ATP Hydrolases Superfamily Protein 1 (ASP1) Maintains Root Stem Cell Niche Identity through Regulating Reactive Oxygen Species Signaling in Arabidopsis
Abstract
1. Introduction
2. Results
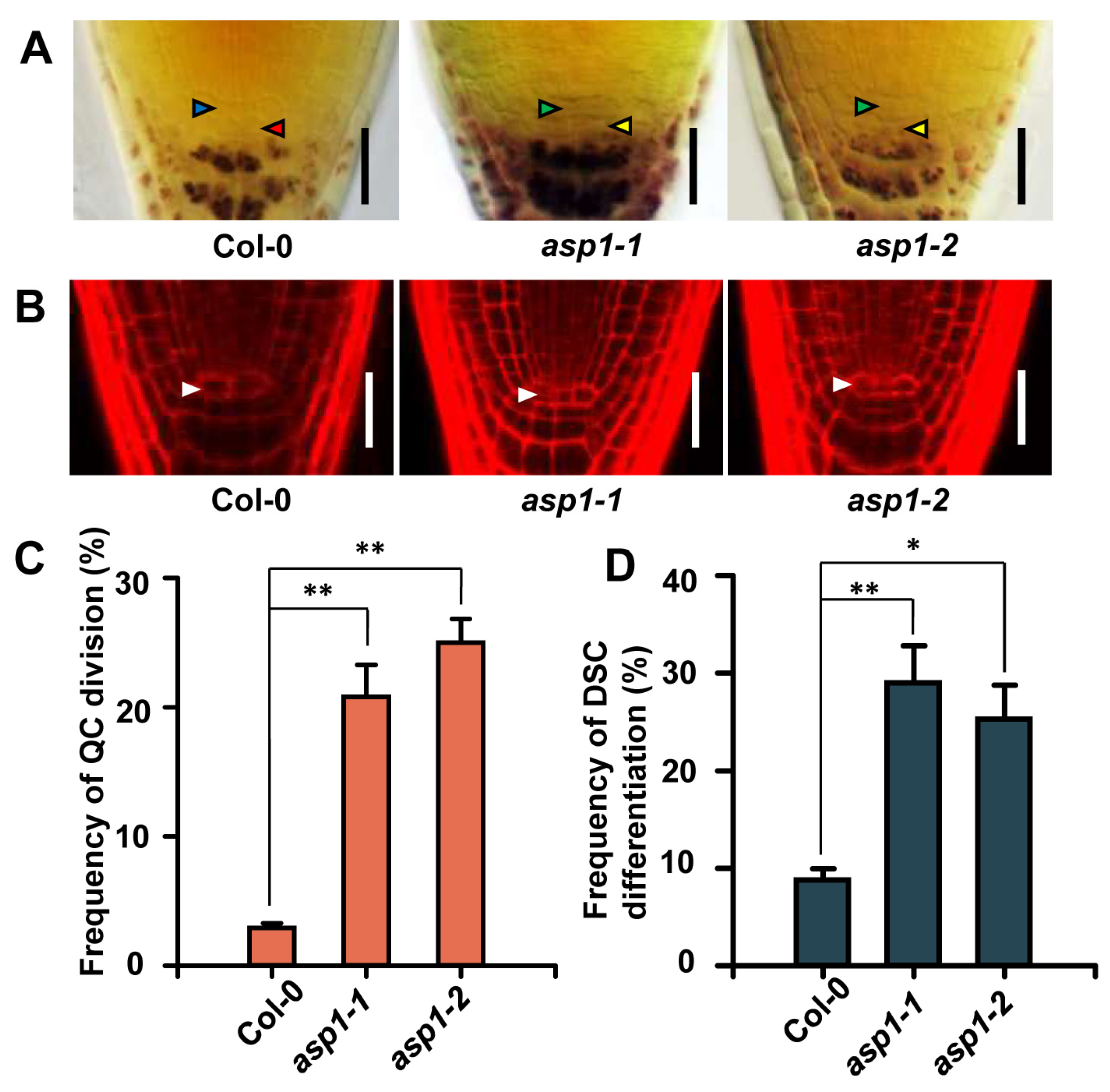
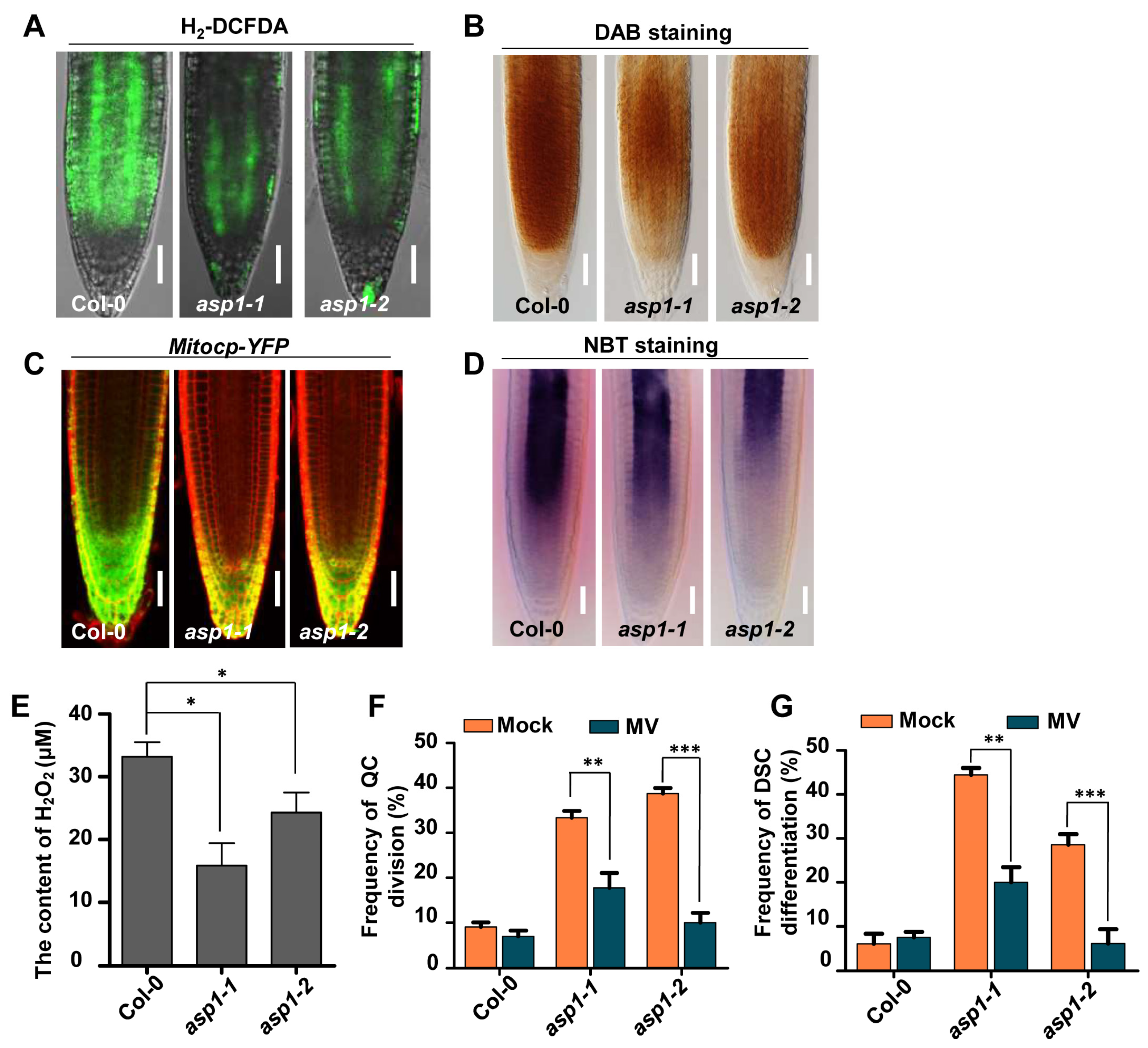
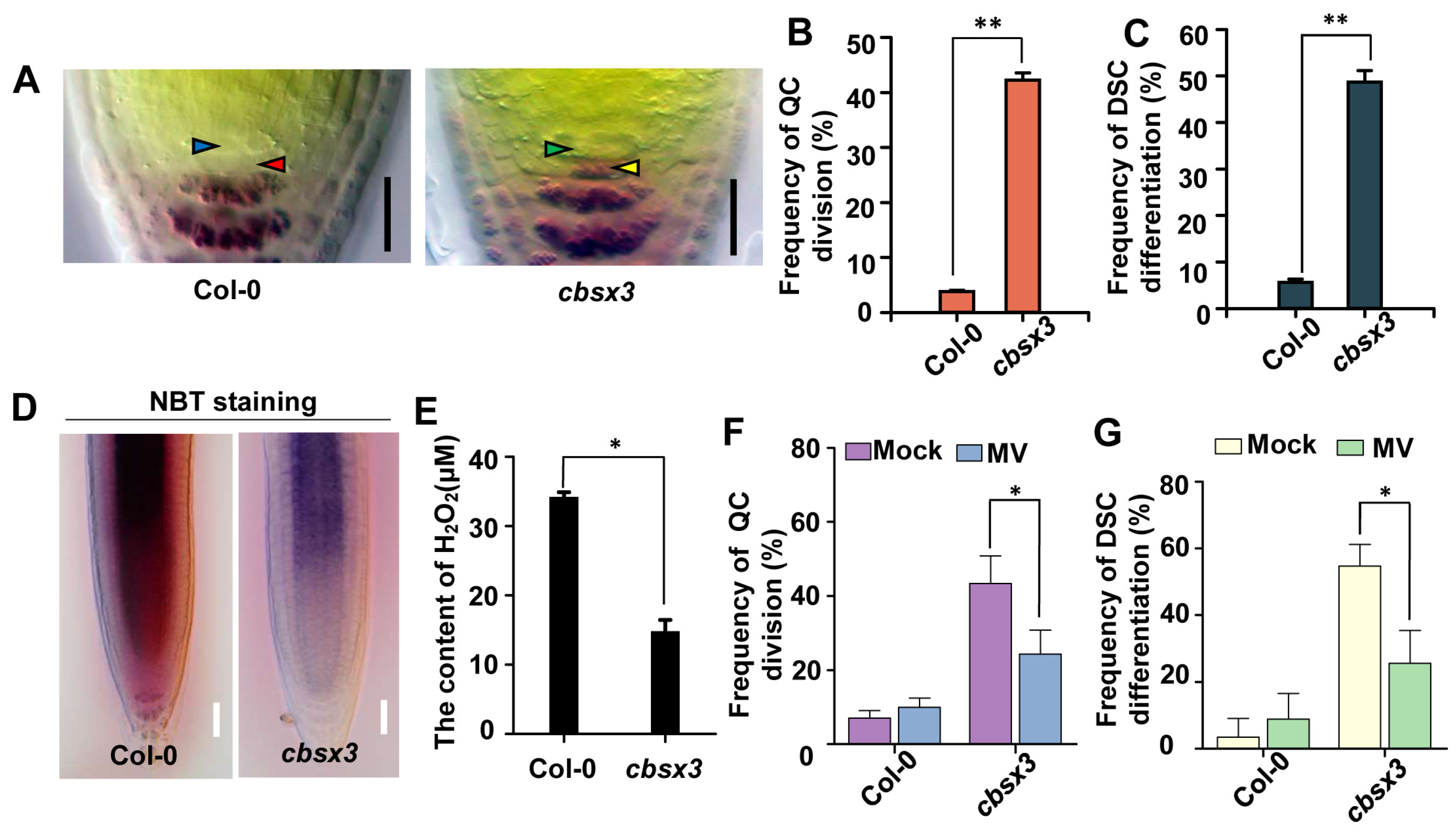
2.1. ASP1 Plays a Role in ROS Maintenance to Control RSCN Identity
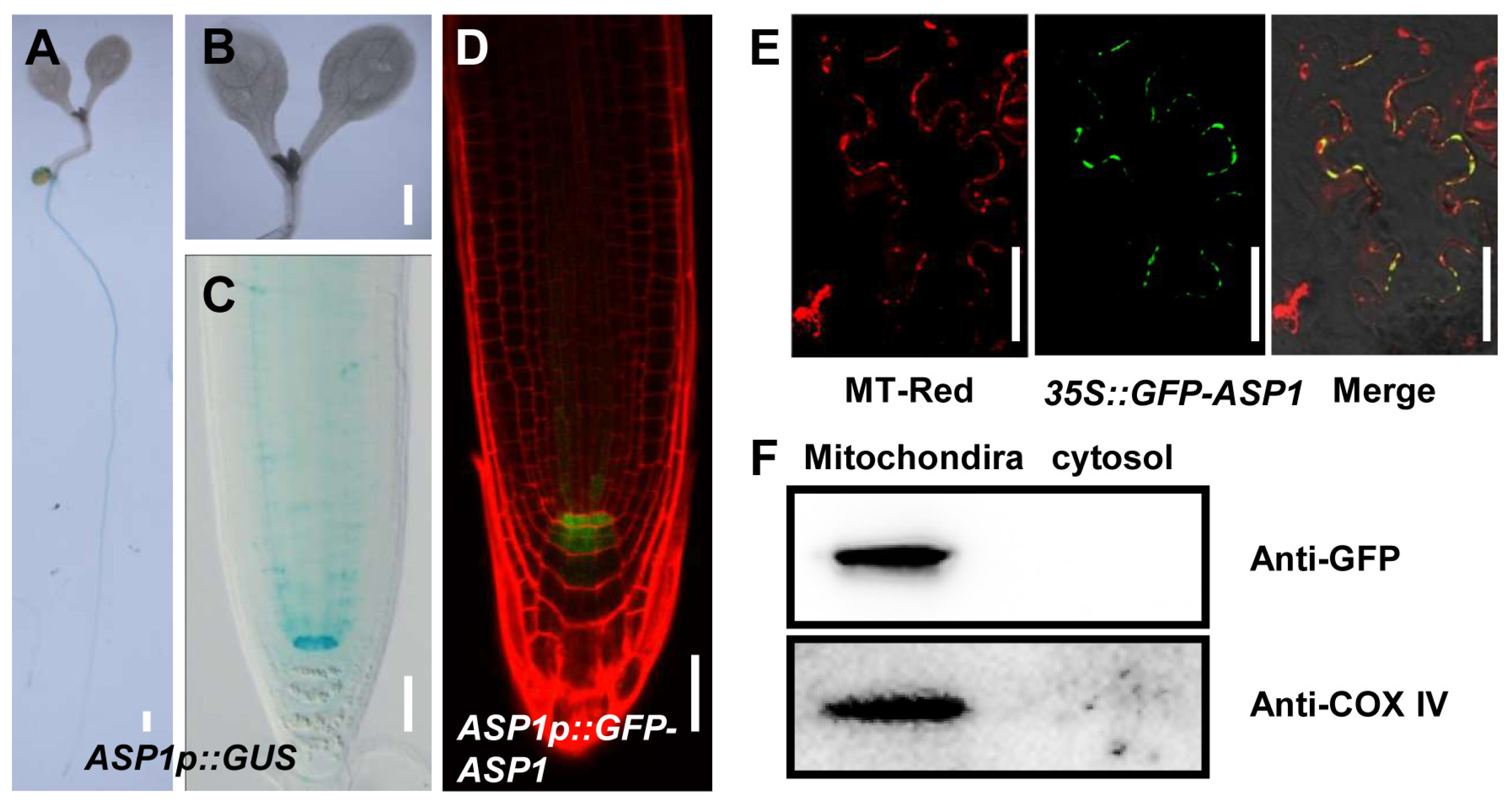
2.2. ASP1 Is Exclusively Expressed in QC Cells and Localized in Mitochondria
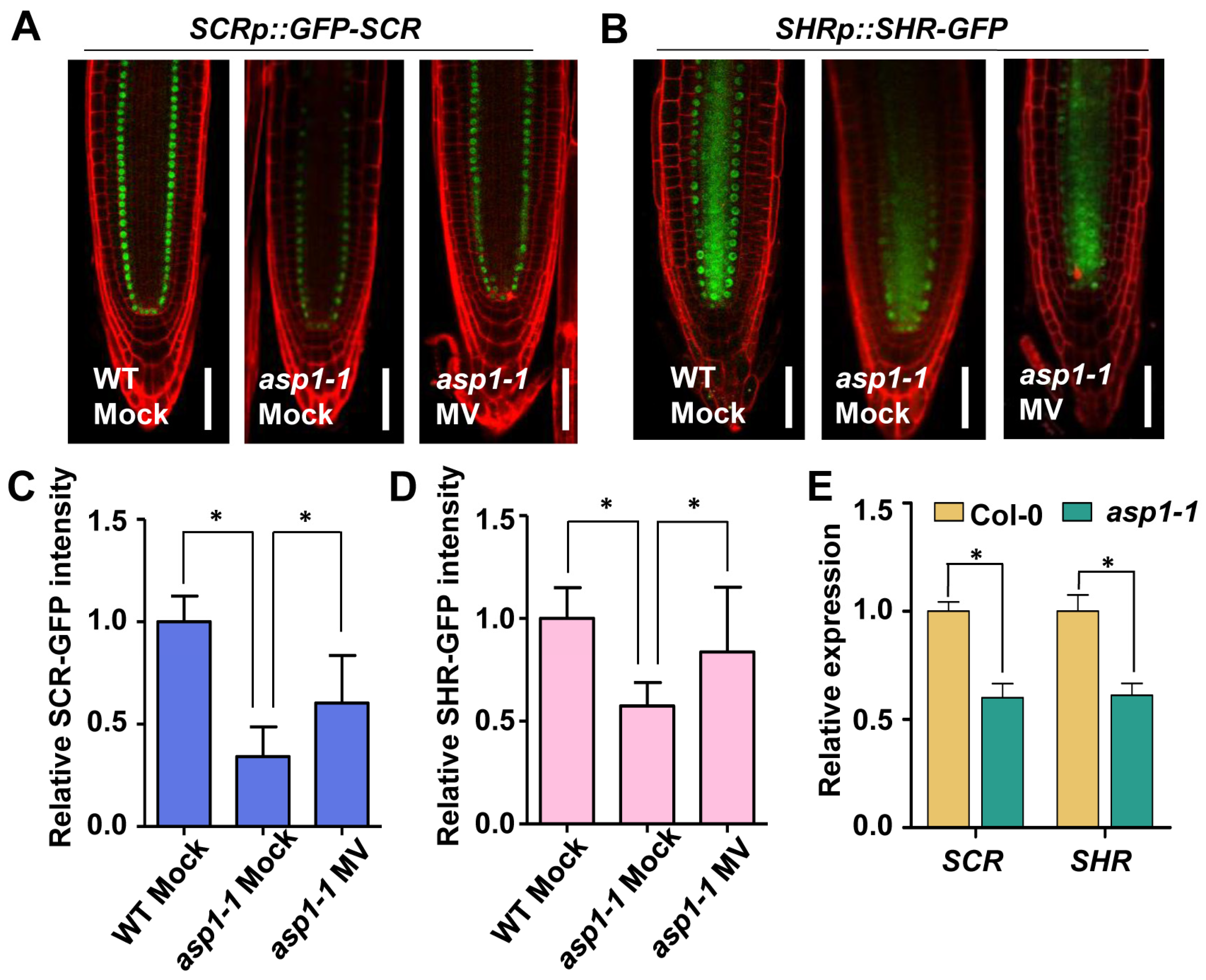
2.3. SCR and SHR Are Implicated in ASP1-Mediated ROS Homeostasis Regulating the SCN Maintenance
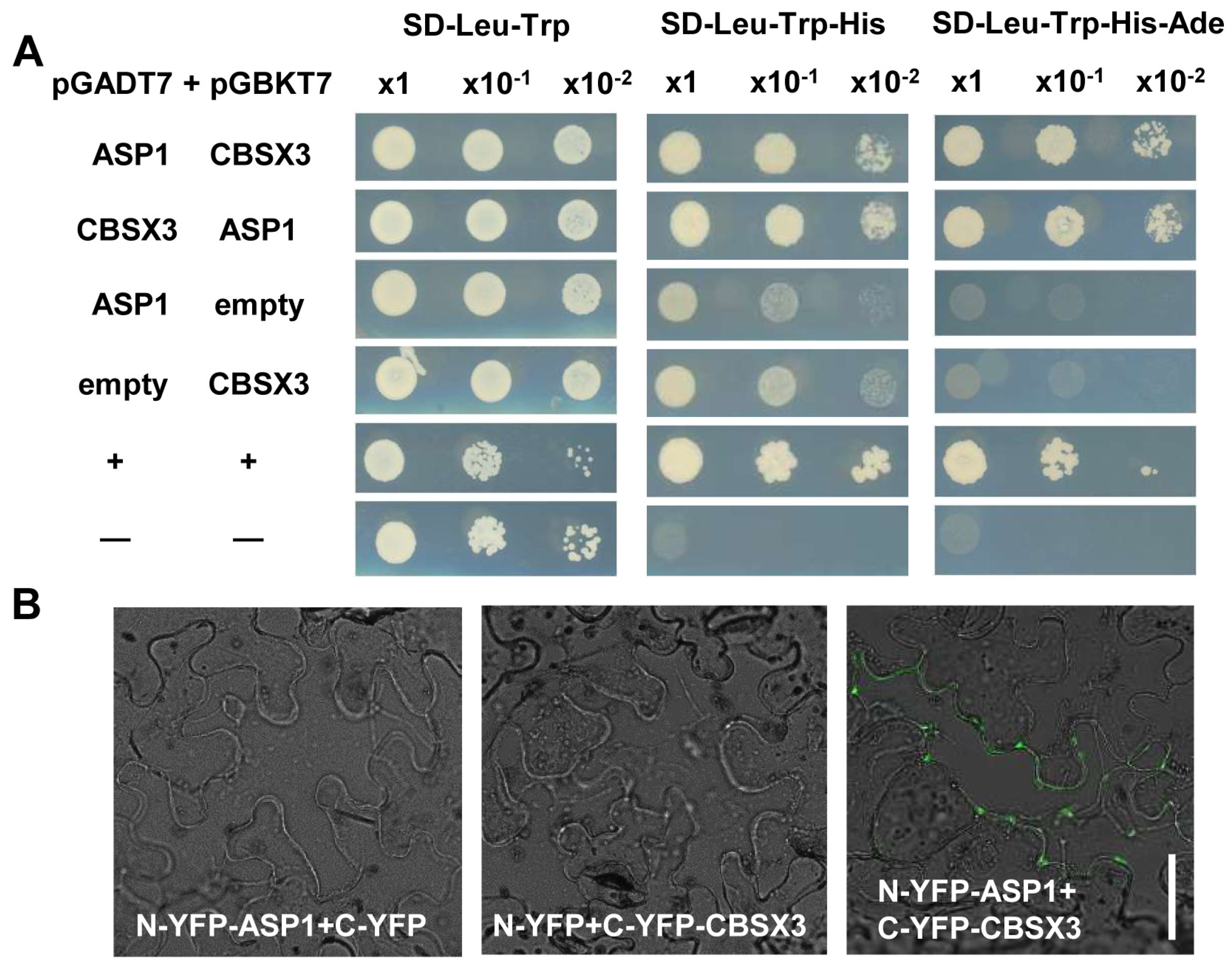
2.4. ASP1 Interacts Directly with CBSX3 to Maintain Root SCN Stability via Manipulating ROS Generation
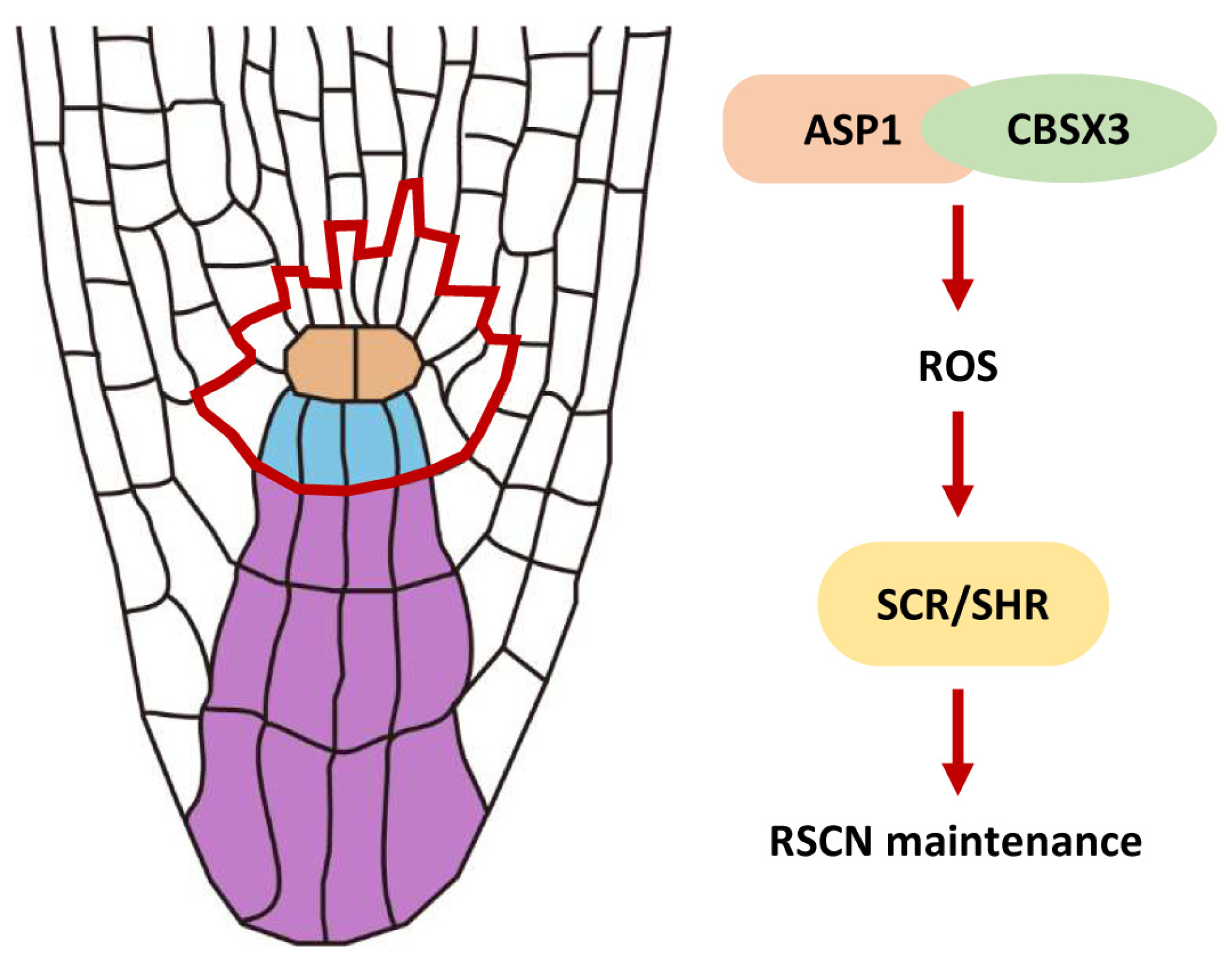
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Histochemical Assays and Microscopy
4.3. Western Blotting
4.4. Quantitative RT-PCR
4.5. Yeast Two-Hybrid Assay
4.6. BiFC Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ubogoeva, E.V.; Zemlyanskaya, E.V.; Xu, J.; Mironova, V. Mechanisms of stress response in the root stem cell niche. J. Exp. Bot. 2021, 72, 6746–6754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Feldman, L.J. Regulation of root apical meristem development. Annu. Rev. Cell Dev. Biol. 2005, 21, 485–509. [Google Scholar] [CrossRef] [PubMed]
- Scheres, B. Stem-cell niches: Nursery rhymes across kingdoms. Nat. Rev. Mol. Cell Biol. 2007, 8, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Rahni, R.; Efroni, I.; Birnbaum, K.D. A Case for Distributed Control of Local Stem Cell Behavior in Plants. Dev. Cell 2016, 38, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Drisch, R.C.; Stahl, Y. Function and regulation of transcription factors involved in root apical meristem and stem cell maintenance. Front. Plant Sci. 2015, 6, 505. [Google Scholar] [CrossRef] [PubMed]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.S.; Amasino, R.; Scheres, B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Heidstra, R.; Wildwater, M.; Scheres, B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes. Dev. 2003, 17, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Shimotohno, A.; Heidstra, R.; Blilou, I.; Scheres, B. Root stem cell niche organizer specification by molecular convergence of PLETHORA and SCARECROW transcription factor modules. Genes Dev. 2018, 32, 1085–1100. [Google Scholar] [CrossRef]
- Kong, X.; Lu, S.; Tian, H.; Ding, Z. WOX5 is Shining in the Root Stem Cell Niche. Trends Plant Sci. 2015, 20, 601–603. [Google Scholar] [CrossRef]
- Burkart, R.C.; Strotmann, V.I.; Kirschner, G.K.; Akinci, A.; Czempik, L.; Dolata, A.; Maizel, A.; Weidtkamp-Peters, S.; Stahl, Y. PLETHORA-WOX5 interaction and subnuclear localization control Arabidopsis root stem cell maintenance. EMBO Rep. 2022, 23, e54105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xiang, Y.; Li, C.; Yu, G. Modulatory Role of Reactive Oxygen Species in Root Development in Model Plant of Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 485932. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, Q.; Zhang, Y.; Jia, Y.; Wan, S.; Kong, X.; Ding, Z. ROS: The Fine-Tuner of Plant Stem Cell Fate. Trends Plant Sci. 2018, 23, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Tian, H.; Yue, K.; Liu, J.; Zhang, B.; Li, X.; Ding, Z. A P-Loop NTPase Regulates Quiescent Center Cell Division and Distal Stem Cell Identity through the Regulation of ROS Homeostasis in Arabidopsis Root. PLoS Genet. 2016, 12, e1006175. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Han, X.; Benfey, P.N. RGF1 controls root meristem size through ROS signalling. Nature 2020, 577, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Tian, H.; Yu, Q.; Zhang, F.; Wang, R.; Gao, S.; Xu, W.; Liu, J.; Shani, E.; Fu, C.; et al. PHB3 Maintains Root Stem Cell Niche Identity through ROS-Responsive AP2/ERF Transcription Factors in Arabidopsis. Cell Rep. 2018, 22, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H.; Zhao, X.; Zhou, J.; Qin, G.; Liu, Y.; Kou, X.; Zhao, Z.; Wu, T.; Zhu, J.K.; et al. SYNTAXIN OF PLANTS81 regulates root meristem activity and stem cell niche maintenance via ROS signaling. Plant Physiol. 2023, 191, 1365–1382. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; He, J.; Qin, Y.; Hua, D.; Duan, Y.; Chen, Z.; Gong, Z. ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet. 2014, 10, e1004791. [Google Scholar] [CrossRef]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, M.; Qin, Z.; Lv, H.; Wang, M.; Zhang, Z.; Zhou, W.; Zhao, N.; Li, X.; Han, C.; et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Wang, Z.; Rong, D.; Chen, D.; Xiao, Y.; Liu, R.; Wu, S.; Yamamuro, C. Salicylic acid promotes quiescent center cell division through ROS accumulation and down-regulation of PLT1, PLT2, and WOX5. J. Integr. Plant Biol. 2021, 63, 583–596. [Google Scholar] [CrossRef]
- Sheftel, A.D.; Stehling, O.; Pierik, A.J.; Netz, D.J.; Kerscher, S.; Elsasser, H.P.; Wittig, I.; Balk, J.; Brandt, U.; Lill, R. Human ind1, an iron-sulfur cluster assembly factor for respiratory complex I. Mol. Cell Biol. 2009, 29, 6059–6073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rubio, V.; Lieberman, M.W.; Shi, Z.Z. OLA1, an Obg-like ATPase, suppresses antioxidant response via nontranscriptional mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 15356–15361. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Ahn, J.H.; Blazquez, M.A.; Borevitz, J.O.; Christensen, S.K.; Fankhauser, C.; Ferrandiz, C.; Kardailsky, I.; Malancharuvil, E.J.; Neff, M.M.; et al. Activation tagging in Arabidopsis. Plant Physiol. 2000, 122, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.I.; Whiteheart, S.W. AAA+ proteins: Have engine, will work. Nat. Rev. Mol. Cell Biol. 2005, 6, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Leipe, D.D.; Koonin, E.V.; Aravind, L. Evolutionary history and higher order classification of AAA+ ATPases. J. Struct. Biol. 2004, 146, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Frickey, T.; Lupas, A.N. Phylogenetic analysis of AAA proteins. J. Struct. Biol. 2004, 146, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Muralla, R.; Lloyd, J.; Meinke, D. Molecular foundations of reproductive lethality in Arabidopsis thaliana. PLoS ONE 2011, 6, e28398. [Google Scholar] [CrossRef]
- Girard, C.; Chelysheva, L.; Choinard, S.; Froger, N.; Macaisne, N.; Lemhemdi, A.; Mazel, J.; Crismani, W.; Mercier, R. AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms. PLoS Genet. 2015, 11, e1005369. [Google Scholar] [CrossRef]
- Baek, K.; Seo, P.J.; Park, C.M. Activation of a mitochondrial ATPase gene induces abnormal seed development in Arabidopsis. Mol. Cells 2011, 31, 361–369. [Google Scholar] [CrossRef]
- Vinegra de la Torre, N.; Vayssieres, A.; Obeng-Hinneh, E.; Neumann, U.; Zhou, Y.; Lazaro, A.; Roggen, A.; Sun, H.; Stolze, S.C.; Nakagami, H.; et al. FLOWERING REPRESSOR AAA(+) ATPase 1 is a novel regulator of perennial flowering in Arabis alpina. New Phytol. 2022, 236, 729–744. [Google Scholar] [CrossRef]
- Ueda, M.; Matsui, K.; Ishiguro, S.; Sano, R.; Wada, T.; Paponov, I.; Palme, K.; Okada, K. The HALTED ROOT gene encoding the 26S proteasome subunit RPT2a is essential for the maintenance of Arabidopsis meristems. Development 2004, 131, 2101–2111. [Google Scholar] [CrossRef]
- Yang, S.; Li, C.; Zhao, L.; Gao, S.; Lu, J.; Zhao, M.; Chen, C.Y.; Liu, X.; Luo, M.; Cui, Y.; et al. The Arabidopsis SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA Targets Directly to PINs and Is Required for Root Stem Cell Niche Maintenance. Plant Cell 2015, 27, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Li, K.; Wang, J.; Zhao, C.Z.; Zhao, S.Z.; Hou, L.; Xia, H.; Ma, C.L.; Wang, X.J. The AAA-ATPase MIDASIN 1 Functions in Ribosome Biogenesis and Is Essential for Embryo and Root Development. Plant Physiol. 2019, 180, 289–304. [Google Scholar] [CrossRef]
- Yoo, K.S.; Ok, S.H.; Jeong, B.C.; Jung, K.W.; Cui, M.H.; Hyoung, S.; Lee, M.R.; Song, H.K.; Shin, J.S. Single cystathionine beta-synthase domain-containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plant Cell 2011, 23, 3577–3594. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; So, W.M.; Kim, S.Y.; Noh, M.; Hyoung, S.; Yoo, K.S.; Shin, J.S. CBSX3-Trxo-2 regulates ROS generation of mitochondrial complex II (succinate dehydrogenase) in Arabidopsis. Plant Sci. 2020, 294, 110458. [Google Scholar] [CrossRef]
- Dinneny, J.R.; Benfey, P.N. Plant stem cell niches: Standing the test of time. Cell 2008, 132, 553–557. [Google Scholar] [CrossRef]
- Di Laurenzio, L.; Wysocka-Diller, J.; Malamy, J.E.; Pysh, L.; Helariutta, Y.; Freshour, G.; Hahn, M.G.; Feldmann, K.A.; Benfey, P.N. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 1996, 86, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Helariutta, Y.; Fukaki, H.; Wysocka-Diller, J.; Nakajima, K.; Jung, J.; Sena, G.; Hauser, M.T.; Benfey, P.N. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 2000, 101, 555–567. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Paquette, A.J.; Nakajima, K.; Benfey, P.N. Mechanisms regulating SHORT-ROOT intercellular movement. Curr. Biol. 2004, 14, 1847–1851. [Google Scholar] [CrossRef]
- Wang, W.; Fang, H.; Groom, L.; Cheng, A.; Zhang, W.; Liu, J.; Wang, X.; Li, K.; Han, P.; Zheng, M.; et al. Superoxide flashes in single mitochondria. Cell 2008, 134, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Sena, G.; Nawy, T.; Benfey, P.N. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 2001, 413, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Kornet, N.; Scheres, B. Members of the GCN5 histone acetyltransferase complex regulate PLETHORA-mediated root stem cell niche maintenance and transit amplifying cell proliferation in Arabidopsis. Plant Cell 2009, 21, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Li, H.; Zhang, B.; Song, Y.; Sun, Y.; Ding, Z. ATP Hydrolases Superfamily Protein 1 (ASP1) Maintains Root Stem Cell Niche Identity through Regulating Reactive Oxygen Species Signaling in Arabidopsis. Plants 2024, 13, 1469. https://doi.org/10.3390/plants13111469
Yu Q, Li H, Zhang B, Song Y, Sun Y, Ding Z. ATP Hydrolases Superfamily Protein 1 (ASP1) Maintains Root Stem Cell Niche Identity through Regulating Reactive Oxygen Species Signaling in Arabidopsis. Plants. 2024; 13(11):1469. https://doi.org/10.3390/plants13111469
Chicago/Turabian StyleYu, Qianqian, Hongyu Li, Bing Zhang, Yun Song, Yueying Sun, and Zhaojun Ding. 2024. "ATP Hydrolases Superfamily Protein 1 (ASP1) Maintains Root Stem Cell Niche Identity through Regulating Reactive Oxygen Species Signaling in Arabidopsis" Plants 13, no. 11: 1469. https://doi.org/10.3390/plants13111469
APA StyleYu, Q., Li, H., Zhang, B., Song, Y., Sun, Y., & Ding, Z. (2024). ATP Hydrolases Superfamily Protein 1 (ASP1) Maintains Root Stem Cell Niche Identity through Regulating Reactive Oxygen Species Signaling in Arabidopsis. Plants, 13(11), 1469. https://doi.org/10.3390/plants13111469





