1. Introduction
Tan spot, caused by the necrotrophic fungus
Pyrenophora tritici-repentis (Died.) Drechs (formerly known as
Drechslera tritici-repentis (Died.) Shoem.) (
Ptr), is a significant wheat disease worldwide [
1]. In the main wheat-producing region of southern Brazil, tan spot stands out as the predominant leaf disease [
2]. Recent studies of the
Ptr-wheat interaction showed that the activation of defense responses differs with the level of susceptibility of wheat cultivars, and the biochemical and histocytological defense responses are more robust in genotypes with moderate resistance [
3,
4]. In moderately resistant cultivars, oxidative burst (H
2O production in the first hour after inoculation) constitutes an efficient strategy to active plant defense mechanisms, resulting in the fast accumulation of hydrogen peroxide (H
2O
2) in the epidermal cells [
5]. Overall, oxidative burst can induce higher activity of the antioxidative system which is governed by enzymatic activity, such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX), as well as defense-related enzymes chitinase (CHI) and phenylalanine ammonia-lyase (PAL), this one leading to an early (<18 h after infection (hai)) and more intense fluorescence of epidermal cells due to the accumulation of phenylpropanoid derivatives at the infection site [
3,
4]. However, in the susceptible cultivar, although some of the same defense response was observed, they occurred later, such the accumulation of H
2O
2 in the epidermal and mesophyll cells as after 24 hai and the fluorescence in epidermal cells after 72 hai [
3,
4]. In the susceptible genotype, the accumulation of H
2O
2 during
Ptr attack is probably the result of toxin action, which is known to be a pathogen-triggered response, inducing cell death in the host [
1].
Nitrogen (N) and silicon (Si) have been mineral elements utilized successfully for disease control in crops [
6,
7]. In wheat plants, besides the use of moderately resistant cultivars, crop rotation, and fungicide-spraying, several studies have reported that N and Si fertilization are considered complementary alternatives for the management of Blast, Fusarium head blight, Powdery mildew, Spot blotch, Stripe rust, and tan spot [
8,
9,
10].
Nitrogen is an essential element for plant growth and plays a key role in health and yield [
11,
12]. Several studies have shown contrasting effects of N fertilization on disease development. In some, N increases disease severity and reduces the incubation period, due to delay in lignin deposition, and reduces the thickness of the secondary cell walls, resulting in a higher severity level [
6]. Nevertheless, other studies have shown decreased severity because an increase in N is probably associated with a healthy area duration (HAD) [
13,
14]. Regarding biochemical plant defense mechanisms, higher N led to lower activity of PAL and reduced concentration of C-based secondary metabolites such as some phytoalexins. On the other hand, there was a positive effect on pathogenesis related (PR) proteins, such as CHI and β-1,3-glucanase, along with increases in amino acid metabolism, which acts as a precursor to many defense-related phytoalexins, and increases in the activity of antioxidant enzymes [
6]. Furthermore, N also influences other compounds involved in the defense against pathogens, such as the production of nitric oxide, polyamines, hormonal signaling molecules, apoplastic sugars and amino acids, but the effect is variable with the N source: nitrate or ammonium [
6,
15].
In turn, Si is considered a non-essential plant nutrient, although it plays a beneficial role in promoting plant growth and increasing yield by alleviating biotic and abiotic stresses in many plant species, especially plants belonging to Poaceae family [
7,
9,
16]. As is well known, Si reduces disease intensity through physical and biochemical mechanisms [
17]. The physical mechanism, restricting or delaying the penetration and spread of fungal hyphae in host tissues, occurs due to the formation of the Si-cuticle double layer, cell wall strengthening, and the formation of papillae [
17,
18,
19]. Furthermore, plants supplied with Si showed priming of biochemical defenses through increased activity of defense enzymes, biosynthesis and accumulation of antimicrobial compounds (phenylpropanoids, flavonoids, phytoalexins, and PR proteins), and the accumulation of H
2O
2 at infection sites when infected by fungi, mainly [
3,
4,
7,
20,
21,
22]. Additionally, Si can influence the effect or movement of effectors, which, in turn, confers stronger plant resistance by keeping effectors from reaching the targets or the plant signals from being recognized by the pathogen [
23].
Nitrogen and Si nutrition has shown significant increments in wheat diseases’ control [
3,
5,
6,
10,
12,
13,
15,
24]; however, few studies have hypothesized synergistic effects associated with N and Si supplementation in combination to reduce disease severity levels. Experiments carried out in wheat fields demonstrated that the presence of N and Si did not result in any significant effect on wheat production, Si absorption, or leaf rust disease development [
25,
26]. For this reason, we hypothesize that Si supply in combination with a higher N fertilization rate in wheat plants may develop a synergistic effect, benefiting a higher effective control of tan spot. Given the essential role of N in wheat production, understanding the intricate interactions between N and Si is crucial for unraveling their combined effects on plant disease. In this study, our aim was to explore and understand the role of Si and its interplay with nitrogen in eliciting histocytological and biochemical defense responses during
Ptr infection in wheat.
3. Discussion
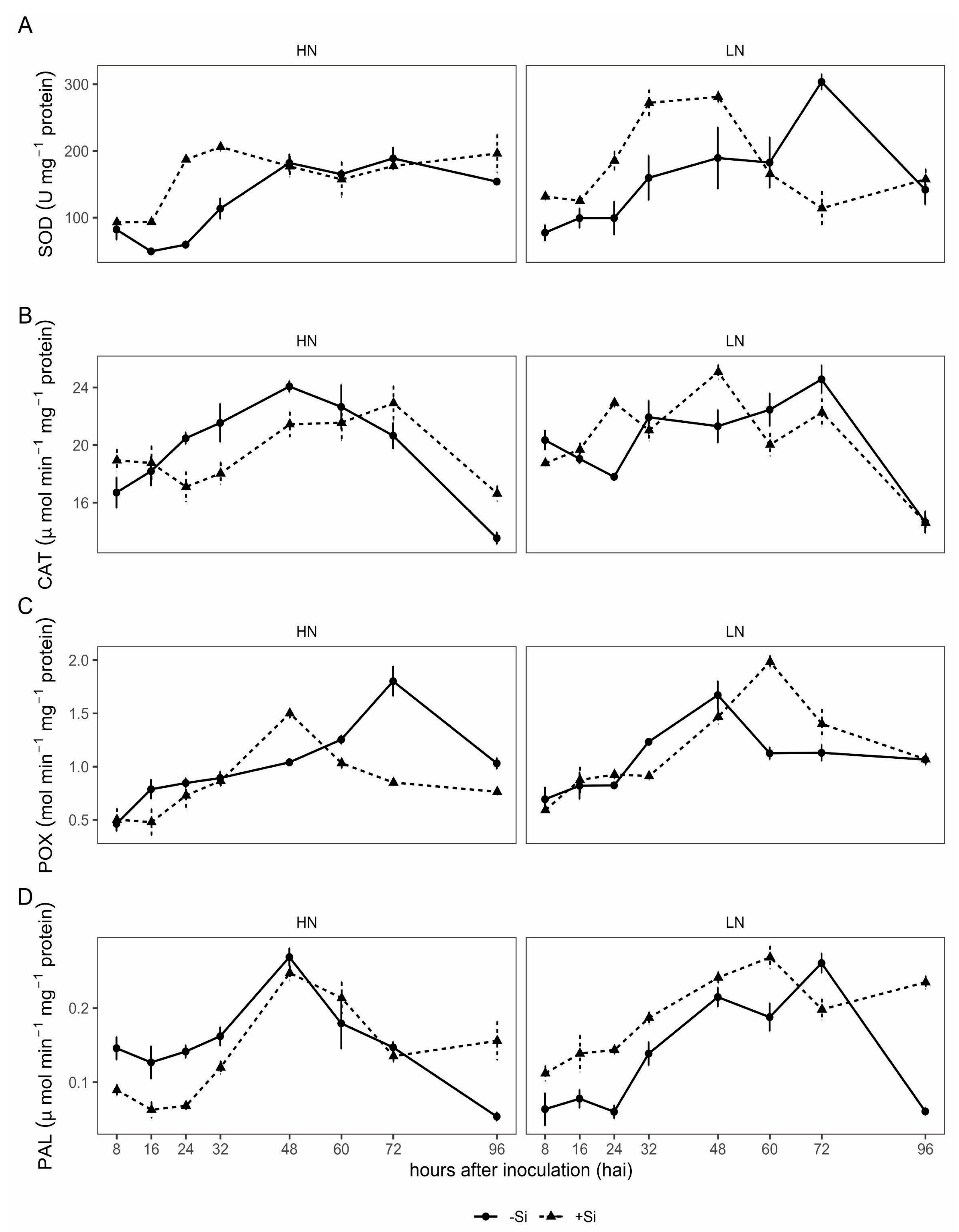
In this study, wheat plants supplied with Si and N in combination displayed a differential response in resistance components to Ptr, affecting plants’ histocytological and biochemical defense responses. A higher Si concentration in wheat plants stimulated earlier and higher H2O2 accumulation, callose deposition, and increased the enzymatic activity of metabolic pathways related to the antioxidative and defense systems, which together caused a reduction in tan spot severity. In addition, the high N concentration interfered with the Si-beneficial action on the plant defense metabolism.
The metabolic benefits of Si supply in plants with low N input were better evidenced compared to higher N input, resulting in earlier and stronger activation of defense responses, indicating a beneficial role of Si with low availability of N in potentiating the plant defenses against
Ptr. A higher SOD activity indicated that +Si plants respond rapidly to pathogen infection, which is associated with increases in the H
2O
2 concentration, resulting in a reduction in NLs per cm². This observation was also reported by Dorneles et al. [
3], who found that earlier accumulation of H
2O
2 in wheat epidermal cells was associated with the plants’ defense mechanism in Si-supplied plants and in cultivars with moderate resistance, as opposed to the later accumulation of H
2O
2, and mainly in the parenchymal cells, in susceptible cultivars. Likewise, the increase in the activity of POX and PAL observed in +Si plants under low N input indicated that the defense mechanism involving the phenylpropanoid metabolism was activated, such as lignin biosynthesis [
27], which may contribute to reducing the lesion size and the final disease severity. PAL is a key enzyme of the phenylpropanoid pathway, which leads to increased synthesis of resistance-related phenols [
28]. Previous studies also reported that phenylpropanoid derivatives and lignin accumulation have important roles in wheat defense against
Ptr [
3,
4]. Interestingly, in plants under low N input, Si nutrition stimulated SOD, POX and PAL activities, starting them earlier and with greater activity, while under high N input, although SOD activation was similar to that of low N input, the changes in the activity of POX and PAL occurred a little later or at a lower intensity. However, there was a reduction in CAT activity, indicating different strategies under high and low N input in the potentiation of defenses by Si leading to an increase in the H
2O
2 and phenylpropanoid derivatives.
Regarding PAL activity, studies have reported that in plants receiving low N input, its activity increases, but only slightly, while at high N input, the activity decreases [
29]. The results of our study partially corroborate that finding; although low and high N inputs increased PAL activity in response to
Ptr, under high N, the change in the enzyme activity was slightly delayed. Despite this difference in PAL activity at high N input, there was less
Ptr damage as indicated by the lower tan spot severity in this study and as was previously reported by Castro et al. [
30] and Fleitas et al. [
24]. In this sense, at high N, although rapid accumulation of H
2O
2 was induced, PAL activity was not the first response to counteract the infection, although it contributed to delayed colonization, inducing resistance to
Ptr.
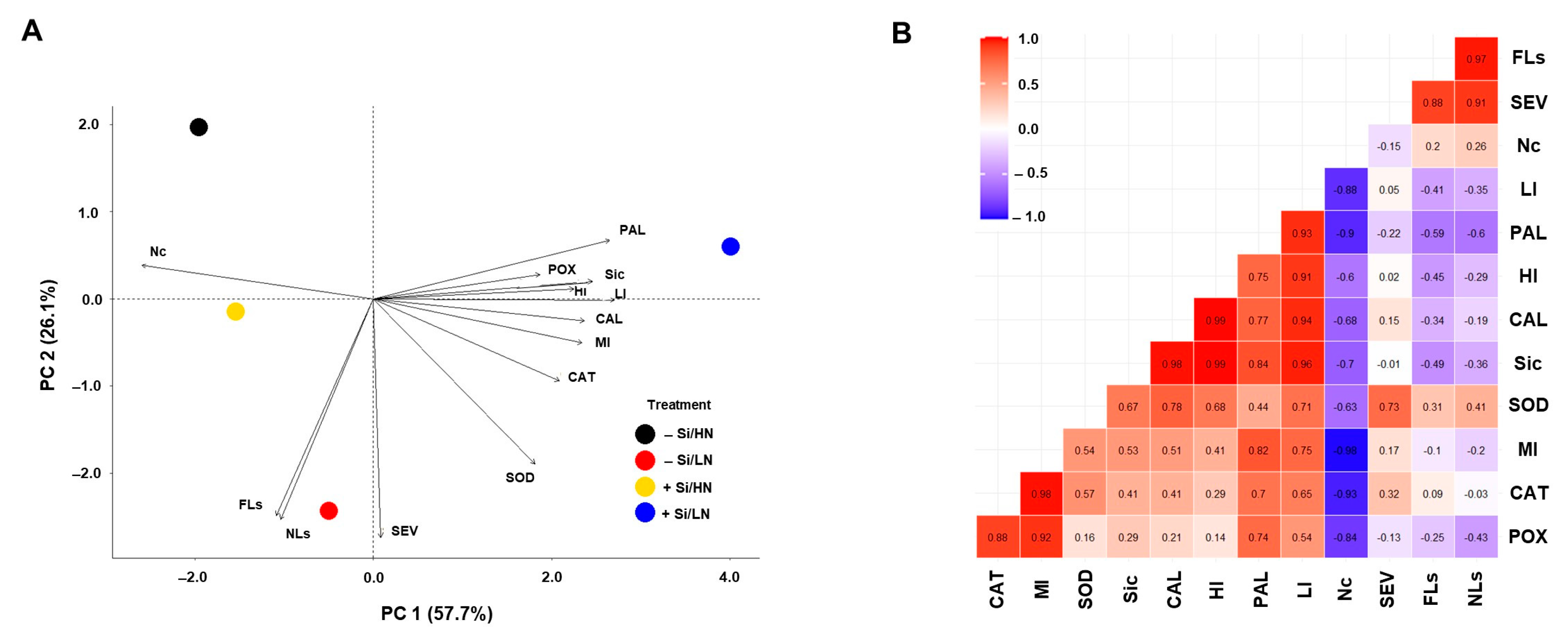
Nevertheless, observations in the enzymatic defense system, H
2O
2 accumulation, and callose deposition may be associated with the effect of +Si. Callose deposition is promoted by indole glucosinolates (IGSs) and reactive oxygen species (ROS) [
31]. Furthermore, the activation of abscisic acid (ABA) signaling induces or primes callose deposition and is influenced by carbohydrate metabolism [
31,
32]. In our study, callose deposition began at 24 hai, reaching the highest peak at 32 hai in plants supplied with +Si/LN input. Indeed, highest callose and H
2O
2 accumulation were observed in +Si leaves infected by
Ptr regardless of N input. For callose formation, a glucose molecule is required [
33], resulting in higher carbon sources that probably come from a sucrose metabolism. In this context, sucrose metabolism plays a pivotal role in the cellular function and biosynthesis of starch, cellulose, callose, and proteins through hexoses and their derivatives produced by the metabolic pathway. Thus, sucrose metabolism stands as a cornerstone process, sustaining the energetic and biosynthetic demands of cellular life [
34]. This observation agrees with the fact that Si impact the source–sink relationship and stimulates amino acid remobilization observed in rice [
35]. For instance, the wheat–
Pyricularia oryzae interaction mediated by Si nutrition improved the source–sink relationship of infected leaves due to an alteration in the activities of the enzymes’ acid invertase and sucrose phosphate synthase in leaves and spikes of wheat challenged by
Pyricularia oryzae [
36], suggesting that in our case, Si incorporation, besides contributing to severity reduction, may perhaps involve maintaining the sucrose’s concentration despite the
Ptr infection and without a direct influence of N concentration.
Associated with this observation, Si concentration in leaves decreased with a high N rate. At least two factors may have influenced this outcome: first, the greater increase in biomass with higher N, which led to the dilution of Si in the plant tissue; and second, changes in the soil pH caused by the higher N rate affected Si uptake. In this context, soil acidification under high N rates in this experiment may have affected Si absorption, which mainly occurs through active transport mechanisms inherent in the roots [
37], resulting in lower Si content at high N. This observation agrees with Murozuka et al. [
38], who reported that differences in leaf Si concentration among wheat genotypes were partially explained by differences in soil pH.
This is the first report of the effect of N × Si interaction in wheat challenged by Ptr. At low N, the Si concentration was higher in the plants, demonstrating the effect on Ptr due to enhancement in plant defenses. The findings of this study suggest that Si and low N input increase callose deposition in a compatible reaction (susceptible host). There were no synergistic effects between the two elements in resistance to tan spot. Despite an effective control of Ptr at high N, N fertilization should be used with caution when Si is incorporated in the soil during crop management. Nitrogen application and soil amended with Si are strategies that activate plant defense mechanisms independently because increasing the N rate affects Si concentration, altering the changes in the plants’ defense responses to Ptr. In conclusion, the Si and N input treatment altered the activities of SOD, CAT, POX, and PAL, improving the defense against Ptr infection. In addition, the greater accumulation of H2O2 and deposition of callose in leaves infected by Ptr suggests that the activation of these defenses was mainly associated with Si.
4. Materials and Methods
4.1. Plant Material and Growth
Wheat cultivar TBIO Tibagi (BIOTRIGO GENETICA®, Passo Fundo/RS—Brazil), susceptible to tan spot, was used in the experiment. Seeds were surface-sterilized in 10% (vol vol−1) NaOCl for 2 min and rinsed in sterile water for 3 min. Pre-germination was conducted in wet/dry cycles of 12 h, at 20 °C, in the dark for 2 days. Then, seven germinated seeds were transferred to biodegradable seed nursery bags (14 × 18 cm) (Huvai®, USA) containing 475 g of soil and 100 g of seed starter (Carolina Soil®, Santa Cruz do Sul/RS, Brazil), and kept in plastic trays (47 × 25 × 10 cm), ten in total. The plants were grown in a greenhouse with relative humidity of 80 ± 5% and temperature of 25 ± 2 °C.
4.2. Soil Characteristics, Silicon Amendments, and Nitrogen Treatment
The soil used in this experiment was collected in the experimental area of the Federal University of Pelotas, Capão do Leão, Rio Grande do Sul, Brazil. The collected soil had the following physicochemical characteristics: 658 g kg−1 sand; 241 g kg−1 clay; 101 g kg−1 silt; 1% organic matter; 1.5 g N kg−1; 6.64 g NO3 kg−1; 1.93 g NH4 kg−1; 11.9 kg P; 143 mg K dm−3; 2.7 cmolc Ca dm−3; 1.4 cmolc Mg dm−3; 0.9 mg Cu dm−3; 0.8 mg Zn dm−3; 12.3 mg Mn dm−3; pH 5.5; cation exchange capacity (CEC), 10.4 cmolc dm−3; H + Al, 0.3 cmolc dm−3; and base saturation, 48.1%.
The concentration of available Si (extracted with 0.01 M CaCl
2) was 6.0 mg dm
−3, determined according to the standard colorimetric analysis method [
39].
The Si source was calcium silicate (Agrosilício®, Agronelli Insumos Agrícolas, Uberaba, Brazil), which is composed of 10.5% Si, 25.0% Ca and 6.0% Mg. Calcium silicate was mixed with the soil at an equivalent rate of 13.2 tons ha−1 to increase the soil’s pH to 6.0. To isolate the effect of Si, we standardized the amount of Ca and Mg supplied to the plants in the calcium silicate treatment. The extra-fine limestone (Dagoberto Barcelos, Caçapava do Sul, Brazil), composed of Ca (26.5%) and Mg (15%) magnesium, was added to the soil at an equivalent rate of 11 tons ha−1, being utilized for plant growth in the control treatment. Calcium carbonate (Synth, Diadema, Brazil) and magnesium carbonate (Synth, Diadema, Brazil) were used to adjust the final concentrations of Ca and Mg, respectively, between treatments. After complete soil homogenization of each treatment, water was added to reach 80% of field capacity, followed by 30 days of incubation in plastic bags, until use.
The nutritional requirements of wheat plants were supplied using an adjusted nutrient solution of phosphorus and potassium. The nutrient solution contained 12.97 g L−1 of KH2PO4 and 15.62 g L−1 of KCl. A volume of 63.5 mL of this nutrient solution described above was applied to each bag at the third and fourth weeks after transplanting. Also, a N requirement was applied as a nutrient solution adjusted to obtain the two doses under study, low N (LN): 70 kg ha−1, and high N (HN): 200 kg ha−1. The two N concentrations were prepared containing 0.147 g L−1 and 0.442 g L−1 for LN and HN, respectively, using granular urea (N = 45%). A volume of 40 mL of the N solution was applied per bag, according to the N rate planed, in the fifth to seventh weeks. Deionized water was utilized to prepare both nutrient solutions and plant irrigation.
4.3. Experimental Design and Treatments
Two experiments were carried out under a completely randomized experimental design in a factorial arrangement of 2 × 2, consisting of two Si treatments [not supplied (11.0 ton of limestone; −Si) and supplied (13.2 ton of calcium silicate; +Si)] and two N rates (LN:70 or HN:200 kg N ha−1), with twelve replications. In each experiment, the fourth and fifth leaves (from the top), at a phenological stage of 37 according to Zadoks Growth Scale, of each plant were marked and used for pathometrical, histocytological, and biochemical analyses. Each experimental unit corresponded to a plastic bag containing seven plants.
4.4. Inoculum Production and Inoculation Procedure
The Pyrenophora tritici-repentis race 1 (BRPtr8), provided by Universidade de Passo Fundo, was used in the experiments. The fungus, preserved as a PDA mycelial disk at −20 °C, was reactivated on PDA for one week, after which mycelial plugs with a diameter of 5 mm were cut from the margin of actively growing colonies and transferred to Petri dishes containing modified V8-agar medium [(3.0 g L−1 of calcium carbonate (Synth, Diadema, Brazil), 150 mL L−1 of tomato sauce (Fugini®, Monte Alto/SP, Brazil), 15 g L−1 of agar (Kasvi, Curitiba, Brazil)] and distilled water.
The inoculum production was performed according to the method described by Dorneles et al. [
3]. Briefly, after fungal growth in the modified V8-agar medium over five days at 25 ± 1 °C in darkness, it was stressed by mycelium scraping. Then, the fungal colony was exposed for 24 h to light at 25 ± 1 °C to allow for the development of conidiophores, followed by a further 24 h of darkness at 15 ± 1 °C, necessary for conidia formation. After seven days, conidia were carefully removed from the Petri dishes with a soft bristle brush using water containing 1 drop of Tween 20. The conidial suspension was adjusted to obtain a concentration of 3 × 10
3 conidia mL
−1 as described by Dorneles et al. [
3].
The fourth leaves of 50-day-old wheat plants with the flag leaf just visible (ZGS37) were used in the experiment. For this, leaves were picked and immediately placed in NaOCl at 10% (vol vol−1) for 2 min, followed by washing three times with sterilized water and kept in water for 3 min, and air drying for 5 min. The conidial suspension was applied with a hand-held sprayer (Tecblas, REF: 60 mL/Porto Alegre, Brazil) on the adaxial surface of the leaves. After inoculation, six leaves were transferred to a plastic Petri dish (150 × 15 mm) with water–agar medium. Mock-inoculated leaves (control) were sprayed with distilled water and exposed to the same conditions as Ptr-inoculated ones. The plastic Petri dishes with Ptr-inoculated or mock-inoculated leaves were incubated at 25 ± 1 °C, relative humidity of 80 ± 5%, and a photoperiod of 12 h during the experiment.
4.5. Disease Assessment
Disease assessments were performed at 96 h after inoculation (hai) by quantifying the number of lesions per cm2 (NLs), as well as determining the final lesion size (FLS) and disease severity (SEV). For NLs, four areas (1 cm2) of six leaves were used to quantify the NLs formed. The final lesion size (FLS) was quantified at four points of one cm2 of leaf by randomly measuring at least five lesions with a digital caliper (150 mm, model 02KC4PR7P1995, YuanSen®, Shenzhene, China). The SEV, defined as the percentage of total leaf area affected by the disease (necrotic and chlorotic tissue), was determined in six inoculated leaves per replicate. For this, digital leaf images were obtained at 600 dpi using a scanner (Epson/L395), and the SEV was determined using the digital image analysis software Quant® (Universidade Federal de Viçosa, Viçosa/MG, Brazil).
4.6. Leaf Samples for Detection of Hydrogen Peroxide and Aniline Blue Staining
To determine if N rate and Si supply altered the amount and/or time of hydrogen peroxide accumulation and callose deposition in response to pathogen presence, Ptr-inoculated leaves were sampled at 8, 16, 24, 32, 48, 60, 72 and 96 hai. To verify that hydrogen peroxide or callose was produced in response to the presence of the pathogen, the analysis and image acquisition were only performed after verifying the presence of the pathogen (conidia and germ tube or appressoria) in the plant tissue. Additionally, to prove that the defense responses were only due to inoculation with the pathogen, leaf samples from mock-inoculated plants were sampled at the same time as Ptr-inoculated plants. However, due to the absence of pathogen structures on these plants, they were used only as a control to verify that the alteration in defense responses was not due to environmental factors or manipulation of the leaf tissue.
4.6.1. Detection of Hydrogen Peroxide
Hydrogen peroxide in the plant tissue was detected according to the method described by Shi et al. [
26]. Briefly, fragments of 1 cm
2 were cut from the middle of each leaf, discarding the tip and base of the leaf, and immediately vacuum infiltrated in diaminobenzidine (DAB) (Sigma-Aldrich, Jurubatuba/SP, Brazil ) solution (wt vol
−1) for 15 min (1 mg mL
−1, pH 3.8, adjusted using 0.1 N HCl), which was freshly made in 10 mM phosphate buffer (pH 5). Leaf samples were incubated in the solution at room temperature and exposed to light (fluorescent tube, Osram 40 W) for 8 h. Finally, the leaf samples were discolored in boiling 70% ethanol. After this period, the leaf samples were kept in 5 mL of 70% ethanol in Falcon tubes for further documentation.
Microscopic Analysis, DAB Image Acquisition, and Quantification of Affected Epidermal Cells
For microscopic analysis, 16 leaf samples of 1 cm2 per treatment were examined at each sampling time. Leaf samples were placed adaxial side up on slides with one drop of 20% glycerol and covered by a coverslip, followed by observation under a CX41 biological microscope (Olympus®, Shinjuku-ku/Tokyo, Japan) at 200× magnification. To verify that hydrogen peroxide was produced in response to the presence of the pathogen, image acquisition was only performed after verifying the presence of the pathogen (conidia and germ tube or appressoria). The number of epidermal cells showing brown staining (DAB polymerized), indicating the presence of H2O2, was counted at each infection site. For each sampling time, at least 20 appressorial sites were randomly observed and photographed for further analyses. Leaf image acquisition was performed using a digital camera with 3.1 MP (USB 2.0 Color CMOS Digital Eyepiece Microscope Camera, AmScope, Irvine/CA, USA) linked to the PC via a USB interface. AmScope 4.11 software was used as an interface for DAB image acquisition. All photographs were acquired with 2038 × 1536 resolution and saved as JPEG files.
After this, a set of reference images with a visible H
2O
2 reaction at the infection site caused by
Ptr were selected to determine the intensity profile (value between 0 and 255 RGB). For this, the ‘magic wand’ and histogram tools of the Adobe Photoshop CS6
® 13.0 v64 software were used to manually select visible H
2O
2 reactions and determine the intensity scale (IS) based on a previous categorical color intensity scale according to Dorneles et al. [
3]. In this way, the IS was established considering three color intensities: low intensity or slightly brownish wall of the epidermal cells [(LI); range between 111 and 131 RGB)]; medium intensity or wall of epidermal cells encircled by a dark brownish color [(MI); range between 91 and 110 RGB)]; and high intensity or dark brown entire epidemic cells [(HI); values ≤ 90 RGB)]. Based on the IS from the training images, 12 images for each treatment were evaluated. The histogram analysis tool of ImageJ/Fiji 1.46 was used, which allows for the attainment of an intensity profile for RGB images. For each image, the number of pixels was quantified according to the IS. At the end of these analyses, the IS pixels of the image evaluated were expressed as a percentage of H
2O
2 reaction based on the ratio between the total number of pixels at each IS and the total number of pixels into which each image was decomposed.
4.6.2. Aniline Blue Staining
To detect callose, plant tissue was stained with aniline blue (Sigma-Aldrich, Jurubatuba/SP, Brazil) as described by Schenk and Schikora [
27], with some modifications. Briefly, leaf samples (1 cm
2) were discolored in 1:3 [acetic acid/ethanol 95% (vol vol
−1)]. The saturated destaining solution was replaced every 12 h, if necessary, until all tissues were transparent. Then, leaf samples were washed in 0.07 mM phosphate buffer (pH = 9) for 30 min. Next, the destained leaf samples were incubated in the dark for at least 8 h in a staining solution containing 0.001 mg mL
−1 aniline blue fluorochrome (wt vol
−1) (Sigma-Aldrich, Jurubatuba/SP, Brazil) in 0.07 mM phosphate buffer (pH = 9), and kept in Falcon tubes. After incubation, the leaf samples were washed again in 0.07 mM phosphate buffer (pH = 9), placed in 70% ethanol for fixation, and finally embedded in 50% glycerol (vol vol
−1) in the dark for conservation. After this procedure, leaf segments at each sampling time were placed and fixed with the adaxial side up on slides containing drops of Entellan
TM new rapid mounting medium (Sigma-Aldrich, Jurubatuba/SP, Brazil) with a drop of 50% glycerol (vol vol
−1), and maintained in the dark for further microscopic analysis.
Microscopic Analysis, Image Acquisition, and Callose Quantification
For microscopic analysis, 16 leaf samples of 1 cm2 per treatment at each sampling time were visualized for callose deposition through epifluorescence microscopy (Eclipse Ts2-FL, Nikon, Melville/NY, USA) using a DAPI filter (wavelength of 385 nm and maximum emission of 420 nm). The Capture 2.2 software was used for documentation. The images were acquired at 1300 × 1030 resolution with adjustment of brightness at 300 ms exposure time, and saved as JPEG files. As for hydrogen peroxide, to ensure that the callose was produced in response to the presence of the pathogen, image acquisition was only performed after verifying the presence of the pathogen at the infection site.
Once again, a set of reference photographs was used to determine the intensity profile (value between 0 and 255 RGB). For this purpose, the ‘magic wand’ and histogram tools of Adobe Photoshop CS6® were used to manually select callose intensity, which ranged between 139 and 176 RGB. Based on the intensity range identified from the training images, 12 images for each treatment were evaluated using the histogram analysis tool ImageJ/Fiji 1.46, which quantifies the number of pixels and intensity profile at the range of each RGB image. The callose deposition area was recorded as the corresponding pixels of range intensity (139 and 176 RGB) of the total number of pixels into which each image was decomposed and expressed as a percentage of callose based on the ratio between the number of callose pixels and the total number of image pixels.
4.7. Enzyme Activity
Inoculated leaves (
Ptr-inoculated and mock-inoculated) were collected at 8, 16, 24, 32, 48, 60, 72 and 96 hai, flash-frozen using liquid nitrogen, and subsequently preserved at −80 °C until further examination. For each treatment and each sampling time, four samples were collected. Each leaf sample (300 mg) was ground with a mortar and pestle and the resulting fine powder analyzed for enzyme activity. The crude extract used for enzyme activity determination was obtained according to the method described by Dallagnol et al. [
40].
The activities of catalase, peroxidase and superoxide dismutase were determined according to the methods described by Dallagnol et al. [
40]. The activity of catalase (CAT, EC 1.11.1.6) was determined by quantifying the degradation of hydrogen peroxide (H
2O
2) (Merck, Pinheiros/SP, Brazil) and expressed as micromoles of H
2O
2 degraded min
−1 mg
−1 of protein. Peroxidase activity (POX, EC 1.11.1.7) was determined based on the colorimetric quantification of pyrogallol (Sigma-Aldrich, São Paulo, Brazil) oxidation, and expressed as moles of purpurogallin produced min
−1 mg
−1 of protein using an extinction coefficient of 2.47 mM cm
−1. The superoxide dismutase (SOD, EC 1.15.1.1) activity was estimated based on the colorimetric quantification of the photoreduction of nitroblue tetrazolium (NBT) (Sigma-Aldrich, Jurubatuba/SP, Brazil). The specific activity of SOD was expressed in units of SOD mg
−1 of protein, considering that one unit of SOD was the amount required to inhibit the photoreduction of NBT by 50%. Phenylalanine ammonia-lyase (PAL, EC 4.3.1.5) activity was determined according to the method described by Dorneles et al. [
4] by colorimetric quantification of transcinnamic acid formed from phenylalanine (Sigma-Aldrich Jurubatuba/SP, Brazil) and expressed as µmol of transcinnamic acid produced per min
−1 mg
−1 of protein.
For each enzyme activity, four separate extractions of samples from each treatment were performed. Each extraction was read in a spectrophotometer (model UV-UM51, Bel Engineering srl®, Monza/MB, Italy) in the corresponding wavelength [CAT: 240 nm; POX: 420 nm; SOD: 560 mn; PAL: 290nm]. The soluble protein concentrations of the extracts were measured by the standard Bradford method, using bovine serum albumin as the standard protein wavelength [595 nm].
4.8. Analyses of N Leaf Concentration
Leaf samples (four) were collected from the just visible flag leaves (ZGS37) for each treatment, dried at 60 °C for 72 h, and ground using an R-TE-350 mill. A subsample of 1.0 g was used for determination of leaf concentration (g kg−1) according to the standard micro-Kjeldahl method.
4.9. Analyses of Si Leaf Concentration
Four leaves were collected at the same stage mentioned above for the leaf Si concentration (g kg
−1) determination for each treatment. The samples were rinsed with deionized water, dried for 72 h at 65 °C, and ground to pass through a 40-mesh screen using a mill. The foliar Si concentration was determined by standard colorimetric analysis from 0.1 g of alkali-digested tissue [
39].
4.10. Data Analyses
The data of callose, hydrogen peroxide and enzymatic activity were used to calculate the area under the curve (
AUC) according to the equation of Shaner and Finney (1977).
where
Yi = values (per replication) in each observation;
Xi = is the time (days) of each observation; and
n = total number of observations. The Shapiro–Wilk test was applied to ascertain the normality of the data and the homogeneity of variances was checked with Bartlett’s test. Data obtained from two experiments were analyzed using Hartley’s test (
Fmax test) to determine the homogeneity of variances between experiments and could be combined. The data were analyzed by applying parametric ANOVA, and the means were compared by
F and Tukey’s test (
p ≤ 0.05). Principal components analysis (PCA) was used to determine the relationship among the variables and parameters. Statistical analyses were conducted with R version 4.0.4. through the packages “dplyr”, “FactoMiner”, “factoextra”, “ggplot2”, “ggcorrplot”, “ggpubr”, and “tidyr” [
41].