Exploring Southern Ecuador’s Traditional Medicine: Biological Screening of Plant Extracts and Metabolites
Abstract
1. Introduction
2. Results
2.1. Screening the Extracts
2.2. Determination of the Cytotoxicity of the Selected Extracts
2.3. Description of Recognized Traditional Therapeutic Uses
2.4. Identification of Potential Target for the Selected Extracts
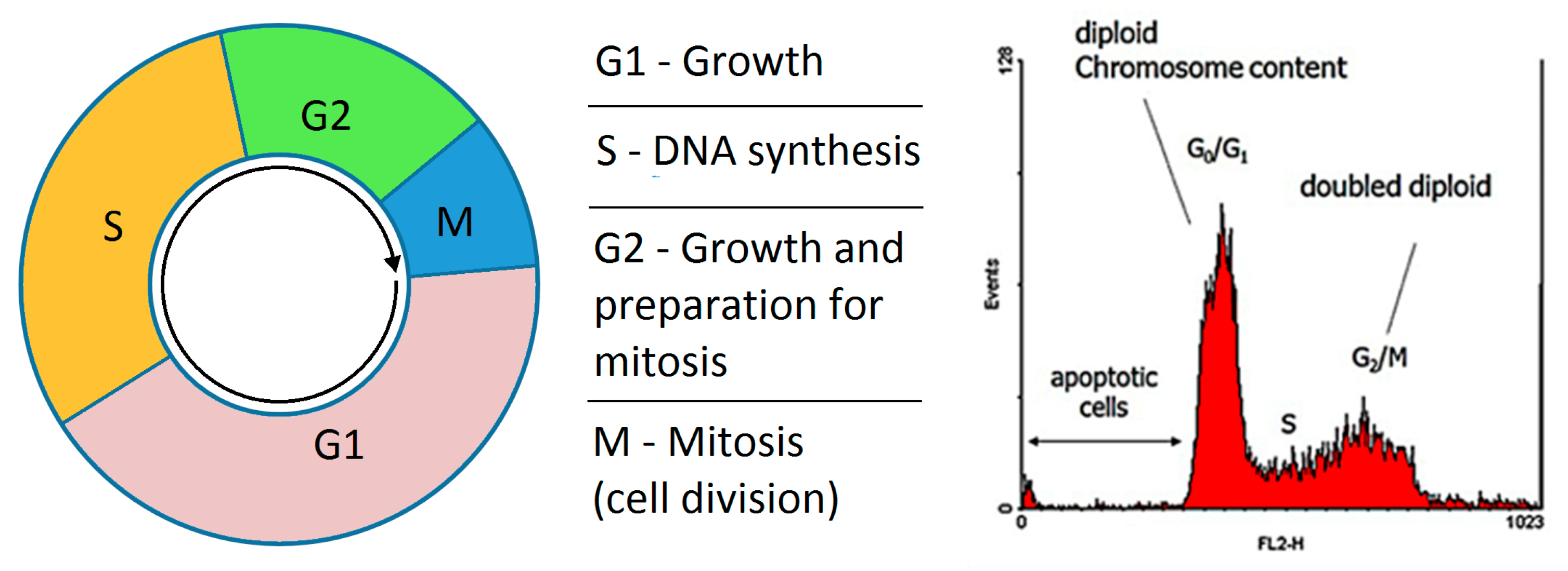
2.4.1. Cell Cycle
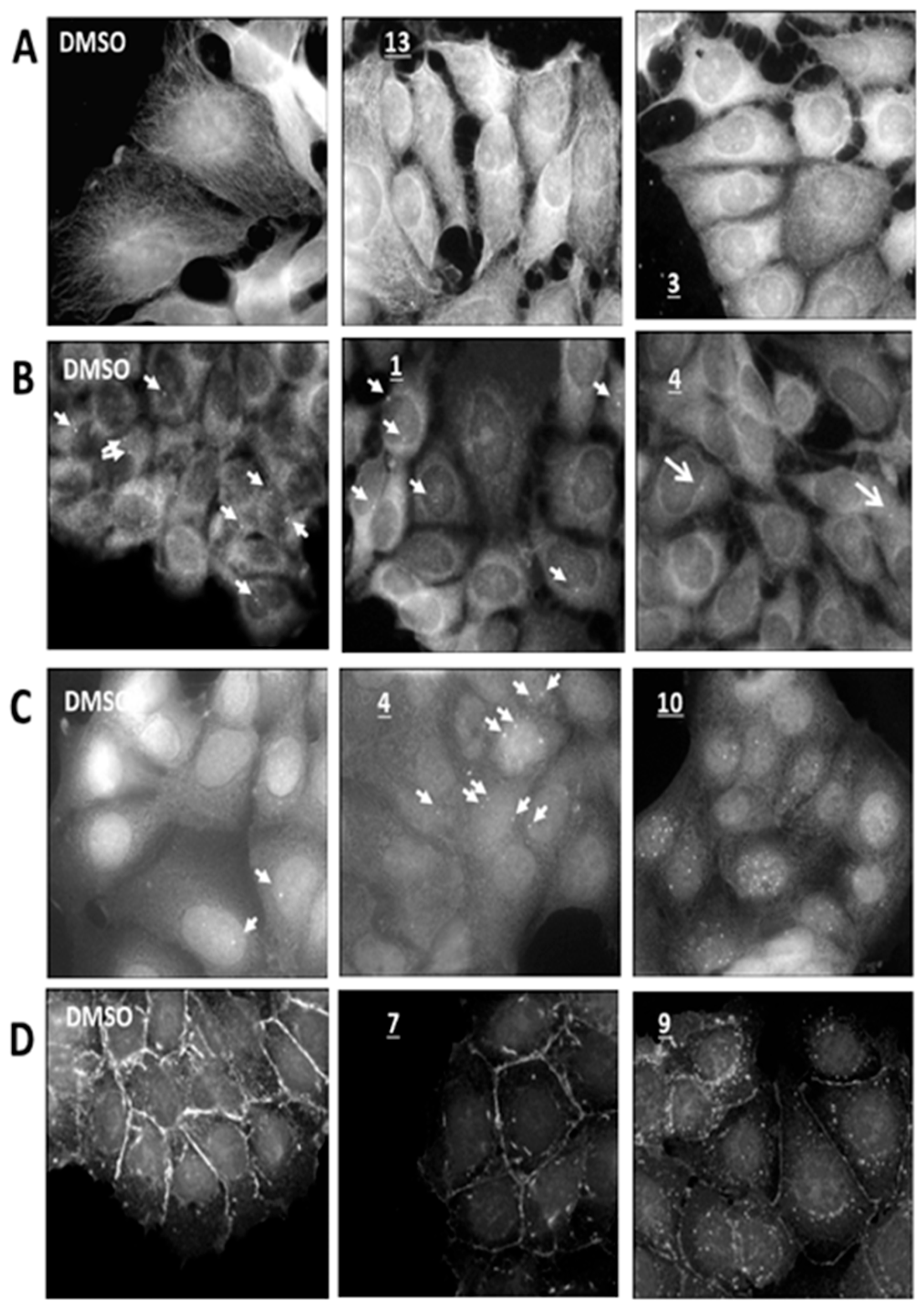
2.4.2. Microtubule
2.4.3. Centrosomes
2.4.4. DNA Damage Analysis
2.4.5. E-Cadherin Test
3. Discussion
4. Materials and Methods
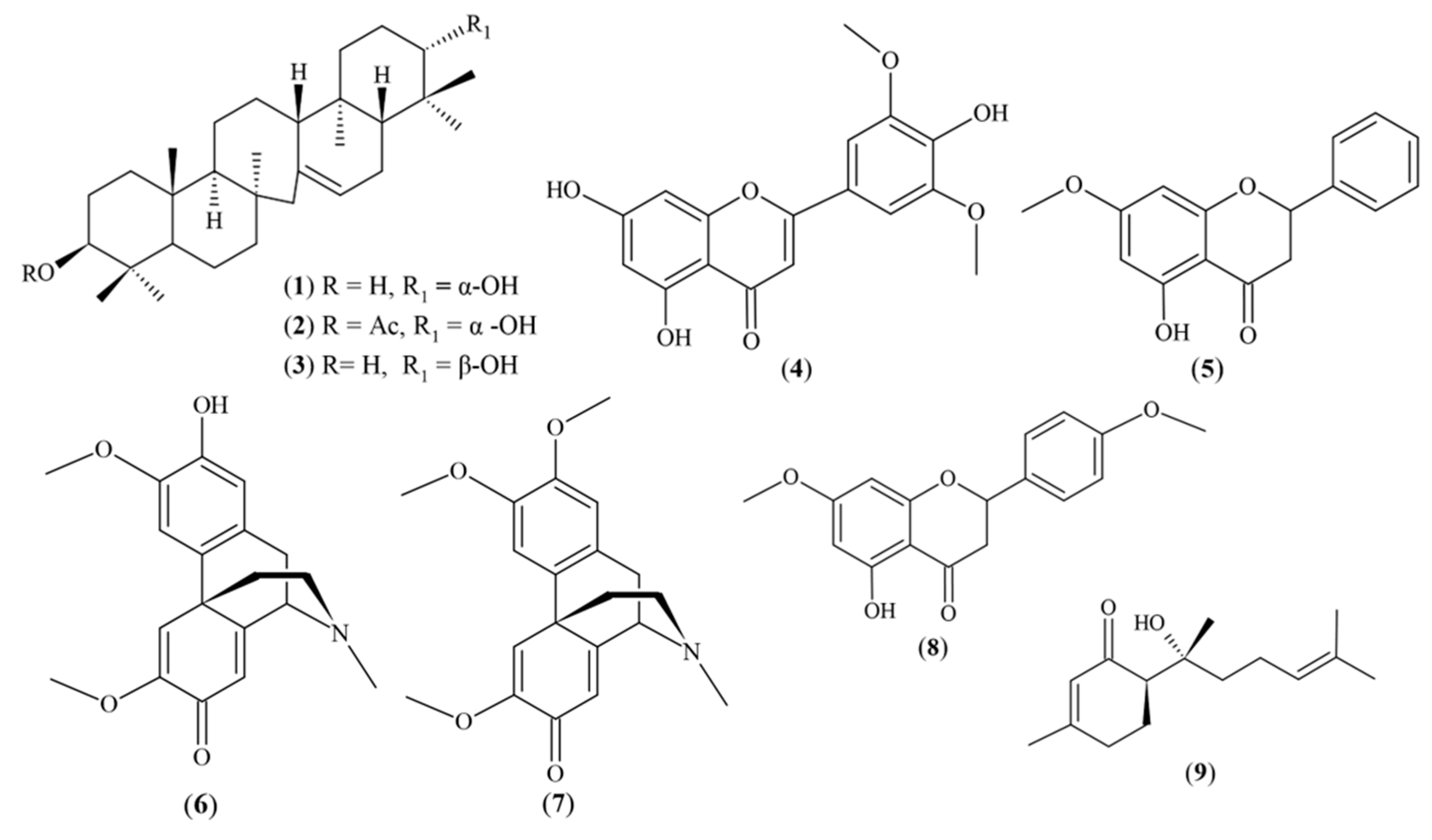
4.1. Chemical Compounds Studied in this Article
4.2. Origin of Plant Material for Obtaining Extract Preparation
4.3. Preparation of Extracts
4.3.1. Crude Extracts
4.3.2. Lyophilized Extracts
4.3.3. Alkaloid Extracts
4.3.4. Pure Compounds
4.4. Biological Experiments
4.4.1. Cell Culture
4.4.2. Cytotoxicity Evaluation
4.4.3. Cell-Cycle Analysis
4.4.4. Immunofluorescence Microscopy Analysis
4.4.5. Evaluation of the Effects on Centrosomes and γH2AX Foci
5. Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Y. History, Present and Prospect of World Traditional Medicine (In 2 Volumes), 1st ed.; World Scientific: Singapore, 2023. [Google Scholar]
- Graves, T.C. Commentary on a Case of Hystero-epilepsy with delayde puberty treated with testicular extract. Lancet 1920, 196, 1134–1135. [Google Scholar] [CrossRef][Green Version]
- Beecher, H.K. The powerful placebo. J. Am. Med. Assoc. 1995, 159, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023, 1st ed.; WHO Library Cataloguing: Hong Kong SAR, China, 2013. [Google Scholar]
- Jones, K. Review of Sangre de Drago (Croton lechleri)—A south American tree sap in the treatment of diarrhea, inflammation, insect bites, viral infections, and wounds: Traditional uses to clinical research. J. Altern. Complement. Med. 2003, 9, 877–896. [Google Scholar] [CrossRef]
- Tene, V.; Malagón, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Malagón, O.; Ramírez, J.; Andrade, M.J.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016, 11, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Quisatagsi, E.V.; Cuenca, M.; Cuenca-Camacho, S.; Bailón-Moscoso, N. The cytotoxic principle of Bejaria resinosa from Ecuador. J. Pharmacogn. Phytochem. 2015, 4, 268–272. [Google Scholar]
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, A.; Liu, Q.; Yuan, X.; Xu, H.; Jiao, D.; Pestell, R.G.; Han, X.; Wu, K. Recent advances of bispecific antibodies in solid tumors. J. Hematol. Oncol. 2017, 10, 155. [Google Scholar] [CrossRef]
- Trenevska, I.; Li, D.; Banham, A.H. Therapeutic Antibodies against Intracellular Tumor Antigens. Front. Immunol. 2017, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Barlesi, B.; Tarroza-David, S.; Friedlander, T. Improved Control of Tyrosine Kinase Inhibitor-Induced Diarrhea with a Novel Chloride Channel Modulator: A Case Report. Oncol. Ther. 2021, 9, 247–253. [Google Scholar] [CrossRef]
- Harrison, F.; Roberts, A.E.; Gabrilska, R.; Rumbaugh, K.P.; Lee, C.; Diggle, S.P. A 1000-Year-Old Antimicrobial Remedy with Antistaphylococcal Activity. mBio 2015, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Dettweiler, M. American Civil War plant medicines inhibit growth, biofilm formation, and quorum sensing by multidrug-resistant bacteria. Sci. Rep. 2019, 9, 7692. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Tan, M.; Pohlmann, P.R.; Swain, S.M. HALT-D: A Phase II Evaluation of Crofelemer for the Prevention and Prophylaxis of Diarrhea in Patients With Breast Cancer on Pertuzumab-Based Regimens. Clin. Breast Cancer. 2017, 17, 76–78. [Google Scholar] [CrossRef][Green Version]
- Zhong, C.; Wall, N.R.; Zu, Y.; Sui, G. Therapeutic Application of Natural Medicine Monomers in Cancer Treatment. Curr. Med. Chem. 2017, 24, 3681–3697. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef]
- Akone, S.H.; Ntie-Kang, F.; Stuhldreier, F.; Ewonkem, M.B.; Noah, A.M.; Mouelle, S.E.M.; Müller, R. Natural Products Impacting DNA Methyltransferases and Histone Deacetylases. Front. Pharmacol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Martínez-Sánchez, S.M.; Jiménez-González, V.; Burgos-Morón, E.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Díaz-Ortega, P.; Garcia, F.; Aparicio, A.; López-Lázaro, M. Screening for Selective Anticancer Activity of 65 Extracts of Plants Collected in Western Andalusia, Spain. Plants 2020, 10, 2193. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Le, T.T.; Wu, M.; Lee, J.H.; Bhatt, N.; Inman, J.T.; Berger, J.M.; Wang, M.D. Etoposide promotes DNA loop trapping and barrier formation by topoisomerase II. Nat. Chem. Biol. 2008, 19, 641–650. [Google Scholar] [CrossRef]
- Pasquier, E.; Kavallaris, M. Microtubules: A dynamic target in cancer therapy. IUBMB Life 2008, 60, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Singh, S.; Skvortsova, I.; Kumar, V. Promising Targets in Anti-cancer Drug Development: Recent Updates. Curr. Med. Chem. 2017, 24, 4629–4752. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Lucero Mosquera, H.; Armijos, C. Ethnobotany of Indigenous Saraguros: Medicinal Plants Used by Community Healers “Hampiyachakkuna” in the San Lucas Parish, Southern Ecuador. BioMed. Res. Int. 2017, 2017, 9343724. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Ponce, J.; Ramírez, J.; Gozzini, D.; Finzi, P.V.; Vidari, G. An Unprecedented High Content of the Bioactive Flavone Tricin in Huperzia Medicinal Species Used by the Saraguro in Ecuador. Nat. Prod. Commun. 2016, 11, 273–274. [Google Scholar]
- Armijos, C.; Cota, I.; González, S. Traditional medicine applied by the Saraguro yachakkuna: A preliminary approach to the use of sacred and psychoactive plant species in the southern region of Ecuador. J. Ethnobiol. Ethnomed. 2014, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, Z.; Yaguana, C.; Merino, B. Plantas Medicinales de la Zona Andina de la Provincia de Loja, 1st ed.; Herbario y Jardín Botánico “Reinaldo Espinosa”: Loja, Ecuador, 2014; p. 193. [Google Scholar]
- De la Torre, L.; Navarette, H.; Muriel, P.; Macia, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador, 1st ed.; Herbario QCA de la Escuela de Ciencias Biologicas de la Pontifica Universidad Católica del Ecuador, Quito, Ecuador & Herbario AAU del Departamento de Ciencias Biologicas de la Universidad Aarhus: Aarhus, Denmark, 2008. [Google Scholar]
- Bussmann, R.W.; Sharon, D. Traditional medicinal plant use in Loja province, Southern Ecuador. J. Ethnobiol. Ethnomed. 2006, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Gilardoni, G.; Amay, L.; Lozano, A.; Bracco, F.; Ramírez, J.; Bec, N.; Larroque, C.; Finzi, P.V.; Vidari, G. Phytochemical and ethnomedicinal study of Huperzia species used in the traditional medicine of Saraguros in Southern Ecuador; AChE and MAO inhibitory activity. J. Ethnopharmacol. 2016, 193, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Hickey, B.J.; Lumsden, A.J.; Cole, A.L.J.; Walker, J.R.L. Antibiotic compounds from new zealand plants: Methyl haematommate, an anti-fungal agent from Stereocaulon ramulosum. N. Z. Nat. Sci. 1990, 17, 49–53. [Google Scholar]
- Compadre, C.M.; Pezzuto, J.M.; Kinghorn, A.D.; Kamath, S.K. Hernandulcin: An intensely sweet compound discovered by review of ancient literature. Science 1985, 227, 417–419. [Google Scholar] [CrossRef]
- Kaneda, N.; Lee, I.S.; Gupta, M.P.; Soejarto, D.D.; Kinghorn, A.D. (+)-4 beta-hydroxyhernandulcin, a new sweet sesquiterpene from the leaves and flowers of Lippia dulcis. J. Nat. Prod. 1992, 55, 1136–1141. [Google Scholar] [CrossRef]
- Armijos, C.; Valarezo, E.; Cartuche, L.; Zaragoza, T.; Finzi, P.V.; Mellerio, G.G.; Vidari, G. Chemical composition and antimicrobial activity of Myrcianthes fragrans essential oil, a natural aromatizer of the traditional Ecuadorian beverage colada morada. J. Ethnopharmacol. 2018, 225, 319–326. [Google Scholar] [CrossRef]
- Jadaun, A.; Subbarao, N.; Dixit, A. Allosteric inhibition of topoisomerase I by pinostrobin: Molecular docking, spectroscopic and topoisomerase I activity studies. J. Photochem. Photobiol. B 2017, 167, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, A.; Lechler, T. Microtubule organization, dynamics and functions in differentiated cells. Development 2017, 144, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650. [Google Scholar] [CrossRef]
- Forth, S.; Kapoor, T.M. The mechanics of microtubule networks in cell division. J. Cell Biol. 2017, 216, 1525–1531. [Google Scholar] [CrossRef]
- Song, S.; Jung, S.; Kwon, M. Expanding roles of centrosome abnormalities in cancers. BMB Rep. 2023, 56, 216–224. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Alhmoud, J.F.; Woolley, J.F.; Al Moustafa, A.E.; Malki, M.I. DNA Damage/Repair Management in Cancers. Cancers 2020, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Haraguchi, M.; Mimaki, Y.; Motidome, M.; Morita, H.; Takeya, K.; Itokawa, H.; Yokosuka, A.; Sashida, Y. Steroidal saponins from the leaves of Cestrum sendtenerianum. Phytochemistry 2000, 55, 715–720. [Google Scholar] [CrossRef]
- Alonso-Castro, Á.J.; Ortíz-Sánchez, E.; Domínguez, F.; López-Toledo, G.; Chávez, M.I.; De Jesús Ortiz-Tello, A.; García-Carrancá, A. Antitumor effect of Croton lechleri Mull. Arg. (Euphorbiaceae). J. Ethnopharmacol. 2012, 140, 438–442. [Google Scholar] [CrossRef]
- Chordia, P.; MacArthur, R.D. Crofelemer, a novel agent for treatment of non-infectious diarrhea in HIV-infected persons. Expert. Rev. Gastroenterol. Hepatol. 2013, 7, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Pona, A.; Cline, A.; Kolli, S.S.; Taylor, S.L.; Feldman, S.R. Review of future insights of Dragon’s Blood in dermatology. Dermatol. Ther. 2019, 32, e12786. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.L.; Núñez-Figueredo, Y.; Tudella, V.G.; Cuesta-Rubio, O.; Rodrigues, F.P.; Pestana, C.R.; Uyemura, S.A.; Leopoldino, A.M.; Alberici, L.C.; Curti, C. The anti-cancer agent guttiferone-A permeabilizes mitochondrial membrane: Ensuing energetic and oxidative stress implications. Toxicol. Appl. Pharmacol. 2011, 253, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N.; Setzer, M.C.; Moriarity, D.M.; Bates, R.B.; Haber, W.A. Biological Activity of the Essential Oil of Myrcianthes sp. nov. “Black Fruit” from Monteverde, Costa Rica. Planta Med. 1999, 65, 468–469. [Google Scholar] [CrossRef]
- Ramírez, J.; Cartuche, L.; Morocho, V.; Aguilar, S.; Malagon, O. Antifungal activity of raw extract and flavanons isolated from Piper ecuadorense from Ecuador. Rev. Bras. Farmacogn. 2013, 23, 370–373. [Google Scholar] [CrossRef]
- Valarezo, E.; Flores-Maza, P.; Cartuche, L.; Ojeda-Riascos, S.; Ramírez, J. Phytochemical profile, antimicrobial and antioxidant activities of essential oil extracted from Ecuadorian species Piper ecuadorense sodiro. Nat. Prod. Res. 2021, 35, 6014–6019. [Google Scholar] [CrossRef]
- Garavito, G.; Rincón, J.; Arteaga, L.; Hata, Y.; Bourdy, G.; Giménez, A.; Pinzón, R.; Deharo, E. Antimalarial activity of some Colombian medicinal plants. J. Ethnopharmacol. 2006, 107, 460–462. [Google Scholar] [CrossRef]
- Matvieieva, N.A.; Pasichnyk, L.A.; Zhytkevych, N.V.; Pabón Garcés Galo, J.; Pidgorskyi, V.S. Antimicrobial activity of extracts from ecuadorian lichens. Mikrobiolohichnyi Zhurnal 2015, 77, 23–27. [Google Scholar] [CrossRef]
- Wei, F.; Zhao, L.; Jing, Y. Mechanisms underlying dimethyl sulfoxide-induced cellular migration in human normal hepatic cells. Environ. Toxicol. Pharmacol. 2020, 80, 103489. [Google Scholar] [CrossRef]
- Jorgensen, P.; Leon-Yanez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Garden Press: St. Louis, MO, USA, 1999. [Google Scholar]
- Plantlist. Plantlist 2013. Available online: http://www.theplantlist.org (accessed on 16 June 2017).
- Herrera, C.; Pérez, Y.; Morocho, V.; Armijos, C.; Malagón, O.; Brito, B.; Tacán, M.; Cartuche, L.; Gilardoni, G. Preliminary phytochemical study of the ecuadorian plant Croton elegans Kunth (Euphorbiaceae). J. Chil. Chem. Soc. 2018, 63, 3875–3877. [Google Scholar] [CrossRef]
- Vidal, D.C.E. Aislamiento, Caracterización y Actividad biológica de Metabolitos Secundarios a Partir de la Sspecie Piper peltatum. Ph.D. Thesis, Biochemistry and Pharmacy, Universidad Tecnica Particular Loja, Loja, Ecuador, 2013. [Google Scholar]
- Vega, M.; Brito, B.; Malagon, O. Application of qNMR in the Characterization of Hernandulcin in the Species Phyla strigulosa; Congresso SILAE: Carthagena, Colombia, 2017. [Google Scholar]
- Hamid, N.; Junaid, M.; Pei, D.S. Combined toxicity of endocrine-disrupting chemicals: A review. Ecotoxicol. Environ. Saf. 2021, 215, 112136. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.Y.; Ngai, S.C.; Goh, B.H.; Lee, L.H.; Htar, T.T.; Chuah, L.H. Is Curcumin the Answer to Future Chemotherapy Cocktail? Molecules 2021, 26, 4329. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lü, J.; Zhao, Q.; Chen, J.; Wang, D.; Lin, M.; Zheng, H. Potential Therapeutic Mechanism of Traditional Chinese Medicine on Diabetes in Rodents: A Review from an NMR-Based Metabolomics Perspective. Molecules 2022, 27, 5109. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, F.; Yang, J.; Pan, J.; Qu, H. Development of a comprehensive method based on quantitative 1H NMR for quality evaluation of Traditional Chinese Medicine injection: A case study of Danshen Injection. J. Pharm. Pharmacol. 2022, 74, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Ren, J.L.; Zhang, A.H.; Sun, H.; Yan, G.L.; Han, Y.; Liu, L. Novel applications of mass spectrometry-based metabolomics in herbal medicines and its active ingredients: Current evidence. Mass. Spectrom. Rev. 2019, 38, 380–402. [Google Scholar] [CrossRef]
- Wen, X.; Shi, J.; Tan, W.; Jiang, H.; Wang, D.; Su, J.; Yang, G.; Zhang, B. Effects of aromatherapy and music therapy on patients’ anxiety during MRI examinations: A randomized controlled trial. Eur. Radiol. 2023, 33, 2510–2518. [Google Scholar] [CrossRef]
| Plants and Compounds Evaluated | Family | Common Name | Traditional Uses | Extraction Condition | Product (µg/mL) | MCF Cell Line Survival Ratio (%) |
|---|---|---|---|---|---|---|
| Control substances: | ||||||
| RPMI media | - | - | - | - | - | 100 |
| DMSO | - | - | - | - | - | 100 |
| TAXOL 1000 nM | - | - | - | - | 0.8 | 18 |
| TAXOL 10 nM | - | - | - | - | 0.008 | 26 |
| TAXOL 1nM | - | - | - | - | 0.0008 | 94 |
| Pure compounds evaluated: | ||||||
| Serratenediol-3-O-acetate (C32H5203) | - | Pure compound isolated from Huperzia crassa species | - | 48 | 96 | |
| Tricin (5, 7, 4′-trihydroxy-3′, 5′-dimethoxyflavone) C17H14O7 | - | Pure compound isolated (Flavone) from various species from Huperzia gender | PubChem CID: 5281702 | 33 | 88 | |
| 5-hydroxy-4′,7-dimethoxyflavone (apigenin 7,4′-dimethyl ether) C17H14O5 | - | Pure compound isolated from Piper peltatum L. | PubChem CID: 5281601 | 30 | 27 | |
| 21-episerratenediol (serrat-14-en-3β,21β-diol) (C30H50O2) | - | Pure compound isolated from Huperzia crassa | PubChem CID: 12309682 | 100 | 47 | |
| 20 | 97 | |||||
| Serratenediol (serrat-14-en-3β,21α- diol) C30H50O2 | Pure compound isolated from Huperzia crassa | PubChem CID: 164947 | 100 | 109 | ||
| 20 | 100 | |||||
| Pinostrobin (2s)-5-hydroxy-7-methoxyflavanone) C16H14O4 | Pure compound isolated from Piper ecuadorense | PubChem CID: 73201 | 100 | 60 | ||
| 20 | 37 | |||||
| Pallidine (2-hydroxy-3,6-dimethoxy-17-methyl-5,6,8,14-tetradehydromorphinan-7-one) C19H21NO4 | Pure compound isolated from Croton elegans | PubChem CID: 12313923 | 100 | 74 | ||
| 20 | 103 | |||||
| O-methylpallidine ((1S,9S)-4,5,13-trimethoxy-17-methyl-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2,4,6,10,13-pentaen-12-one) C20H23NO4 | Pure compound isolated from Croton elegans | PubChem CID: 10405046 | 100 | 108 | ||
| 20 | 107 | |||||
| Hernandulcin ((6S)-6-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-3-methylcyclohex-2-en-1-one) C15H24O2 | Pure compound isolated from Phyla strigulosa | PubChem CID: 125608 | 100 | 10 | ||
| 20 | 67 | |||||
| Species evaluated/Herbarium voucher | ||||||
| Acanthoxanthium spinosum (L.) Fourr./PPN-as-039 | Asteraceae | Casamarucha, cardo de tres puntas | Treat conditions of the prostate and kidneys and urinary tract infection (oral testimony); anti-inflammatory and blood purifier [28] | EtOAc extract (leaves) | 100 | 31 |
| 20 | 103 | |||||
| MeCl2 extract (leaves) | 100 | 14 | ||||
| 20 | 93 | |||||
| Baccharis obtusifolia Kunth/PPN-as-014 | Asteraceae | Chilca, chilca redonda, shadán | Antimycotic, cold, rheumatism [6] | MeOH extract (leaves) | 100 | 84 |
| 20 | 94 | |||||
| Croton elegans Kunth/HUTPL536 | Euphorbiaceae | Mosquera | Anti-inflammatory; powerful purgative; treatment of rheumatism, neuralgia, and bronchitis [9] | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 46 |
| 20 | 80 | |||||
| Cinchona officinalis L./PPN-ry-002 | Rubiaceae | Cascarilla, quina | Stomach pain; fever; malaria; antimycotic [6] | EtOH extract (bark) | 100 | 90 |
| 20 | 93 | |||||
| Clusia alata Triana & Planch./HUTPL5081 | Clusiaceae | Duco | Gastritis [28] | MeOH extract (leaves) | 100 | 18 |
| 20 | 91 | |||||
| Croton lechleri Müll. Arg./PPN-eu-003 | Euphorbiaceae | Sangre de drago | Selected compound | Latex | 100 | 5 |
| 20 | 4 | |||||
| Renealmia alpinia (Rottb.) Maas./HUTPL 11186 | Zingiberaceae | Kumpía | The leaves are used to treat rheumatism; a blue pigment is obtained from the fruit [29] | Lyophilized aqueous fruit extract | 100 | 6 |
| 20 | 71 | |||||
| Garcinia macrophylla Mart./HUTPL3841 | Clusiaceae | Shora | Selected compound | MeOH extract (leaves) | 100 | 14 |
| 20 | 53 | |||||
| Huperzia brevifolia (Grev. & Hook.) Holub/PPNIc-10 | Lycopodiaceae | Waminga verde | Liver and kidney diseases, fever, inflammation, colds [30] | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 4 |
| 20 | 71 | |||||
| Huperzia columnaris B. Øllg./PPNIc-09 | Lycopodiaceae | Waminga oso | Selected compound | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 3 |
| 20 | 42 | |||||
| Huperzia compacta (Hook.) Trevis./PPNIc-02 | Lycopodiaceae | Waminga roja | Acts as a purgative and to treat supernatural diseases [31] | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 90 |
| 20 | 94 | |||||
| Huperzia crassa (Humb. & Bonpl. ex Willd.) Rothm./PPNIc-05 | Lycopodiaceae | Waminga amarilla | To treat the itching of the body [29] | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 82 |
| 20 | 98 | |||||
| Huperzia espinosana B. Øllg/PPNIc-08 | Lycopodiaceae | Waminga oso warmi | Liver and kidney diseases, fever, inflammation, colds [30] | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 4 |
| 20 | 78 | |||||
| Huperzia kuesteri (Nessel) B. Øllg./Ly-HK-001 | Lycopodiaceae | Waminga verde grande | Selected compound | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 4 |
| 20 | 73 | |||||
| Huperzia tetragona (Hook. & Grev.) Trevis./PPNIc-04 | Lycopodiaceae | Trencilla roja | Treatment of elephantiasis and leprosy and to treat supernatural diseases [31] | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 81 |
| 20 | 93 | |||||
| Hypericum lancioides Cuatrec./PP-hy-001 | Hypericaceae | Bura bura | Antidepressant effects; antioxidant, antimicrobial, and antiviral properties | MeCl2 extract (aerial part) | 100 | 23 |
| 20 | 88 | |||||
| Lycopodium complanatum L./Ly-001-08 | Lycopodiaceae | Gateador, trencilla | In bathrooms during the postpartum period for bone pain in children | Alkaloid fraction from MeOH-H2O extract (8:2) (leaves) | 100 | 51 |
| 20 | 76 | |||||
| Loricaria thuyoides (Lam.) Sch. Bip./PPN-as-044 | Asteraceae | Ushkuchaki | Used in baths after childbirth to treat hip pain and cold and treat the mal aire (bad air) [20] | EtOAc extract (leaves and stem) | 100 | 93 |
| 20 | 100 | |||||
| Ludwigia peruviana (L.) H. Hara./PPN-on-003 | Onagraceae | Mejorana | Hepatic pain, diuretic, kidney problems [6] | MeOH-H2O extract (9:1) (leaves and stem) | 100 | 54 |
| 20 | 97 | |||||
| Macrocarpaea lenae J. R. Grant/PPN-gn-003 | Gentianaceae | Tabaco de cerro | Fever or cold caused by cold air or strong winds locally known as mal aire (bad air) [25] | Alkaloid fraction from MeOH-H2O extract (8:2) (flowers and leaves) | 100 | 52 |
| 20 | 93 | |||||
| Sarcorhachis sydowii Trel./Pi-003-010 | Piperaceae | Intiwaska | The infusion of the leaves is drunk to treat stomach pain [29] | MeOH-H2O extract (9:1) (leaves and stem) | 100 | 56 |
| 20 | 96 | |||||
| Lyophilized aqueous leaves and stem extract | 100 | 31 | ||||
| 20 | 93 | |||||
| Oreopanax andreanus Marchal/PPN-ar-003 | Araliaceae | Pumamaki | Disinfectant, healing of wounds, dermatitis [6] | MeOH extract (leaves) | 100 | 102 |
| 20 | 97 | |||||
| Curcuma longa L./HUTPL 14333 | Zingiberaceae | Cúrcuma, urmeric, perenchi | The plant is traditionally known for its fungicidal and bactericidal properties [9] | Lyophilized aqueous tuber extract | 100 | 87 |
| 20 | 85 | |||||
| Piper pseudochurumayu (Kunth) C. DC./PPN-pi-009 | Piperaceae | Matico, ámbarámbar | Selected compound | MeOH extract (leaves and stem) | 100 | 5 |
| 20 | 54 | |||||
| Siparuna eggersii Hieron./PPN-mn-001 | Monimiaceae | Monte del oso | Strokes, diabetes, fractured bones, rheumatism, kidney problems [6] | MeOH extract (leaves and stem) | 100 | 39 |
| 20 | 95 | |||||
| Piper crassinervium Kunth/PPN-pi-002 | Piperaceae | Guabiduca | Diabetes, gastritis, prostate problems [6] | Lyophilized aqueous leaf extract | 100 | 96 |
| 20 | 106 | |||||
| Juglans neotropica Diels/PPN-ju-001 | Juglandaceae | Nogal | Rheumatism, hepatic pain in postpartum bath [25] | Lyophilized aqueous leaf extract | 100 | 5 |
| 20 | 106 | |||||
| Tropaeolum tuberosum Ruiz & Pav./PPN-tr-001 | Tropaeolaceae | Mashua | Prostate [25] | Lyophilized aqueous tuber juice | 100 | 103 |
| 20 | 105 | |||||
| Valeriana pyramidalis Kunth./FT991 | Valerianaceae | Valeriana | To treat nerves, heart, liver, and kidney problems [29] | Lyophilized roots exudate | 100 | 80 |
| 20 | 105 | |||||
| Piper ecuadorense Sodiro/PPN-pi-007 | Piperaceae | Matico grande, tiklilin grande, matico del monte | Selected compound | EtOH-H2O extract (7:3) (leaves) | 100 | 18 |
| 20 | 54 | |||||
| Selected compound | MeOH extract (leaves) | 100 | 24 | |||
| 20 | 100 | |||||
| Alibertia sp. | Rubiaceae | Matiri | The fruits of several Alibertia species are edible [29] | MeOH extract (leaves) | 100 | 43 |
| 20 | 85 | |||||
| Artemisia sodiroi Hieron./PPN-as-021 | Asteraceae | Ajenjo | Internal inflammation, stomach pain, hepatic pain, fever, internal infections, kidney problems, cough [6] | MeOH extract (leaves) | 100 | 71 |
| 20 | 111 | |||||
| Artocarpus altilis (Parkinson) Fosberg/PPN-mo-003 | Moraceae | Fruto del pan | Diabetes, high cholesterol [6] | MeOH extract (leaves) | 100 | 90 |
| 20 | 104 | |||||
| Bejaria resinosa Mutis ex L.f./PPN-er-002 | Ericaceae | Payama, pena pena, pena de cerro | To treat nervous system problems, swollen wounds and inflammations of the genital organs, as well liver diseases and cancer [8] | MeOH extract (leaves) | 100 | 6 |
| 20 | 89 | |||||
| Brugmansia suaveolens (Willd.) Bercht. & J. Presl/PPNso-021 | Solanaceae | Floripondio rosado, guando rosado | To treat rheumatic pain [27] | EtOH-H2O (8:2) (flowers) | 100 | 94 |
| 20 | 110 | |||||
| Brugmansia versicolor Lagerh./PPN-so-027 | Solanaceae | Floripondio, guando | To treat headache and inflammation and swelling from blows and act as psychoactive plant [29] | Alkaloid fraction from MeOH-H2O extract (8:2) (flowers) | 100 | 84 |
| 20 | 125 | |||||
| Centropogon comosus Gleason/HUTPL 11342 | Campanulaceae | Motepela | Wash insect bites (oral testimony) | EtOH-H2O (7:3) (leaves) | 100 | 94 |
| 20 | 107 | |||||
| Cestrum sendtnerianum C. Mart./PPN-so-003 | Solanaceae | Sauco negro | Selected compound | EtOH-H2O (7:3) (flowers) | 100 | 6 |
| 20 | 47 | |||||
| Purgative, head pain, stomach pain, fever, gangrene, influenza, internal infections, rheumatism, cough [6] | MeOH extract (leaves and flowers) | 100 | 149 | |||
| 20 | 115 | |||||
| Clusia alata Triana & Planch/HUTPL5081 | Clusiaceae | Duco | Gastritis [28] | MeOH extract (fruits) | 100 | 9 |
| 20 | 85 | |||||
| Gallesia integrifolia (Spreng.) Harms/PPN-ph-001 | Phytolaccaceae | Palo de ajo | Arthritis, strokes, rheumatism [6] | MeOH extract (bark) | 100 | 7 |
| 20 | 88 | |||||
| MeOH extract (leaves) | 100 | 106 | ||||
| 20 | 107 | |||||
| Gaiadendron punctatum (Ruiz & Pav.) G. Don/PPN-lo-001 | Loranthaceae | Violeta de cerro, violeta de campo | Strong cough [25] | EtOH extract (flowers) | 100 | 28 |
| 20 | 73 | |||||
| Selected compound | EtOH extract (leaves) | 100 | 5 | |||
| 20 | 22 | |||||
| Gaultheria erecta Vent/PPN-er-008 | Ericaceae | Mote pela | The fruits are edible [25] | EtOH-H2O (7:3) (flowers) | 100 | 14 |
| 20 | 78 | |||||
| Huperzia weberbaueri (Hieron. & Herter ex Nessel) Holub/PPNIc-07 | Lycopodiaceae | Waminga suca | Purgative and to treat supernatural diseases [31] | Hexane extract (aerial part) | 100 | 75 |
| 20 | 93 | |||||
| MeOH extract (aerial part) | 100 | 58 | ||||
| 20 | 100 | |||||
| Hesperomeles ferruginea (Pers.) Benth./HUTPL4010 | Rosaceae | Quique | The fruits can be used as foods [29] | EtOH-H2O (7:3) (fruits) | 100 | 73 |
| 20 | 100 | |||||
| EtOH-H2O (7:3) (leaves) | 100 | 17 | ||||
| 20 | 100 | |||||
| Ilex guayusa Loes./PPN-aq-001 | Aquifoliaceae | Guayusa | Gastritis, relaxant, increasing woman’s fertility [6] | EtOH-H2O (7:3) (leaves) | 100 | 5 |
| 20 | 92 | |||||
| Iresine herbstii Hook./PPN-am-001 | Amaranthaceae | Escancel | Fever, relaxant, kidney [6] | Lyophilized aqueous (leaves and stems) | 100 | 8 |
| 20 | 78 | |||||
| EtOH-H2O (7:3) (leaves and stems) | 100 | 5 | ||||
| 20 | 93 | |||||
| Lupinus semperflorens Hartw. ex Benth./HUTPL4786 | Fabaceae | Chocho silvestre, taure de cerro, aspa chocho | Fever and stomach pain | MeOH extract (leaves and stems) | 100 | 56 |
| 20 | 127 | |||||
| Salvia pichinchensis Benth/PPN-la-014 | Lamiaceae | Matico negro, matico grande de cerro | To treat the infection of external wounds and for curing kidney and liver disorders [9] | EtOH-H2O (7:3) (leaves and stems) | 100 | 9 |
| 20 | 122 | |||||
| Myrcianthes fragrans (Sw.) McVaugh/PPN-my-008 | Myrtaceae | Arrayán aromático, saco, wawall (kichwa) | Selected compound | MeOH extract (leaves) | 100 | 6 |
| 20 | 63 | |||||
| Oreopanax ecuadorensis Seem./PPN-ar-001 | Araliaceae | Pumamaqui | Headache [6] | MeOH extract (leaves) | 100 | 87 |
| 20 | 100 | |||||
| Oreopanax eriocephalus Harms/HUTPL 4901 | Araliaceae | Maqui-maqui | Anti-inflammatory and antibacterial properties [9] | MeOH extract (leaves and flowers) | 100 | 87 |
| 20 | 96 | |||||
| Otholobium mexicanum (L. f.) J.W. Grimes/PPN-fa-005 | Fabaceae | Culén, teculén | Stomach pain, diarrhea, indigestions, contraceptive [6] | EtOAc extract (leaves and flowers) | 100 | 4 |
| 20 | 93 | |||||
| Phyla strigulosa (M. Martens & Galeotti) Moldenke/MT-KN-111 | Verbenaceae | Buscapina, novalgina | Selected compound | EtOAc extract | 100 | 6 |
| 20 | 61 | |||||
| Selected compound | Hexane extract (leaves and flowers) | 100 | 7 | |||
| 20 | 15 | |||||
| Stomachache [29], cramps, diarrhea in children, and intestinal infections; to act as tonic | Lyophilized aqueous leaves and flowers extract | 100 | 13 | |||
| 20 | 102 | |||||
| Selected compound | MeOH extract (leaves and flowers) | 100 | 6 | |||
| 20 | 38 | |||||
| Cestrum racemosum Ruiz & Pav./PPN-so-010 | Solanaceae | Sauco, sauco de montaña, sauco blanco | Tooth decay, headache, stomach pain, fever, gastritis [6] | MeOH extract (leaves and stem) | 100 | 24 |
| 20 | 91 | |||||
| Stereocaulon ramulosum (Sw.) Raeusch./MUTPL-AB-0650 | Stereocaulaceae | Musgo | External infections, antibiotic [32] | EtOAc extract (aerial part) | 100 | 7 |
| 20 | 71 | |||||
| Selected compound | MeCl2 (aerial part) | 100 | 7 | |||
| 20 | 61 | |||||
| Echinopsis pachanoi (Britton & Rose) Friedrich & G.D. Rowley/PPN-cb-001 | Cactaceae | San Pedro cactus with 5 ribs/San pedrillo | To induce visions (oral and inhaled administration), to act as a purgative, to treat supernatural diseases, to treat anxiety, and serve as an anti-inflammatory or wound disinfectant [27] | Lyophilized from the aqueous extract | 100 | 41 |
| 20 | 94 | |||||
| San Pedro cactus with 7 ribs/San pedrillo | Lyophilized from the aqueous extract | 100 | 7 | |||
| 20 | 68 | |||||
| San Pedro cactus with 9 ribs/San pedrillo | Lyophilized from the aqueous extract | 100 | 19 | |||
| 20 | 66 | |||||
| New Label | Plant Identity | Common Name | Extraction Mode | IC50 vs. MCF7 Cell Line (µg/mL) | Error Bar (µg/mL) | Error Bar (%) |
|---|---|---|---|---|---|---|
| 1 | Cestrum sendtnerianum C. Mart. | Sauco negro | EtOH-H2O (70:30) | 36.80 | 3.62 | 9.82 |
| 2 | Croton lechleri Müll. Arg. | Sangre de drago | Latex | 5.63 | 0.00 | 0.00 |
| 3 | Gaiadendron punctatum (Ruiz & Pav.) G. Don | Violeta de campo, violeta de cerro | EtOH | 15.62 | 0.20 | 1.28 |
| 4 | Garcinia macrophylla Mart. | Shora | MeOH | 36.72 | 1.20 | 3.28 |
| 5 | Huperzia columnaris B. Øllg. | Waminga oso | Alkaloid fraction | 27.35 | 1.81 | 6.62 |
| 6 | Huperzia kuesteri (Nessel) B. Øllg. | Waminga verde grande | EtOAc | 5.39 | 3.23 | 59.80 |
| 7 | Hernandulcin ((6S)-6-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-3-methylcyclohex-2-en-1-one) | - | Pub Chem CID: 125608 | 29.95 | 4.64 | 15.50 |
| 8 | Myrcianthes fragrans (Sw.) McVaugh | Arrayán aromático, saco, wawall | MeOH | 36.02 | 5.62 | 15.60 |
| 9 | Phyla strigulosa (M. Martens & Galeotti) Moldenke | Novalgina, buscapina | EtOAc | 39.53 | 1.03 | 2.59 |
| 10 | Hexane | 10.27 | 0.23 | 2.21 | ||
| 11 | MeOH | 28.83 | 5.66 | 19.64 | ||
| 12 | Pinostrobin ((2s)-5-hydroxy-7-methoxyflavone) | - | Pub Chem CID:73201 | 23.85 | 3.82 | 16.01 |
| 13 | Piper ecuadorense Sodiro | Matico grande, tiklilin grande, matico del monte | EtOH-H2O (70:30) | 79.82 | 2.32 | 2.90 |
| 14 | MeOH | 30.69 | 21.88 | 71.28 | ||
| 15 | Piper pseudochurumayu (Kunth) C. DC. | Matico, ámbar ámbar | MeOH | 21.39 | 1.14 | 5.31 |
| 16 | Stereocaulon ramulosum (Sw.) Raeusch. | Musgo | MeCl2 | 19.26 | 3.26 | 16.91 |
| Extract From: | Label | G0/G1 | S | G2/M | Apoptosis |
|---|---|---|---|---|---|
| RPMI | 63.0 | 16.2 | 20.9 | 0.6 | |
| DMSO (1%) | 76.4 | 10.7 | 11.5 | 1.8 | |
| Cestrum sendtnerianum (flower) | 1 | 74.4 | 12.1 | 12.5 | 1.7 |
| Croton lechleri | 2 | 73.0 | 12.9 | 13.4 | 1.5 |
| G. punctatum (levaes) | 3 | 72.8 | 12.4 | 14.3 | 0.9 |
| Garcinia macrophylla | 4 | 82.6 | 7.9 | 5.9 | 3.8 |
| H. columnaris | 5 | 83.2 | 7.8 | 7.4 | 2.0 |
| H. kuesteri | 6 | 64.4 | 15.3 | 9.8 | 12.7 |
| Hernandulcin | 7 | 75.5 | 11.9 | 6.2 | 9.4 |
| Myrcianthes fragrans | 8 | 66.9 | 16.3 | 7.5 | 12.6 |
| Phyla strigulosa | 9 | 73.0 | 13.1 | 8.2 | 8.1 |
| Phyla strigulosa | 10 | 70.0 | 14.4 | 10.7 | 6.8 |
| Phyla strigulosa | 11 | 70.6 | 15.4 | 8.8 | 8.1 |
| Pinostrobin | 12 | 69.8 | 14.5 | 6.4 | 11.8 |
| Piper ecuadorense | 13 | 69.0 | 15.0 | 7.5 | 12.0 |
| Piper ecuadorense | 14 | 72.4 | 13.8 | 7.8 | 8.6 |
| Piper pseudochurumayu | 15 | 69.7 | 14.2 | 9.5 | 9.1 |
| Stereocaulon ramulosum | 16 | 72.5 | 13.2 | 9.1 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bec, N.; Larroque, C.; Armijos, C. Exploring Southern Ecuador’s Traditional Medicine: Biological Screening of Plant Extracts and Metabolites. Plants 2024, 13, 1422. https://doi.org/10.3390/plants13101422
Bec N, Larroque C, Armijos C. Exploring Southern Ecuador’s Traditional Medicine: Biological Screening of Plant Extracts and Metabolites. Plants. 2024; 13(10):1422. https://doi.org/10.3390/plants13101422
Chicago/Turabian StyleBec, Nicole, Christian Larroque, and Chabaco Armijos. 2024. "Exploring Southern Ecuador’s Traditional Medicine: Biological Screening of Plant Extracts and Metabolites" Plants 13, no. 10: 1422. https://doi.org/10.3390/plants13101422
APA StyleBec, N., Larroque, C., & Armijos, C. (2024). Exploring Southern Ecuador’s Traditional Medicine: Biological Screening of Plant Extracts and Metabolites. Plants, 13(10), 1422. https://doi.org/10.3390/plants13101422





