The Effect of Different Temperatures on the Viability and Senescence of Plum Ovules (Prunus domestica L.)
Abstract
1. Introduction
2. Results
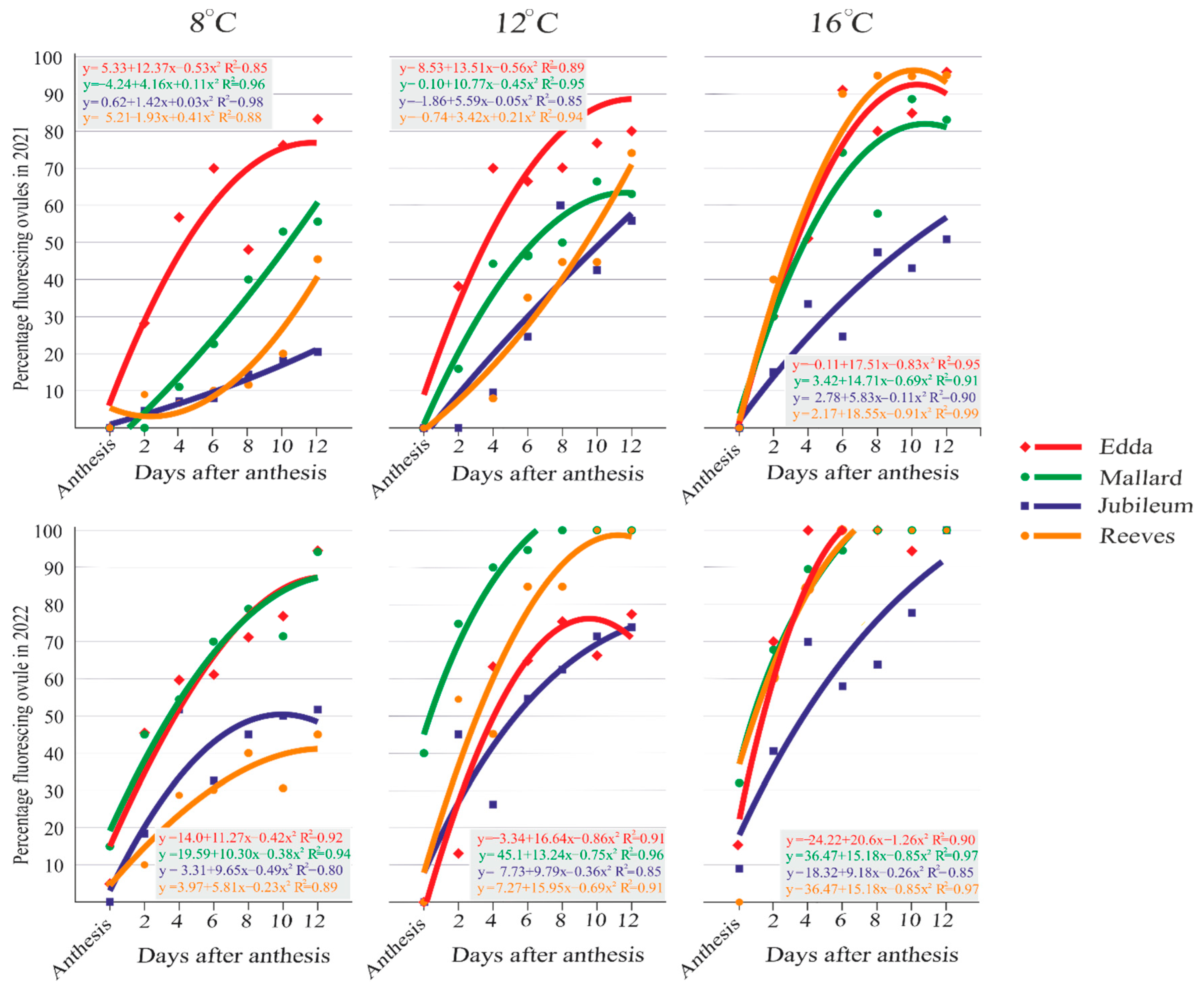
2.1. Viability of Primary Ovules
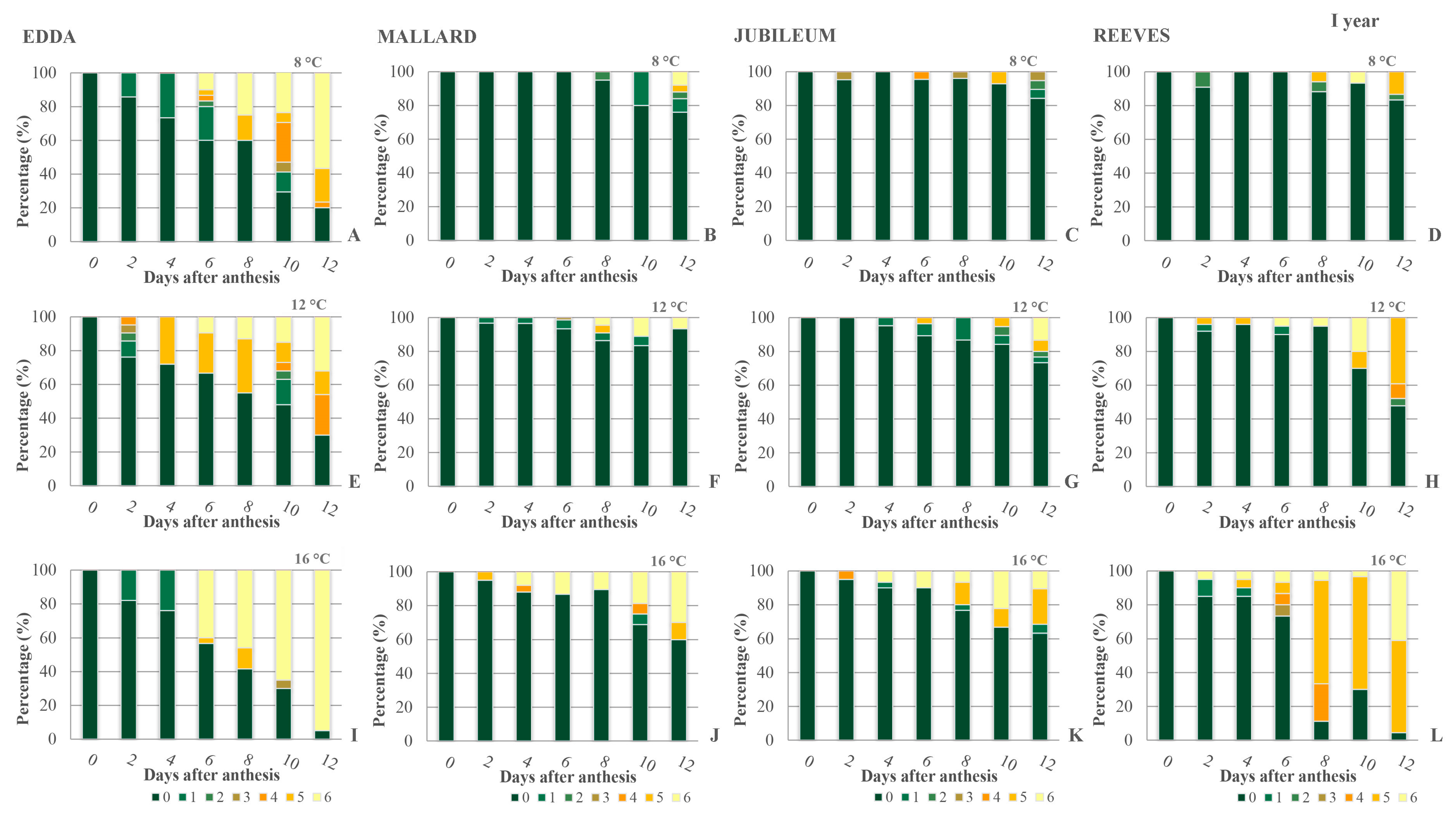
2.2. Callose Detection in Primary Ovules
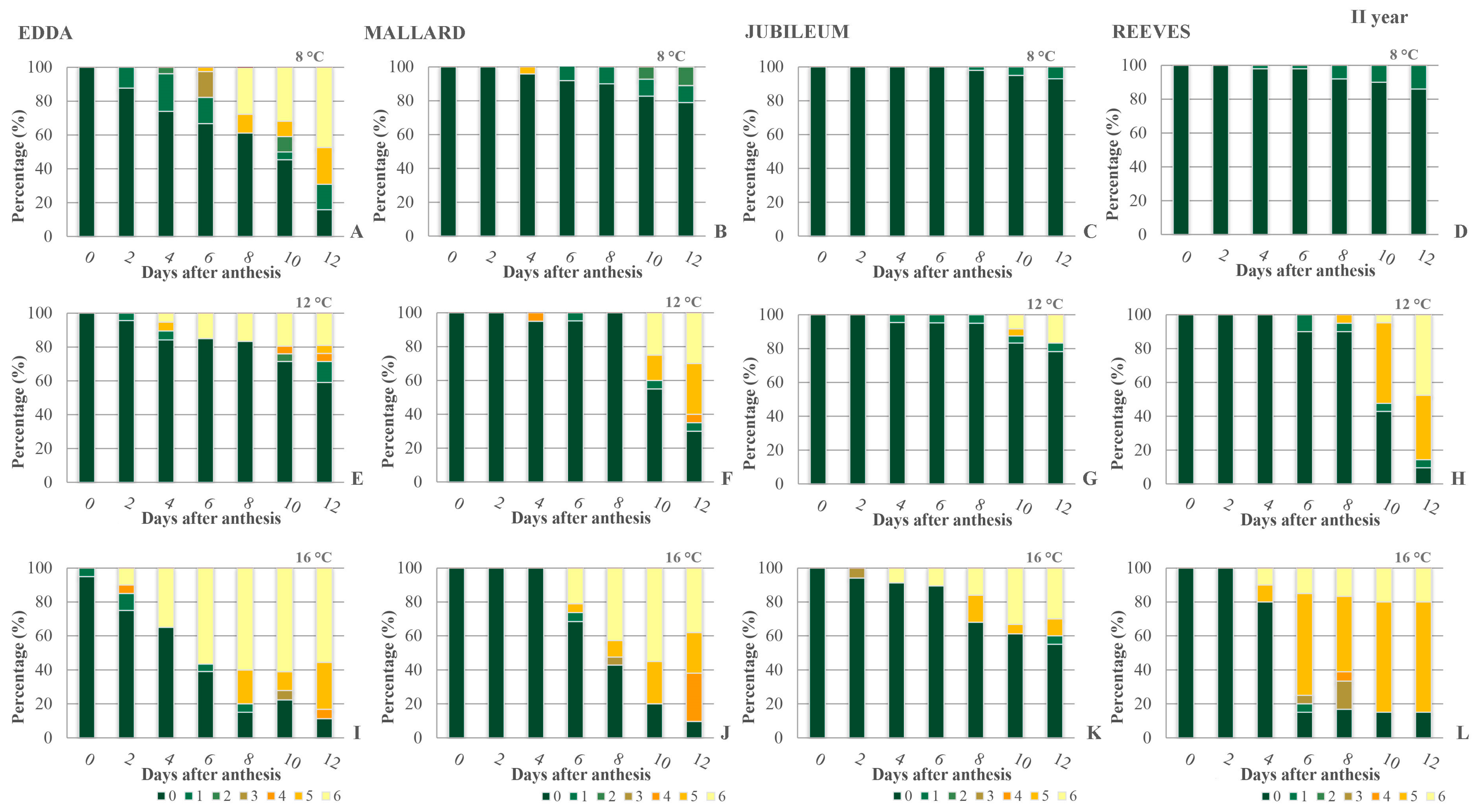
2.3. Secondary Ovules—Viability and Callose Detection
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Designs, Staining, and Sample Preparation
4.3. The Stages of Ovule Fluorescence during Its Senescence
4.4. Statistical Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Fotirić Akšić, M.; Cerović, R.; Hjeltnes, S.H.; Meland, M. The effective pollination period of European plum (Prunus domestica L.) Cultivars in Western Norway. Horticulturae 2022, 8, 55. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.; Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C.A.; Dardick, C. Genetic characterization of worldwide Prunus domestica (plum) germplasm using sequence-based genotyping. Hortic. Res. 2019, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak-Tomczak, D. Characteristics of plums as a raw material with valuable nutritive and dietary properties—A review. Polish J. Food Nutr. Sci. 2008, 58, 401–405. [Google Scholar]
- Fotirić Akšić, M.; Tešić, Ž.; Kalaba, M.; Ćirić, I.; Pezo, L.; Lončar, B.; Gašić, U.; Dojčinović, B.; Tosti, T.; Meland, M. Breakthrough Analysis of Chemical Composition and Applied Chemometrics of European Plum Cultivars Grown in Norway. Horticulturae 2023, 9, 477. [Google Scholar] [CrossRef]
- FaoStat. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 April 2024).
- Redalen, G. Plum growing in Norway at 60° N. Acta Hortic. 2002, 577, 385–389. [Google Scholar] [CrossRef]
- Sekse, L. Plum production in Norway. Acta Hortic. 2007, 734, 23–28. [Google Scholar] [CrossRef]
- Meland, M. Performance of six European plum cultivars on four plum rootstock growing in a northern climate. Acta Agric. Scand.-B Soil Plant Sci. 2010, 60, 381–387. [Google Scholar] [CrossRef]
- Endress, P.K. Angiosperm ovules: Diversity, development, evolution. Ann. Bot. 2011, 107, 1465–1489. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Rodrigo, J.; Herrero, M. Effects of pre-blossom temperatures on flower development and fruit set in apricot. Sci. Hortic. 2002, 92, 125–135. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.; Herrero, M. Warm temperatures at bloom reduce fruit set in sweet cherry. J. Appl. Bot. Food Qual. 2007, 81, 158–164. [Google Scholar]
- Carvalho, D.; Cardoso Pereira, S.; Rocha, A. Future surface temperatures over Europe according to CMIP6 climate projections: An analysis with original and bias-corrected data. Clim. Chang. 2021, 167, 10. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Irenaeus, K.S.T.; Mitra, S.K. Understanding the pollen and ovule characters and fruit set of fruit crops in relation to temperatures and genotype-a review. J. Appl. Bot. Food Qual. 2014, 87, 157–167. [Google Scholar] [CrossRef]
- Cerović, R.; Ružić, Đ. Senescence of the ovules at different temperatures and their effect on the behaviour of pollen tubes in sour cherry. Sci. Hortic. 1992, 51, 321–327. [Google Scholar] [CrossRef]
- Egea, J.; Burgos, L.; Zoroa, N.; Egea, L. Influence of temperature on the in vitro germination of pollen of apricot (Prunus armeniaca L.). J. Hortic. Sci. 1992, 67, 247–250. [Google Scholar] [CrossRef]
- Pirlak, L. The effect of temperature on pollen germination and pollen tube growth of apricot and sweet cherry. Gartenbauwissenschaft 2002, 67, 61–64. [Google Scholar]
- Nava, G.A.; Dalmago, G.A.; Bergamaschi, H.; Paniz, R.; Santos, R.P.; Marodin, G.A.B. Effect of high temperatures in the pre-blooming and blooming periods on ovule formation, pollen grains and yield of ‘Granada’ peach. Sci. Hortic. 2009, 122, 37–44. [Google Scholar] [CrossRef]
- Sorkheh, K.; Azimkhani, R.; Mehri, N.; Chaleshtori, M.H.; Halasz, J.; Ercisli, S.; Koubouris, G.C. Interactive effects of temperature and genotype on almond (Prunus dulcis L.) pollen germination and tube length. Sci. Hortic. 2018, 227, 162–168. [Google Scholar] [CrossRef]
- Raja, M.M.; Vijayalakshmi, G.; Naik, M.L.; Basha, P.O.; Sergeant, K.; Hausman, J.F.; Khan, P.S.S.V. Pollen development and function under heat stress: From effects to responses. Acta Physiol. Plant. 2019, 41, 47. [Google Scholar] [CrossRef]
- Sato, S.; Peet, M.M.; Thomas, J.F. Determining critical pre- and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. J. Exp. Bot. 2002, 53, 1187–1195. [Google Scholar] [CrossRef]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef]
- Cecchetti, V.; Celebrin, D.; Napoli, N.; Ghelli, R.; Brunetti, P.; Costantino, P.; Cardarelli, M. An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytol. 2017, 213, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.P.; Crone, D.E. Pollen and the heat shock response. Sex. Plant Reprod. 1996, 9, 370–374. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. The effect of temperature on stigmatic receptivity in sweet cherry (Prunus avium L.). Plant Cell Environ. 2003, 26, 1673–1680. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Burgos, L.; Berenguer, T.; Egea, J. Self- and cross-compatibility among apricot cultivars. Hortscience 1993, 28, 148–150. [Google Scholar] [CrossRef]
- Cerović, R.; Ružić, Ð.; Mićić, N. Viability of plum ovules at different temperatures. Ann. Appl. Biol. 2000, 137, 53–59. [Google Scholar] [CrossRef]
- Ünal, M.; Vardar, F.; Aytürk, Ö. Chapter 14: Callose in Plant Sexual Reproduction. In Current Progress in Biological Research; Silva-Opps, M., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Chen, X.Y.; Kim, J.Y. Callose synthesis in higher plants. Plant Signal Behav. 2009, 4, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Brzezicka, E.; Kozieradzka-Kiszkurno, M. Callose deposition analysis with special emphasis on plasmodesmata ultrastructure during megasporogenesis in Sedum (Crassulaceae). Protoplasma 2024, 261, 31–41. [Google Scholar] [CrossRef]
- Pimienta, E.; Polito, V.S. Ovule abortion in ‘Nonpareil’ almond (Prunus dulcis [Mill.] D.A. Webb). Am. J. Bot. 1983, 69, 913–920. Available online: https://www.jstor.org/stable/2442888 (accessed on 7 April 2024). [CrossRef]
- Rodrigo, J.; Herrero, M. Influence of intraovular reserves on ovule fate in apricot (Prunus armeniaca L.). Sex. Plant Reprod. 1998, 11, 86–93. [Google Scholar] [CrossRef]
- Stösser, R.; Anvari, S.F. On the senescence of ovules in cherries. Sci. Hortic. 1982, 16, 29–38. [Google Scholar] [CrossRef]
- Postweiler, K.; Stösser, R.; Anvari, S.F. The effect of different temperatures on the viability of ovules in cherries. Sci. Hortic. 1985, 25, 235–239. [Google Scholar] [CrossRef]
- Burgos, L.; Egea, J.; Dicenta, F. Effective pollination period in apricot (Prunus persica L.) cultivars. Ann. Appl. Biol. 1991, 119, 533–539. [Google Scholar] [CrossRef]
- Zhang, L.; Ferguson, F.; Whiting, M. Temperature effects on pistil viability and fruit set in sweet cherry. Sci. Hortic. 2018, 241, 8–17. [Google Scholar] [CrossRef]
- Radičević, S.; Ognjanov, V.; Marić, S.; Barać, G. The effect of genotype and temperature interaction on pollen performance in the pistils of autochthonous sour cherry cultivar ‘Feketićka’. Zemdirbyste 2021, 108, 271–278. [Google Scholar] [CrossRef]
- Đorđević, M. Cytoembryological Aspects of Fertilisation of Plum Cultivar “Pozna Plava“ (Prunus domestica L.). Doctoral Dissertation, The Faculty of Agriculture, University of Belgrade, Belgrade, Serbia, 2016. Available online: https://refri.institut-cacak.org/handle/123456789/87 (accessed on 9 April 2024).
- Anvari, S.F.; Stösser, R. Fluoreszenmikroskpische untersuchungen des pollenschlauchwachstums und des zustands der samenanlagen bei sauerkirschen. Mitteilungen Klosterneubg. 1978, 28, 23–30. [Google Scholar]
- Guerra, M.E.; Wünsch, A.; López-Corrales, M.; Rodrigo, J. Flower emasculation as the cause for lack of fruit set in Japanese plum crosses. J. Am. Soc. Hortic. Sci. 2010, 135, 556–562. [Google Scholar] [CrossRef]
- Ruiz, D.; Campoy, J.A.; Egea, J. Ovule development at anthesis in Japanese plum (Prunus salicina Lindl.) cultivars. Span. J. Agric. Res. 2010, 8, 151–158. [Google Scholar] [CrossRef]
- Verma, D.P.; Hong, Z. Plant callose synthase complexes. Plant Mol Biol. 2001, 47, 693–701. [Google Scholar] [CrossRef]
- Nishikawa, S.; Zinkl, G.M.; Swanson, R.J.; Maruyama, D.; Preuss, D. Callose (β-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Gonkiewicz, A.; Nosal, K. Anatomical Changes in Plum Fruit Prunus domestica L. after Fruit Thinning Using Potassium Salt of Alpha-Naphtaleneacetic Acid. EJPAU 2006, 9, 50. Available online: http://www.ejpau.media.pl/volume9/issue4/art-50.html (accessed on 12 April 2024).
- Van Durme, M.; Olvera Carrillo, Y.; Pfeiffer, M.; Doll, N.; De Winter, F.; Lin, Z.; Nowack, M. Fertility loss in senescing Arabidopsis ovules is controlled by the maternal sporophyte via a NAC transcription factor triad. Proc. Natl. Acad. Sci. USA 2023, 120, e2219868120. [Google Scholar] [CrossRef]
- Cerović, R.; Fotirić Akšić, M.; Đorđević, M.; Meland, M. Viability of embryo sacs and fruit set in different plum (Prunus domestica L.) cultivars grown under Norwegian climatic conditions. Plants 2022, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth stages of mono- and dicotyledonous plants. In Federal Biological Research Centre for Agriculture and Forestry, 2nd ed.; BBCH Monograph; Blackwell Wissenschafts-Verlag: Berlin, Germany, 2001; pp. 1–158. [Google Scholar]
- Anvari, S.F.; Stösser, R. Eine neue fluoreszenzmikroskopische Methode zur Beurteilung der Befruchtungsfährigkeit der Samenanlagen bei Prunus. Zertschrift Pflanzenzüchtung 1978, 81, 333–336. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đorđević, M.; Cerović, R.; Meland, M.; Akšić, M.F. The Effect of Different Temperatures on the Viability and Senescence of Plum Ovules (Prunus domestica L.). Plants 2024, 13, 1359. https://doi.org/10.3390/plants13101359
Đorđević M, Cerović R, Meland M, Akšić MF. The Effect of Different Temperatures on the Viability and Senescence of Plum Ovules (Prunus domestica L.). Plants. 2024; 13(10):1359. https://doi.org/10.3390/plants13101359
Chicago/Turabian StyleĐorđević, Milena, Radosav Cerović, Mekjell Meland, and Milica Fotirić Akšić. 2024. "The Effect of Different Temperatures on the Viability and Senescence of Plum Ovules (Prunus domestica L.)" Plants 13, no. 10: 1359. https://doi.org/10.3390/plants13101359
APA StyleĐorđević, M., Cerović, R., Meland, M., & Akšić, M. F. (2024). The Effect of Different Temperatures on the Viability and Senescence of Plum Ovules (Prunus domestica L.). Plants, 13(10), 1359. https://doi.org/10.3390/plants13101359






