Investigation of a Perspective Urban Tree Species, Ginkgo biloba L., by Scientific Analysis of Historical Old Specimens
Abstract
1. Introduction
1.1. Origin of Ginkgo biloba (L.) and Its Emergence in the European Carpathian Basin
1.2. The Unique Feature of Ginkgo biloba (L.)
1.3. Ploidity and Genetic Background of Ginkgo biloba (L.)
1.4. Foliar Macromorphology and Histology
2. Results
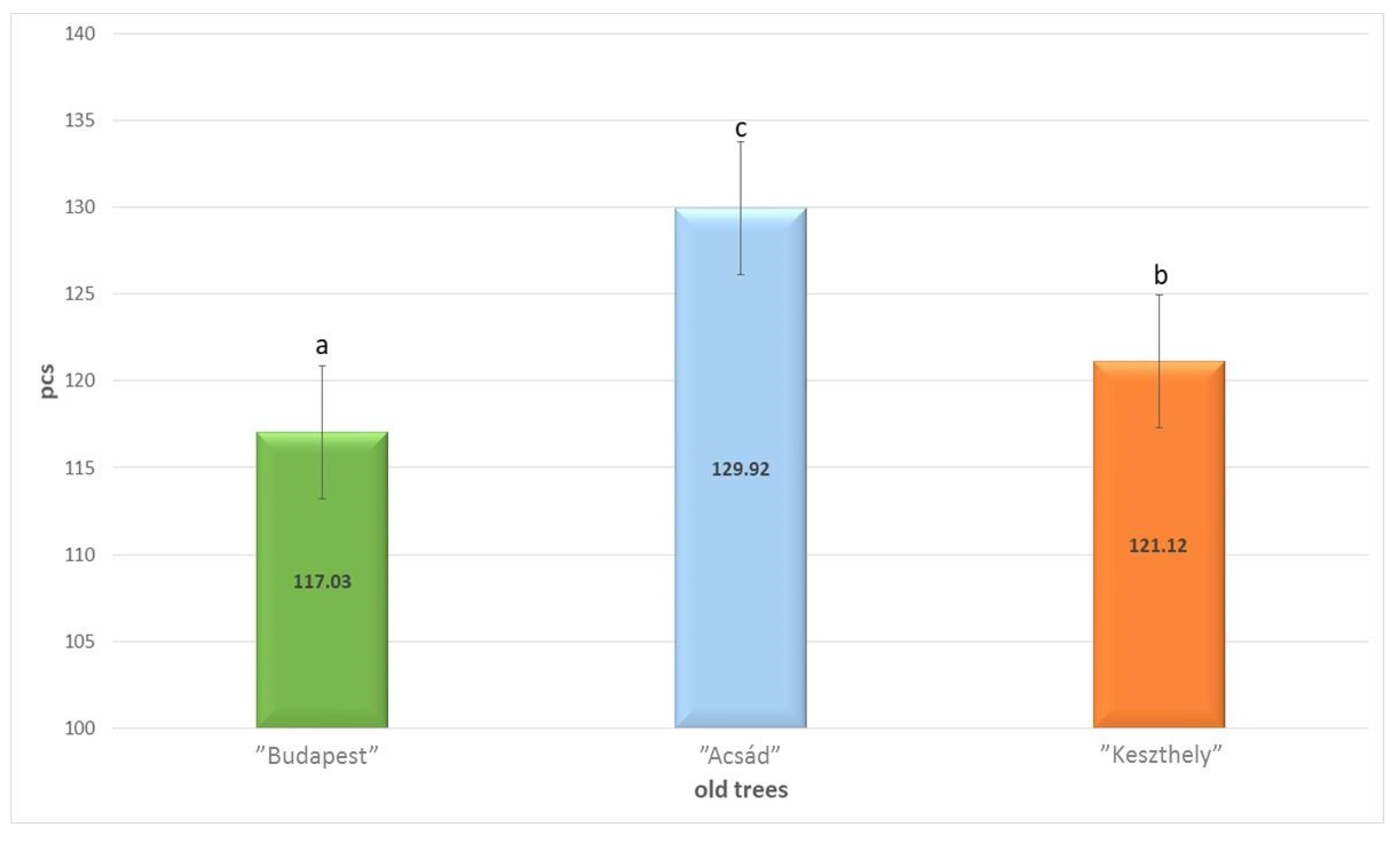
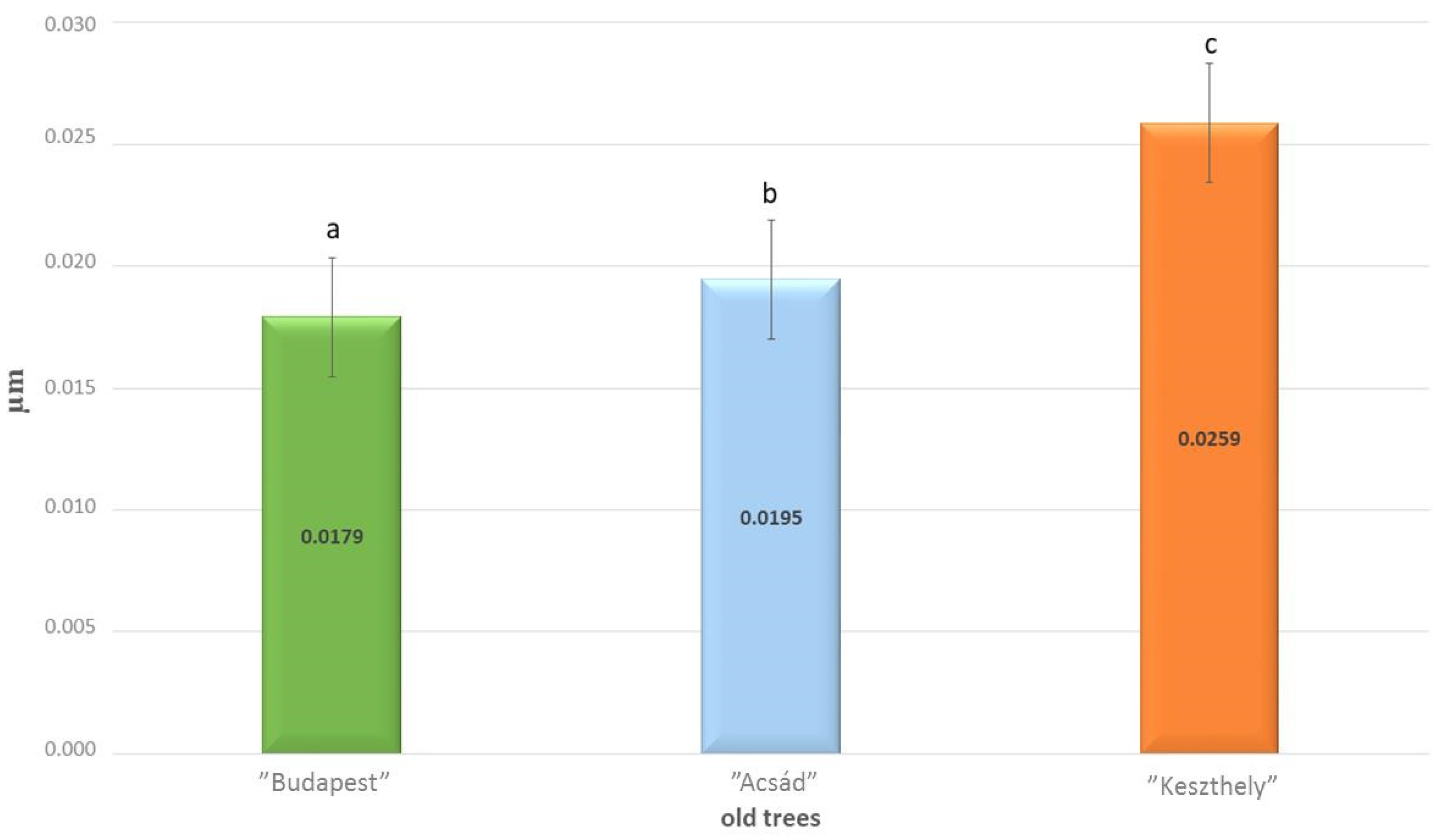
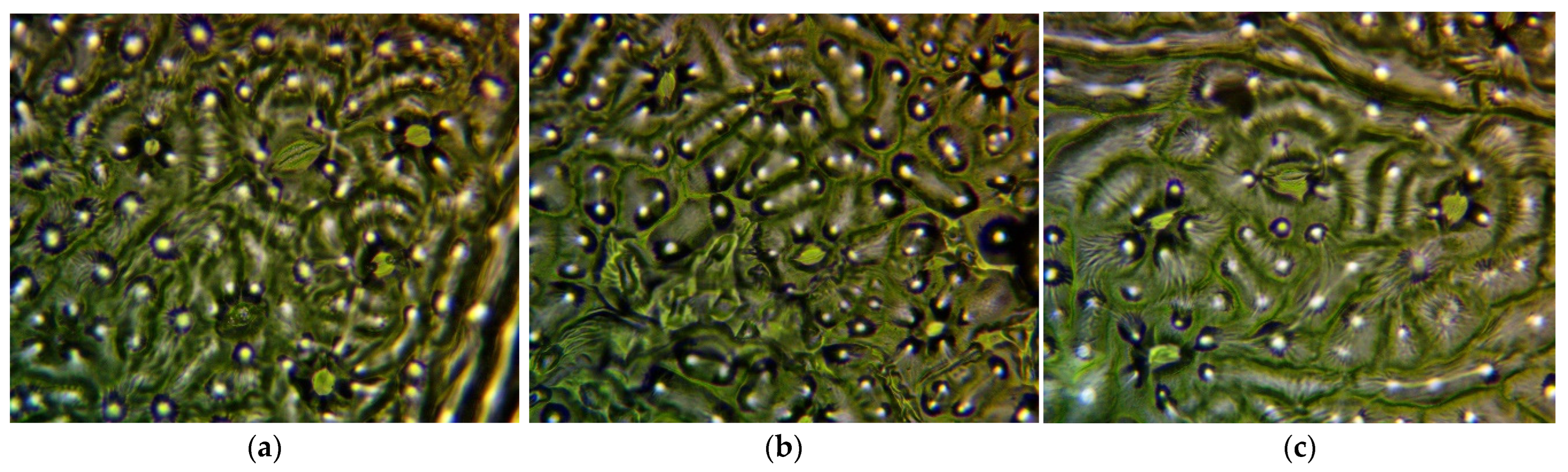
2.1. Micromorphology
2.2. Air Pollution Tolerance Index (APTI)
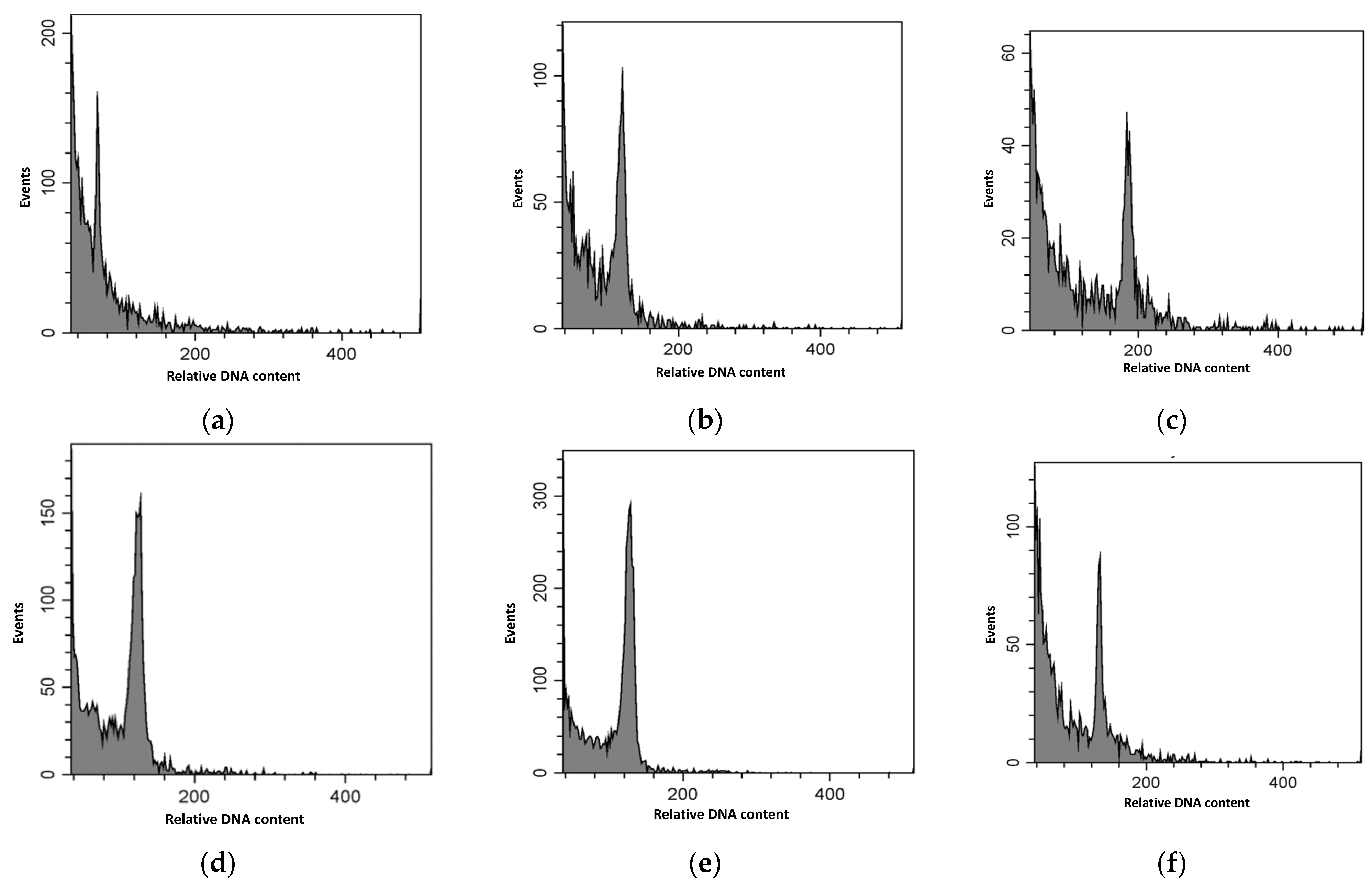
2.3. Determination of Ploidy Level in Gingko biloba Specimens
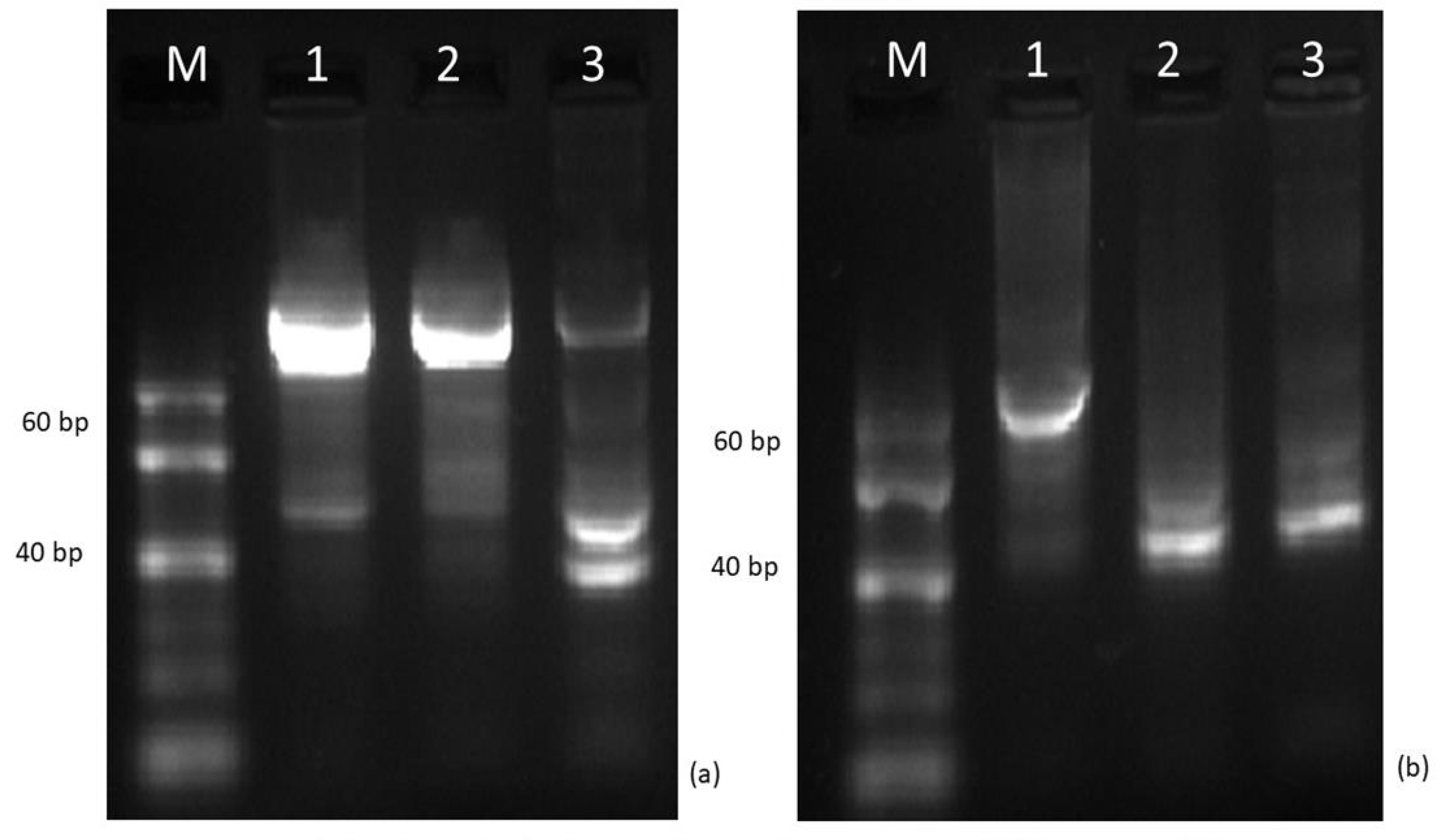
2.3.1. Genetic Evaluation Based on miRNA Markers
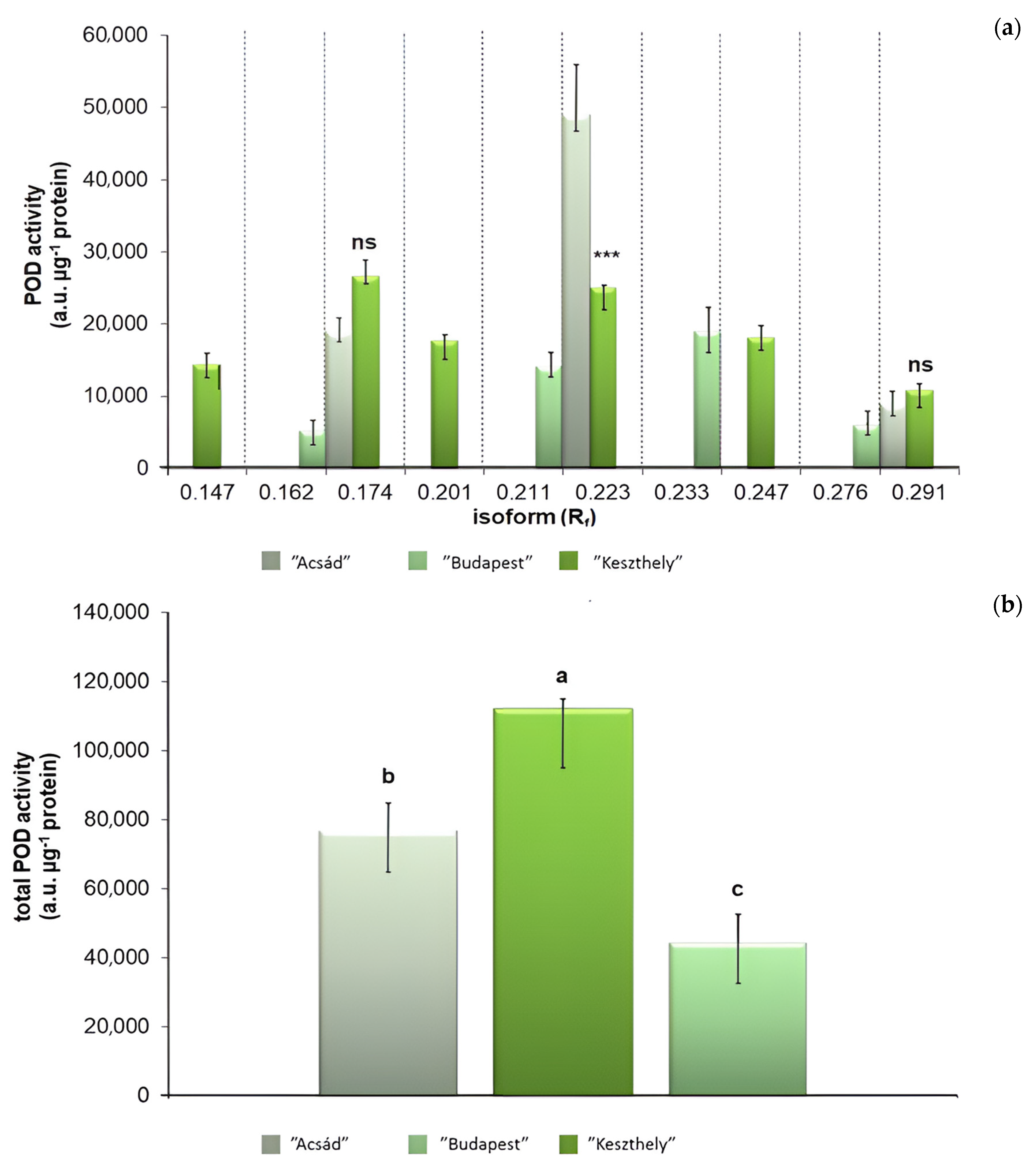
2.3.2. Determination of Stress Enzyme Activity
3. Discussion
3.1. Health and Physiological Status of the Old Trees
3.2. Origin of the Ginkgo biloba Individuals
3.3. Urban Stress Tolerance of Old the Ginkgo biloba Trees
4. Materials and Methods
4.1. Investigated Specimens and Their Locations
4.2. Density and Size of Stomata
4.3. Determination of Stress Enzyme Activity
4.4. Calculation of Air Pollution Tolerance Index
4.5. Analysis of miRNA-Based Markers
4.6. Flow Cytometric Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, C.Q.; Yang, Y.; Ohsawa, M.; Yi, S.R.; Momohara, A.; Su, W.H.; Wang, H.-C.; Zhang, Z.-Y.; Peng, M.-C.; Wu, Z.-L. Evidence for the persistence of wild Ginkgo biloba (Ginkgoaceae) populations in the Dalou Mountains, southwestern China. Am. J. Bot. 2012, 99, 1408–1414. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Fan, G.; Yin, P.P.; Sun, S.; Li, N.; Hong, X.; Hu, G.; Zhang, H.; Zhang, F.M.; Han, J.D.; et al. Resequencing 545 Ginkgo genomes across the world reveals the evolutionary history of the living fossil. Nat Commun. 2019, 10, 4201. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.X.; Xiang, Z.; Xiang, Y.H. Report on wild Ginkgo biloba in Qianzhong Altiplano–Guizhou ancient Ginkgo biloba germplasm resources investigation VIII. Guizhou Sci. 2007, 25, 47–55. [Google Scholar]
- Crane, P. Ginkgo: The Tree That Time Forgot; Yale University Press: New Haven, CT, USA, 2013; ISBN 978-0-300-18751-9. [Google Scholar]
- Fekete, A.; Sárospataki, M.; Takács, K. Landscape ecological and visual significance of dendrological gardens in the Carpathian Basin. Acta Univ. Sapientiae Agric. Environ. 2014, 6, 57–68. [Google Scholar]
- Papp, L. Az ELTE Füvészkert Története Alapításának 250. Évfordulójára; ELTE: Budapest, Hungary, 2021; ISBN 978-963-489-383-7. [Google Scholar]
- Csoma, Z. Kertészet és Polgárosodás, 1st ed.; Centrál Európa Alapítvány: Budapest, Hungary, 1997. [Google Scholar]
- Nakanishi, K. Terpene trilactones from Gingko biloba: From ancient times to the 21st century. BMC 2005, 13, 4987–5000. [Google Scholar]
- Gong, W.; Filatov, D.A. Evolution of the sex-determining region in Ginkgo biloba. Philos. Trans. R Soc. B 2022, 377, 20210229. [Google Scholar] [CrossRef] [PubMed]
- Trémouillaux-Guiller, J.; Rohr, T.; Rohr, R.; Huss, V.A. Discovery of an endophytic alga in Ginkgo biloba. Am. J. Bot. 2002, 89, 727–733. [Google Scholar] [CrossRef]
- Ražná, K.; Hrubík, P. Cultural extension of Ginkgo biloba L. in Slovakia. Agrobiodivers. Improv. Nutr. Health Life Qual. 2021, 5. [Google Scholar] [CrossRef]
- Crane, P.R. An evolutionary and cultural biography of Ginkgo. Plants People Planet 2019, 1, 32–37. [Google Scholar] [CrossRef]
- Teja, I.; Madhavi, M. Ginkgo-Facts About Living Fossil Tree. Krishi Sci. 2021, 2, 9–11. [Google Scholar]
- Hizume, M. Chromosomes of Ginkgo biloba. In Ginkgo Biloba, A Global Treasure: From Biology to Medicine, 1st ed.; Hori, T., Ridge, R.W., Tulecke, W., Del Tredici, P., Trémouillaux-Guiller, J., Tobe, H., Eds.; Springer Repring: London, UK, 1997; ISBN 978-4431684183. [Google Scholar]
- Guan, R.; Zhao, Y.; Zhang, H.; Fan, G.; Liu, X.; Zhou, W.; Shi, C.; Wang, J.; Liu, W.; Liang, X.; et al. Draft genome of the living fossil Ginkgo biloba. Gigascience 2016, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Wang, G.; Cui, P.; Wu, S.; Ai, C.; Hu, N.; Li, A.; He, B.; Shao, X.; et al. The nearly complete genome of Ginkgo biloba illuminates gymnosperm evolution. Nat. Plants 2021, 7, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Li, W.H.; Lin, C.F.; Wu, H.R.; Zhao, Y.P. International biological flora: Ginkgo biloba. J. Ecol. 2022, 110, 951–982. [Google Scholar] [CrossRef]
- Šmarda, P.; Veselý, P.; Šmerda, J.; Bureš, P.; Knápek, O.; Chytrá, M. Poliploidia egy „élő kövületben” a Ginkgo biloba. New Phitologist 2016, 212, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Šmarda, P.; Horová, L.; Knápek, O.; Dieck, H.; Dieck, M.; Ražná, K.; Hrubík, P.; Orlóci, L.; Papp, L.; Veselá, K.; et al. Multiple haploids, triploids, and tetraploids found in modern-day “living fossil” Ginkgo biloba. Hortic. Sci. 2018, 5, 55. [Google Scholar] [CrossRef]
- Zhao, Y.; Paule, J.; Fu, C.; Koch, M.A. Out of China: Distribution history of Ginkgo biloba L. Taxon 2010, 59, 495–504. [Google Scholar] [CrossRef]
- Chinn, E.; Silverthorne, J. Light-dependent chloroplast development and expression of a light-harvesting chlorophyll a/b-binding protein gene in the gymnosperm Ginkgo biloba. Plant Physiol. 1993, 103, 727–732. [Google Scholar] [CrossRef]
- Palmer, J.D.; Stein, D.B. Conservation of chloroplast genome structure among vascular plants. Curr. Genet. 1986, 10, 823–833. [Google Scholar] [CrossRef]
- Bainian, S.; Dilcher, D.L.; Beerling, D.J.; Chengjun, Z.; Defei, Y.; Elizabeth, K. Variation in Ginkgo biloba L. leaf characters through a climatic gradient in China. Proc. Nat. Acad. Sci. USA 2003, 100, 7141–7146. [Google Scholar]
- Kiani-Pouya, A.; Rasouli, F.; Bazihizina, N.; Zhang, H.; Hedrich, R.; Shabala, S. A large-scale screening of quinoa accessions reveals an important role of epidermal bladder cells and stomatal patterning in salinity tolerance. EEB 2019, 168, 103885. [Google Scholar] [CrossRef]
- Panda, A.; Sahu, N.; Behera, S.; Sayama, T.; Sahu, L.; Avtar, R.; Singh, R.B.; Yamada, M. Impact of climate variability on crop yield in Kalahandi, Bolangir, and Koraput districts of Odisha, India. Climate 2019, 7, 126. [Google Scholar] [CrossRef]
- Maácz, G.J. Über die Blattepidermis von Ginkgo biloba L.; Akadémiai Kiadó: Cambridge, MA, USA, 1957. [Google Scholar]
- Kausik, S.B. The stomata of Ginkgo biloba L., with comments on some noteworthy features. Bot. J. Linn. Soc. 1974, 69, 137–146. [Google Scholar] [CrossRef]
- Chen, L.Q.; Li, C.S.; Chaloner, W.G.; Beerling, D.J.; Sun, Q.G.; Collinson, M.E.; Mitchell, P.L. Assessing the potential for the stomatal characters of extant and fossil Ginkgo leaves to signal atmospheric CO2 change. Am. J. Bot. 2001, 88, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Conde, G.D. Stomatal Index of Ginkgo biloba as a Proxy for Atmospheric CO2. Ph.D. Thesis, University of Oregon, Eugene, OR, USA, 2016. [Google Scholar]
- de Freitas, J.M.B.; Kuhn, A.W.; Essi, L.; Tedesco, S.B. Differences in stomatal and pollen grain dimensions and pollen viability between Paspalum rawitscheri populations. Ciência Nat. 2020, 42, e46. [Google Scholar] [CrossRef]
- Hong, T.; Lin, H.; He, D. Characteristics and correlations of leaf stomata in different Aleurites montana provenances. PLoS ONE 2018, 13, e0208899. [Google Scholar] [CrossRef] [PubMed]
- Bheemanahalli, R.; Wang, C.; Bashir, E.; Chiluwal, A.; Pokharel, M.; Perumal, R.; Moghimi, N.; Ostmeyer, T.; Caragea, D.; Jagadish, S.K. Classical phenotyping and deep learning concur on genetic control of stomatal density and area in sorghum. Plant Physiol. 2021, 186, 1562–1579. [Google Scholar] [CrossRef] [PubMed]
- Dittberner, H.; Korte, A.; Mettler-Altmann, T.; Weber, A.P.; Monroe, G.; de Meaux, J. Natural variation in stomata size contributes to the local adaptation of water-use efficiency in Arabidopsis thaliana. Mol. Ecol. 2018, 27, 4052–4065. [Google Scholar] [CrossRef] [PubMed]
- Gond, M.; Dwivedi, D.H.; Maji, S.; Kishor, S. Leaf Morphology and Stomatal Anatomy Indicates Inter-Varietal Variability in Water Chestnut (Trapa natans var. bispinosa Roxb). Int. J. Minor Fruits Med. Aromat. Plants 2019, 5, 27–32. [Google Scholar]
- Ganguly, S.; Das, M.; Mukherjee, A. Anticipated Performance Index (API) of some selected phanerophytes considered for Green Belt Development. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 525–532. [Google Scholar]
- Singh, S.; Rao, D.; Agrawal, M.; Pandey, J.; Naryan, D. Air pollution tolerance index of plants. J. Environ. Manag. 1991, 32, 45–55. [Google Scholar] [CrossRef]
- Neutelings, G.; Fénart, S.; Lucau-Danila, A.; Hawkins, S. Identification and characterization of miRNAs and their potential targets in flax. J. Plant Physiol. 2012, 169, 1754–1766. [Google Scholar] [CrossRef]
- Barvkar, V.T.; Pardeshi, V.C.; Kale, S.M.; Qiu, S.Q.; Rollins, M.; Datla, R.; Gupta, V.S.; Kadoo, N.Y. Genome-wide identification and characterization of microRNA genes and their targets in flax (Linum usitatissimum). Planta 2013, 237, 1149–1161. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, G.; Yuan, H.; Cheng, L.; Zhao, D.; Huang, W.; Zhang, S.; Zhang, L.; Chen, H.; Zhang, J.; et al. Identification and characterization of miRNAs and targets in flax (Linum usitatissimum) under saline, alkaline, and saline-alkaline stresses. BMC Plant Biol. 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Ma, B.; Mason, A.S.; Xiao, M.; Wei, L.; An, Z. MicroRNA-based molecular markers: A novel PCR-based genotyping technique in Brassica species. Plant Breed. 2013, 132, 375–381. [Google Scholar] [CrossRef]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.L.; Hlavačková, L.; Miklášová, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant characterization, antimicrobial activities, and genomic MicroRNA based marker fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.H.; Shen, T.T.; Liu, W.J.; Wang, X.Q. Evolution of the bHLH genes involved in stomatal development: Implications for the expansion of developmental complexity of stomata in land plants. PLoS ONE 2013, 8, e78997. [Google Scholar] [CrossRef]
- Edwards, D.; Kerp, H.; Hass, H. Stomata in early land plants: An anatomical and ecophysiological approach. J. Exp. Bot. 1998, 49, 255–278. [Google Scholar] [CrossRef]
- Sapra, V.T.; Hughes, J.L.; Sharma, G.C. Frequency, size, and distribution of stomata in triticale leaves. Crop Sci. 1975, 15, 356–358. [Google Scholar] [CrossRef]
- Yadav, C.B.Y.; Muthamilarasan, M.; Pandey, G.; Prasad, M. Development of novel microRNA-based genetic markers in foxtail millet for genotyping applications in related grass species. Mol. Breed. 2014, 34, 2219–2224. [Google Scholar] [CrossRef]
- Solti, Á.; Sárvári, É.; Szöllősi, E.; Tóth, B.; Mészáros, I.; Fodor, F.; Szigeti, Z. Stress hardening under long-term cadmium treatment is correlated with the activation of antioxidative defence and iron acquisition of chloroplasts in Populus. ZNC 2016, 71, 323–334. [Google Scholar] [CrossRef]
- Hill, K.E.; Guerin, G.R.; Hill, R.S.; Watling, J.R. Temperature influences stomatal density and maximum potential water loss through stomata of Dodonaea viscosa subsp. angustissima along a latitude gradient in southern Australia. Aust. J. Bot. 2015, 62, 657–665. [Google Scholar]
- Sárospataki, M. Dendrological Gardens in 19th Century Garden Architecture in Hungary. Doktori. Ph.D. Thesis, BCE, Tájépítészeti és Tájökológiai Doktori Iskola, Budapest, Hungary, 2014. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, É.; Gellén, G.; Sági-Kazár, M.; Schlosser, G.; Solymosi, K.; Solti, Á. Qualitative and quantitative evaluation of thylakoid complexes separated by Blue Native PAGE. Plant Methods 2022, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Molnár, V.É.; Simon, E.; Tóthmérész, B.; Ninsawat, S.; Szabó, S. Air pollution induced vegetation stress—The Air Pollution Tolerance Index as a quick tool for city health evaluation. Ecol. Ind. 2020, 113, 106234. [Google Scholar] [CrossRef]
- Molnár, V.É.; Tőzsér, D.; Szabó, S.; Tóthmérész, B.; Simon, E. Use of leaves as bioindicator to assess air pollution based on composite proxy measure (APTI), dust amount and elemental concentration of metals. Plants 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Molnár, V.É.; Lajtos, D.; Bibi, D.; Tóthmérész, B.; Szabó, S. Usefulness of Tree Species as Urban Health Indicators. Plants 2021, 10, 2797. [Google Scholar] [CrossRef]
- Htwe, N.M.P.S.; Luo, Z.Q.; Jin, L.G.; Nadon, B.; Wang, K.J.; Qiu, L.J. Functional marker development of miR1511- InDel and allelic diversity within the genus glycine. MBC Genom. 2015, 16, 467. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Ashman, T.L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Zhang, M.; Li, W.; Luo, K.; Lu, Z.; Zhang, C.; Jin, B. Identification and characterization of microRNA expression in Ginkgo biloba L. leaves. Tree Genet. Genomes 2015, 11, 76. [Google Scholar] [CrossRef]



| Specimen | RWC | AAC | pH | Chlorophyll | APTI | Ganguly et al. [35] | Singh et al. [36] |
|---|---|---|---|---|---|---|---|
| “Acsád” | 73.384 | 4.625 | 4.28 | 15.653 | 17 | intermediate | intermediate |
| “Budapest” | 82 | 2.635 | 4.517 | 17.710 | 14 | sensitive | sensitive |
| “Keszthely” | 83.513 | 3.787 | 4.70 | 21.982 | 18 | intermediate | intermediate |
| Markers | Sequences |
|---|---|
| lus miR168_F | CACGCATCGCTTGGTGCAGGT |
| lus miR168_R | CCAGTGCAGGGTCCGAGGTA |
| lus miR408_F | GGCTGGGAACAGACAGAGCATGGA |
| lus miR408_R | GGGAAAAAGGCCAGGGAAGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisvarga, S.; Hamar-Farkas, D.; Horotán, K.; Gyuricza, C.; Ražná, K.; Kučka, M.; Harenčár, Ľ.; Neményi, A.; Lantos, C.; Pauk, J.; et al. Investigation of a Perspective Urban Tree Species, Ginkgo biloba L., by Scientific Analysis of Historical Old Specimens. Plants 2024, 13, 1470. https://doi.org/10.3390/plants13111470
Kisvarga S, Hamar-Farkas D, Horotán K, Gyuricza C, Ražná K, Kučka M, Harenčár Ľ, Neményi A, Lantos C, Pauk J, et al. Investigation of a Perspective Urban Tree Species, Ginkgo biloba L., by Scientific Analysis of Historical Old Specimens. Plants. 2024; 13(11):1470. https://doi.org/10.3390/plants13111470
Chicago/Turabian StyleKisvarga, Szilvia, Dóra Hamar-Farkas, Katalin Horotán, Csaba Gyuricza, Katarína Ražná, Matúš Kučka, Ľubomír Harenčár, András Neményi, Csaba Lantos, János Pauk, and et al. 2024. "Investigation of a Perspective Urban Tree Species, Ginkgo biloba L., by Scientific Analysis of Historical Old Specimens" Plants 13, no. 11: 1470. https://doi.org/10.3390/plants13111470
APA StyleKisvarga, S., Hamar-Farkas, D., Horotán, K., Gyuricza, C., Ražná, K., Kučka, M., Harenčár, Ľ., Neményi, A., Lantos, C., Pauk, J., Solti, Á., Simon, E., Bibi, D., Mukherjee, S., Török, K., Tilly-Mándy, A., Papp, L., & Orlóci, L. (2024). Investigation of a Perspective Urban Tree Species, Ginkgo biloba L., by Scientific Analysis of Historical Old Specimens. Plants, 13(11), 1470. https://doi.org/10.3390/plants13111470










