Overcoming Dormancy in Prunus Species under Conditions of Insufficient Winter Chilling in Israel
Abstract
1. Introduction
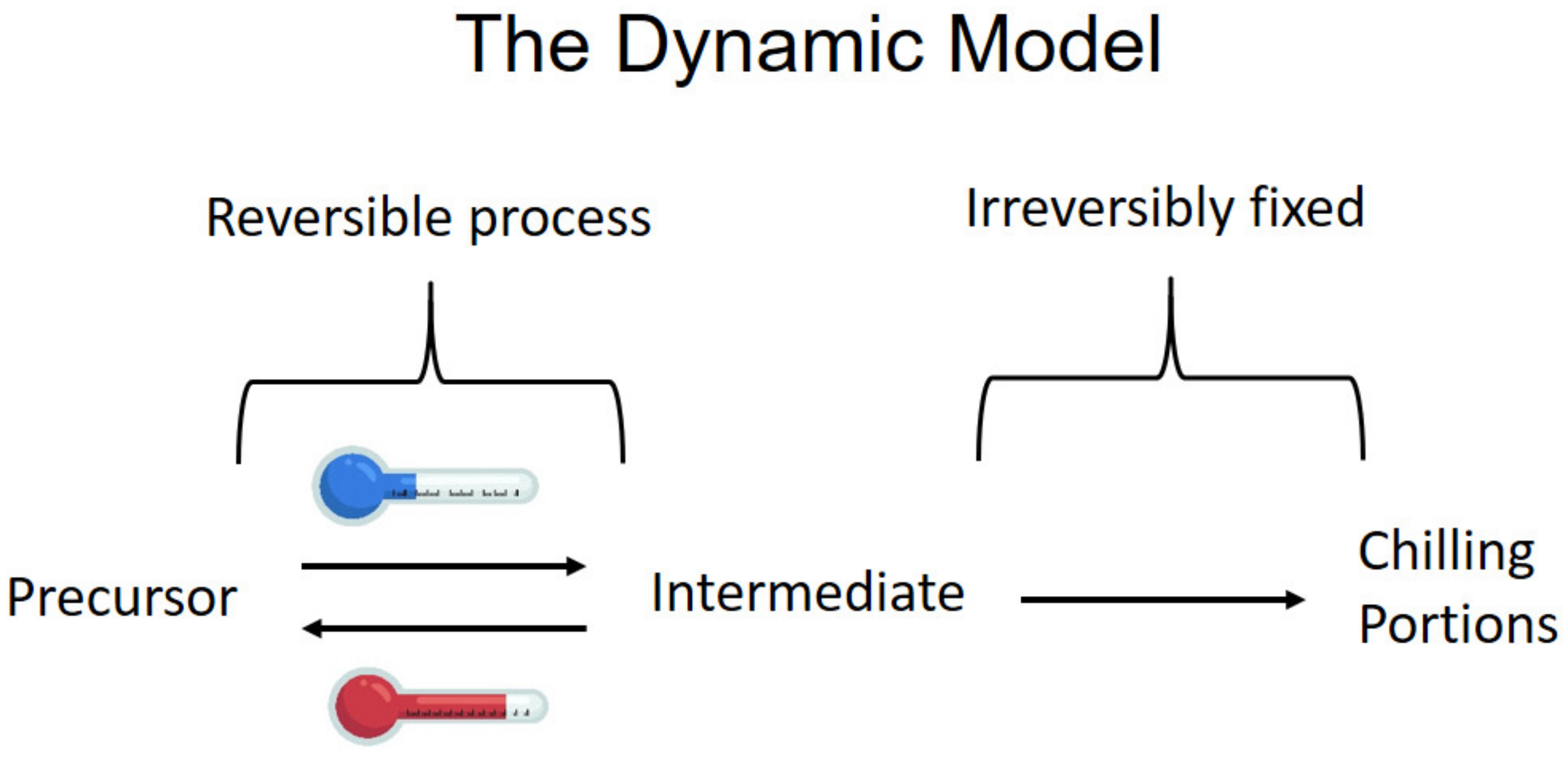
2. Temperatures Effect on Dormancy
- Effective temperatures to break dormancy in the peach are between −2 °C and 13 °C, and the most effective being 4–8 °C with reduced efficiency at higher and lower temperatures.
- Moderate temperatures between 13 °C and 16 °C that will not break dormancy alone, when occurring in a daily cycle after previous chilling, enhance the effect of chilling. On the other hand, temperatures higher than 18 °C in a daily cycle will nullify former chilling.
- This negative effect of high temperatures increases the longer the duration and the higher the temperature. However, when cycles are longer than a day, the chilling effect is final and cannot be nullified by high temperatures.
3. Light Effects on Dormancy
4. Action on the Orchard Level to Improve Bud Breaks under Conditions of Lack of Winter Chilling
4.1. Horticultural Means
4.2. Physical Means
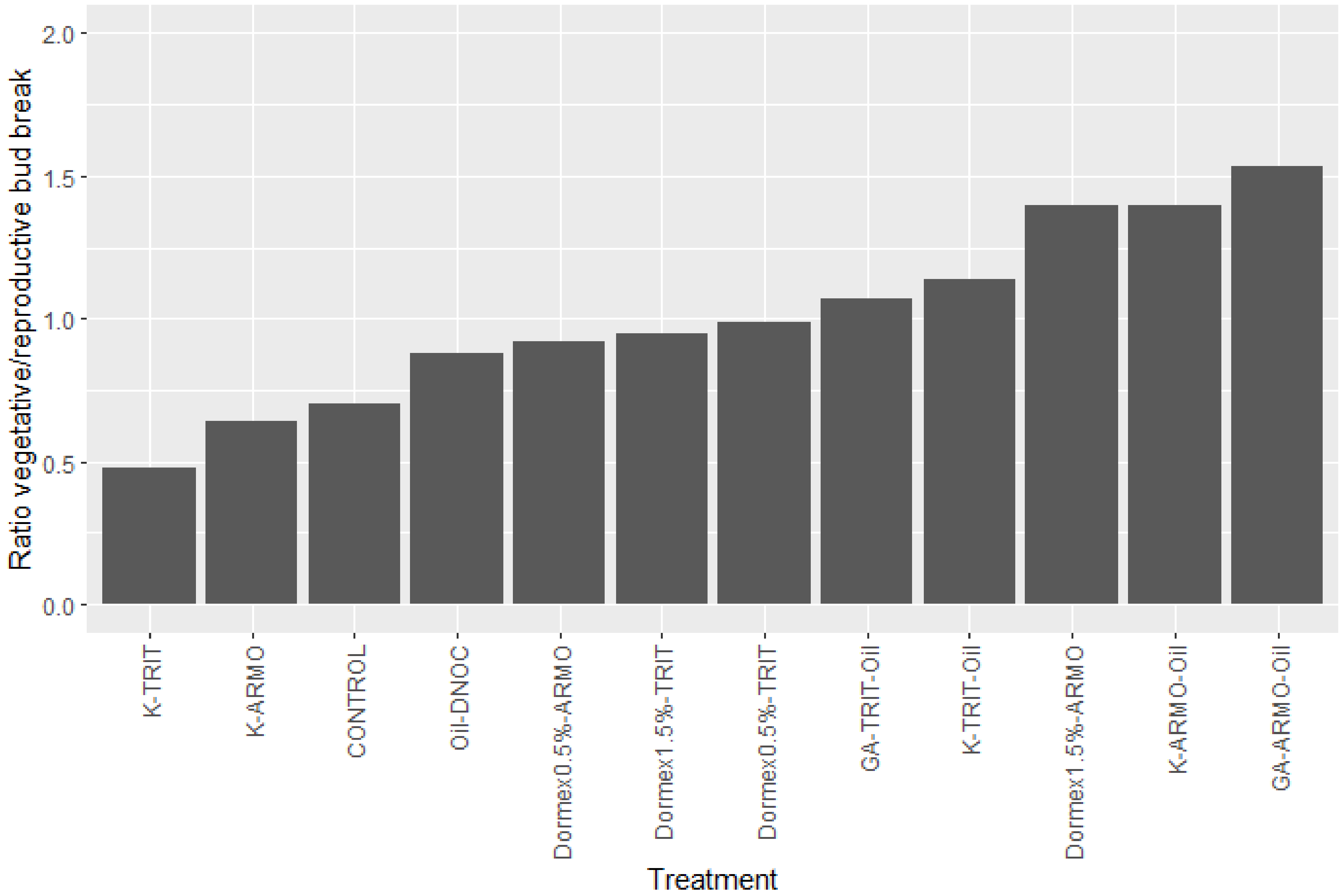
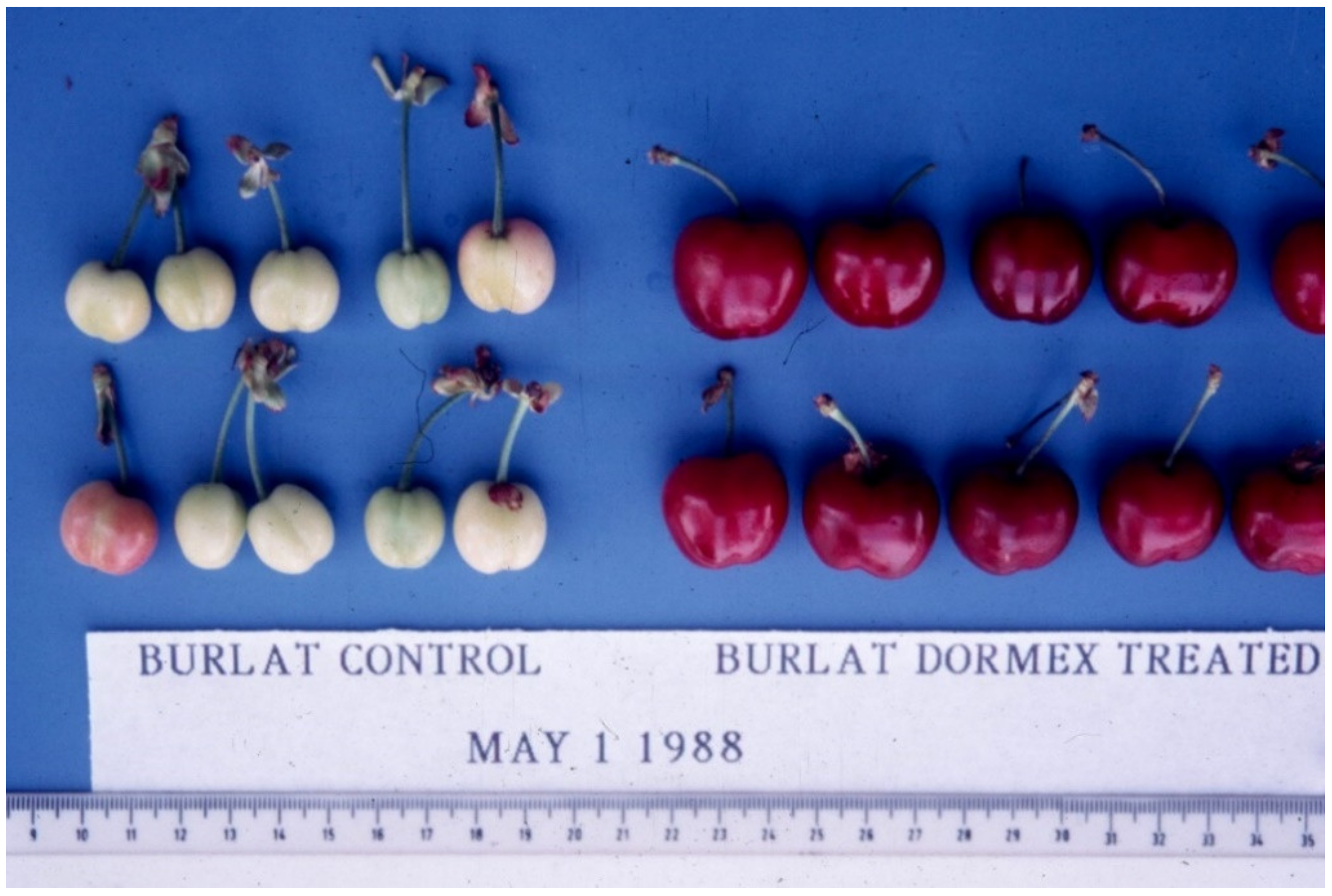
4.3. Chemical Means
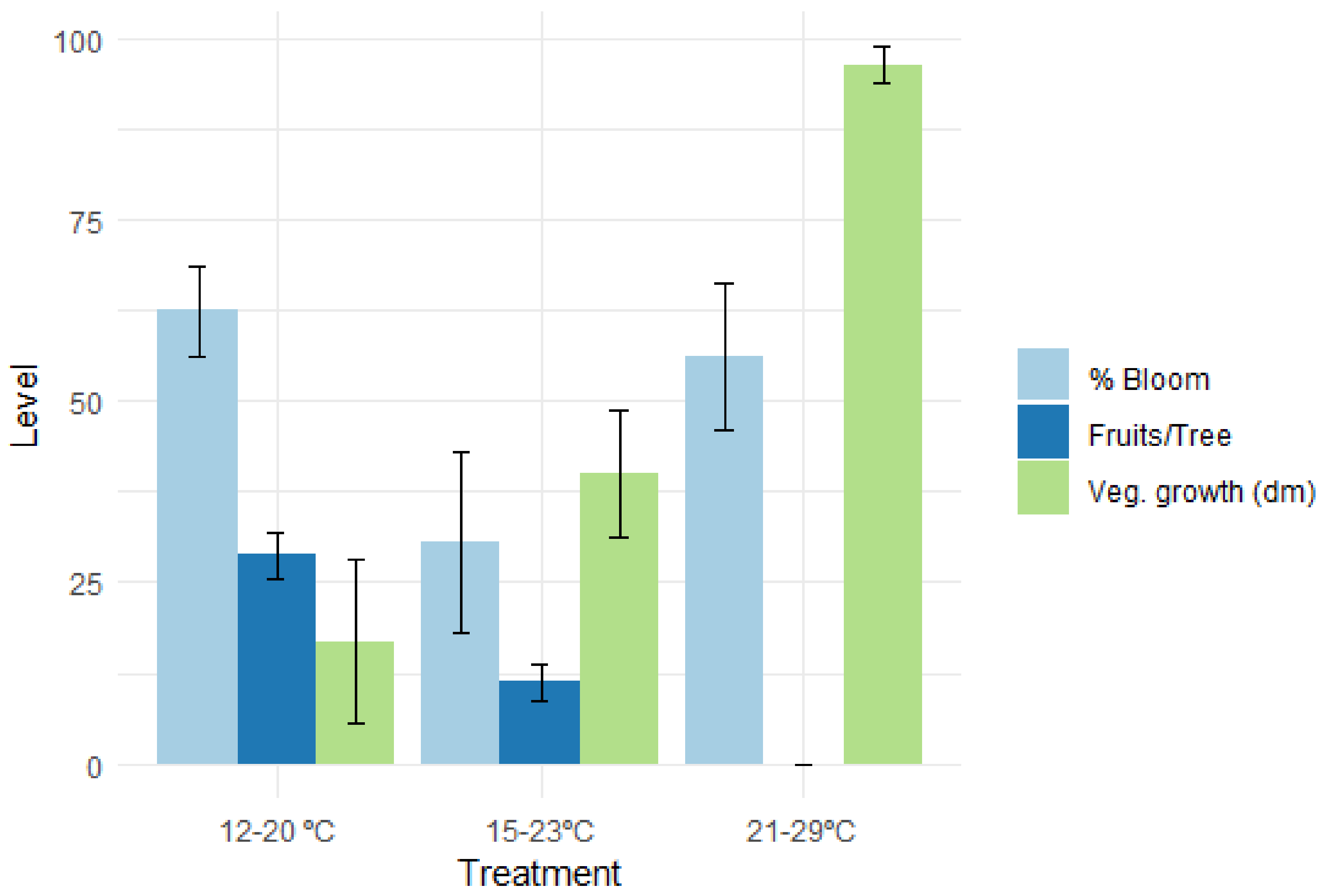
4.4. Dormancy Avoidance
5. Specific Heat Effect on Stone Fruit Flower Buds
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samish, R.M. The use of dinitrocresol-mineral oil sprays for the control of prolonged rest in apple orchards. J. Pomol. Hortic. Sci. 1945, 21, 164–179. [Google Scholar] [CrossRef]
- Samish, R.M.; Lavee, S. The chilling requirement of fruit trees. Proc. XVI Int. Hort. Cong. 1962, 5, 372–388. [Google Scholar]
- Erez, A. Means to compensate for insufficient chilling to improve bloom and leafing. Acta Hortic. 1995, 395, 81–96. [Google Scholar] [CrossRef]
- Erez, A.; Lavee, S. Breaking the dormancy of deciduous fruit trees in subtropical climates. Proc. XIX Inter. Hort. Cong. 1974, 3, 69–78. [Google Scholar]
- Erez, A.; Zur, A. Breaking the rest of apple buds by narrow-distillation-range oil and dinitro-o-cresol. Sci. Hortic. 1981, 14, 47–54. [Google Scholar] [CrossRef]
- Erez, A.; Yablowitz, Z. Effect of dormancy agents with armobreak in the peach. Acta Hort. 1997, 441, 183–190. [Google Scholar] [CrossRef]
- Erez, A.; Yablowitz, Z.; Aronovitz, A.; Hadar, A. Dormancy breaking chemicals: Efficiency with reduced phytotoxicity. Acta Hort. 2008, 772, 105–112. [Google Scholar] [CrossRef]
- Crane, O.; Sar Shalom, A.; Erez, A. Coping with global warming effects on reduced winter chilling for deciduous fruit trees. Acta Hort. 2022, 1346, 91–100. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Faust, M.; Erez, A.; Rowland, L.J.; Wang, S.Y.; Norman, H.A. Bud dormancy in perennial fruit trees; Physiological basis for dormancy induction maintenance and release. HortScience 1997, 32, 623–629. [Google Scholar] [CrossRef]
- Rinne, P.L.H.; van der Schoot, C. Cell-Cell Communication as a Key Factor in Dormancy Cycling. J. Crop. Improv. 2004, 10, 113–156. [Google Scholar] [CrossRef]
- Erez, A.; Couvillon, G.A.; Hendershott, C.H. Quantitative chilling enhancement and negation in peach buds by high temperatures in a daily cycle1. J. Am. Soc. Hortic. Sci. 1979, 104, 536–540. [Google Scholar] [CrossRef]
- Erez, A.; Couvillon, G.A.; Hendershott, C.H. The effect of cycle length on chilling negation by high temperatures in dormant peach leaf buds1. J. Am. Soc. Hortic. Sci. 1979, 104, 573–576. [Google Scholar] [CrossRef]
- Erez, A.; Couvillon, G.A. Characterization of the influence of moderate temperatures on rest completion in peach. J. Am. Soc. Hort. Sci. 1987, 112, 677–680. [Google Scholar] [CrossRef]
- Couvillon, G.A.; Erez, A. Effect of level and duration of high temperatures on rest in the peach. J. Am. Soc. Hortic. Sci. 1985, 110, 579–581. [Google Scholar] [CrossRef]
- Weinberdger, J.H. Chilling requirements of peach varieties. Proc. Amer. Soc. Hort. Sci. 1950, 56, 122–128. [Google Scholar]
- Richardson, E.A.; Seeley, S.D.; Walker, D.R. A model for estimating the completion of rest for Redhaven and Elberta peach trees. Hortscience 1974, 9, 331–332. [Google Scholar] [CrossRef]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Mathematical analysis of a two-step model involving a cooperative transition. J. Theor. Biol. 1987, 124, 473–483. [Google Scholar] [CrossRef]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Simulation of processes studied under controlled temperatures. J. Theor. Biol. 1987, 126, 309–322. [Google Scholar] [CrossRef]
- Erez, A.; Couvillon, G.A. Evaporative cooling to improve rest breaking of nectarine buds by counteracting high daytime temperatures. HortScience 1983, 18, 480–481. [Google Scholar] [CrossRef]
- Luedeling, E.; Zhang, M.; McGranahn, G.; Leslie, C. Validation of winter chill models using historic records of walnut phenology. Agric. Forest Meteor. 2009, 149, 1854–1864. [Google Scholar] [CrossRef]
- Luedeling, E. Climate change impacts on winter chill for temperate fruit and nut production: A review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef]
- Erez, A.; Samish, R.M.; Lavee, S. The role of light in leaf and flower bud break of the peach (Prunus persica). Physiol. Plant. 1966, 19, 650–659. [Google Scholar] [CrossRef]
- Erez, A.; Lavee, S.; Samish, R.M. The Effect of limitation in light during the rest period on leaf bud break of the peach (Prunus persica). Physiol. Plant. 1968, 21, 759–764. [Google Scholar] [CrossRef]
- Byrne, D.H.; Sherman, W.B.; Bacon, T.A. Stone fruits Genetic pool and its exploitation for growing under warm winter conditions. In Temperate Fruit Crops in Warm Climates; Erez, A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 157–230. [Google Scholar]
- Erez, A. Bud dormancy, phenomenon, problems and solutions in the tropics and subtropics. In Temperate Fruit Crops in Warm Climates; Erez, A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 17–48. [Google Scholar]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Samish, R.M. Dormancy in woody plants. Annu. Rev. Plant Physiol. 1954, 5, 183–204. [Google Scholar] [CrossRef]
- Erez, A. Chemical control of bud break. Invited symposium paper. HortScience 1987, 22, 1240–1243. [Google Scholar] [CrossRef]
- Erez, A. The Effect of temperature on the activity of oil + dinitro-o-cresol sprays to break the rest of apple buds 1. HortScience 1979, 14, 141–142. [Google Scholar] [CrossRef]
- Shulman, Y.; Nir, G.; Fanberstein, L.; Lavee, S. The effect of cyanamide on the release from dormancy of grapevine buds. Sci. Hortic. 1983, 19, 97–104. [Google Scholar] [CrossRef]
- Erez, A.; Yablowitz, Z.; Korcinski, R. Temperature and chemical effects on competing sinks in peach bud break. Acta Hortic. 2000, 514, 51–58. [Google Scholar] [CrossRef]
- Edwards, G.R. Production of temperate-zone fruit at low latitudes, avoiding rest and the chilling requirement. HortScience 1987, 22, 1236–1240. [Google Scholar] [CrossRef]
- Erez, A. Use of the rest avoidance technique in peaches in Israel. Second International Workshop on Temperate Zone Fruits in the Tropics and Subtropics. Acta Hortic. 1987, 199, 137–144. [Google Scholar] [CrossRef]
- Erez, A.; Lerner, H. Means to improve leafing using rest-avoidance technique in peaches in Israel. Acta Hort. 1990, 279, 239–246. [Google Scholar] [CrossRef]
- Erez, A. Dormancy completion- a dual response. HortScience 1999, 34, 542–543. [Google Scholar] [CrossRef]




| Treatment | Bloom Level (1–10) | Leafing Level (1–10) | Total Harvest Until April 22 (Kg/Ha) |
|---|---|---|---|
| Control | 0.13 b | 0 c | 500 b |
| Oil–DNOC | 1.00 b | 0 c | 1130 b |
| Oil–DNOC + Dormex 0.5% | 2.25 b | 0.6 c | 3510 b |
| Oil + TDZ, 75 ppm | 4.88 a | 3.6 b | 13,910 a |
| Oil + TDZ, 100 ppm | 5.63 a | 4.8 a | 14,540 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erez, A. Overcoming Dormancy in Prunus Species under Conditions of Insufficient Winter Chilling in Israel. Plants 2024, 13, 764. https://doi.org/10.3390/plants13060764
Erez A. Overcoming Dormancy in Prunus Species under Conditions of Insufficient Winter Chilling in Israel. Plants. 2024; 13(6):764. https://doi.org/10.3390/plants13060764
Chicago/Turabian StyleErez, Amnon. 2024. "Overcoming Dormancy in Prunus Species under Conditions of Insufficient Winter Chilling in Israel" Plants 13, no. 6: 764. https://doi.org/10.3390/plants13060764
APA StyleErez, A. (2024). Overcoming Dormancy in Prunus Species under Conditions of Insufficient Winter Chilling in Israel. Plants, 13(6), 764. https://doi.org/10.3390/plants13060764







