Research Progress on Plant Shaker K+ Channels
Abstract
1. Introduction
2. Study History of Shaker K+ Channel
2.1. The Initial Discovery of Shaker K+ Channels
2.2. Cloning of the Shaker K+ Channel Genes
2.3. Cloning of the First Plant Shaker K+ Channel Gene
2.4. Visualization of Shaker K+ Channel Protein Structures
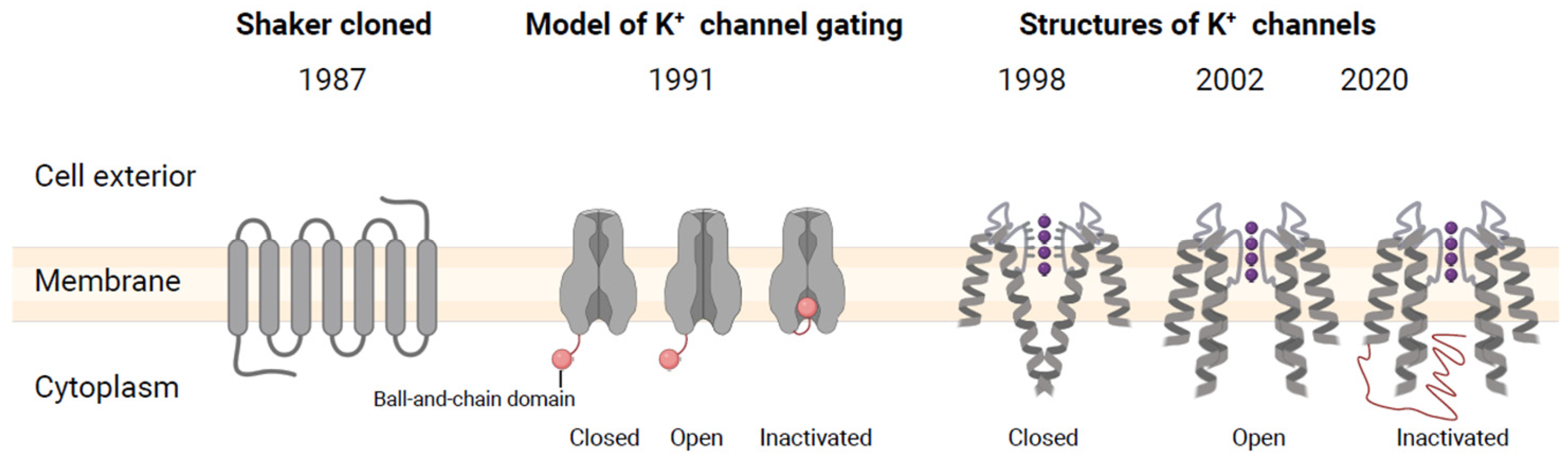
3. Shaker K+ Channel Protein Structure Characteristics and K+ Uptake Mechanisms
3.1. Structural Features
3.2. Voltage Sensing and Channel Opening Mechanism
3.3. Selectivity Filter and Permeability
3.4. Comparison among Shaker and Other K+ Channel Families
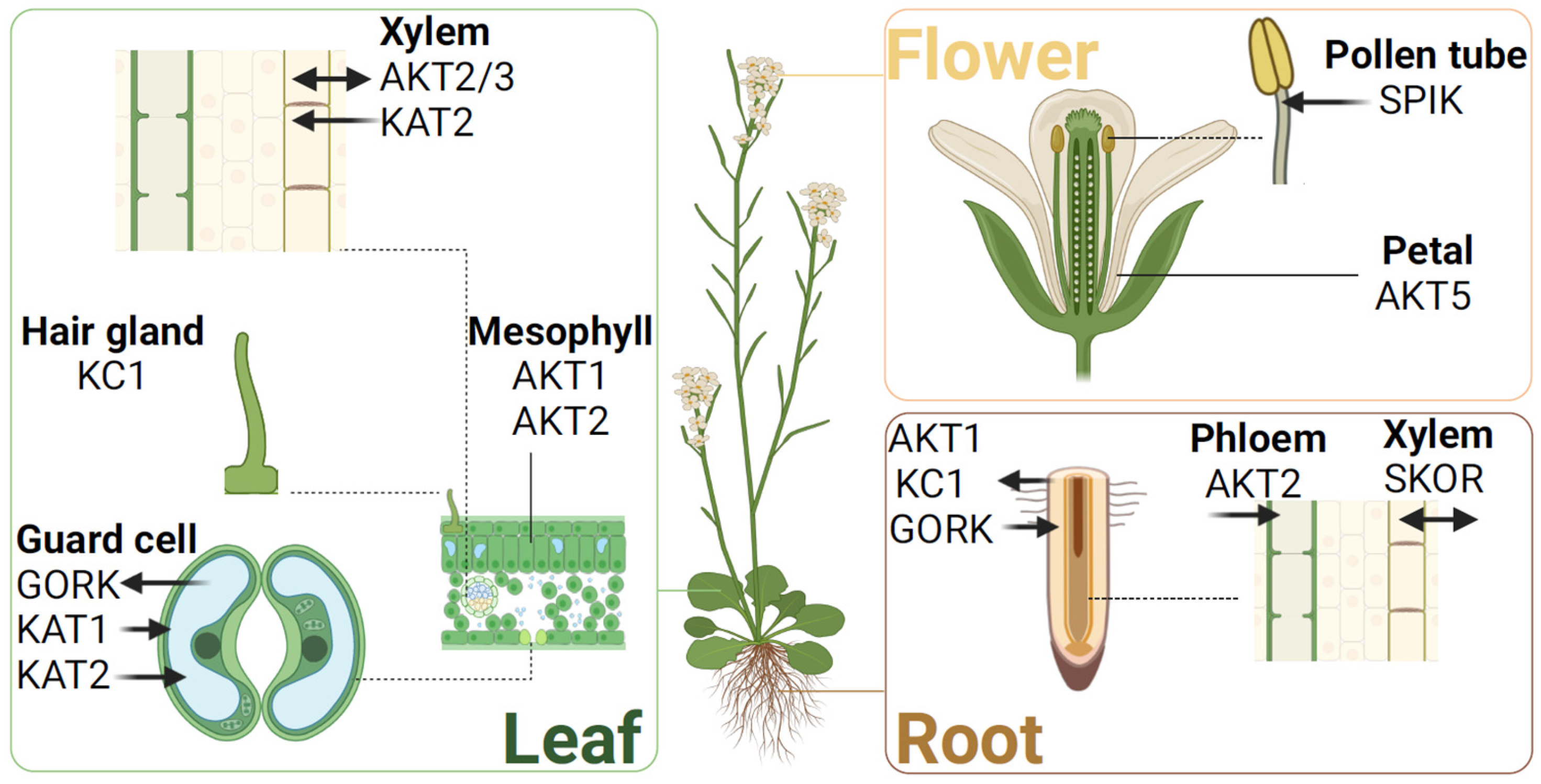
4. Advances in Function of Plant Shaker K+ Channels
4.1. Group I
4.2. Group II
4.3. Group III
4.4. Group IV
4.5. Group V
5. Regulation of Shaker K+ Channels
5.1. Regulation at Transcriptional Level
5.2. Post-Translational Regulation
5.2.1. Regulation by Regulatory Proteins
5.2.2. Regulation by Assembly
5.2.3. Regulation in Protein Localization
5.2.4. Regulation by pH
5.2.5. Voltage Regulation
6. Strategies and Research Methods for Plant K+ Channels
6.1. Bioinformatics Prediction and Analysis
6.2. Heterologous Expression System
6.2.1. Evaluating Yeast Strains for Low K+ Tolerance through Genetic Expression Analysis
6.2.2. Physiological Assessment of Heterologous K+ Channel Expression in Xenopus Oocytes
6.2.3. Model Plant Expression and Phenotypic Identification
6.3. Detection of K+ Channel Activity by Electrophysiological Technology
6.3.1. Two-Electrode Voltage Clamp Technology
6.3.2. Patch-Clamp Technique
7. Prospectives
Author Contributions
Funding
Conflicts of Interest
References
- Yadav, B.; Jogawat, A.; Lal, S.K.; Mehta, N.L.S.; Shabek, N.; Narayan, O.P. Plant mineral transport systems and the potential for crop improvement. Planta 2021, 2, 253. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.; Boudker, O. Shared Molecular Mechanisms of Membrane Transporters. Annu. Rev. Biochem. 2016, 85, 543–572. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Mishra, M.; Patil, G.; Mulkey, S.; Ramawat, N.; Pratap Singh, V.; Deshmukh, R.; Kumar Tripathi, D.; Nguyen, H.T.; Sharma, S. Avenues of the membrane transport system in adaptation of plants to abiotic stresses. Crit. Rev. Biotechnol. 2019, 39, 861–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W.H. Potassium Transport and Signaling in Higher Plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, L.; Tan, P.; Deng, W.; Huang, C.; Liu, D.; Lin, W.; Su, Y. Multiple High-Affinity K+ Transporters and ABC Transporters Involved in K+ Uptake/Transport in the Potassium-Hyperaccumulator Plant Phytolacca acinosa Roxb. Plants 2020, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Rasool, S.; Mir, M.A.; Wani, W.; Masoodi, K.Z.; Ahmad, P. Ion homeostasis for salinity tolerance in plants: A molecular approach. Physiol. Plant. 2020, 171, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Jan, Y.N.; Jan, L.i.Y. Two mutations of synaptic transmission in Drosophila. Print. Great Br. 1977, 198, 87–108. [Google Scholar]
- Kamb, A.; Iverson, L.E.; Tanouye, M.A. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell 1987, 50, 405–413. [Google Scholar] [CrossRef]
- Papazian, D.M.; Schwarz, T.L.; Tempel, B.L.; Jan, Y.N.; Jan, L.Y. Cloning of Genomic and Complementary DNA from Shaker, a Putative Potassium Channel Gene from Drosophila. Science 1987, 237, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Tempel, B.L.; Papazian, D.M.; Schwarz, T.L.; Jan, Y.N.; Jan, L.Y. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science 1987, 237, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Huprikar, S.S.; Kochiant, L.V.; Lucasf, W.J.; Gaber, R.F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Science 1992, 89, 3736–3740. [Google Scholar] [CrossRef] [PubMed]
- Sentenac, H.; Bonneaud, N.; Minet, M.; Lacroute, F.; Salmon, J.-M.; Gaymard, F.; Grignon, C. Cloning and expression in yeast of a plant potassium ion transport system. Science 1992, 256, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Chérel, I. Regulation of K+ channel activities in plants: From physiological to molecular aspects. J. Exp. Bot. 2004, 55, 337–351. [Google Scholar] [CrossRef]
- Doyle, D.A.; Cabral, J.M.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Sukomon, N.; Flood, E.; Rheinberger, J.; Allen, T.W.; Nimigean, C.M. Ball-and-chain inactivation in a calcium-gated potassium channel. Nature 2020, 580, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Nimigean, C.M. 35 years of channelling potassium ions. Nature 2022, 608, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Gambale, F.; Uozumi, N. Properties of Shaker-type Potassium Channels in Higher Plants. J. Membr. Biol. 2006, 210, 1–19. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Chavanieu, A.; Jeanguenin, L.; Alcon, C.; Szponarski, W.; Estaran, S.; Chérel, I.; Zimmermann, S.; Sentenac, H.; Gaillard, I. Distinct Amino Acids in the C-Linker Domain of the Arabidopsis K+ Channel KAT2 Determine Its Subcellular Localization and Activity at the Plasma Membrane. Plant Physiol. 2014, 164, 1415–1429. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M.; Hollingworth, S. A perspective on Na and K channel inactivation. J. Gen. Physiol. 2018, 150, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, O. Recent Advances in the Physiology of Ion Channels in Plants. Annu. Rev. Plant Biol. 2021, 72, 463–495. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.-D.; Chen, L.-Q.; Wang, Y.; Liu, L.-L.; He, L.; Wu, W.-H. A Protein Kinase, Interacting with Two Calcineurin B-like Proteins, Regulates K+ Transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Lefoulon, C. The bare necessities of plant K+ channel regulation. Plant Physiol. 2021, 187, 2092–2109. [Google Scholar] [CrossRef] [PubMed]
- Basset, M.; Conejero, G.; Lepetit, M.; Fourcroy, P.; Sentenac, H. Organization and expression of the gene coding for the potassium transport system AKT1 of Arabidopsis thaliana. Plant Mol. Biol. 1995, 29, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, D.; Basset, M.; Lepetit, M.; Conejero, G.; Gaymard, F.; Astruc, S.; Grignon, C. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J. 1996, 9, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.E.; Lewis, B.D.; Spalding, E.P.; Sussman, M.R. A Role for the AKT1 Potassium Channel in Plant Nutrition. Science 1998, 280, 918–921. [Google Scholar] [CrossRef]
- Mouline, K.; Véry, A.-A.; Gaymard, F.; Boucherez, J.; Pilot, G.; Devic, M.; Bouchez, D.; Thibaud, J.-B.; Sentenac, H. Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev. 2002, 16, 339–350. [Google Scholar] [CrossRef]
- Lacombe, B.; Pilot, G.; Michard, E.; Gaymard, F.; Sentenac, H.; Thibaud, J.-B. A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 2000, 12, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.L.; William, L.; McKendree, J.; Hirsch, R.E.; Sedbrook, J.C.; Caber, R.F.; Sussman, M.R. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995, 109, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Pilot, G.; Lacombe, B.; Gaymard, F.; Chérel, I.; Boucherez, J.; Thibaud, J.-B.; Sentenac, H. Guard Cell Inward K+ Channel Activity in Arabidopsis Involves Expression of the Twin Channel Subunits KAT1 and KAT2. J. Biol. Chem. 2001, 276, 3215–3221. [Google Scholar] [CrossRef]
- Lebaudy, A.; Pascaud, F.; Véry, A.-A.; Alcon, C.; Dreyer, I.; Thibaud, J.-B.; Lacombe, B. Preferential KAT1-KAT2 Heteromerization Determines Inward K+ Current Properties in Arabidopsis Guard Cells. J. Biol. Chem. 2010, 285, 6265–6274. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ward, J.M.; Kelly, W.B.; Ichida, A.M.; Richard, F.C.; Anderson, J.A.; Uozumi, N.; Schroeder, J.I.; Crawford, N.M. Multiple genes, tissue specificity, and expression-dependent modulationcontribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 1995, 109, 1093–1106. [Google Scholar] [CrossRef]
- Marten, I.; Hoth, S.; Deeken, R.; Ache, P.; Ketchum, K.A.; Hoshi, T.; Hedrich, R. AKT3, a phloem-localized K+ channel, is blocked by protons. Proc. Natl. Acad. Sci. USA 1999, 96, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Deeken, R.; Geiger, D.; Fromm, J.; Koroleva, O.; Ache, P.; Langenfeld-Heyser, R.; Sauer, N.; May, S.T.; Hedrich, R. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 2002, 216, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Michard, E.; Lacombe, B.; Thibaud, J.-B. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or leak current. FEBS Lett. 2001, 505, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Reintanz, B.; Szyroki, A.; Ivashikina, N.; Ache, P.; Godde, M.; Beckert, D.; Palme, K.; Hedricht, R. AtKC1, a silent Arabidopsis potassium channel α-subunit modulates root hair K+ influx. Proc. Natl. Acad. Sci. USA 2002, 99, 4079–4084. [Google Scholar] [CrossRef]
- Geiger, D.; Becker, D.; Vosloh, D.; Gambale, F.; Palme, K.; Rehers, M.; Anschuetz, U.; Dreyer, I.; Kudla, J.; Hedrich, R. Heteromeric AtKC1·AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J. Biol. Chem. 2009, 284, 21288–21295. [Google Scholar] [CrossRef]
- Jeanguenin, L.; Alcon, C.; Duby, G.; Boeglin, M.; Cherel, I.; Gaillard, I.; Zimmermann, S.; Sentenac, H.; Very, A.-A. AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J. 2011, 67, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Antunes, S.; Hoshi, T.; Muller-R6ber, B.; Palme, K.; Pongs, O.; Reintanz, B.; Hedrich, R. Plant K+ Channel α-Subunits Assemble Indiscriminately. Biophys. J. 1997, 72, 2143–2150. [Google Scholar] [CrossRef]
- Geoffrey, D.; Eric, H.; Cecile, F.; Carine, A.; Alex, C.; Herve, S.; Jean-Baptiste, T. AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+ channels. Plant J. 2008, 53, 115–123. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Li, H.-D.; Xu, J.; Wu, W.-H. Potassium channel α-subunit AtKC1 negatively regulates AKT1-mediated K+ uptake in Arabidopsis roots under low-K+ stress. Cell Res. 2010, 20, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Boucherez, J.; Michaux-Ferriere, N.; Thibaud, J.-B.; Sentenac, H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef]
- Ache, P.; Becker, D.; Ivashikina, N.; Dietrich, P.; Roelfsema, M.R.G.; Hedrich, R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 2000, 486, 93–98. [Google Scholar] [CrossRef]
- Hosy, E.; Vavasseur, A.; Mouline, K.; Dreyer, I.; Gaymard, F.; Porée, F.; Boucherez, J.; Lebaudy, A.; Bouchez, D.; Véry, A.A.; et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 2003, 100, 7418. [Google Scholar] [CrossRef]
- Ivashikina, N.; Beckera, D.; Achea, P.; Meyerhoff, O.; Felle, H.H.; Hedrich, R. K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 2011, 508, 463–469. [Google Scholar] [CrossRef]
- Pilot, G.; Gaymard, F.; Mouline, K.; Cherel, I.; Sentenac, H. Regulated expression of Arabidopsis shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003, 51, 773–787. [Google Scholar] [CrossRef]
- Buschmann, P.H.; Vaidyanathan, R.; Gassmann, W.; Schroeder, J.I. Enhancement of Na+ Uptake Currents, Time-Dependent Inward-Rectifying K+ Channel Currents, and K+Channel Transcripts by K+ Starvation in Wheat Root Cells. Plant Physiol. 2000, 122, 1387–1397. [Google Scholar] [CrossRef]
- Philippar, K.; Fuchs, I.; Luthen, H.; Hoth, S.; Bauer, C.S.; Haga, K.; Gerhard Thiel, K.L.; Sandberg, G.; Bottger, M.; Becker, D.; et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci. USA 1999, 96, 12186–12191. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, I.; Stölzle, S.; Ivashikina, N.; Hedrich, R. Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta 2004, 221, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Q.H.; Yu, Y.N.; Qiao, Y.M.; Haq, S.U.; Gong, Z.H. The CBL-CIPK Pathway in Plant Response to Stress Signals. Int. J. Mol. Sci. 2020, 21, 5668. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Long, Y.; Schmitz-Thom, I.; Wang, X.P.; Zhang, C.; Li, H.; Steinhorst, L.; Manishankar, P.; Ren, X.L.; Offenborn, J.N.; et al. Two spatially and temporally distinct Ca2+ signals convey Arabidopsis thaliana responses to K+ deficiency. New Phytol. 2017, 213, 739–750. [Google Scholar] [CrossRef]
- Lara, A.; Rodenas, R.; Andres, Z.; Martinez, V.; Quintero, F.J.; Nieves-Cordones, M.; Botella, M.A.; Rubio, F. Arabidopsis K+ transporter HAK5-mediated high-affinity root K+ uptake is regulated by protein kinases CIPK1 and CIPK9. J. Exp. Bot. 2020, 71, 5053–5060. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-L.; Tang, R.-J.; Wang, C.; Luan, S. Potassium nutrient status drives posttranslational regulation of a low-K response network in Arabidopsis. Nat. Commun. 2023, 14, 360. [Google Scholar] [CrossRef]
- Lan, W.Z.; Lee, S.C.; Che, Y.F.; Jiang, Y.Q.; Luan, S. Mechanistic analysis of AKT1 regulation by the CBL-CIPK-PP2CA interactions. Mol. Plant 2011, 4, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Pascaud, F.; Eckert, C.; Gajdanowicz, P.; Hashimoto, K.; Corratge-Faillie, C.; Offenborn, J.N.; Lacombe, B.; Dreyer, I.; Thibaud, J.B.; et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011, 21, 1116–1130. [Google Scholar] [CrossRef]
- Tagliani, A.; Tran, A.N.; Novi, G.; Mambro, R.D.; Pesenti, M.; Sacchi, G.A.; Perata, P.; Pucciariello, C. The calcineurin β-like interacting protein kinase CIPK25 regulates potassium homeostasis under low oxygen in Arabidopsis. J. Exp. Bot. 2020, 71, 2678–2689. [Google Scholar] [CrossRef]
- Liese, A.; Romeis, T. Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim. Biophys. Acta 2013, 1833, 1582–1589. [Google Scholar] [CrossRef]
- Huimin, R.; Hussain, J.; Wenjie, L.; Fenyong, Y.; Junjun, G.; Youhan, K.; Shenkui, L.; Guoning, Q. The expression of constitutively active CPK3 impairs potassium uptake and transport in Arabidopsis under low K+ stress. Cell Calcium 2021, 98, 102447. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.N.; Yao, F.Y.; Ren, H.M.; Sun, S.J.; Hussain, J.; Huang, C.F.; Wang, Y.F. Constitutive activation of calcium-dependent protein kinase 3 confers a drought tolerance by inhibiting inward K+ channel KAT1 and stomatal opening in Arabidopsis. Sci. Bull. 2018, 63, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.-N.; Shen, L.-K.; Zhang, W.-Z.; Zhang, W.; Wang, Y.; Wu, W.-H. Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 2013, 25, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Ronzier, E.; Corratgé-Faillie, C.; Sanchez, F.; Prado, K.; Brière, C.; Leonhardt, N.; Thibaud, J.-B.; Xiong, T.C. CPK13, a noncanonical Ca2+-dependent protein kinase, specifically inhibits KAT2 and KAT1 shaker K+ channels and reduces stomatal opening. Plant Physiol. 2014, 166, 314–326. [Google Scholar] [CrossRef]
- Ronzier, E.; Corratgé-Faillie, C.; Sanchez, F.; Brière, C.; Xiong, T.C. Ca2+-Dependent Protein Kinase 6 Enhances KAT2 Shaker Channel Activity in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 1596. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-L.; Qi, G.-N.; Feng, H.-Q.; Zhao, S.; Zhao, S.-S.; Wang, Y.; Wu, W.-H. Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J. 2013, 74, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Chérel, I.; Michard, E.; Platet, N.; Mouline, K.; Alcon, C.; Sentenac, H.; Thibaud, J.-B. Physical and Functional Interaction of the Arabidopsis K+ Channel AKT2 and Phosphatase AtPP2CA. Plant Cell 2002, 14, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Sottocornola, B.; Visconti, S.; Orsi, S.; Gazzarrini, S.; Giacometti, S.; Olivari, C.; Camoni, L.; Aducci, P.; Marra, M.; Abenavoli, A.; et al. The potassium channel KAT1 is activated by plant and animal 14-3-3 proteins. J. Biol. Chem. 2006, 281, 35735–35741. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Ullah, H.; Jones, A.M.; Assmann, S.M. G Protein Regulation of Ion Channels and Abscisic Acid Signaling in Arabidopsis Guard Cells. Science 2001, 292, 2070–2072. [Google Scholar] [CrossRef]
- Locascio, A.; Marqués, M.C.; García-Martínez, G.; Corratgé-Faillie, C.; Andrés-Colás, N.; Rubio, L.; Fernández, J.A.; Véry, A.-A.; Mulet, J.M.; Yenush, L. BCL2-ASSOCIATED ATHANOGENE4 Regulates the KAT1 Potassium Channel and Controls Stomatal Movement. Plant Physiol. 2019, 181, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Pilot, G.; Pratelli, R.; Gaymard, F.; Meyer, Y.; Sentenac, H. Five-group distribution of the Shaker-like K+ channel family in higher plants. J. Mol. Evol. 2003, 56, 418–434. [Google Scholar] [CrossRef]
- Xicluna, J.; Lacombe, B.; Dreyer, I.; Alcon, C.; Jeanguenin, L.; Sentenac, H.; Thibaud, J.-B.; Chérel, I. Increased functional diversity of plant K+ channels by preferential heteromerization of the shaker-like subunits AKT2 and KAT2. J. Biol. Chem. 2007, 282, 486–494. [Google Scholar] [CrossRef]
- Grefen, C.; Chen, Z.; Honsbein, A.; Donald, N.; Hills, A.; Blatt, M.R. A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant Cell 2010, 22, 3076–3092. [Google Scholar] [CrossRef] [PubMed]
- Sutter, J.-U.; Campanoni, P.; Tyrrell, M.; Blatt, M.R. Selective Mobility and Sensitivity to SNAREs Is Exhibited by the Arabidopsis KAT1 K+ Channel at the Plasma Membrane. Plant Cell 2006, 18, 935–954. [Google Scholar] [CrossRef]
- Hoth, S.; Geiger, D.; Becker, D.; Hedrich, R. The pore of plant K+ channels is involved in voltage and pH sensing domain-swapping between different K+ channel alpha-subunits. Plant Cell 2001, 13, 943–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moshelion, M.; Becker, D.; Czempinski, K.; Mueller-Roeber, B.; Attali, B.; Hedrich, R.; Moran, N. Diurnal and circadian regulation of putative potassium channels in a leaf moving organ. Plant Physiol. 2002, 128, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Pantha, P.; Dassanayake, M. Living with Salt. Innovation 2020, 1, 100050. [Google Scholar] [CrossRef]
- Zei, P.C.; Aldrich, R.W. Voltage-dependent Gating of Single Wild-Type and S4 Mutant KAT1 Inward Rectifier Potassium Channels. J. Biol. Chem. 1998, 112, 679–713. [Google Scholar] [CrossRef]
- Czempinski, K.; Zimmermann, S.; Ehrhardt, T.; Müller-Röber, B. New structure and function in plant K+ channels KCO1, an outward rectifier with a steep Ca2+ dependency. EMBO J. 1997, 16, 2565–2575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bañuelos, M.A.; Garciadeblas, B.; Cubero, B.; Rodríguez-Navarro, A. Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol. 2002, 130, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y.; Wang, H.; Wang, X.; Lv, M.; Yang, P.; Zhang, L. Identification and Characterization of Shaker K+ Channel Gene Family in Foxtail Millet (Setaria italica) and Their Role in Stress Response. Front. Plant Sci. 2022, 13, 907635. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; He, C.; Wang, Y.; Xu, H.; Xu, K.; Zhao, Y.; Yao, B.; Zhang, Y.; Zhao, Y.; Carther, K.F.I.; et al. Genome-wide identification of soybean Shaker K+ channel gene family and functional characterization of GmAKT1 in transgenic Arabidopsis thaliana under salt and drought stress. J. Plant Physiol. 2021, 266, 153529. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhang, A.; Sun, J.; Chen, X.; Liu, M.; Zhao, P.; Jiang, W.; Tang, Z. Identification of Shaker K+ channel family members in sweetpotato and functional exploration of IbAKT1. Gene 2021, 768, 145311. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, X.; Eneji, A.E.; Li, Z. Functional characterization of GhAKT1, a novel Shaker-like K+ channel gene involved in K+ uptake from cotton (Gossypium hirsutum). Gene 2014, 545, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Long, Y.; Qi, G.-N.; Li, J.; Xu, Z.-J.; Wu, W.-H.; Wang, Y. The Os-AKT1 Channel Is Critical for K+ Uptake in Rice Roots and Is Modulated by the Rice CBL1-CIPK23 Complex. Plant Cell 2014, 26, 3387–3402. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Wu, W.H.; Wang, Y. The K+ Channel KZM2 is Involved in Stomatal Movement by Modulating Inward K+ Currents in Maize Guard Cells. Plant J. 2017, 92, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, M.; Mo, C.; Li, W.; Ji, Y.; Xie, Q.; Jiang, X. MeCIPK10 regulates the transition of the K+ transport activity of MeAKT2 between low- and high-affinity molds in cassava. J. Plant Physiol. 2023, 280, 153861. [Google Scholar] [CrossRef]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice Shaker Potassium Channel OsKAT1 Confers Tolerance to Salinity Stress on Yeast and Rice Cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef]
- Braun, N.; Sheikh, Z.P.; Pless, S.A. The current chemical biology tool box for studying ion channels. J. Physiol. 2020, 598, 4455–4471. [Google Scholar] [CrossRef]
- O’Connor, E.C.; Kambara, K.; Bertrand, D. Advancements in the use of xenopus oocytes for modelling neurological disease for novel drug discovery. Expert Opin. Drug Discov. 2024, 19, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Ji, Y.; Wu, W.; Cheng, J.K.; Feng, H.Q.; Wang, Y. ZMK1 Is Involved in K+ Uptake and Regulated by Protein Kinase ZmCIPK23 in Zea mays. Front. Plant Sci. 2021, 12, 517742. [Google Scholar] [CrossRef] [PubMed]
- Ache, P.; Becker, D.; Deeken, R.; Dreyer, I.; Weber, H.; Fromm, J.; Hedrich, R. VFK1, a Vicia faba K+ Channel Involved in Phloem Unloading. Plant J. 2009, 27, 571–580. [Google Scholar] [CrossRef]
- Su, Y.H.; North, H.; Grignon, C.; Thibaud, J.B.; Sentenac, H.; Very, A.A. Regulation by external K+ in a maize inward shaker channel targets transport activity in the high concentration range. Plant Cell 2005, 17, 1532–1548. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Lei, H.; Jin, M.; Yue, G.; Su, Y. Functional identification of a GORK potassium channel from the ancient desert shrub Ammopiptanthus mongolicus (Maxim.) Cheng f. Plant Cell Rep. 2016, 35, 803–815. [Google Scholar] [CrossRef]



| Gene Family Name | Shaker | TPK | Kir-like | CNGC |
|---|---|---|---|---|
| Topological structure | 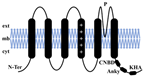 | 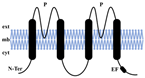 | 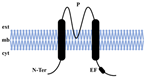 | 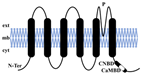 |
| Structural characteristics | TM1-6 1 Pore | TM1-4 2 Pores | TM1-2 1 Pore | TM1-6 1 Pore |
| Subgroup divisions and representative members | Ⅰ: AKT1, AKT5, AKT6 Ⅱ:KAT1, KAT2 Ⅲ: AKT2 Ⅳ: AtKC1 Ⅴ: GORK, SKOR | AtTPK1, AtTPK2, AtTPK3, AtTPK4, AtTPK5, | AtKCO3 | AtCNGC1 AtCNGC2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, G.; Nong, T.; Hunpatin, O.S.; Shi, C.; Su, X.; Wang, Q.; Liu, H.; Dai, P.; Ning, Y. Research Progress on Plant Shaker K+ Channels. Plants 2024, 13, 1423. https://doi.org/10.3390/plants13101423
Yuan G, Nong T, Hunpatin OS, Shi C, Su X, Wang Q, Liu H, Dai P, Ning Y. Research Progress on Plant Shaker K+ Channels. Plants. 2024; 13(10):1423. https://doi.org/10.3390/plants13101423
Chicago/Turabian StyleYuan, Guang, Tongjia Nong, Oluwaseyi Setonji Hunpatin, Chuhan Shi, Xiaoqing Su, Qian Wang, Haobao Liu, Peigang Dai, and Yang Ning. 2024. "Research Progress on Plant Shaker K+ Channels" Plants 13, no. 10: 1423. https://doi.org/10.3390/plants13101423
APA StyleYuan, G., Nong, T., Hunpatin, O. S., Shi, C., Su, X., Wang, Q., Liu, H., Dai, P., & Ning, Y. (2024). Research Progress on Plant Shaker K+ Channels. Plants, 13(10), 1423. https://doi.org/10.3390/plants13101423





