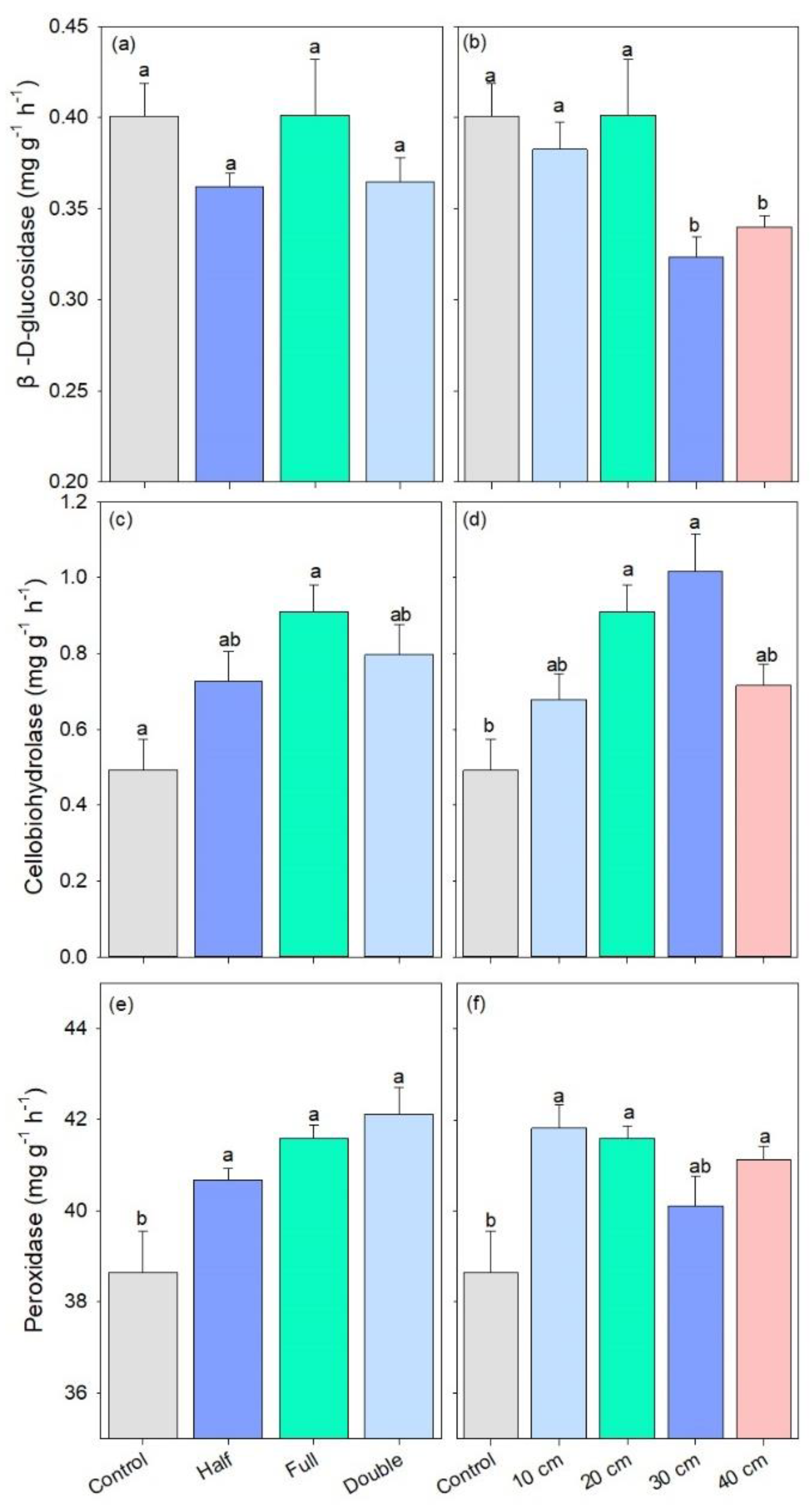
The activity of β-D-glucosidase remained stable among straw return amount treatments. However, it was lower under straw buried at 30 and 40 cm as compared to other treatments (p < 0.05, Figure 5a,b). DB-SR with full amount increased the activity of cellobiohydrolase by 32% compared to control, and cellobiohydrolase activity was also 28–54% higher under DB-SR at 20 and 30 cm relative to control (p < 0.01, Figure 5c,d). In addition, the activity of peroxidase was higher under DB-SR treatments than control, regardless of straw amount or burial depth (p < 0.05, Figure 5e,f).
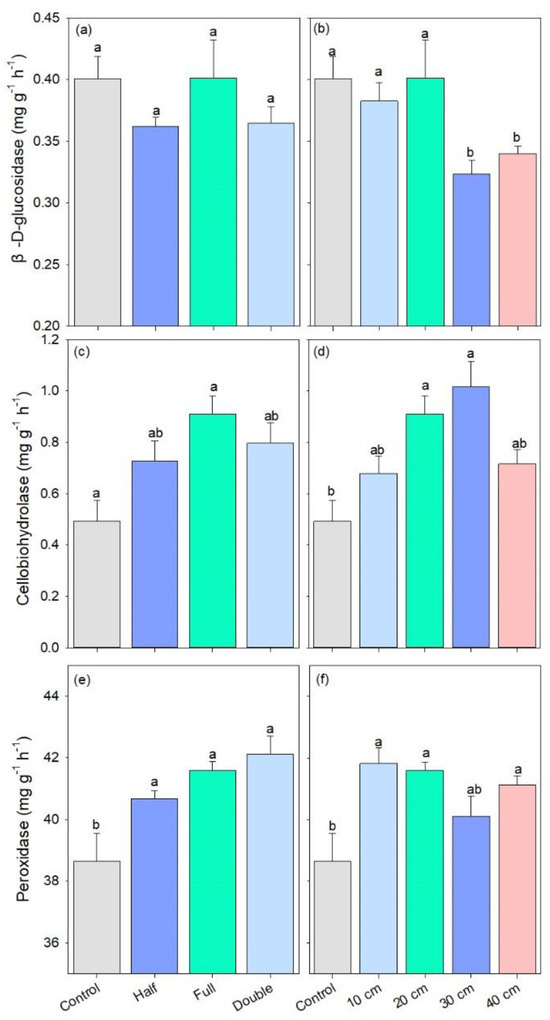
Figure 5.
C-degrading enzymatic activity under different straw management practices: (a,b) β-D-glucosidase; (c,d) cellobiohydrolase; (e,f) peroxidase. Control, no tillage and no straw return; 10 cm, straw return with full amount return at a depth of 10 cm; 20 cm, straw return with full amount return at a depth of 20 cm; 30 cm, straw return with full amount return at a depth of 30 cm; 40 cm, straw return with full amount return at a depth of 40 cm; Half, straw return with half amount; Full, straw return with full amount; Double, straw return with double amount. Different letters indicate significant differences between treatments (p < 0.05).
4. Discussions
Fungi can decompose organic materials or induce plant disease; as a consequence, the shift in fungal community structure (Figure 1) may influence soil health and crop productivity [15]. Our findings suggested that ditch-buried straw return did not affect fungal diversity (Figure S1), but different burial amounts or depths under ditch-buried straw return structured distinct fungal communities. In particular, saprotrophic and plant pathogenic fungi showed strongly diverse responses to different ditch-buried straw return strategies.
4.1. Influences of Ditch-Buried Straw Amount and Depth on Saprotrophic Fungi
Our results showed that DB-SR with full amount increased the abundance of Ascomycota as compared to other straw amount treatments (Figure 2a), which has been demonstrated to be capable of degrading cellulose and lignocellulose [34]. Indeed, Ascomycota is the prominent saprotrophic fungi in agroecosystems [35]. In soils with higher C contents, DB-SR with a full amount of straw return created a niche that allowed Ascomycota to better utilize easily degradable substrates [36]. This explanation is in accordance with Wang et al. [37], who suggested that dense populations of Ascomycota mainly affected crop residue decomposition. Similarly, we further observed higher abundances of class Dothideomycetes and Sordariomycetes under DB-SR with full relative to half and double amounts (Figure S2). These taxa can produce cellulolytic enzymes [38,39]. As a consequence, cellobiohydrolase activity was higher under DB-SR with full amount return relative to half and full (Figure 5c). This is further supported by the positive correlation between the abundance of Ascomycota and cellobiohydrolase activity (Figure S3a). These patterns may result from the C demand for fungal growth after straw incorporation.
In agroecosystems, saprotrophic fungi are usually C-limited as abundant N from fertilizers is available [40]. Straw incorporation may reduce C-limitation and therefore induce saprotrophic fungi to secrete more C-acquiring enzymes to degrade such organic residues for their growth [41]. It has been documented that Sordariomycetes are involved in straw decomposition and facilitate the transformation of straw to soil organic C by secreting cellulase [42]. From this aspect, DB-SR with the full amount of straw return would increase the exudation of microbial byproducts and subsequently the accumulation of microbial necromass, which facilitates the formation of stable soil C pool [36]. During the process of straw decomposition, nutrients would be released from straw residues. This can be supported by the increased NO3- and TN contents under DB-SR with full amounts, as shown in our previous results performed in a similar field [28]. Similarly, our RDA results showed that Ascomycota is positively correlated with NO3− (Figure 4).
However, with deeper straw burial, the abundance of Ascomycota and the activity of β-D-glucopyranoside were lower (Figure 2 and Figure 5), which was contrary to our second hypothesis. This indicates that deeper straw burial may inhibit soil fungal growth and subsequent enzyme production and consequently decrease straw decomposition. Although the growth of soil fungi and the production of enzymes were inhibited when straw returned to deeper soils under DB-SR tillage, it increased the abundance of Eurotiales (Figure 2), which might stimulate N2O emissions, since the order Eurotiales has been demonstrated to be capable of denitrification and therefore potentially producing N2O [43]. In short, a larger amount of straw return and deeper buried soil depth would hamper the decomposition of straw as well as the release of energy and nutrients to soils.
4.2. Influences of Ditch-Buried Straw Amount and Depth on Plant Pathogenic Fungi
Straw return could provide suitable environments for fungal pathogen growth, reproduction, and accumulation and thus may lead to crop diseases [44]. Here, we found higher relative abundance of Alternaria and the predicted plant fungal pathogens under DB-SR with a larger amount and deeper burial (Figure 2c). The genus Alternaria consists of plant pathogenic species, which may result in the extensive spoilage of crops [45]. Thus, DB-SR with a larger straw amount or at deeper burial depth may have higher crop disease potential. This could be explained by the fact that a high rate of straw return could influence soil hydrothermal processes [46], which might be favorable to the growth of pathogenic fungi, such as Alternaria in soils [45]. On the other hand, many allelochemicals can be released from straw decomposition, such as phenolic acids and short- and long-chain fatty acids, which could decrease specific soil-borne fungal pathogens under a full amount of straw return [47]. Similarly, Zhen et al. [44] reported that the indexes of wheat soil-borne diseases were increased remarkably when the amendment rate increased to 15,000 kg ha−1. This was further supported by the higher abundance of dominant Zygomycota species like Mortierella under full compared to that under the double amount of straw return, since Mortierella performs a role in the suppression of disease [48,49].
Moreover, Fusarium is the fungal pathogen most responsible for inducing major damage to the production of wheat and maize [50]. Since straw buried below 20 cm increased the abundance of Fusarium relative to other buried depth treatments, it may increase the risk of plant pathogens. The results of fungal function prediction determined by the Fungild approach also suggested that the predicted plant pathogens were larger whilst arbuscular mycorrhizal fungi were lower under the double amount of straw return and deeper straw (Figure S4). These findings indicate that straw return with the full amount and buried depth less than 30 cm could be beneficial to soil health and crop growth.
Although our case studies showed that ditch-buried straw return with the full amount and buried at a depth less than 30 cm may improve soil nutrient cycles and health and could be beneficial to crop production, higher machinery costs from ditch-buried straw return may exceed the yield benefits. Therefore, it might be beneficial to further explore the economic aspects of implementing deep tillage and straw return, considering the potential costs and farmers’ acceptance.
5. Conclusions
Straw return amount and depth under ditch-buried managements influenced the fungal groups with different ecological functions. As compared to half and double burial amounts, DB-SR with a full amount increased the abundance of Ascomycota and cellobiohydrolase activity. This in turn, enhanced straw decomposition and subsequently caused higher N availability in soils. Furthermore, the abundance of Ascomycota and β-D-glucopyranoside activity were lower with deeper burial depth. Finally, plant pathogenic fungi (i.e., genus Alternaria and Fusarium) increased with straw burial amount and depth, which may have a negative effect on other fungal community members. Thus, ditch-buried straw return with the full amount and buried at a depth less than 30 cm may improve soil nutrient cycles and health and could be beneficial to crop production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13131738/s1, Figure S1. Shannon diversity (a,b) and chao 1 (c,d) of soil fungi at the phylum and genus level under different straw management practices. Control, no tillage and no straw return; 10 cm, straw return with full amount return at a depth of 10 cm; 20 cm, straw return with full amount return at a depth of 20 cm; 30 cm, straw return with full amount return at a depth of 30 cm; 40 cm, straw return with full amount return at a depth of 40 cm; Half, straw return with half amount; Full, straw return with full amount; Double, straw return with double amount. Different letters indicate significant differences between treatments (p < 0.05). Figure S2. Relative abundance of Class Sordariomycetes (a,b) and Dothideomycetes (c,d) under straw management practices. Control, no tillage and no straw return; 10 cm, straw return with full amount return at a depth of 10 cm; 20 cm, straw return with full amount return at a depth of 20 cm; 30 cm, straw return with full amount return at a depth of 30 cm; 40 cm, straw return with full amount return at a depth of 40 cm; Half, straw return with half amount; Full, straw return with full amount; Double, straw return with double amount. Figure S3. Relative abundance of soil fungal functional groups under different straw management practices at guild level according to FUNGuild: (a,c) Plant pathogen; (b,d) Arbuscular mycorrhizal fungi. Control, no tillage and no straw return; 10 cm, straw return with full amount return at a depth of 10 cm; 20 cm, straw return with full amount return at a depth of 20 cm; 30 cm, straw return with full amount return at a depth of 30 cm; 40 cm, straw return with full amount return at a depth of 40 cm; Half, straw return with half amount; Full, straw return with full amount; Double, straw return with double amount. Different letters indicate significant differences between treatments (p < 0.05). Table S1 The relative abundance of soil fungal community composition at phylum level under different straw management practices. Control, no tillage and no straw return; 10 cm, straw return with full amount return at a depth of 10 cm; 20 cm, straw return with full amount return at a depth of 20 cm; 30 cm, straw return with full amount return at a depth of 30 cm; 40 cm, straw return with full amount return at a depth of 40 cm; Half, straw return with half amount; Full, straw return with full amount; Double, straw return with double amount. Different letters indicate significant differences between treatments (p < 0.05). Figure S4. Correlation between relative abundance of Ascomycete and cellobiohydrolase; and peroxidase across straw management practices.
Author Contributions
Conceptualization, J.Z. and H.Y.; Methodology, Y.L., Y.W. and H.Y.; Validation, Y.X.; Formal analysis, J.L. (Jiawen Lou); Investigation, Y.L.; Writing—original draft, J.Z.; Writing—review & editing, Y.L., Z.K., R.W.N., F.L., J.L. (Jian Liu), K.D., Y.X., H.Y. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (31770483, 42207388).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Yuekai Wang was employed by the company Longkang Farm, Anhui Agricultural Reclamation Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lal, R. Enhancing eco-efficiency in agro-ecosystems through soil carbon sequestration. Crop Sci. 2010, 50, S-120–S-131. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Pedraza-Zapata, D.C.; Sánchez-Garibello, A.M.; Quevedo-Hidalgo, B.; Moreno-Sarmiento, N.; Gutiérrez-Rojas, I. Promising cellulolytic fungi isolates for rice straw degradation. J. Microbiol. 2017, 55, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Vosátka, M.; Cai, B.; Ding, J.; Lu, C.; Xu, J.; Yan, W.; Li, Y.; Liu, C. The role of arbuscular mycorrhiza fungi in the decomposition of fresh residue and soil organic carbon: A mini-review. Soil Sci. Soc. Am. J. 2019, 83, 511–517. [Google Scholar] [CrossRef]
- Baumann, K.; Marschner, P.; Smernik, R.J.; Baldock, J.A. Residue chemistry and microbial community structure during decomposition of eucalypt, wheat and vetch residues. Soil Biol. Biochem. 2009, 41, 1966–1975. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Y.; Blagodatskaya, E.; Berauer, B.J.; Schuchardt, M.; Holz, M.; Shi, L.; Dannenmann, M.; Kiese, R.; Jentsch, A.; et al. Response of microbial growth and enzyme activity to climate change in European mountain grasslands: A translocation study. Catena 2024, 239, 107956. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, T.; Shi, L.; Kurganova, I.; de Gerenyu, V.L.; Kalinina, O.; Giani, L.; Kuzyakov, Y. Organic carbon accumulation and microbial activities in arable soils after abandonment: A chronosequence study. Geoderma 2023, 435, 116496. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Rillig, M.C.; Shi, L.; Dippold, M.A.; Zeng, Z.; Kuzyakov, Y.; Zang, H.; Jones, D.L.; Blagodatskaya, E. Restricted power: Can microorganisms maintain soil organic matter stability under warming exceeding 2 degrees? Glob. Ecol. Biogeogr. 2023, 32, 919–930. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Yang, H.; Meng, Y.; Feng, J.; Li, Y.; Zhai, S.; Liu, J. Direct and indirect effects of long-term ditch-buried straw return on soil bacterial community in a rice–wheat rotation system. Land Degrad. Dev. 2020, 31, 851–867. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Q.; Liu, D.; Hu, C.; Sun, J.; Wang, X.; Liang, G.; Zhou, W. Composition, predicted functions, and co-occurrence networks of fungal and bacterial communities_ Links to soil organic carbon under long-term fertilization in a rice-wheat cropping system. Eur. J. Soil Biol. 2020, 100, 103226. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Yang, S.; Yang, X.; Shi, L. Fungal interactions matter: Tricholoma matsutake domination affect fungal diversity and function in mountain forest soils. Biology 2021, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, A.; Von Oheimb, G.; Härdtle, W.; Wilken, C.; Gutknecht, J.L.M. Effects of anthropogenic disturbances on soil microbial communities in oak forests persist for more than 100 years. Soil Biol. Biochem. 2014, 70, 79–87. [Google Scholar] [CrossRef]
- Coyle, D.R.; Nagendra, U.J.; Taylor, M.K.; Campbell, J.H.; Cunard, C.E.; Joslin, A.H.; Mundepi, A.; Phillips, C.A.; Callaham, M.A., Jr. Soil fauna responses to natural disturbances, invasive species, and global climate change: Current state of the science and a call to action. Soil Biol. Biochem. 2017, 110, 116–133. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Evolutionary ecology of plant–microbe interactions: Soil microbial structure alters selection on plant traits. New Phytol. 2011, 192, 215–224. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Mitchell, J.I.; Zuccaro, A. Sequences, the environment and fungi. Mycologist 2006, 20, 62–74. [Google Scholar] [CrossRef]
- Hannula, S.E.; Ma, H.K.; Pérez-Jaramillo, J.E.; Pineda, A.; Bezemer, T.M. Structure and ecological function of the soil microbiome affecting plant–soil feedbacks in the presence of a soil-borne pathogen. Environ. Microbiol. 2020, 22, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pan, H.; Wang, Q.; Ge, Y.; Liu, W.; Christie, P. Enrichment of the soil microbial community in the bioremediation of a petroleum-contaminated soil amended with rice straw or sawdust. Chemosphere 2019, 224, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, G.; Li, Y.; Wang, Q.; Dang, P.; Qin, X.; Zou, Y.; Chen, Y.; Siddique, K.H. Straw incorporation with ridge–furrow plastic film mulch alters soil fungal community and increases maize yield in a semiarid region of China. Appl. Soil Ecol. 2021, 167, 104038. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Zhang, W.; Li, S.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Wang, J. Subsoiling tillage with straw incorporation improves soil microbial community characteristics in the whole cultivated layers: A one-year study. Soil Tillage Res. 2022, 215, 105188. [Google Scholar] [CrossRef]
- Song, K.; Sun, Y.; Qin, Q.; Sun, L.; Zheng, X.; Terzaghi, W.; Lv, W.; Xue, Y. The effects of earthworms on fungal diversity and community structure in farmland soil with returned straw. Front. Microbiol. 2020, 11, 594265. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fang, C.; Meng, Y.; Dai, Y.; Liu, J. Long-term ditch-buried straw return increases functionality of soil microbial communities. Catena 2021, 202, 105316. [Google Scholar] [CrossRef]
- Yang, H.; Zhai, S.; Li, Y.; Zhou, J.; He, R.; Liu, J.; Xue, Y.; Meng, Y. Waterlogging reduction and wheat yield increase through long-term ditch-buried straw return in a rice—Wheat rotation system. Field Crops Res. 2017, 209, 189–197. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Feng, J.; Zhai, S.; Chen, W.; Liu, J.; Bian, X. Ditch-buried straw return: A novel tillage practice combined with tillage rotation and deep ploughing in rice-wheat rotation systems. Adv. Agron. 2019, 154, 257–290. [Google Scholar]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols–A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Xu, T.L.; Veresoglou, S.D.; Hu, H.W.; Hao, Z.P.; Hu, Y.J.; Liu, L.; Deng, Y.; Rillig, M.C.; Chen, B.D. Plant diversity represents the prevalent determinant of soil fungal community structure across temperate grasslands in northern China. Soil Biol. Biochem. 2017, 110, 12–21. [Google Scholar] [CrossRef]
- Salo, K.; Domisch, T.; Kouki, J. Forest wildfire and 12 years of post-disturbance succession of saprotrophic macrofungi (Basidiomycota, Ascomycota). For. Ecol. Manag. 2019, 451, 117454. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, X.; Zhang, M.; Liu, X.; Song, X.; Lu, J.; Wang, B.; van Groenigen, K.J.; Li, S. Linking soil microbial community traits and organic carbon accumulation rate under long-term conservation tillage practices. Soil Tillage Res. 2022, 220, 105360. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Wang, X.; Wei, X.; Wei, J. Lichen-associated fungal community in Hypogymnia hypotrypa (Parmeliaceae, Ascomycota) affected by geographic distribution and altitude. Front. Microbiol. 2016, 7, 1231. [Google Scholar] [CrossRef] [PubMed]
- Phosri, C.; Polme, S.; Taylor, A.F.; Koljalg, U.; Suwannasai, N.; Tedersoo, L. Diversity and community composition of ectomycorrhizal fungi in a dry deciduous dipterocarp forest in Thailand. Biodivers. Conserv. 2012, 21, 2287–2298. [Google Scholar] [CrossRef]
- Freedman, L.P.; Cockburn, I.M.; Simcoe, T.S. The economics of reproducibility in preclinical research. PLoS Biol. 2015, 13, e1002165. [Google Scholar] [CrossRef] [PubMed]
- Grandy, T.H.; Werkle-Bergner, M.; Chicherio, C.; Schmiedek, F.; Lövdén, M.; Lindenberger, U. Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology 2013, 50, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, L.K.; Billings, S.A. Changes in variability of soil moisture alter microbial community C and N resource use. Soil Biol. Biochem. 2011, 43, 1837–1847. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, L.; Li, Y.; Li, J.; Zhang, C.; Ma, D.; Zhou, G.; Zhang, J. Nitrogen input level modulates straw-derived organic carbon physical fractions accumulation by stimulating specific fungal groups during decomposition. Soil Tillage Res. 2023, 225, 105560. [Google Scholar] [CrossRef]
- Mothapo, N.; Chen, H.; Cubeta, M.A.; Grossman, J.M.; Fuller, F.; Shi, W. Phylogenetic, taxonomic and functional diversity of fungal denitrifiers and associated N2O production efficacy. Soil Biol. Biochem. 2015, 83, 160–175. [Google Scholar] [CrossRef]
- Zhen, W.; Wang, S.; Zhang, C.; Ma, Z. Influence of maize straw amendment on soil-borne diseases of winter wheat. Front. Agric. China 2009, 3, 7–12. [Google Scholar] [CrossRef]
- Li, F.Q.; Yoshizawa, T. Alternaria mycotoxins in weathered wheat from China. J. Agric. Food Chem. 2000, 48, 2920–2924. [Google Scholar] [CrossRef]
- Yuan, G.; Huan, W.; Song, H.; Lu, D.; Chen, X.; Wang, H.; Zhou, J. Effects of straw incorporation and potassium fertilizer on crop yields, soil organic carbon, and active carbon in the rice–wheat system. Soil Tillage Res. 2021, 209, 104958. [Google Scholar] [CrossRef]
- Qi, Y.Z.; Zhen, W.C.; Li, H.Y. Allelopathy of decomposed maize straw products on three soil-born diseases of wheat and the analysis by GC-MS. J. Integr. Agric. 2015, 14, 88–97. [Google Scholar] [CrossRef]
- Wani, Z.A.; Kumar, A.; Sultan, P.; Bindu, K.; Riyaz-Ul-Hassan, S.; Ashraf, N. Mortierella alpina CS10E4, an oleaginous fungal endophyte of Crocus sativus L. enhances apocarotenoid biosynthesis and stress tolerance in the host plant. Sci. Rep. 2017, 7, 8598. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.; Wu, H.; Jousset, A.; Shen, Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla fusarium wilt disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Summerell, B.A.; Leslie, J.F. Fifty years of Fusarium: How could nine species have ever been enough? Fungal Divers. 2011, 50, 135–144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).