Studies on the Phosphorus-Solubilizing Ability of Isaria cateinannulata and Its Influence on the Growth of Fagopyrum tataricum Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid Medium Preparation and Determination of Phosphorus-Solubilization Index of Strains
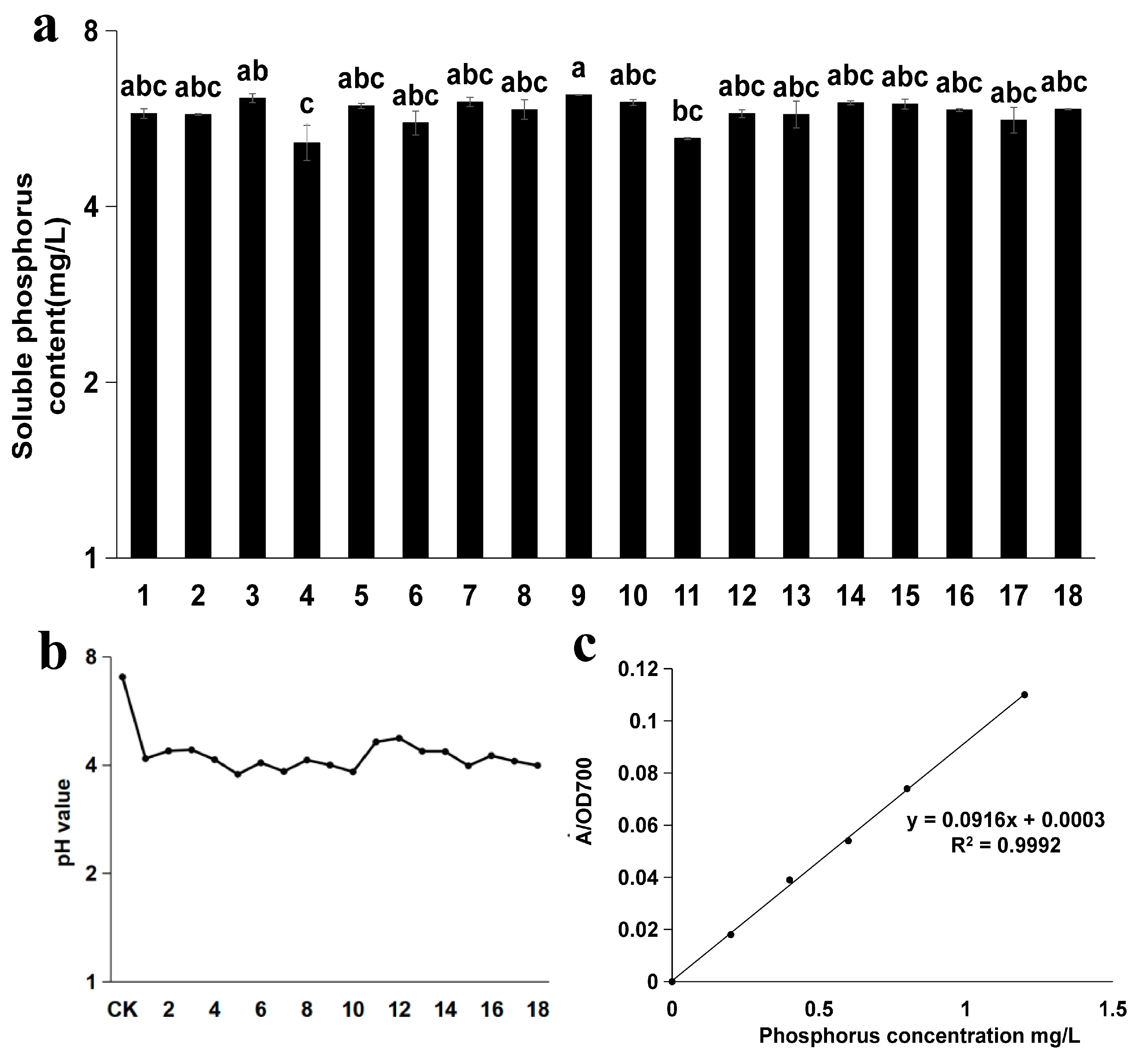
2.2. Preparation of Liquid Medium and Determination of Soluble Phosphorus Content of Strains
2.3. Determination of Phosphorus Solubilization Amount by I. cateinannulata
2.4. Field Test Ring I. cateinannulata Strain on the Growth of F. tataricum
2.4.1. I. cateinannulata Roots Irrigation for F. tataricum
2.4.2. Determination of Growth Index of F. tataricum
2.4.3. Chlorophyll Determination
2.4.4. Determination of Soluble Phosphorus Content in F. tataricum Plants
2.5. Data Processing and Analysis
3. Results and Discussion
3.1. Analysis of Phosphorus-Solubilization Ability among Different Strains of I. cateinannulata
3.2. Analysis of Soluble Phosphorus Content and pH in Different Strains of I. cateinannulata
3.3. The Effect of I. cateinannulata on the Soluble Phosphorus Content in F. tataricum Plants
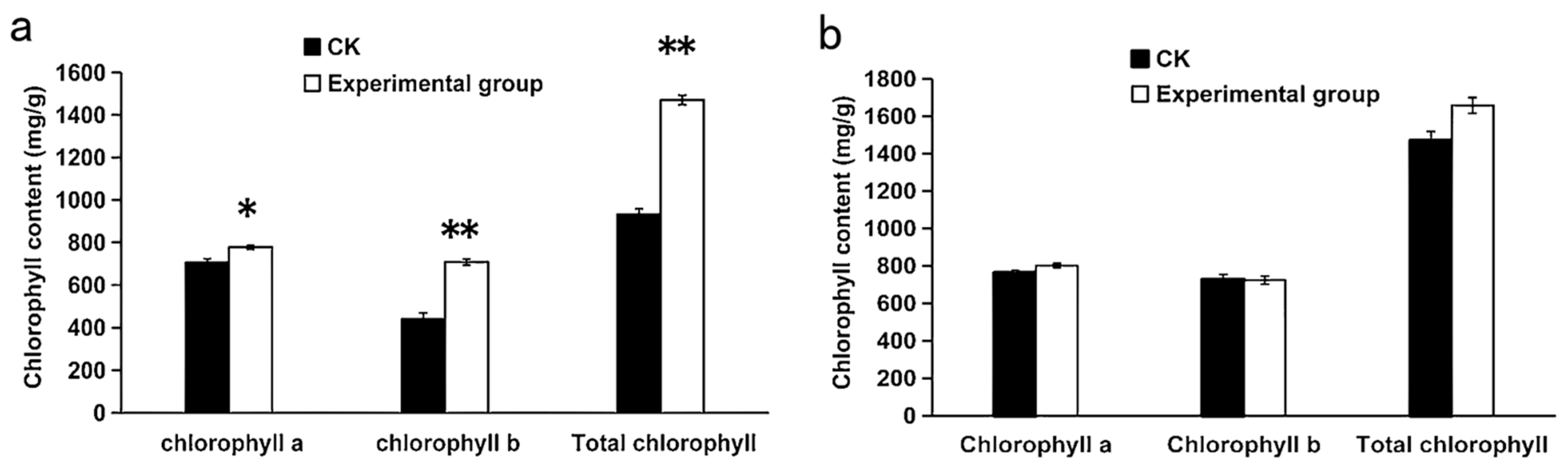
3.4. Effect of Rhizosphere Irrigation with I. cateinannulata on Chlorophyll Content of F. tataricum
3.5. The Effect of I. cateinannulata on the Growth of F. tataricum at Different Stages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Guo, X.; Chen, Y.; Fan, C.; Hu, Z.; Zhang, X. Response Strategies and Molecular Mechanisms of Phosphate Deficiency in Plants. Mol. Plant Breed. 2023, 1–12. Available online: https://link.cnki.net/urlid/46.1068.S.20230906.1318.002 (accessed on 17 March 2024).
- Shenoy, V.V.; Kalagudi, G.M. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005, 23, 501–513. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: Status and needs. Front. Bioeng. Biotechnol. 2020, 7, 425. [Google Scholar] [CrossRef]
- de Barros Mesquita, C.; Garcia, É.L.; Bolfarini, A.C.B.; Leonel, S.; Franco, C.M.L.; Leonel, M. Phosphate fertilization changes the characteristics of ‘Maçã’ banana starch. Int. J. Biol. Macromol. 2018, 112, 1138–1145. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishiyama, K.; Yoon, D.K.; Takegahara-Tamakawa, Y.; Kondo, E.; Suganami, M.; Makino, A. Suppression of chloroplast triose phosphate isomerase evokes inorganic phosphate-limited photosynthesis in rice. Plant Physiol. 2022, 188, 1550–1562. [Google Scholar] [CrossRef]
- CY, K.; SF, F.; Chou, F.C.; Chen, R.Y.; Chou, J.Y. Phosphate-solubilizing characteristics of yeasts. Mycosphere 2018, 9, 1117–1131. [Google Scholar]
- Yang, Q.; Han, J.; Li, Y.; Xiao, K.; Liu, Y. Effects of different phosphorus levels on leaf photosynthetic performance and yield traits of wheat. J. Plant Nutr. Fertil. 2006, 12, 816–821. [Google Scholar]
- Wang, X. Effects of Phosphorus on Yield and Quality of Wheat and Its Physiological Basis. Ph.D. Thesis, Shandong Agricultural University, Taian, China, 2003. [Google Scholar]
- Tao, P. Study on the Photosynthetic Characteristics and Leaf Proteomics of Maize under Low Phosphorus Stress. Ph.D. Thesis, Shandong University, Taian, China, 2009. [Google Scholar]
- Luthar, Z.; Golob, A.; Germ, M.; Vombergar, B.; Kreft, I. Tartary buckwheat in human nutrition. Plants 2021, 10, 700. [Google Scholar] [CrossRef]
- Xiang, D.B.; Song, Y.; Song, C.; Wan, Y.; Ye, X.L.; Liu, C.Y.; Zhao, G. Seed Setting and Its Spatial Characteristics in Tartary Buckwheat (Fagopyrum tataricum). Phyton 2022, 91, 1659–1669. [Google Scholar] [CrossRef]
- Song, Y. Fruitful Characteristics of Different Varieties of Bitter Buckwheat and Their Response to Source-Sink Regulation. Ph.D. Thesis, Chengdu University, Chengdu, China, 2019. [Google Scholar]
- Li, Z.; Wang, Y.; Wu, X.; Zhou, L.; Li, Z.; Huang, K. Effects of phosphorus fertilizer treatment on filling characteristics and solidification of bitter buckwheat. Shanghai J. Agric. Sci. 2020, 36, 29–34. [Google Scholar]
- Liu, S.; Li, X. High-yield cultivation techniques of bitter buckwheat. Nong Jia Can Mou 2021, 48–50. [Google Scholar]
- Wang, X. Mechanism of Fertilizer and Exogenous Hormone Regulation on Filling and Yield Formation in Thin-Shelled Bitter Buckwheat. Ph.D. Thesis, Guizhou Normal University, Guiyang, China, 2023. [Google Scholar]
- Qarni, A.; Billah, M.; Hussain, K.; Shah, S.H.; Ahmed, W.; Alam, S.; Khan, N. Isolation and characterization of phosphate solubilizing microbes from rock phosphate mines and their potential effect for sustainable agriculture. Sustainability 2021, 13, 2151. [Google Scholar] [CrossRef]
- Barrow, N.J. The effects of pH on phosphate uptake from the soil. Plant Soil 2017, 410, 401–410. [Google Scholar] [CrossRef]
- Xie, J.; Yan, Z.; Chen, D. A bacterium isolated from soil in a karst rocky desertification region has efficient phosphate-solubilizing and plant growth-promoting ability. Front. Microbiol. 2021, 11, 625450. [Google Scholar] [CrossRef]
- Rashid, M.; Khalil, S.; Ayub, N.; Alam, S.; Latif, F. Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in vitro conditions. Pak. J. Biol. Sci. 2004, 7, 187–196. [Google Scholar] [CrossRef]
- Kucey, R.M.N.; Janzen, H.H.; Leggett, M.E. Microbially mediated increases in plant-available phosphorus. Adv. Agron. 1989, 42, 199–228. [Google Scholar]
- Lin, Q.; Wang, H.; Zhao, X.; Zhao, Z. Preliminary investigation on phosphatases activity and mechanism of some bacteria and fungi. Microbiol. Bull. 2001, 26–30. [Google Scholar] [CrossRef]
- Yang, L.; Guo, L.; Zhou, Y.; Wang, J.; Liang, C.; Xu, Y.; Huang, J. Screening and phosphate-solubilizing activity evaluation of multifunctional phosphate-solubilizing fungi. J. Trop. Crops 2023, 44, 1662–1670. [Google Scholar]
- Yu, H.; Zhou, F.; Li, F.; Zhang, G.; Zhou, H.; Zhang, X. Research progress on phosphate-solubilizing microorganisms and their application in soil pollution prevention and control. Environ. Sci. Technol. 2020, 43, 44–51. [Google Scholar]
- Qiao, H.; Sun, X.R.; Wu, X.Q.; Li, G.E.; Wang, Z.; Li, D.W. The phosphate-solubilizing ability of Penicillium guanacastense and its effects on the growth of Pinus massoniana in phosphate-limiting conditions. Biol. Open 2019, 8, bio046797. [Google Scholar]
- Kaur, G.; Reddy, M.S. Improvement of crop yield by phosphate-solubilizing Aspergillus species in organic farming. Arch. Agron. Soil Sci. 2017, 63, 24–34. [Google Scholar] [CrossRef]
- Maharana, R.; Das, S.; Dhal, N.K.; Dinda, S.; Singh, B.M. Characterization and Mechanisms of Biosolubilization of Rock Phosphate by Microbes Isolated from Mahanadi Estuary, Odisha, India. Int. J. Environ. Res. 2021, 15, 335–348. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, P.S.; Zhang, B.X.; Wang, Y.P.; Meng, J.; Gao, Y.F.; Hu, X.M. Identification of phosphate-solubilizing microorganisms and determination of their phosphate-solubilizing activity and growth-promoting capability. BioResources 2020, 15, 2560–2578. [Google Scholar] [CrossRef]
- Majumder, M.S.I.; Islam, M.K.; Akamine, H.; Sano, A.; Onjo, M.; Hossain, M.A. Comparative study of phosphate solubilization potential of Talaromyces pinophilus strains. Appl. Ecol. Environ. Res. 2019, 17, 14973–14984. [Google Scholar] [CrossRef]
- Coutinho, F.P.; Felix, W.P.; Yano-Melo, A.M. Solubilization of phosphates in vitro by Aspergillus spp. and Penicillium spp. Ecol. Eng. 2012, 42, 85–89. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahmad, E. Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. In Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 31–62. [Google Scholar]
- Ram, H.; Malik, S.S.; Dhaliwal, S.S.; Kumar, B.; Singh, Y. Growth and productivity of wheat affected by phosphorus-solubilizing fungi and phosphorus levels. Plant Soil Environ. 2015, 61, 122–126. [Google Scholar] [CrossRef]
- Bektas, I.; Kusek, M. Biological control of onion basal rot disease using phosphate solubilising rhizobacteria. Biocontrol Sci. Technol. 2021, 31, 190–205. [Google Scholar] [CrossRef]
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, S. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Xu, S.; Zhao, W.; Liu, H.; Li, Q.; Huang, Z. Screening of phosphate solubilizing fungi and analysis of their salt tolerance characteristics. Microbiol. Bull. 2018, 45, 2142–2151. [Google Scholar]
- Wang, X.L.; Qiu, S.Y.; Zhou, S.Q.; Xu, Z.H.; Liu, X.T. Phosphate-Solubilizing Capacity of Paecilomyces lilacinus PSF7 and Optimization Using Response Surface Methodology. Microorganisms 2023, 11, 454. [Google Scholar] [CrossRef]
- Erkfeldt, S. Slow and steady phosphate solubilization by a psychrotolerant strain of Paecilomyces hepiali (MTCC9621). World J. Microbiol. Biotechnol. 2011, 27, 1055–1062. [Google Scholar]
- Hernández-Leal, T.I.; Carrión, G.; Heredia, G. In vitro phosphate solubilization by a strain of Paecilomyces lilacinus (Thom) Samson. Agrociencia. 2011, 45, 881–892. [Google Scholar]
- Peng, X. The Influence of the Dynamic Colonization of Nematodospora coryli on Enzyme Activity and Metabolic Products during Buckwheat Seed Germination. Ph.D. Thesis, Guizhou Normal University, Guiyang, China, 2023. [Google Scholar]
- Liang, Z. Two new species of Aspergillus. Acta Microbiol. Sin. 1981, 21, 31–34+132. [Google Scholar]
- Zhang, X.; Peng, X.; Yang, G.; Chen, Q.; Jin, D. The colonization and effect of Isaria cateinannulata on buckwheat sprouts. Plants 2022, 12, 145. [Google Scholar]
- Qiao, L.; Liu, J.; Zhou, Z.; Li, Z.; Zhou, Y.; Xu, S.; Zou, X. Positive effects of Cordyceps cateniannulata colonization in tobacco: Growth promotion and resistance to abiotic stress. Front. Microbiol. 2023, 14, 1131184. [Google Scholar] [CrossRef]
- Xu, S. The Influence of Nematodospora coryli Colonization on Tobacco Growth and Rhizosphere Microbial Diversity. Ph.D. Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar]
- Guan, J. Construction of Nematodospora coryli and Tomato Symbionts and Their Effects on Tetranychus urticae. Ph.D. Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Zhang, L.; Zhang, G.; Wang, X.; Du, A.; Li, J.; Chang, Y.; Xu, N. Screening of Strains for Organophosphorus-degradation in Soil. J. Shandong Agric. Univ. 2019, 50, 774–777. [Google Scholar]
- Arnen, D.I. Copper enzymes in isolated chloroplasts: Polyphenol oxidase in Beta dulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, J.; Jiang, T. Study on the content of phytic acid in winter and spring wheat bran in Xinjiang. J. Urumqi Adult Educ. Inst. 1997, 1, 55–58. [Google Scholar]
- Hu, X.; Wu, Q.; Huang, B.; Yan, Z.; Tang, X.; Dong, Q. Determination of phospholipid in different germplasm of Polygonummultiflorum by molybdenum blue colorimetry. Pop. Technol. 2014, 16, 92–94. [Google Scholar]
- Rfaki, A.; Zennouhi, O.; Aliyat, F.Z.; Nassiri, L.; Ibijbijen, J. Isolation, selection and characterization of root-associated rock phosphate solubilizing bacteria in moroccan wheat (Triticum aestivum L.). Geomicrobiol. J. 2020, 37, 230–241. [Google Scholar] [CrossRef]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; He, D. Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 2020, 11, 510229. [Google Scholar] [CrossRef]
- Sun, Q.; He, Z.; Ouyang, J.; Zhang, P.; Ji, X.; Chen, Y.; He, G. Composition and phosphorus solubilization ability of rhizosphere phosphate solubilizing bacteria in wild chestnut. Agric. Sci. Technol. 2023, 24, 56–64. (In English) [Google Scholar]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid.-Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef]
- Kin, K.Y.; Mcdonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352. [Google Scholar]
- Li, X.; Liu, C.; Zhao, H.; Gao, F.; Ji, G.; Hu, F.; Li, H. Similar positive effects of beneficial bacteria, nematodes and earthworms on soil quality and productivity. Appl. Soil Ecol. 2018, 130, 202–208. [Google Scholar]
- Ye, J.; Wei, X.; Guo, M.; Jiang, Y.; Dai, J. Preliminary screening of a phosphorus-solubilizing bacterium that does not produce “transparent zone” and its preliminary determination of phosphorus-solubilizing ability. Anhui Agric. Sci. Bull. 2016, 22, 38–39. [Google Scholar]
- Tian, X.; Han, X.; Huang, P.; Li, W.; Yang, Q.; Yang, M. Study on phosphate-solubilizing ability of a high efficiency phosphate-solubilizing bacterium in rhizosphere of Parashorea chinensis Wang Hsie. J. Hubei Agric. Sci. 2023, 62, 51–56. [Google Scholar]
- Sun, Y.; Ye, S.; Fan, G.; Zhang, Y.; Liu, X.; Wu, D.; Wang, F. Screening of Phosphate-solubilizing Bacteria in Wheat Field Soil and Its Phosphate-solubilizing Ability. Appl. Acta Agric. Boreali-Occi-Dent. Sin. 2022, 31, 379–387. [Google Scholar]
- Lin, Y.; Liu, J.; Lu, H.; Ding, Y.; Yan, L. Screening phosphate-solubilizing fungi from the mangrove rhizosphere and their effect on Aegiceras corniculatum seedling growth. J. Agro-Environ. Sci. 2022, 41, 950–958. [Google Scholar]
- Jiang, H.; Zhang, J.; Liang, Y.; Fan, Y.; He, H. Isolation and identification of phosphate-solubilizing fungi and analysis of their phosphate-solubilizing ability. Mod. Agric. Sci. Technol. 2024, 170–172. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, P.; Wang, T.; Chen, M.; Chi, X.; Wang, M.; Chen, N.; Pan, L. Isolation, Identification and Biological Identification of Phosphate-solubilizing Fungi (PSF). China Soil Agric. 2018, 49, 856–861. [Google Scholar]
- Li, X.; Zhang, D.; Zhao, F.; Jiang, Y.; Zeng, X.; Wang, X.; Cheng, L. Screening and Analysis of Two Strains of High Efficiency Phosphor-Soluble Bacteria. J. Microbiol. 1–10. Available online: http://kns.cnki.net/kcms/detail/21.1186.Q.20231116.1438.004.html (accessed on 17 March 2024).
- Liu, Z.; Li, Y.C.; Zhang, S.; Fu, Y.; Fan, X.; Patel, J.S.; Zhang, M. Characterization of phosphate-solubilizing bacteria isolated from calcareous soils. Appl. Soil Ecol. 2015, 96, 217–224. [Google Scholar] [CrossRef]
- Yang, T.; Li, L.; Shi, F. Isolation, mutagenesis, and organic acid secretion of a highly efficient Phosphate-solubilizing fungus. Front. Microbiol. 2022, 13, 793122. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.G.; Maqbool, F. Capability of Penicillium oxalicum y2 to release phosphate from different insoluble phosphorus sources and soil. Folia Microbiol. 2020, 66, 69–77. [Google Scholar] [CrossRef]
- He, D.; Geng, L.; Guo, J.; Lu, X.; Liu, W.; Li, B. Ability and mechanism of Penicillium oxalicum HB1solubilizing phosphates. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2020, 36, 255–265. [Google Scholar]
- Tian, D.; Wang, W.; Su, M.; Zheng, J.; Wu, Y.; Wang, S.; Hu, S. Remediation of lead-contaminated water by geological fluorapatite and fungus Penicillium oxalicum. Environ. Sci. Poll. 2018, 25, 21118–21126. [Google Scholar] [CrossRef]
- Rodrogues, K.; Rodrogues, B. Development of carrier based in vitro produced arbuscular mycorrhizal (AM) fungal inocula for organic agriculture. Ann. Adv. Agric. Sciences. 2017, 18, 471–483. [Google Scholar]
- Ling, Q.; Zhao, H.; Zhao, X. The Characteristics of dolubilizing rock phosphate by four isolates of bacteria and fungi. Microbiology 2002, 24–28. [Google Scholar] [CrossRef]
- Wang, W. Screening of Phosphorus-Solubilizing Fungus and Analysis of Its Phosphorus-Solubilizing Mechanism. J Trop. Agric. Sci. 2018, 38, 64–67. [Google Scholar]
- Qiao, X.; Wu, Z.; Wang, X. Phosphate-solubilizing characteristic of a Penicillium pinophilum strain JP-NJ4. Microbiol. Bull. 2014, 41, 1741–1748. [Google Scholar]
- LI, T.; Fu, Z.; LI, X. Effects of Inoculation of Arbuscular Mycorrhizal Fungi and Phosphate-solubilizing Bacteria on Maize Growth and Phosphorous Nutrient Uptake in Low Phosphorous Field. Chin. J. Soil Sci. 2017, 48, 922–929. [Google Scholar]
- Yadav, R.S.; Tarafdar, J.C. Phytase and phosphatase producing fungi in arid and semi-arid soils and their efficiency in hydrolyzing different organic P compounds. Soil Biol. Biochem. 2003, 35, 745–751. [Google Scholar] [CrossRef]
- Zhan, X.; Han, X.; Yang, J.; Liu, X.; Ma, L. Effects of different nitrogen, phosphorus and potassium application rates on the dynamic changes of dry matter accumulation in maize source and sink. Soil Bulletin. 2007, 495–499. [Google Scholar] [CrossRef]
- Geng, X.; Wu, H.; Fu, P.; Liu, G.; Gao, Z. Effect of Phosphate Fertilizer on Seed and Hay Yield of Avena sativa in Linxia of Gansu Province. Acta Agrestia Sin. 2023, 31, 813–818. [Google Scholar]
- Li, D.; Shuang, H.; Wei, F.; Na, N.; Yan, L. Screening, identification, and phosphate solubilizing characteristics of a new efficient phosphate solubilizing fungus. Chin. J. Appl. Ecol. 2019, 30, 2384–2392. [Google Scholar]
- Wu, H.; Yu, M.; Feng, J.; Liu, H. Effect of Phosphorus Solubilizing Bio-fertilizer on Soil Phosphorus Availability and Pepper Yield in Greenhouse. J. Agric. Sci. Technol. 2023, 25, 189–197. [Google Scholar]
- Xu, H.; Lv, J.; Yu, C. Combined phosphate-solubilizing microorganisms jointly promote Pinus massoniana growth by modulating rhizosphere environment and key biological pathways in seedlings. Ind. Crops Products. 2023, 191, 116005. [Google Scholar] [CrossRef]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef]
- Wooley, S.C.; Paine, T.D. Infection by mycorrhizal fungi increases natural enemy abundance on tobacco (Nicotiana rustica). Environ. Entomol. 2011, 40, 36–41. [Google Scholar] [CrossRef]
- Zhao, X.; Deng, Y.; Chen, S.; Zhang, B. Effects of Nitrogen Combined with Phosphate Applying on Yield and Water and Fertilizer Use Efficiency of Tatary Buckwheat in Dryland. J. North China Agric. Sci. 2016, 31, 350–355. [Google Scholar]
- Cruz-Ramírez, A.; Oropeza-Aburto, A.; Razo-Hernández, F.; Ramírez-Chávez, E.; Herrera-Estrella, L. Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2006, 103, 6765–6770. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. (Eds.) Biochemistry and Molecular Biology of Plants; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Yang, S.; Yu, W.; He, Y.; Zhu, Q.; Wu, C.; Lin, H.; Li, J. Effects of three phosphate-solubilizing bacteria on photosynthetic physiological characteristics of Chinese fir seedlings. J. Xiamen Univ. (Nat. Sci. ) 2023, 62, 865–872. [Google Scholar]
- Song, W.; Gong, S.; Wu, Y. Effects of N, P, K fertilizers on pigment content of Coleus blumei. Mod. Hortic. 2012, 6. [Google Scholar] [CrossRef]
- Koczorski, P.; Furtado, B.U.; Baum, C.; Weih, M.; Ingvarsson, P.; Hulisz, P.; Hrynkiewicz, K. Large effect of phosphate-solubilizing bacteria on the growth and gene expression of Salix spp. at low phosphorus levels. Front. Plant Sci. 2023, 14, 1218617. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y.; Wang, S.; Liang, J. Effects of phosphate solubilizing bacteria on the growth, photosynthesis, and nutrient uptake of Camellia oleifera Abel. Forests 2019, 10, 348. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, F. Study on root morphological characteristics of wheat under phosphorus deficiency stress. Chin. J. Appl. Ecolocy 2002, 13, 295–299. [Google Scholar]
- Anuardha, M.; Narayanan, A. Promotion of root elongation by phosphorus deficiency. Plant Soil 1991, 136, 273–275. [Google Scholar] [CrossRef]
- Ueda, Y.; Yanagisawa, S. Perception, transduction, and integration of nitrogen and phosphorus nutritional signals in the transcriptional regulatory network in plants. J. Exp. Bot. 2019, 70, 3709–3717. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wu, X.; Zhou, L.; Li, Z.; Huang, K. Effects of phosphate fertilizer on filling characteristics and plumpness of tartary buckwheat. Acta Agric. Shanghai 2020, 36, 29–34. [Google Scholar]
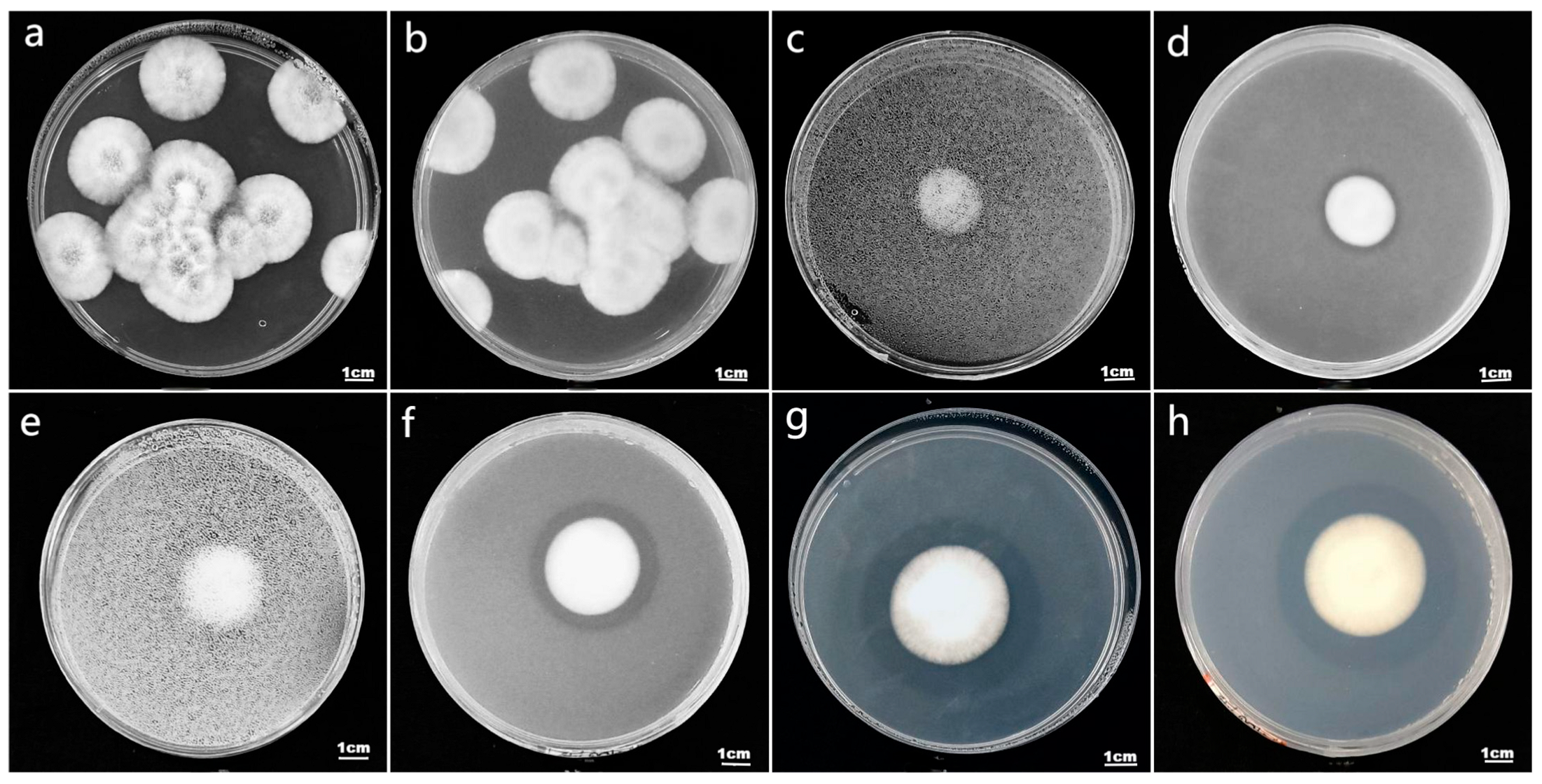


| Code in Paper | PSI | ||
|---|---|---|---|
| 6 d | 8 d | 10 d | |
| 1 | 1.14 ± 0.05 b | 1.21 ± 0.00 cd | 1.52 ± 0.08 ab |
| 2 | 1.58 ± 0.02 a | 1.84 ± 0.11 a | 1.48 ± 0.24 ab |
| 3 | 1.27 ± 0.27 ab | 1.24 ± 0.24 bcd | 1.27 ± 0.27 bc |
| 4 | 1.11 ± 0.03 b | 1.12 ± 0.06 d | 1.24 ± 0.08 bc |
| 5 | 1.05 ± 0.02 b | 1.11 ± 0.07 d | 1.21 ± 0.11 bc |
| 6 | 1.53 ± 0.03 a | 1.56 ± 0.11 abc | 1.00 ± 0.00 bc |
| 7 | 1.32 ± 0.11 ab | 1.58 ± 0.11 ab | 1.86 ± 0.07 a |
| 8 | 1.14 ± 0.04 b | 1.18 ± 0.07 cd | 1.19 ± 0.05 bc |
| 9 | 1.07 ± 0.04 b | 1.16 ± 0.09 d | 1.25 ± 0.13 bc |
| 10 | 1.03 ± 0.03 b | 1.08 ± 0.05 d | 1.13 ± 0.09 bc |
| 11 | 1.56 ± 0.28 a | 1.60 ± 0.30 ab | 1.33 ± 0.33 bc |
| 12 | 1.12 ± 0.04 b | 1.20 ± 0.07 cd | 1.27 ± 0.10 bc |
| 13 | 1.02 ± 0.02 b | 1.05 ± 0.02 d | 1.07 ± 0.04 bc |
| 14 | 1.08 ± 0.02 b | 1.14 ± 0.05 d | 1.13 ± 0.05 bc |
| 15 | 0.00 ± 0.00 b | 0.00 ± 0.00 e | 0.00 ± 0.00 d |
| 16 | 1.11 ± 0.00 b | 1.19 ± 0.01 cd | 1.29 ± 0.04 bc |
| 17 | 1.56 ± 0.05 a | 1.70 ± 0.03 a | 1.84 ± 0.10 a |
| 18 | 1.11 ± 0.03 b | 1.20 ± 0.10 cd | 1.18 ± 0.09 bc |
| Stage | Height (cm) | Stem Thick (mm) | Number of Stems | Number of Branches | Taproot Long (cm) | Leaf Area (cm2) | F. tataricum Yield (kg/m2) | |
|---|---|---|---|---|---|---|---|---|
| Seeding stage | CK | 48.23 ± 1.39 | 5.48 ± 0.14 | 10.00 ± 0.23 | 8.78 ± 0.40 | 87.06 ± 3.65 | 522.99 ± 20.09 | — |
| EG | 56.28 ± 0.56 * | 5.36 ± 0.17 | 10.33 ± 0.24 | 8.78 ± 0.40 | 108.06 ± 1.9 * | 610.96 ± 30.70 | — | |
| Filling stage | CK | 99.65 ± 1.29 | 6.81 ± 0.28 | 18.56 ± 0.18 | 10.44 ± 0.18 | 89.82 ± 3.82 | 3228.45 ± 153.81 | — |
| EG | 106.23 ± 1.58 * | 6.96 ± 0.19 | 19.00 ± 0.33 | 10.67 ± 0.24 | 115.81 ± 3.66 ** | 3429.11 ± 184.94 | — | |
| Maturation stage | CK | 104.16 ± 0.72 | 6.94 ± 0.29 | 20.00 ± 0.24 | 11.11 ± 0.31 | 98.45 ± 1.14 | 1340.7 ± 81.76 | 0.35 ± 0.01 |
| EG | 113.05 ± 1.2 ** | 6.62 ± 0.16 | 20.22 ± 0.40 | 11.11 ± 0.35 | 116.01 ± 1.96 ** | 1566.25 ± 108.05 | 0.79 ± 0.05 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Liu, C.; Gu, L.; Chen, Q.; Zhang, X. Studies on the Phosphorus-Solubilizing Ability of Isaria cateinannulata and Its Influence on the Growth of Fagopyrum tataricum Plants. Plants 2024, 13, 1694. https://doi.org/10.3390/plants13121694
Yang G, Liu C, Gu L, Chen Q, Zhang X. Studies on the Phosphorus-Solubilizing Ability of Isaria cateinannulata and Its Influence on the Growth of Fagopyrum tataricum Plants. Plants. 2024; 13(12):1694. https://doi.org/10.3390/plants13121694
Chicago/Turabian StyleYang, Guimin, Can Liu, Lingdi Gu, Qingfu Chen, and Xiaona Zhang. 2024. "Studies on the Phosphorus-Solubilizing Ability of Isaria cateinannulata and Its Influence on the Growth of Fagopyrum tataricum Plants" Plants 13, no. 12: 1694. https://doi.org/10.3390/plants13121694
APA StyleYang, G., Liu, C., Gu, L., Chen, Q., & Zhang, X. (2024). Studies on the Phosphorus-Solubilizing Ability of Isaria cateinannulata and Its Influence on the Growth of Fagopyrum tataricum Plants. Plants, 13(12), 1694. https://doi.org/10.3390/plants13121694





