Reactive Oxygen and Related Regulatory Factors Involved in Ethylene-Induced Petal Abscission in Roses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Observation of Abscission Phenotype in Rose Petal
2.3. Collection of Abscission Zone Samples from Roses
2.4. Observation of the Cells in AZ of Rose Petal by Scanning Electron Microscopy
2.5. Detection of ROS Contents
2.6. Quantifying the ROS Content
2.7. RNA Extraction and RT-qPCR
2.8. Statistical Analysis and Image Processing
3. Results
3.1. Ethylene Promotes Petal Abscission by Affecting Cells of AZ and Related Physiological Indicators
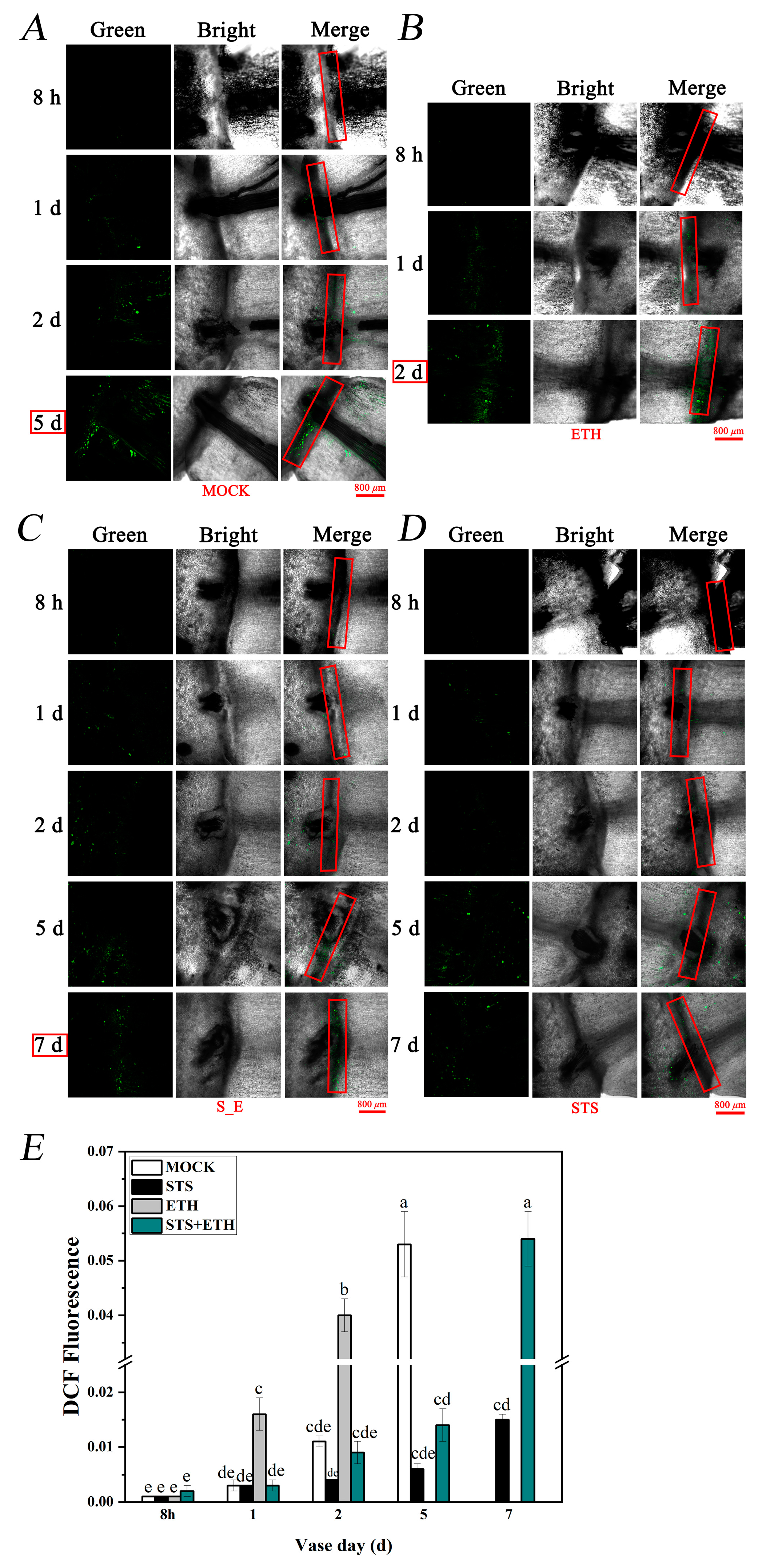
3.2. Ethylene Enhanced the Accumulation of H2O2 and O2− in Rose Petals
3.3. Ethylene Promoted H2O2 Production in the AZ of Rose Petals
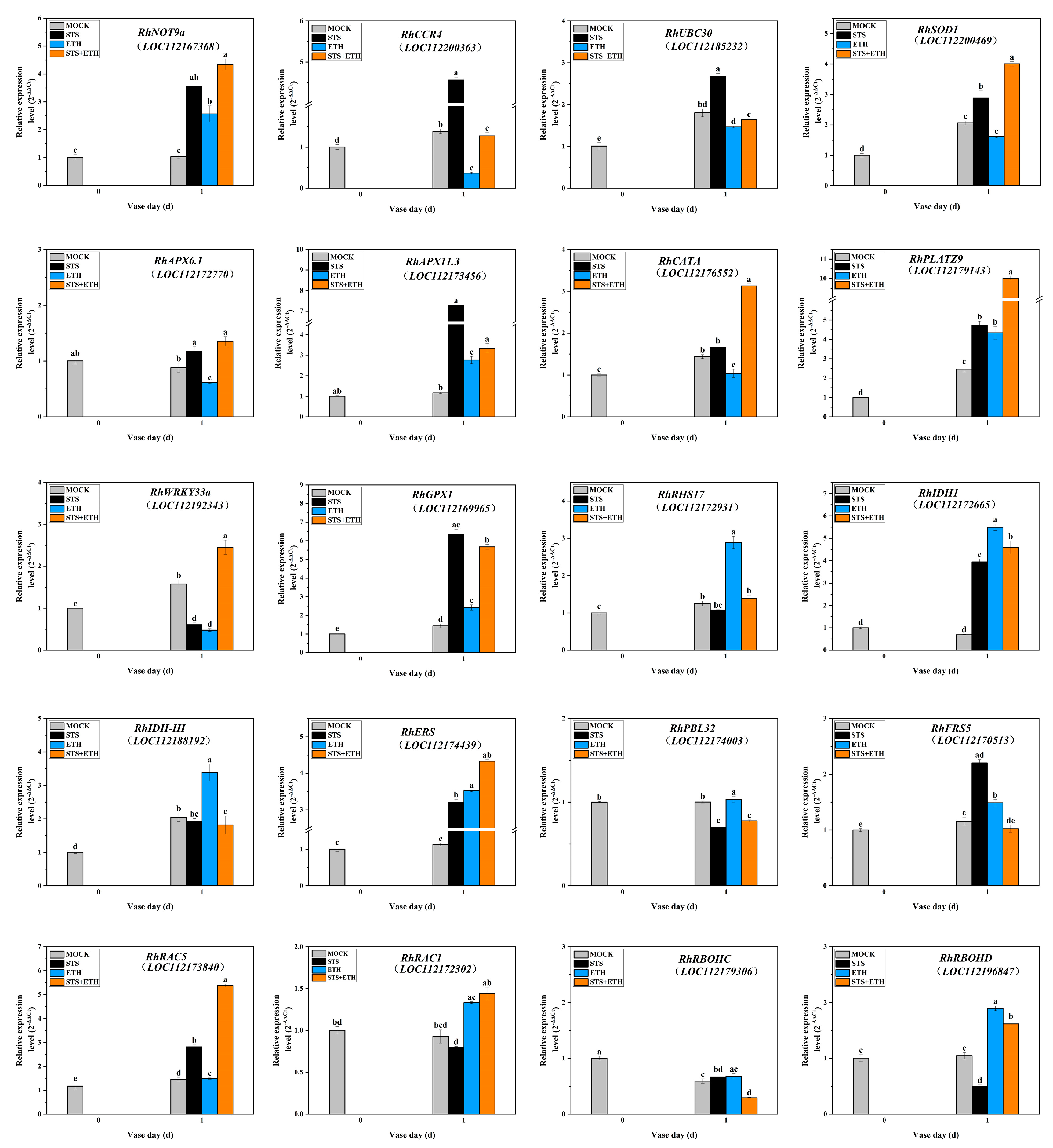
3.4. Ethylene Regulated the Expressions of Key Genes Related to ROS Production and ROS Scavenging in the AZ of Rose Petals
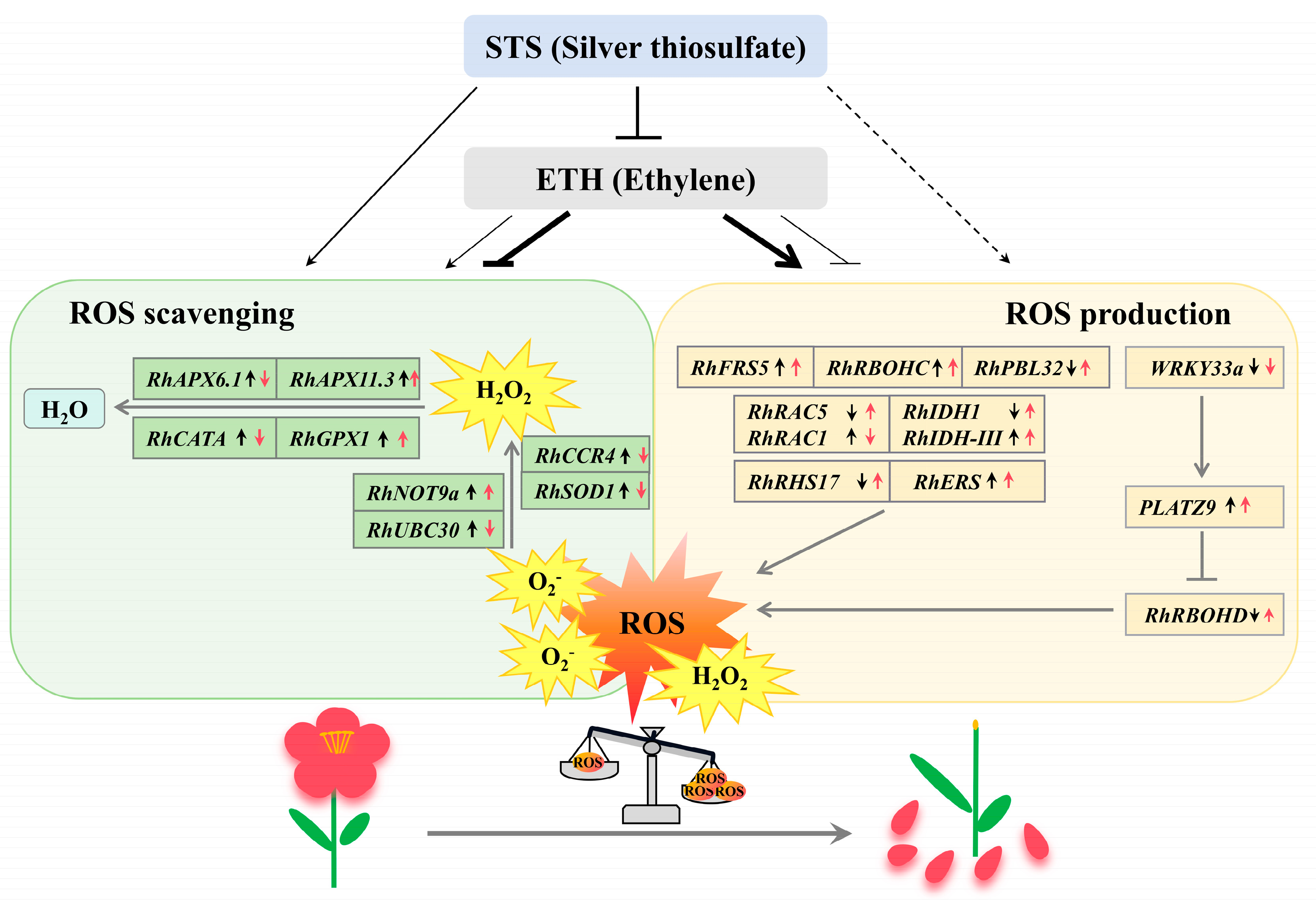
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Chun, J.P.; Tucker, M.L. Transcriptional Regulation of Abscission Zones. Plants 2019, 8, 154. [Google Scholar] [CrossRef]
- Beno-Moualem, D.; Gusev, L.; Dvir, O.; Pesis, E.; Meir, S.; Lichter, A. The effects of ethylene, methyl jasmonate and 1-MCP on abscission of cherry tomatoes from the bunch and expression of endo-1,4-β-glucanases. Plant Sci. 2004, 167, 499–507. [Google Scholar] [CrossRef]
- Pei, H.X.; Ma, N.; Tian, J.; Luo, J.; Chen, J.W.; Li, J.; Zheng, Y.; Chen, X.; Fei, Z.J.; Gao, J.P. An NAC transcription factor controls ethylene-regulated cell expansion in flower petals. Plant Physiol. 2013, 163, 775–791. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhang, Y.Y.; He, Y.M.; Du, T.T.; Shan, D.X.; Fan, H.D.; Wang, W.Y.; Qin, Z.; Xin, C.H.; Pei, H.X. Metabolome and transcriptome integration reveals insights into the process of delayed petal abscission in rose by STS. Front. Plant Sci. 2022, 13, 1045270. [Google Scholar] [CrossRef]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–339. [Google Scholar] [CrossRef]
- Patterson, S.E.; Bleecker, A.B. Ethylene-dependent and -independent processes associated with floral organ abscission in Arabidopsis. Plant Physiol. 2004, 134, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Whitelaw, C.A.; Lyssenko, N.N.; Chen, L.; Zhou, D.; Mattoo, A.K.; Tucker, M.L. Delayed abscission and shorter Internodes correlate with a reduction in the ethylene receptor LeETR1 transcript in transgenic tomato. Plant Physiol. 2002, 128, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Asamizu, E.; Saito, T.; Matsukura, C.; Ariizumi, T.; Brès, C.; Rothan, C.; Mizoguchi, T.; Ezura, H. Tomato TILLING technology: Development of a reverse genetics tool for the efficient isolation of mutants from Micro-Tom mutant libraries. Plant Cell Physiol. 2011, 52, 1994–2005. [Google Scholar] [CrossRef]
- Borda, M.A.; Clark, G.D.; Huber, J.D.; Welt, B.A.; Nell, T.A. Effects of ethylene on volatile emission and fragrance in cut roses: The relationship between fragrance and vase life. Postharvest Biol. Technol. 2011, 59, 245–252. [Google Scholar] [CrossRef]
- Fanourakis, D.; Pieruschka, R.; Savvides, A.; Macnish, A.J.; Sarlikioti, V.; Woltering, E.J. Sources of vase life variation in cut roses: A review. Postharvest Biol. Technol. 2013, 78, 1–15. [Google Scholar] [CrossRef]
- Khatami, F.; Najafi, F.; Yari, F.; Khavari-Nejad, R.A. Expression of etr1-1 gene in transgenic Rosa hybrida L. increased postharvest longevity through reduced ethylene biosynthesis and perception. Sci. Hortic. 2020, 263, 109103. [Google Scholar] [CrossRef]
- Cai, S.Q.; Lashbrook, C.C. Stamen abscission zone transcriptome profiling reveals new candidates for abscission control: Enhanced retention of floral organs in transgenic plants overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiol. 2008, 146, 1305–1321. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Tripathi, S.K.; Nath, P.; Sane, A.P. Petal abscission in rose is associated with the differential expression of two ethylene-responsive xyloglucan endotransglucosylase/hydrolase genes, RbXTH1, and RbXTH2. J. Exp. Bot. 2011, 62, 5091–5103. [Google Scholar] [PubMed]
- Gao, Y.R.; Liu, Y.; Liang, Y.; Lu, J.Y.; Jiang, C.Y.; Fei, Z.J.; Jiang, C.Z.; Ma, C.; Gao, J.P. Rosa hybrida RhERF1 and RhERF4 mediate ethylene and auxin regulated petal abscission by influencing pectin degradation. Plant J. 2019, 99, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.k.; He, C.Z. Regulation of plant reactive oxygen species (ROS) in stress responses: Learning from AtRBOHD. Plant Cell Rep. 2016, 35, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Nam, H.G.; Lim, P.O. Plant leaf senescence and death—Regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 2013, 126, 4823–4833. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.; Wang, X.; Wu, L.; Wang, F.; Wu, Z.; Yang, H.; Chen, Y.; Wen, D.; Liu, X. Silicon improves growth and alleviates oxidative stress in rice seedlings (Oryza sativa L.) by strengthening antioxidant defense and enhancing protein metabolism under arsanilic acid exposure. Ecotoxicol. Environ. Saf. 2018, 158, 266–273. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z.L. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B.; et al. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Roles of ethylene production and ethylene receptor expression in regulating apple fruitlet abscission. Plant Physiol. 2015, 169, 125–137. [Google Scholar] [CrossRef]
- Sakamoto, M.; Munemura, I.; Tomita, R.; Kobayashi, K. Reactive oxygen species in leaf abscission signaling. Plant Signal. Behav. 2008, 3, 1014–1015. [Google Scholar] [CrossRef]
- Goldental-Cohen, S.; Burstein, C.; Biton, I.; Ben Sasson, S.; Sadeh, A.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Mugira, Y.; Schneider, D.; et al. Ethephon induced oxidative stress in the olive leaf abscission zone enables development of a selective abscission compound. BMC Plant Biol. 2017, 17, 87. [Google Scholar] [CrossRef]
- Yin, X.D.; Ma, K.Y.; Wang, Y.L.; Sun, Y.; Shang, X.F.; Zhao, Z.M.; Wang, R.X.; Chen, Y.J.; Zhu, J.K.; Liu, Y.Q. Design, Synthesis and Antifungal Evaluation of 8-hydroxyquinoline Metal Complexes against Phytopathogenic Fungi. J. Agric. Food Chem. 2020, 68, 11096–11104. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, C.; Liu, Y.; Gao, Y.; Lu, J.; Aiwaili, P.; Fei, Z.; Jiang, C.Z.; Hong, B.; Ma, C.; et al. Auxin Regulates Sucrose Transport to Repress Petal Abscission in Rose (Rosa hybrida). Plant Cell. 2020, 32, 3485–3499. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, K.; Zhang, T.; Chen, J.; Zhao, S.; Cui, Y.; Zhou, W.; Yu, Y.; Chen, S.; Wang, C.; et al. The RhWRKY33a-RhPLATZ9 regulatory module delays petal senescence by suppressing rapid reactive oxygen species accumulation in rose flowers. Plant J. 2023, 114, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, H.; Borochov, A.; Mayak, S. Age-related changes in petal membranes from attached and detached rose flowers. Plant Physiol. 1990, 94, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Leshem, Y.Y. Plant senescence processes and free radicals. Free Radic. Biol. Med. 1988, 5, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. In Vitro 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Sakamoto, T.; Kawasaki, T.; Umemura, K.; Shimamoto, K. Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 2004, 135, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.E. Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 2001, 126, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Botton, A.; Ruperti, B. The Yes and No of the Ethylene Involvement in Abscission. Plants 2019, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.; Hazama, Y.; Hayakawa, T.; Totsuka, T. Simulation of effects of atmospheric ethylene on tree leaf shedding. Environ. Pollut. 1989, 61, 173–185. [Google Scholar] [CrossRef]
- Khan, A.R.; Azhar, W.; Wu, J.; Ulhassan, Z.; Salam, A.; Zaidi, S.H.R.; Yang, S.; Song, G.; Gan, Y. Ethylene participates in zinc oxide nanoparticles induced biochemical, molecular and ultrastructural changes in rice seedlings. Ecotoxicol. Environ. Saf. 2021, 226, 112844. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Wang, L.; Li, D.; Liu, L.; Li, M. An ethylene response factor AcERF116 identified from A. catechu is involved in fruitlet abscission. Plant Sci. 2024, 344, 112091. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, J.; Yi, J. Impact of silver nanoparticles on marine diatom Skeletonema costatum. J. Appl. Toxicol. 2016, 36, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Nagar, P.; Rawat, A.; Mustafiz, A. Phytosulfokine receptor 1 (AtPSKR1) acts as a positive regulator of leaf senescence by mediating ROS signaling in Arabidopsis thaliana. Environ. Exp. Bot. 2024, 220, 105674. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, P.; Liang, W.; Cheng, Q.; Mu, B.; Niu, F.; Yan, J.; Liu, C.; Xie, H.; Kav, N.N.V.; et al. A Rapeseed WRKY Transcription Factor Phosphorylated by CPK Modulates Cell Death and Leaf Senescence by Regulating the Expression of ROS and SA-Synthesis-Related Genes. J. Agric. Food Chem. 2020, 68, 7348–7359. [Google Scholar] [CrossRef] [PubMed]
- San, B.; Li, Z.; Hu, Q.; Reighard, G.L.; Luo, H. Adventitious shoot regeneration from in vitro cultured leaf explants of peach rootstock Guardian® is significantly enhanced by silver thiosulfate. Plant Cell Tissue Organ Cult. 2015, 120, 757–765. [Google Scholar] [CrossRef]
- Martínez, T.M.; Corredoira, E.; Vieitez, M.A.; Cernadas, J.M.; Montenegro, R.; Ballester, A.; Vieitez, J.F.; San José, C.M. Micropropagation of mature Quercus ilex L. trees by axillary budding. Plant Cell Tissue Organ Cult. 2017, 131, 499–512. [Google Scholar] [CrossRef]
- Adal, A.M.; Doshi, K.; Holbrook, L.; Mahmoud, S.S. Comparative RNA-seq analysis reveals genes associated with masculinization in female Cannabis sativa. Planta 2021, 253, 17. [Google Scholar] [CrossRef]
- Naing, A.H.; Soe, M.T.; Yeum, J.H.; Kim, C.K. Ethylene acts as a negative regulator of the stem-bending mechanism of different cut snapdragon cultivars. Front. Plant Sci. 2021, 12, 745038. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Zhang, J.; Wang, W.; Zhang, S.; Qin, Z.; Pei, H. Reactive Oxygen and Related Regulatory Factors Involved in Ethylene-Induced Petal Abscission in Roses. Plants 2024, 13, 1718. https://doi.org/10.3390/plants13131718
Han S, Zhang J, Wang W, Zhang S, Qin Z, Pei H. Reactive Oxygen and Related Regulatory Factors Involved in Ethylene-Induced Petal Abscission in Roses. Plants. 2024; 13(13):1718. https://doi.org/10.3390/plants13131718
Chicago/Turabian StyleHan, Siwen, Jingjing Zhang, Wenyu Wang, Siying Zhang, Zhe Qin, and Haixia Pei. 2024. "Reactive Oxygen and Related Regulatory Factors Involved in Ethylene-Induced Petal Abscission in Roses" Plants 13, no. 13: 1718. https://doi.org/10.3390/plants13131718
APA StyleHan, S., Zhang, J., Wang, W., Zhang, S., Qin, Z., & Pei, H. (2024). Reactive Oxygen and Related Regulatory Factors Involved in Ethylene-Induced Petal Abscission in Roses. Plants, 13(13), 1718. https://doi.org/10.3390/plants13131718





