The Slow Growth of Adventitious Roots in Tetraploid Hybrid Poplar (Populus simonii × P. nigra var. italica) May Be Caused by Endogenous Hormone-Mediated Meristem Shortening
Abstract
1. Introduction
2. Results
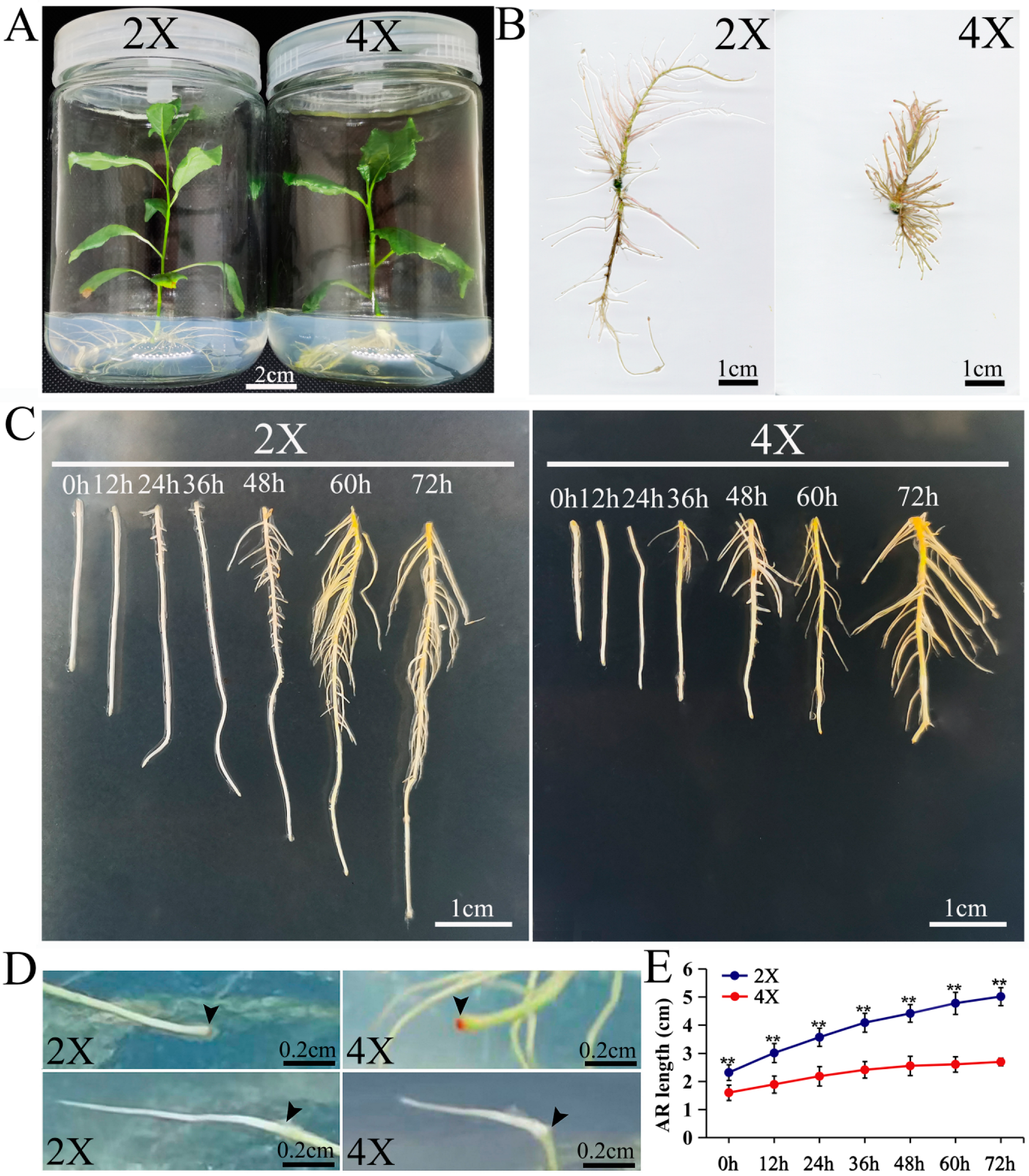
2.1. Comparison of the Development of ARs between Diploids and Tetraploids
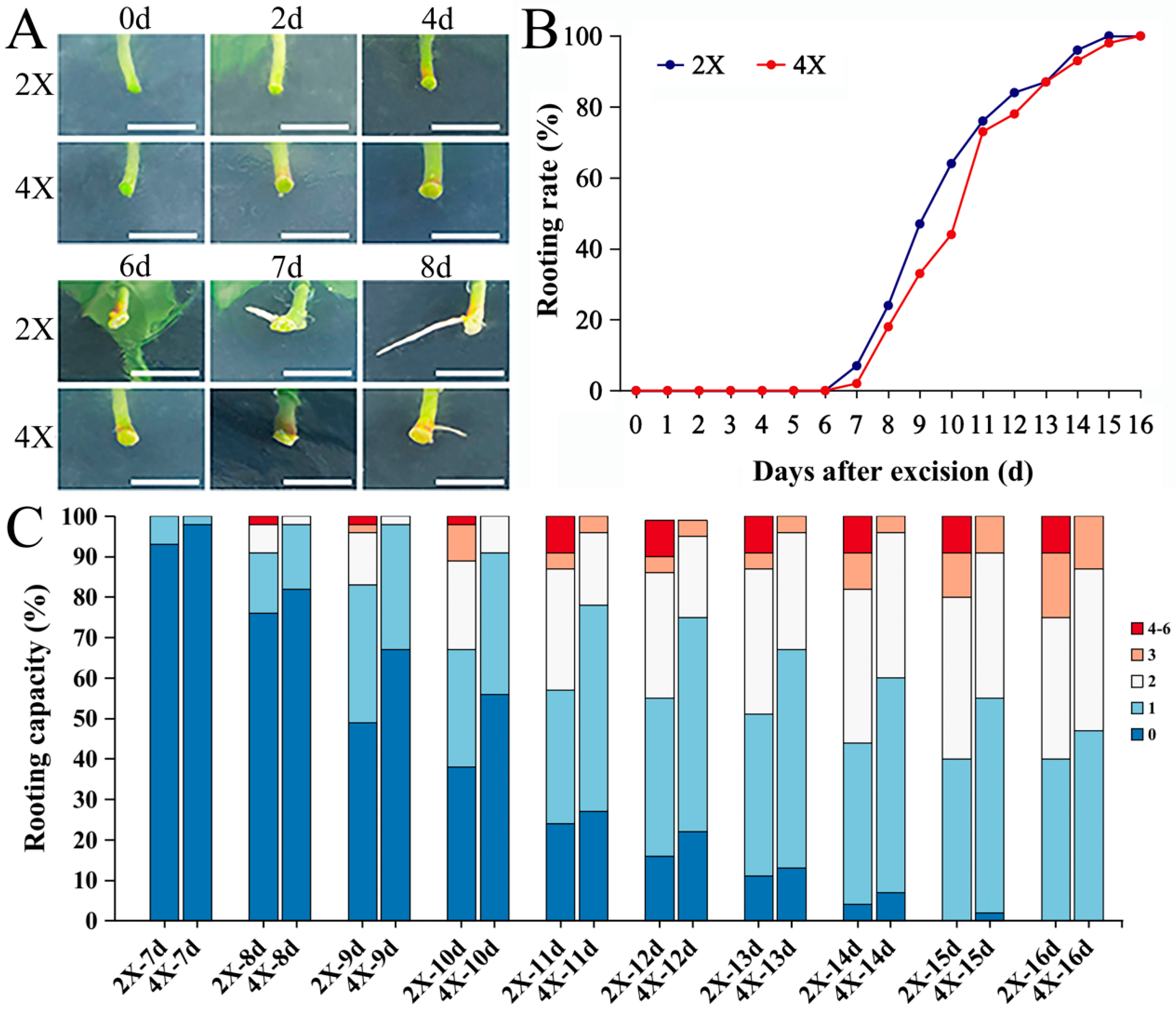
2.2. Comparison of AR Formation Capacities of Diploids and Tetraploids
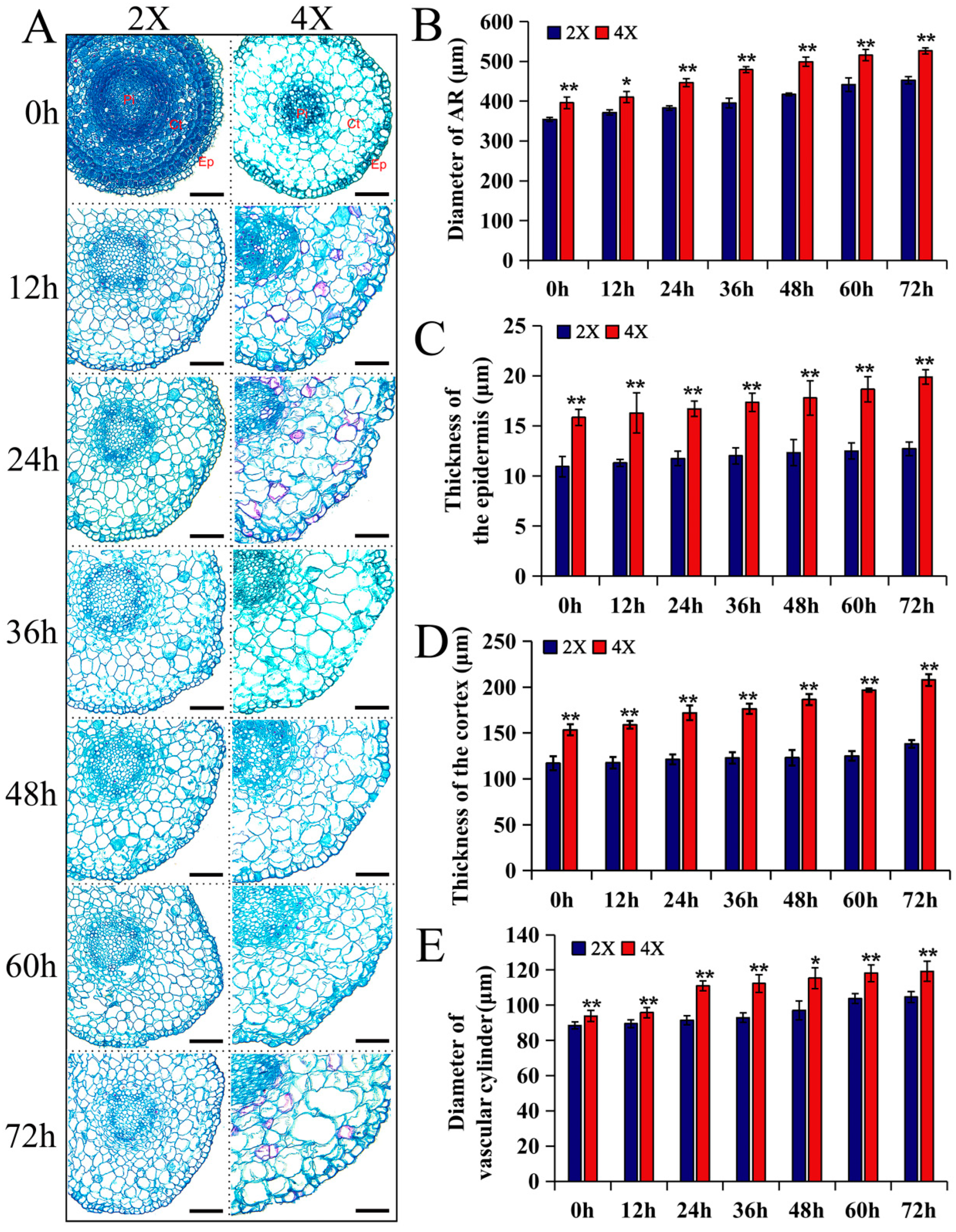
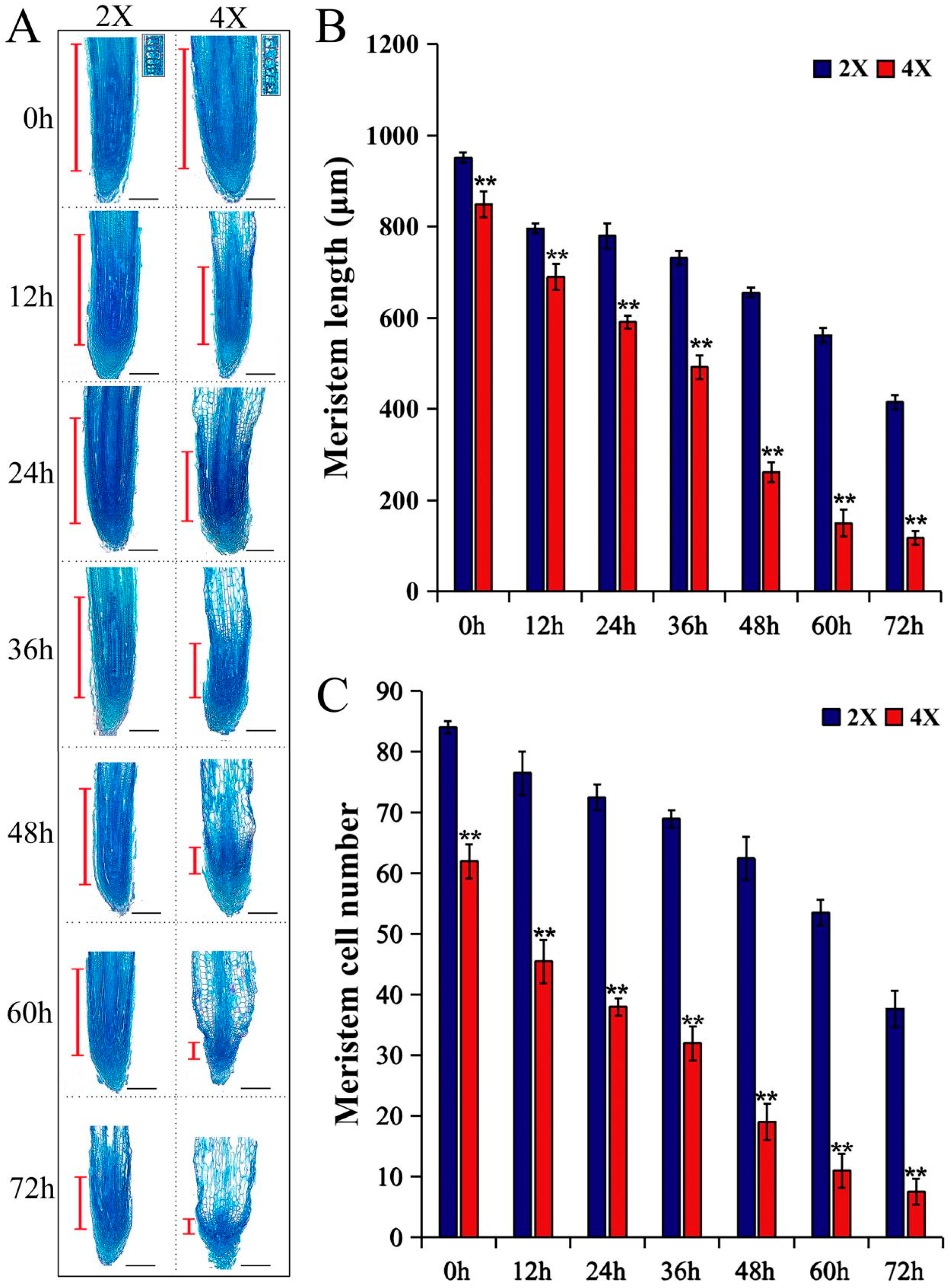
2.3. Histological Observation of ARs of Diploids and Tetraploids
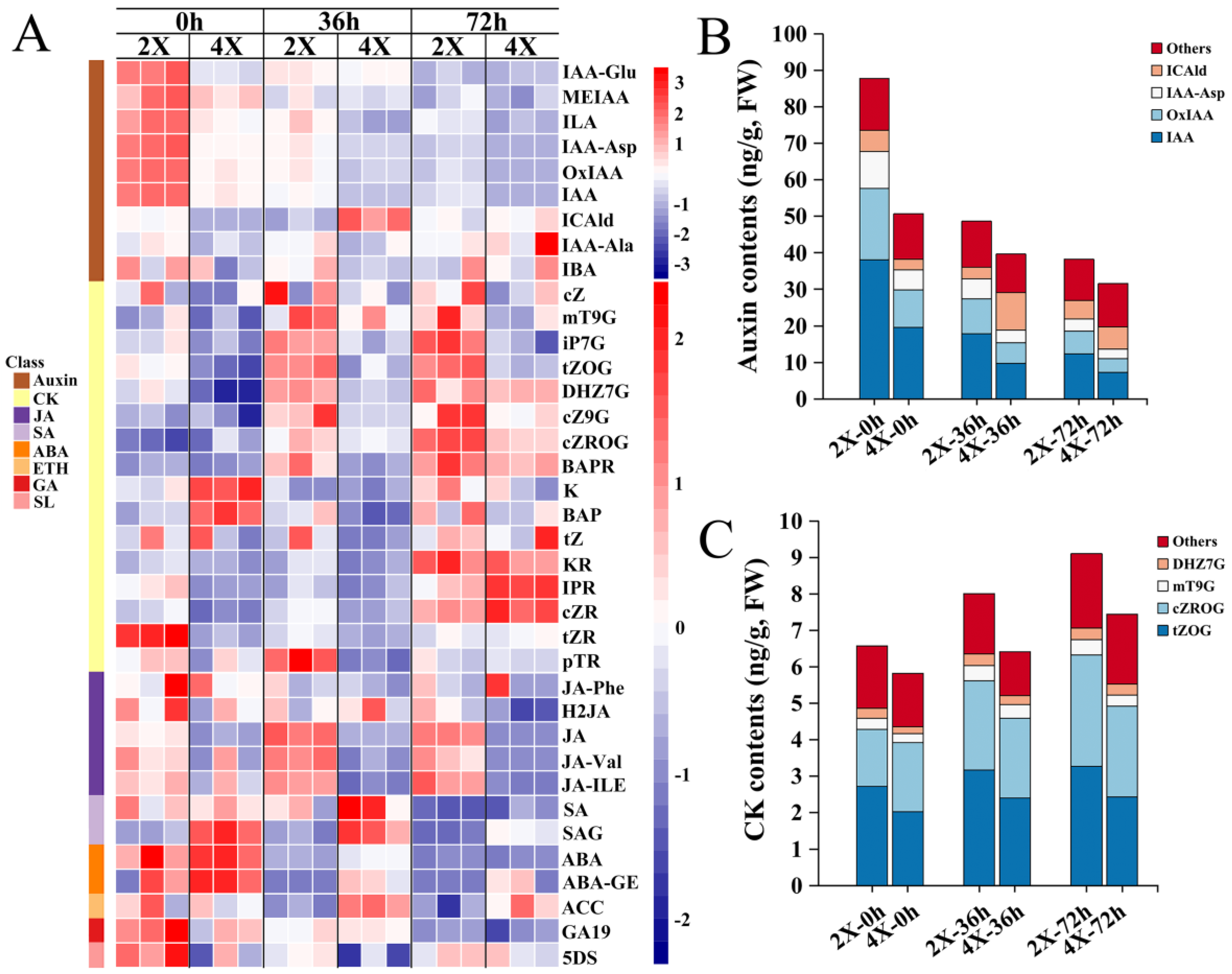
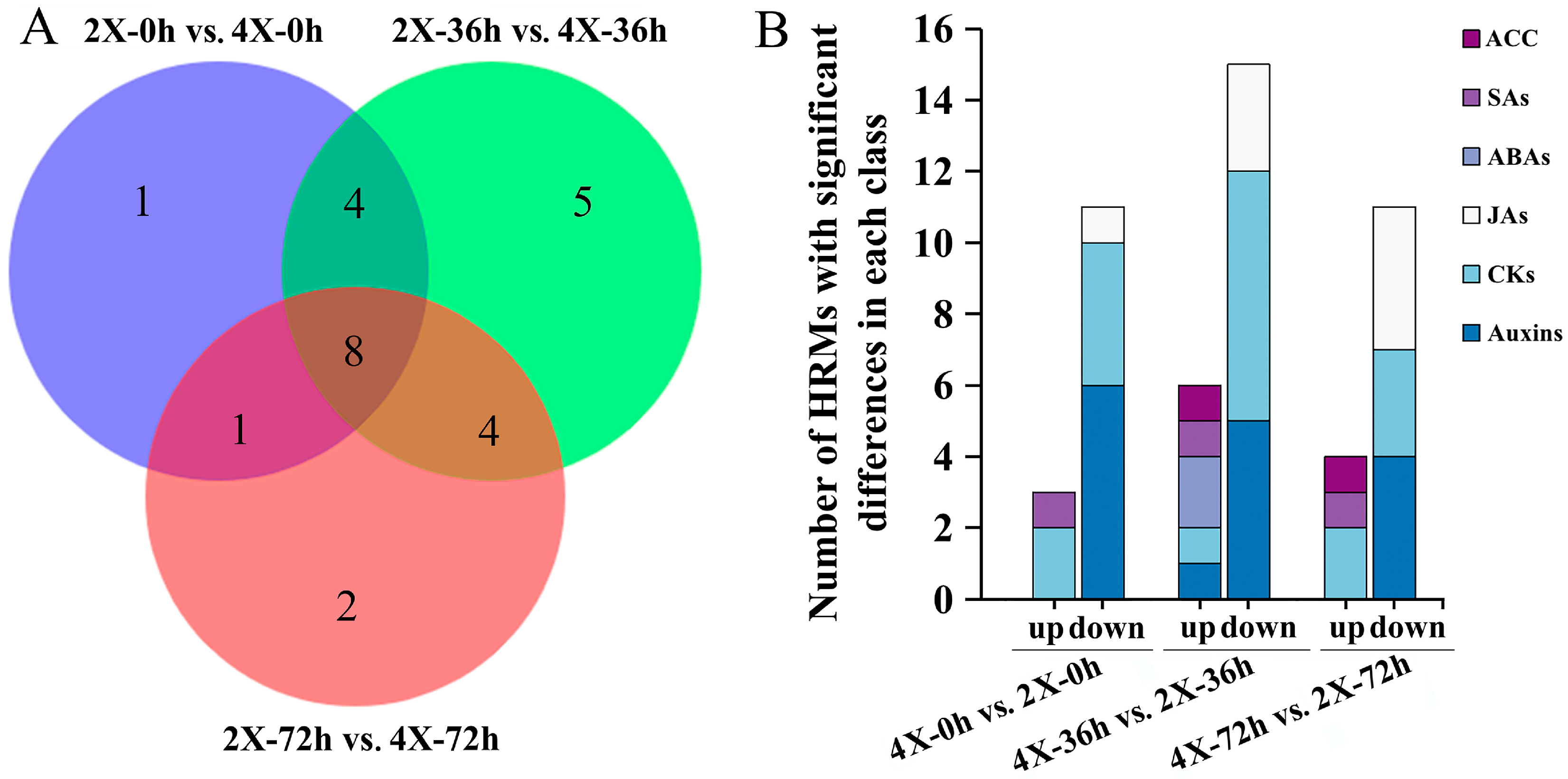
2.4. Changes in HRM Contents during AR Development
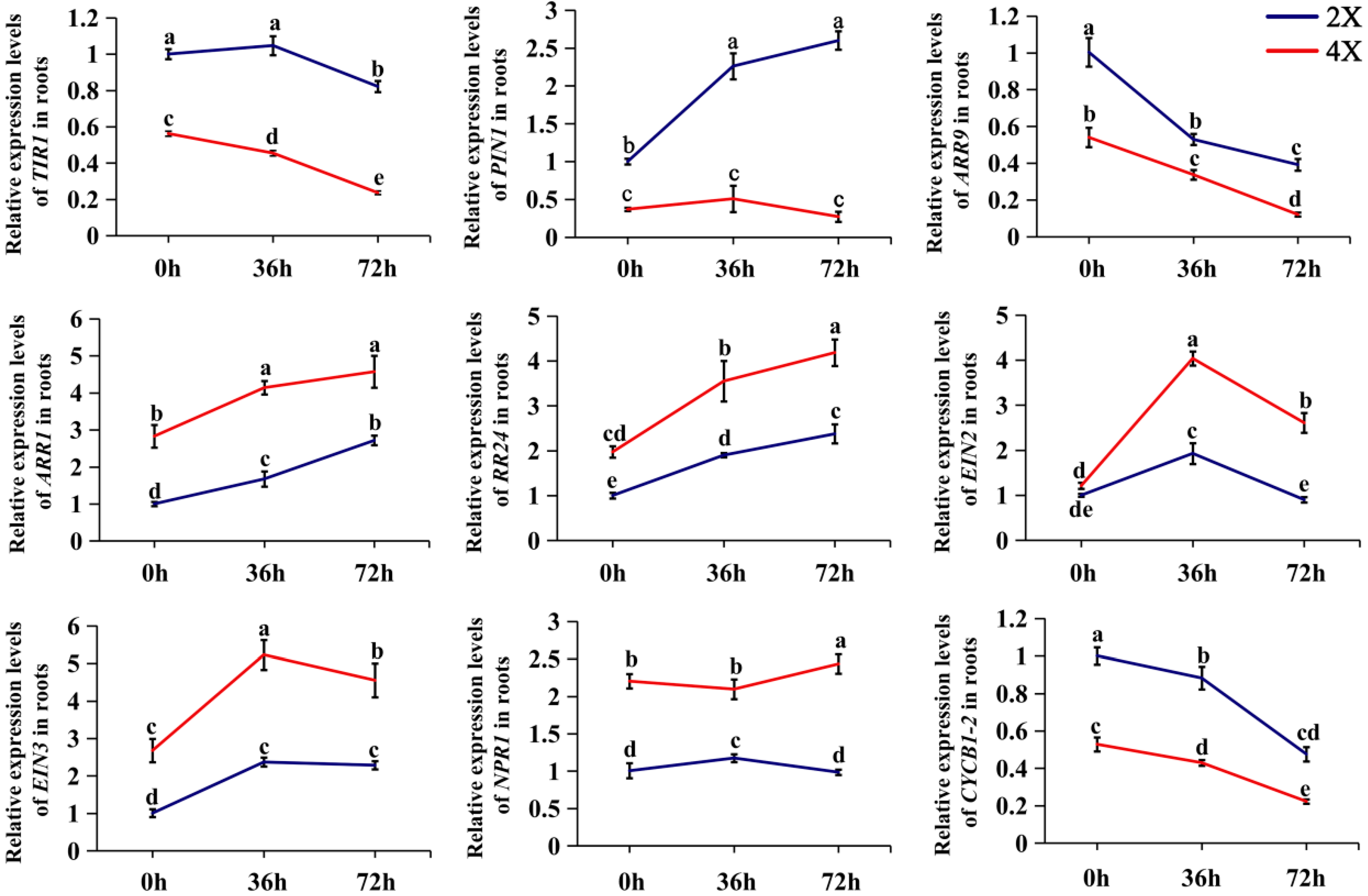
2.5. Expression Analysis of Genes Related to Hormonal Signaling during AR Development
3. Discussion
3.1. Variation in AR Morphology of Tetraploid Poplars
3.2. HRMs Content Changes during Tetraploid AR Development
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. AR Phenotypic Analysis
4.3. Anatomical Analysis
4.4. Measurement of Plant HRMs Contents
4.5. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR (qPCR) Analysis
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mayrose, I.; Zhan, S.H.; Rothfels, C.J.; Magnuson-Ford, K.; Barker, M.S.; Rieseberg, L.H.; Otto, S.P. Recently formed polyploid plants diversify at lower rates. Science 2011, 333, 1257. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Chen, Z.J. Epigenetic and developmental regulation in plant polyploids. Curr. Opin. Plant Biol. 2015, 24, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Svačina, R.; Sourdille, P.; Kopecký, D.; Bartoš, J. Chromosome pairing in polyploid grasses. Front. Plant Sci. 2020, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.L.; Alcock, T.D.; Graham, N.S.; Hayden, R.; Matterson, S.; Wilson, L.; Young, S.D.; Dupuy, L.X.; White, P.J.; Hammond, J.P.; et al. Root morphology and seed and leaf lonomic traits in a Brassica napus L.: Diversity panel show wide phenotypic variation and are characteristic of crop habit. BMC Plant Biol. 2016, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lai, H.; Li, R.; Yao, Y.; Liu, J.; Yuan, S.; Fu, S.; Hu, X.; Guo, J. Character changes and Transcriptomic analysis of a cassava sexual Tetraploid. BMC Plant Biol. 2021, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, X.; Cai, Q.; Han, R.; Tigabu, M.; Jiang, T.; Zhao, X. Variations in growth and photosynthetic traits of polyploid poplar hybrids and clones in northeast China. Genes 2022, 13, 2161. [Google Scholar] [CrossRef]
- Miller, M.; Zhang, C.; Chen, Z.J. Ploidy and hybridity effects on growth vigor and gene expression in Arabidopsis thaliana hybrids and their parents. G3 Genes|Genomes|Genetics 2012, 2, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Cheng, S.; Zhu, X.; Min, Y.; Kang, X. Effects of triploid status on growth, photosynthesis, and leaf area in Populus. Trees-Struct. Funct. 2016, 30, 1137–1147. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, B.; Tian, J.R.; Chen, M.M.; Zhang, Y.Y.; Zhang, Z.H.; Ma, Y. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’ apple trees. Sci. Hortic. 2017, 225, 277–285. [Google Scholar] [CrossRef]
- Pacey, E.K.; Maherali, H.; Husband, B.C. The influence of experimentally induced polyploidy on the relationships between endopolyploidy and plant function in Arabidopsis thaliana. Ecol. Evol. 2019, 10, 198–216. [Google Scholar] [CrossRef]
- Ruiz, M.; Oustric, J.; Santini, J.; Morillon, R. Synthetic Polyploidy in Grafted Crops. Front. Plant Sci. 2020, 11, 540894. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Suzuki, Y.; Hoshino, Y. Phenotypic analysis of polyploid and aneuploid haskap (Lonicera caerulea L. subsp. Edulis (Turcz. ex Herder) Hultén) plants and their progeny production. Euphytica 2023, 219, 52. [Google Scholar] [CrossRef]
- Milo, J.; Levy, A.; Palevitch, D.; Ladizinsky, G. The baine content and yield in induced tetraploid and triploid plants of Papaver bracteatum LINDL. Euphytica 1987, 36, 361–367. [Google Scholar] [CrossRef]
- Xu, C.-G.; Tang, T.-X.; Chen, R.; Liang, C.-H.; Liu, X.-Y.; Wu, C.-L.; Yang, Y.-S.; Yang, D.-P.; Wu, H. A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea purpurea (L.) Moench. Plant Cell Tissue Organ Cult. 2014, 116, 323–332. [Google Scholar] [CrossRef]
- Ostonen, I.; Helmisaari, H.; Borken, W.; Tedersoo, L.; Kukumägi, M.; Bahram, M.; Lindroos, A.; Nöjd, P.; Uri, V.; Merilä, P.; et al. Fine root foraging strategies in Norway spruce forests across a European climate gradient. Glob. Chang. Biol. 2011, 17, 3620–3632. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C. Sampling roots to capture plant and soil functions. Funct. Ecol. 2017, 31, 1506–1518. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, S.; Lu, X.; He, N.; Gao, L.; Dou, J.; Bie, Z.; Liu, W. Genome duplication improves the resistance of watermelon root to salt stress. Plant Physiol. Biochem. 2018, 133, 11–21. [Google Scholar] [CrossRef]
- Dudits, D.; Cseri, A.; Török, K.; Vankova, R.; Dobrev, P.I.; Sass, L.; Steinbach, G.; Kelemen-Valkony, I.; Zombori, Z.; Ferenc, G.; et al. Manifestation of Triploid Heterosis in the Root System after Crossing Diploid and Autotetraploid Energy Willow Plants. Genes 2023, 14, 1929. [Google Scholar] [CrossRef]
- Ruiz, M.; Quiñones, A.; Martínez-Cuenca, M.R.; Aleza, P.; Morillon, R.; Navarro, L.; Primo-Millo, E.; Martínez-Alcántara, B. Tetraploid enhances the ability to exclude chloride from leaves in carrizo citrange seedings. J. Plant Physiol. 2016, 205, 1–10. [Google Scholar] [CrossRef]
- Tavan, M.; Sarikhani, H.; Mirjalili, M.H.; Rigano, M.M.; Azizi, A. Triterpenic and phenolic acids production changed in Salvia officinalis via in vitro and in vivo polyploidization: A consequence of altered genes expression. Phytochemistry 2021, 189, 112803. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yang, N.; Li, L.; Qin, G.; Ren, K.; Wang, H.; Deng, J.; Ding, D. Tetraploidy in Citrus wilsonii enhances drought tolerance via synergistic regulation of photosynthesis, phosphorylation, and hormonal changes. Front. Plant Sci. 2023, 14, 1269493. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhao, Y.; Ai, S.; Chen, X.; Liu, X.; Wang, H.; Han, Y.; Ma, F.; Li, C. Induction of polyploid Malus prunifolia and analysis of its salt tolerance. Tree Physiol. 2022, 42, 2100–2115. [Google Scholar] [CrossRef] [PubMed]
- Perilli, S.; Di Mambro, R.; Sabatini, S. Growth and development of the root apical meristem. Curr. Opin. Plant Biol. 2011, 15, 17–23. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Golin, S.; Hardtke, C.S.; Lo Schiavo, F.; Zottini, M. The co-chaperone p23 controls root development through the modulation of auxin distribution in the Arabidopsis root meristem. J. Exp. Bot. 2015, 66, 5113–5122. [Google Scholar] [CrossRef]
- Numata, T.; Sugita, K.; Rahman, A.A.; Rahman, A. Actin isovariant ACT7 controls root meristem development in Arabidopsis through modulating auxin and ethylene responses. J. Exp. Bot. 2022, 73, 6255–6271. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ren, Y.; Liu, J.; Zhu, J.K.; Zhao, C. A novel mitochondrial protein is required for cell wall integrity, auxin accumulation and root elongation in Arabidopsis under salt stress. Stress Biol. 2022, 2, 13. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zhang, Y.; Zhang, T.; Zhan, D.J.; Pang, Z.W.; Zhao, J.; Zhang, J.F. Comparative transcriptomic, anatomical and phytohormone analyses provide new insights into hormone-mediated tetraploid dwarfing in hybrid sweetgum (Liquidambar styraciflua × L. formosana). Front Plant Sci. 2022, 13, 924044. [Google Scholar] [CrossRef] [PubMed]
- Dudits, D.; Török, K.; Cseri, A.; Paul, K.; Nagy, A.V.; Nagy, B.; Sass, L.; Ferenc, G.; Vankova, R.; Dobrev, P.; et al. Response of organ structure and physiology to autotetraploidization in early development of energy willow Salix viminalis. Plant Physiol. 2016, 170, 1504–1523. [Google Scholar] [CrossRef]
- Chen, T.; Sheng, Y.; Hao, Z.; Long, X.; Fu, F.; Liu, Y.; Tang, Z.; Ali, A.; Peng, Y.; Lu, L.; et al. Transcriptome and proteome analysis suggest enhanced photosynthesis in tetraploid Liriodendron sino-americanum. Tree Physiol. 2021, 41, 1953–1971. [Google Scholar] [CrossRef]
- Jansson, S.; Douglas, C.J. Populus: A model system for plant biology. Annu. Rev. Plant Biol. 2007, 58, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie-Nodoushan, H.; Ghamari-Zare, A.; Rad, F.T.; Youseffard, M. Inducing geneticvariation in growth related characteristics of poplar germplasm, by producing inter-specific hybrids between P. alba and P. euphratica. Silvae Genet. 2015, 64, 239–248. [Google Scholar] [CrossRef]
- Zanewich, K.P.; Pearce, D.W.; Rood, S.B. Heterosis in poplar involves phenotypic stability: Cottonwood hybrids outperform their parental species at suboptimal temperatures. Tree Physiol. 2018, 38, 789–800. [Google Scholar] [CrossRef]
- Liu, W.; Song, S.; Li, D.; Lu, X.; Liu, J.; Zhang, J.; Wang, J. Isolation of diploid and tetraploid cytotypes from mixoploids based on adventitious bud regeneration in Populus. Plant Cell Tissue Organ Cult. 2020, 140, 1–10. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, X.; Tan, Y.; Huang, J.; Zheng, Z.; Tao, L. Phosphoethanolamine N-methyltransferase 1 contributes to maintenance of root apical meristem by affecting ROS and auxin-regulated cell differentiation in Arabidopsis. New Phytol. 2019, 224, 258–273. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, H.; Ruan, W.; Deng, M.; Wang, F.; Peng, J.; Luo, J.; Chen, Z.; Yi, K. Abnormal inflorescence meristem1 functions in salicylic acid biosynthesis to maintain proper reactive oxygen species levels for root meristem activity in rice. Plant Cell 2017, 29, 560–574. [Google Scholar] [CrossRef]
- Chen, R.; Yang, Y.S.; Wu, H. A comparative study on rooting of in vitro regenerated shoots in haploid, diploid and tetraploid purple coneflower (Echinacea purpurea L.). Biotechnol. Biotec. Equip. 2016, 30, 44–48. [Google Scholar] [CrossRef]
- Gao-Takai, M.; Katayama-Ikegami, A.; Nakano, S.; Matsuda, K.; Motosugi, H. Vegetative growth and fruit quality of ‘Ruby Roman’ grapevines grafted on two species of rootstock and their tetraploids. Hortic. J. 2017, 86, 171–182. [Google Scholar] [CrossRef]
- Denaeghel, H.E.R.; Van Larea, K.; Leus, L.; Lootens, P.; Van Huylenbroeck, J.; Van Labeke, M.C. The variable effect of polyploidization on the phenotype in Escallonia. Front Plant Sci. 2018, 9, 354. [Google Scholar] [CrossRef]
- Ioio, R.D.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Dello Ioio, R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef]
- von Rad, U.; Klein, I.; Dobrev, P.I.; Kottova, J.; Zazimalova, E.; Fekete, A.; Hartmann, A.; Schmitt-Kopplin, P.; Durner, J. Response of Arabidopsis thaliana to N-hexanoyl-dl-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 2008, 229, 73–85. [Google Scholar] [CrossRef]
- Moradtalab, N.; Ahmed, A.; Geistlinger, J.; Walker, F.; Hoeglinger, B.; Ludewig, U.; Neumann, G. Synergisms of microbial consortia, N forms, and micronutrients alleviate oxidative damage and stimulate hormonal cold stress adaptations in maize. Front. Plant Sci. 2020, 11, 396. [Google Scholar] [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal interactions in the regulation of plant development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Ruzicka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Swarup, R.; Perry, P.; Hagenbeek, D.; Van Der Straeten, D.; Beemster, G.T.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M.J. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Street, I.H.; Aman, S.; Zubo, Y.; Ramzan, A.; Wang, X.; Shakeel, S.N.; Kieber, J.J.; Schaller, G.E. Ethylene inhibits cell Proliferation of the arabidopsis root meristem. Plant Physiol. 2015, 169, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Zdarska, M.; Cuyacot, A.R.; Tarr, P.T.; Yamoune, A.; Szmitkowska, A.; Hrdinová, V.; Gelová, Z.; Meyerowitz, E.M.; Hejátko, J. ETR1 integrates response to ethylene and cytokinins into a single multistep phosphorelay pathway to control root growth. Mol. Plant 2019, 12, 1338–1352. [Google Scholar] [CrossRef]
- Vandecasteele, M.; Depaepe, T.; Abdallah, M.F.; De Saeger, S.; Höfte, M.M.; Haesaert, G.; van der Straeten, D.; Audenaert, K. Ethylene production during Alternaria infections on potato plants and its antagonistic role in virulence of different Alternaria species. Plant Pathol. 2023, 73, 535–547. [Google Scholar] [CrossRef]
- Ma, B.; Yin, C.-C.; He, S.-J.; Lu, X.; Zhang, W.-K.; Lu, T.-G.; Chen, S.-Y.; Zhang, J.-S. Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLOS Genet. 2014, 10, e1004701. [Google Scholar] [CrossRef] [PubMed]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The biphasic root growth response to abscisic acid in arabidopsis involves interaction with ethylene and auxin signalling pathways. Front. Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef]
- Dong, T.; Yin, X.; Wang, H.; Lu, P.; Liu, X.; Gong, C.; Wu, Y. ABA-INDUCED expression 1 is involved in ABA-inhibited primary root elongation via modulating ROS homeostasis in Arabidopsis. Plant Sci. 2021, 304, 110821. [Google Scholar] [CrossRef]
- Wang, S.; Ichii, M.; Taketa, S.; Xu, L.; Xia, K.; Zhou, X. Lateral root formation in rice (Oryza sativa): Promotion effect of jasmonic acid. J. Plant Physiol. 2002, 159, 827–832. [Google Scholar] [CrossRef]
- Fujisawa, H.; Seto, H.; Yoshida, S.; Kamuro, Y. Promoting effects of jasmonic acid analog, N-propyl dihydrojasmonate (PDJ), on plant growth. In Proceedings of the 23th Annual Meeting of Plant Growth Regulation Society of America, Calgary, AB, Canada, 14–18 July 1996; pp. 111–116. [Google Scholar]
- Chen, Q.; Sun, J.Q.; Zhai, Q.Z.; Zhou, W.K.; Qi, L.L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.L.; Qi, J.; et al. The basic helix-loop-helix transcription factor myc2 directly represses plethora expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Pour, A.P.; Farahbakhsh, H.; Saffari, M.; Keramat, B. Effects of seed priming on germination and seedling growth under salinity stress in fenugreek. Int. J. Agric. Crop Sci. 2012, 4, 779–786. [Google Scholar]
- Bouallègue, A.; Souissi, F.; Nouairi, I.; Souibgui, M.; Abbes, Z.; Mhadhbi, H. Salicylic acid and hydrogen peroxide pretreatments alleviate salt stress in faba bean (Vicia faba) seeds during germination. Seed Sci. Technol. 2017, 45, 675–690. [Google Scholar] [CrossRef]
- Bouallègu, A.; Souissi, F.; Nouairi, I.; Souibgui, M.; Abbes, Z.; Mhadhbi, H. Physiological and biochemicals changes modulated by seeds’ priming of lentil (Lens culinaris L.) under salt stress at germination stage. Acta Sci. Pol-Hortoru. Cultus. 2019, 18, 27–38. [Google Scholar] [CrossRef]
- Singh, P.K.; Chaturvedi, V.K.; Bose, B. Effects of salicylic acid on seedling gowth and nitrogen metabolism in cucumber (Cucumis sativus L.). J. Stress Physiol. Biochem. 2010, 6, 102–113. [Google Scholar]
- Agtuca, B.; Rieger, E.; Hilger, K.; Song, L.; Robert, C.A.M.; Erb, M.; Karve, A.; Ferrieri, R.A. Carbon-11 reveals opposing roles of auxin and salicylic acid in regulating leaf physiology, leaf metabolism, and resource allocation patterns that impact root growth in Zea mays. J. Plant Growth Regul. 2014, 33, 328–339. [Google Scholar] [CrossRef]
- Safari, H.; Hosseini, S.M.; Azari, A.; Rafsajani, M.H. Effects of seed priming with ABA and SA on seed germination and seeding growth of sesame (Sesamum indicum L.) under saline condition. Aust. J. Crop Sci. 2018, 12, 1385–1392. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Šimášková, M.; O’brien, J.A.; Khan, M.; Van Noorden, G.; Ötvös, K.; Vieten, A.; De Clercq, I.; Van Haperen, J.M.A.; Cuesta, C.; Hoyerová, K.; et al. Cytokinin response factors regulate PIN-FORMED auxin transporters. Nat. Commun. 2015, 6, 8717. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, L.; Zhang, F.; Ou, C.; Wang, F.; Zhang, Z. Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus × domestica). Sci. Rep. 2016, 6, 26719. [Google Scholar] [CrossRef]
- Fan, G.; Zhai, X.; Niu, S.; Ren, Y. Dynamic expression of novel and conserved microRNAs and their targets in diploid and tetraploid of Paulownia tomentosa. Biochimie 2014, 102, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Li, Y.; Chen, J. Combined analysis of microRNAs and target genes revealed miR156-SPLs and miR172-AP2 are involved in a delayed flowering phenomenon after chromosome doubling in black goji (Lycium ruthencium). Front. Genet. 2021, 12, 706930. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, Y.; Cong, P.; Zhu, Y. Functional characterization of an apple (Malus x domestica) LysM domain receptor encoding gene for its role in defense response. Plant Sci. 2018, 269, 56–65. [Google Scholar] [CrossRef]
- de Vries, J.; Fischer, A.M.; Roettger, M.; Rommel, S.; Schluepmann, H.; Bräutigam, A.; Carlsbecker, A.; Gould, S.B. Cytokinin-induced promotion of root meristem size in the fern Azolla supports a shoot-like origin of euphyllophyte roots. New Phytol. 2015, 209, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Qin, H.; Zhou, J.; Quan, R.; Lu, X.; Huang, R.; Zhang, H. OsERF2 controls rice root growth and hormone responses through tuning expression of key genes involved in hormone signaling and sucrose metabolism. Plant Mol. Biol. 2015, 90, 293–302. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Ren, Y.; Wang, X.; Zhang, Y.; Wang, J. The Slow Growth of Adventitious Roots in Tetraploid Hybrid Poplar (Populus simonii × P. nigra var. italica) May Be Caused by Endogenous Hormone-Mediated Meristem Shortening. Plants 2024, 13, 1430. https://doi.org/10.3390/plants13111430
Wu L, Ren Y, Wang X, Zhang Y, Wang J. The Slow Growth of Adventitious Roots in Tetraploid Hybrid Poplar (Populus simonii × P. nigra var. italica) May Be Caused by Endogenous Hormone-Mediated Meristem Shortening. Plants. 2024; 13(11):1430. https://doi.org/10.3390/plants13111430
Chicago/Turabian StyleWu, Lixia, Yuxin Ren, Xuefang Wang, Yuntong Zhang, and Jun Wang. 2024. "The Slow Growth of Adventitious Roots in Tetraploid Hybrid Poplar (Populus simonii × P. nigra var. italica) May Be Caused by Endogenous Hormone-Mediated Meristem Shortening" Plants 13, no. 11: 1430. https://doi.org/10.3390/plants13111430
APA StyleWu, L., Ren, Y., Wang, X., Zhang, Y., & Wang, J. (2024). The Slow Growth of Adventitious Roots in Tetraploid Hybrid Poplar (Populus simonii × P. nigra var. italica) May Be Caused by Endogenous Hormone-Mediated Meristem Shortening. Plants, 13(11), 1430. https://doi.org/10.3390/plants13111430





