Application of Silicon Influencing Grain Yield and Some Grain Quality Features in Thai Fragrant Rice
Abstract
1. Introduction
2. Results
2.1. Grain Yield and Yield Component
2.2. Silicon Concentration
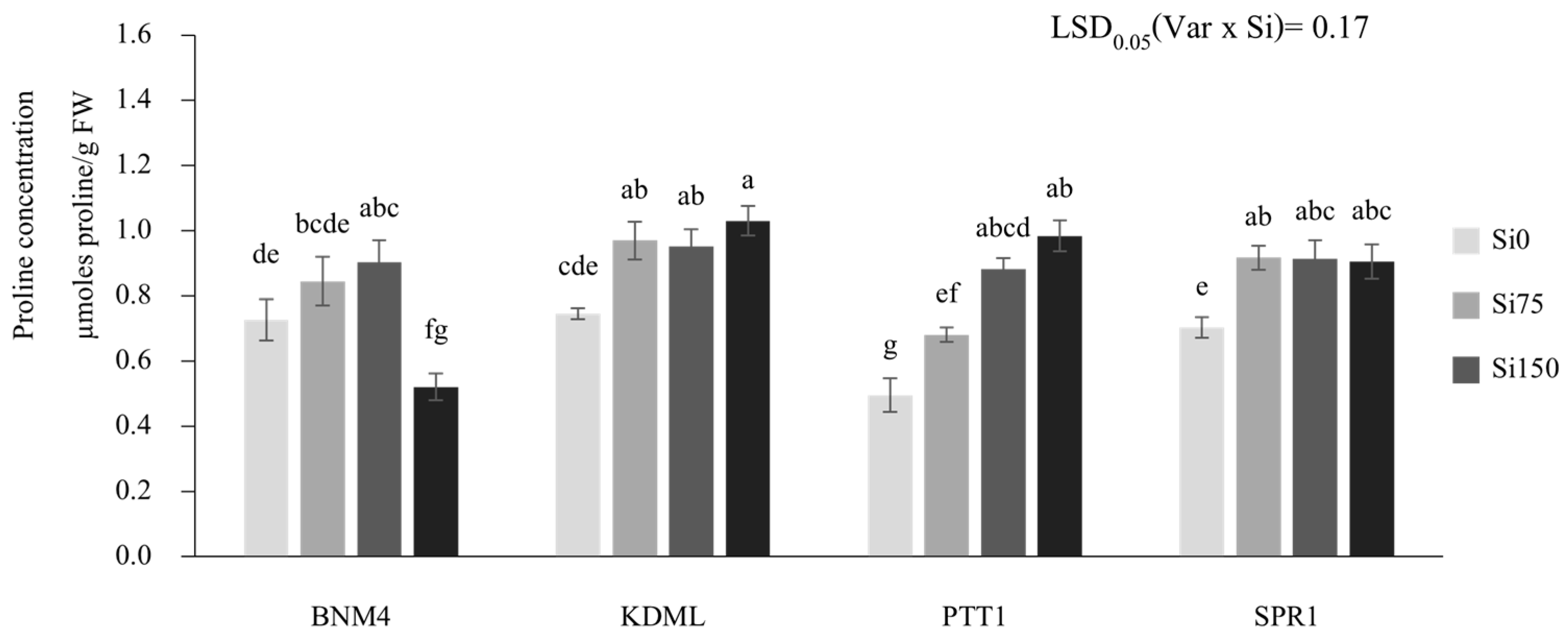
2.3. Proline Concentration
2.4. Grain Quality
2.4.1. Aroma–2AP Concentration
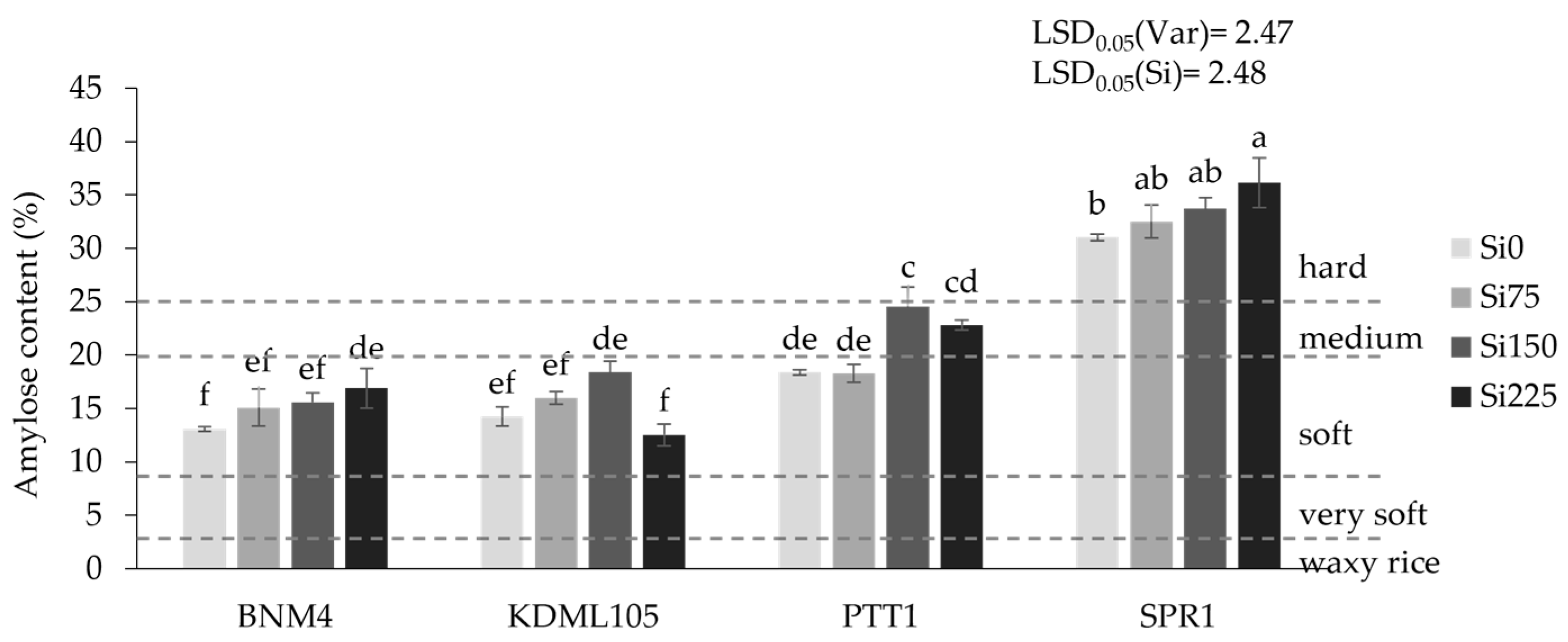
2.4.2. Amylose Content (%)
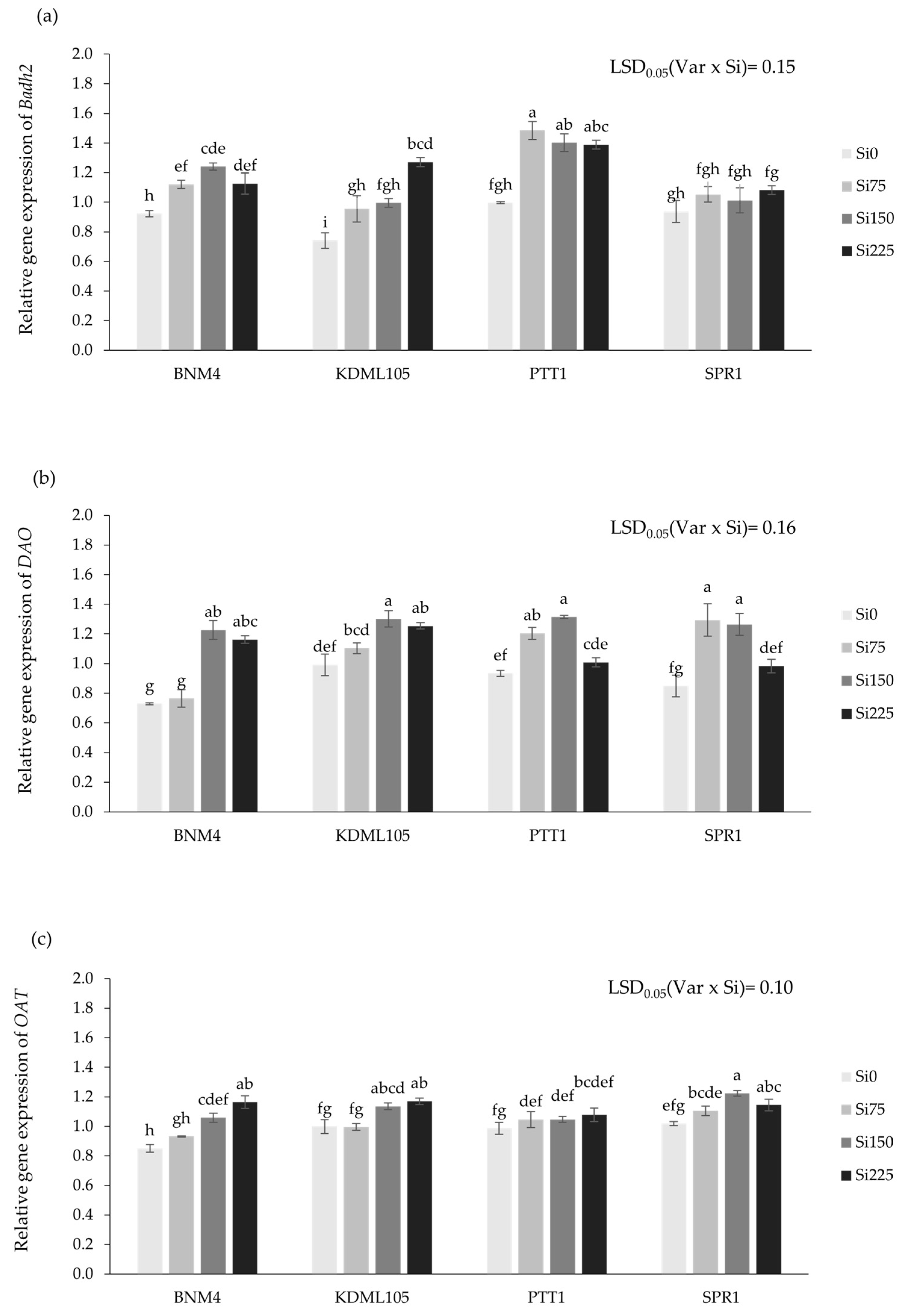
2.5. Gene Expression
3. Discussion
3.1. Yield Response and Expression of Si Transport Gene
3.2. Effects of Silicon on the Aroma (2AP)
4. Materials and Methods
4.1. Plant Culture and Experimental Design
4.2. Yield and Yield Components
4.3. Silicon Concentrations Analysis
4.4. Proline Concentration
4.5. Grain Quality
4.5.1. Aroma–2AP Concentration
4.5.2. Amylose Content
4.6. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, G.B.; Pi, P.Y.; Syriac, E.K. Silicon nutrition in rice: A review. J. Pharmac. Phytochem. 2017, 6, 390–392. [Google Scholar]
- Liang, Y.; Nikolav, B.; Bélanger, R.; Gong, H.; Song, A. Effect of Silicon on Crop Growth, Yield and Quality. In Silicon in Agriculture II; Springer Science + Business Media: Dordrecht, The Netherlands, 2015; pp. 209–224. [Google Scholar]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. Stud. Plant Sci. 2001, 8, 17–39. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Lei, S.; Ashraf, U.; Khan, I.; Li, Y.; Pan, S.; Duan, M.; Tian, H.; Tang, X. Silicon fertilization modulates 2-acetyl-1-pyrroline content, yield formation and grain quality of aromatic rice. J. Cereal Sci. 2017, 75, 17–24. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Berahim, Z.; Omar, M.H.; Zakaria, N.I.; Ismail, M.R.; Rosle, R.; Roslin, N.A.; Che’Ya, N.N. Silicon improves yield performance by enhancement in physiological responses, crop imagery, and leaf and culm sheath morphology in new rice line, PadiU Putra. BioMed Res. Int. 2021, 2021, 6679787. [Google Scholar] [CrossRef] [PubMed]
- Savant, N.K.; Snyder, G.H.; Datnoff, L.E. Silicon management and sustainable rice production. Adv. Agron. 1996, 58, 151–199. [Google Scholar] [CrossRef]
- Meena, V.D.; Dotaniya, M.L.; Coumar, V.; Rajendiran, S.; Ajay; Kundu, S.; Rao, A.S. A case for silicon fertilization to improve crop yields in tropical soils. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 505–518. [Google Scholar] [CrossRef]
- Chaiwong, N.; Prom-u-thai, C. Significant roles of silicon for improving crop productivity and factors affecting silicon uptake and accumulation in rice: A review. J. Soil Sci. Plant Nut. 2022, 22, 1970–1982. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Signifcance of silicon uptake transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani-Ueno, N. Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B 2001, 87, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Tamai, K.; Mitani, N. Genotypic difference in silicon uptake and expression of silicon transporter genes in rice. Plant Physiol. 2007, 145, 919–924. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Shivaraj, S.M.; Isenring, P.; Bélanger, R.R. Lsi2: A black box in plant silicon transport. Plant Soil. 2021, 466, 1–20. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Gómez-Trejo, L.F.; Escobar-Sepúlveda, H.F.; Gómez-Merino, G.M. The genetics of silicon accumulation in plants. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Etesami, H., Al-Saeedi, A.H., El-Ramady, H., Fujita, M., Pessarakli, M., Hossain, M.A., Eds.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2022; pp. 67–75. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Huang, S.; Yoshioka, Y.; Miyaji, T.; Ma, J.F. A silicon transporter gene required for healthy growth of rice on land. Nat. Commun. 2023, 14, 6522. [Google Scholar] [CrossRef]
- Gaur, S.; Kumar, J.; Kumar, D.; Chauhan, D.K.; Prasad, S.M.; Srivastava, P.K. Fascinating impact of silicon and silicon transporters in plants: A review. Ecotox. Environ. Saf. 2020, 202, 110885. [Google Scholar] [CrossRef]
- Hosseini, S.A. Silicon uptake, acquisition, and accumulation in plants. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Etesami, H., Al-Saeedi, A.H., El-Ramady, H., Fujita, M., Pessarakli, M., Hossain, M.A., Eds.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2022; pp. 37–42. [Google Scholar] [CrossRef]
- Huang, S.; Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Konishi, N.; Ma, J.F. A pericycle-localized silicon transporter for efficient xylem loading in rice. New Phytol. 2022, 234, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Ma, J.F. A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 2009, 21, 2878–2883. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.C.; Nikolic, M.; YE, M.J.; Xiao, Z.X.; Liang, Y.C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Wangkaew, B.; Prom-u-thai, C.; Jamjod, S.; Rerkasem, B.; Pusadee, T. Silicon concentration and expression of silicon transport genes in two Thai rice Varieties. CMU J. Nat. Sci. 2019, 18, 358–372. [Google Scholar] [CrossRef]
- Chaiwong, N.; Rerkasem, B.; Pusadee, T.; Prom-u-thai, C. Silicon application improves caryopsis development and yield in rice. J. Sci. Food Agric. 2021, 101, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, N.K.; Ziska, L.H. Rice: Importance for global nutrition. J Nutr Sci Vitaminol. 2019, 65, S2–S3. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Hashim, N.; Shamsudin, R.; Che Man, H. Rice for food security: Revisiting its production, diversity, rice milling process and nutrient content. Agriculture 2022, 12, 741. [Google Scholar] [CrossRef]
- Shao, G.N.; Tang, A.; Tang, S.Q.; Luo, J.; Jiao, G.A.; Wu, J.L.; Hu, P.S. A new deletion mutation of fragrant gene and the development of three molecular markers for fragrance in rice. Plant Breed. 2011, 130, 172–176. [Google Scholar] [CrossRef]
- Shan, Q.; Zhang, Y.; Chen, K.; Zhang, K.; Gao, C. Creation of fragrant rice by targeted knockout of the OsBADH2 gene using TALEN technology. Plant Biotechnol. J. 2015, 13, 791–800. [Google Scholar] [CrossRef]
- FAO, Markets and Trade. 2023. Available online: https://www.fao.org/markets-and-trade/commodities/rice/fao-rice-price-update/en/ (accessed on 18 July 2023).
- Shi, W.W.; Yang, Y.; Chen, S.H.; Xu, M.L. Discovery of a new fragrance allele and the development of functional markers for the breeding of fragrant rice varieties. Mol. Breed. 2018, 22, 185–192. [Google Scholar] [CrossRef]
- Singh, V.; Singh, V.; Singh, S.; Khanna, R. Effect of zinc and silicon on growth and yield of aromatic rice (Oryza Sativa) in north-western plains of India. J. Rice Res. Dev. 2020, 3, 82–86. [Google Scholar] [CrossRef]
- Paul, N.C.; Tasmim, M.T.; Imran, S.; Mahamud, M.A.; Chakrobortty, J.; Rabbi, R.H.M.; Sarkar, S.K.; Paul, S.K. Nutrient management in fragrant rice: A review. Agric. Sci. 2021, 12, 1538–1554. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O.; Turnbaugh, J.G. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar] [CrossRef]
- Mahatheeranont, S.; Keawsa-ard, S.; Dumri, K. Quantification of the rice aroma compound, 2-acetyl-1-pyrroline, in uncooked Khao Dawk Mali 105 brown rice. J. Agric. Food Chem. 2001, 49, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Shewfelt, R.L.; Lee, K.S.; Kays, S.J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 2008, 56, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, C.; Liu, K.; Liu, Q. Volatile Organic Compounds, Evaluation Methods and Processing Properties for Cooked Rice Flavor. Rice 2022, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Teng, C.S.; Chang, J.L.; Chuang, H.S.; Ho, C.T.; Wu, M.L. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J. Agric. Food Chem. 2008, 56, 7399–7404. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ashraf, U.; Tian, H.; Mo, Z.; Pan, S.; Anjum, S.A.; Duan, M.; Tang, X. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice. Plant Physiol. Biochem. 2016, 103, 167–175. [Google Scholar] [CrossRef]
- Bao, G.; Huang, S.; Ashraf, U.; Qiao, J.; Zheng, A.; Zhou, Q.; Li, L.; Wan, X. Insights of improved aroma under additional nitrogen application at booting stage in fragrant rice. Genes 2022, 13, 2092. [Google Scholar] [CrossRef]
- Imran, M.; Shafiq, S.; Ilahi, S.; Ghahramani, A.; Bao, G.; Dessoky, E.S.; Widemann, E.; Pan, S.; Mo, Z.; Tang, X. Post-transcriptional regulation of 2-acetyl-1-pyrroline (2-AP) biosynthesis pathway, silicon, and heavy metal transporters in response to Zn in fragrant rice. Front Plant Sci. 2022, 13, 948884. [Google Scholar] [CrossRef]
- Bradbury, L.M.T.; Gillies, S.A.; Brushett, D.J.; Waters, D.L.E.; Henry, R.J. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 2008, 68, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, T.L.; Waters, D.L.E.; Henry, R.J. The effect of salt on betaine aldehyde dehydrogenase transcript levels and 2-acetyl-1-pyrroline concentration in fragrant and non-fragrant rice (Oryza sativa). Plant Sci. 2008, 175, 539–546. [Google Scholar] [CrossRef]
- Sakthivel, K.; Sundaram, R.M.; Rani, N.S.; Balachandran, S.M.; Neeraja, C.N. Genetic and molecular basis of fragrance in rice. Biotechnol. Adv. 2009, 27, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Ashraf, U.; Wang, C.; He, L.; Wei, X.; Zheng, A.; Mo, Z.; Tang, X. Molecular basis for increased 2-acetyl-1-pyrroline contents under alternate wetting and drying (AWD) conditions in fragrant rice. Plant Physiol. Biochem. 2018, 133, 149–157. [Google Scholar] [CrossRef]
- Renuka, N.; Barvkar, V.T.; Ansari, Z.; Zhao, C.F.; Wang, C.L.; Zhang, Y.D.; Nadaf, A.B. Co-functioning of 2AP precursor amino acids enhances 2-acetyl-1-pyrroline under salt stress in aromatic rice (Oryza sativa L.) cultivars. Sci. Rep. 2022, 12, 3911. [Google Scholar] [CrossRef]
- Hinge, V.R.; Patil, H.B.; Nadaf, A.B. Aroma volatile analyses and 2AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (Oryza sativa L.) cultivars. Rice 2016, 9, 38. [Google Scholar] [CrossRef]
- Abdullah, E.H.E.; Misran, A.; Yaapar, M.N.; Yusop, M.R.; Ramli, A. The potential of silicon in improving rice yield, grain quality, and minimising chalkiness: A review. Pertanika J. Trop. Agric. Sci. 2021, 44, 655–672. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, X.; Sun, A.; Bai, C.; Li, Y.; Nuo, M.; Shen, X.; Li, W.; Wang, D.; Tian, P.; et al. Silicon nutrition improves the quality and yield of rice under dry cultivation. J. Sci. Food Agric. 2024, 104, 1897–1908. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Song, X.; Zhao, L.; Zhao, Q.; Chen, T.; Lu, K.; Zhu, Z.; Huang, S.; Wang, C. Silicon and Zinc Fertilizer Application Improves Grain Quality and Aroma in the japonica Rice Variety Nanjing 46. Foods 2024, 13, 152. [Google Scholar] [CrossRef]
- Praseartkul, P.; Taota, K.; Tisarum, R.; Sakulleerungroj, K.; Sotesaritkul, T.; Panya, A.; Phonsatta, N.; Cha-um, S. Foliar Silicon application regulates 2-acetyl-1-pyrroline enrichment and improves physio-morphological responses and yield attributes in Thai jasmine rice. Silicon 2022, 14, 6945–6955. [Google Scholar] [CrossRef]
- Chen, Y.J.; Dai, L.; Cheng, S.; Ren, Y.; Deng, H.; Wang, X.; Li, Y.; Wang, Z.; Mo, Z. Regulation of 2-acetyl-1-pyrroline and grain quality of early-season indica fragrant rice by nitrogen–silicon fertilization under different plantation methods. J. Integr. Agric. 2024, 23, 511–535. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Li, Y.; Xie, W.; Li, W.; Tang, X.; Asheaf, U.; Kong, L.; Wu, L.; Wang, S.; et al. Selenium-silicon (Se-Si) induced modulations in physio-biochemical responses, grain yield, quality, aroma formation and lodging in fragrant rice. Ecotox. Environ. Saf. 2020, 196, 110525. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Wang, W.; Jiang, S.; Xie, Y.; Cheng, Y.; Xu, J.; Xing, D. Silicon and selenium fertilizer management improved productivity and aroma of fragrant rice. Crop Sci. 2021, 61, 936–946. [Google Scholar] [CrossRef]
- Vanavichit, A.; Kamolsukyeunyong, W.; Siangliw, M.; Siangliw, J.L.; Traprab, S.; Ruengphayak, S.; Chaichoompu, E.; Saensuk, C.; Phuvanartnarubal, E.; Toojinda, T.; et al. Thai Hom Mali Rice: Origin and Breeding for Subsistence Rainfed Lowland Rice System. Rice 2018, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Chan-in, P.; Jamjod, S.; Yimyam, N.; Rerkasem, B.; Pusadee, T. Grain quality and allelic variation of the Badh2 gene in Thai fragrant rice landraces. Agronomy 2020, 10, 779. [Google Scholar] [CrossRef]
- Juliano, B.O.; Villareal, C.P. Grain Quality Evaluation of World Rices; International Rice Research Institute: Manila, Philippines, 1993. [Google Scholar]
- Dela, C.N.; Khush, G.S. Rice Grain Quality Evaluation Procedures. In Aromatic Rices; Singh, R.K., Singh, U.S., Khush, G.S., Eds.; Oxford and IBH Pulishing Co. Pvt. Ltd: New Delhi, India, 2000; pp. 16–28. [Google Scholar]
- Cuong, T.X.; Ullah, H.; Datta, A.; Hanh, T.C. Effects of silicon-based fertilizer on growth, yield and nutrient uptake of rice in tropical zone of Vietnam. Rice Sci. 2017, 24, 283–290. [Google Scholar] [CrossRef]
- Chaiwong, N.; Pusadee, T.; Jamjod, S.; Prom-U-Thai, C. Silicon application promotes productivity, silicon accumulation and upregulates silicon transporter gene expression in rice. Plants 2022, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.; Sanglard, L.M.V.P.; Reis, J.V.; Detmann, E.; Rodrigues, F.A.; Nunes-Nesi, A.; Fernie, A.R.; DaMatta, F.M. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef]
- Tamai, K.; Ma, J.F. Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 2008, 307, 21–27. [Google Scholar] [CrossRef]
- Lavinsky, A.O.; Detmann, K.C.; Reis, J.V.; Ávila, R.T.; Sanglard, M.L.; Pereira, L.F.; Sanglard, L.M.V.P.; Rodrigues, F.A.; Araújo, W.L.; DaMatta, F.M. Silicon improves rice grain yield and photosynthesis specifically when supplied during the reproductive growth stage. J. Plant Physiol. 2016, 206, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Ma, J.F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11401–11406. [Google Scholar] [CrossRef] [PubMed]
- Tejakum, P.; Khumto, S.; Jamjod, S.; Yimyam, N.; Pusadee, T. Yield, grain quality and fragrance of a highland fragrant rice landrace variety, Bue Ner Moo. Khon Kaen Agric. J. 2019, 47, 317–326. [Google Scholar]
- Leesawatwong, M.; Jamjod, S.; Rerkasem, B. Determinants of a premium priced special quality rice. Int. Rice Res. Notes 2003, 28, 34. [Google Scholar]
- Leesawatwong, M.; Jamjod, S.; Kuo, J.; Dell, B.; Rerkasem, B. Nitrogen fertilizer increases seed protein and milling quality of rice. Cereal Chem. 2005, 82, 588–593. [Google Scholar] [CrossRef]
- OAE. Agricultural Economic Information by Crop 2563 (In Thai). Available online: https://www.oae.go.th (accessed on 18 August 2023).
- Boontakham, P.; Sookwong, P.; Jongkaewwattana, S.; Wangtueai, S.; Mahatheeranont, S. Comparison of grain yield and 2-acetyl-1-pyrroline (2AP) content in leaves and grain of two Thai fragrant rice cultivars cultivated at greenhouse and open-air conditions. Aust. J. Crop Sci. 2019, 13, 159–169. [Google Scholar] [CrossRef]
- Mattioli, R.; Falasca, G.; Sabatini, S.; Altamura, M.M.; Costantino, P.; Trovato, M. The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol. Plantarum. 2009, 137, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, L.M.T.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.; Waters, D.L.E. The gene for fragrance in rice. Plant Biotechnol. J. 2005, 3, 363–370. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Shi, W.; Ji, Q.; He, F.; Zhang, Z.; Cheng, Z.; Liu, X.; Xu, M. Badh2, Encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar] [CrossRef]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Du, B.; He, L.; He, J.; Hu, L.; Pan, S.; Tang, X. Exogenous application of zinc (Zn) at the heading stage regulates 2-acetyl-1-pyrroline (2-AP) biosynthesis in different fragrant rice genotypes. Sci. Rep. 2019, 9, 19513. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.M.; Zhang, K.Q.; Duan, B.W.; Sun, C.X.; Zheng, K.L.; Cai, R.; Jiieyun, Z. Rapid determination of silicon content in rice. Rice Sci. 2005, 12, 145–147. [Google Scholar]
- Elliott, C.L.; Snyder, G.H. Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J. Agric. Food Chem. 1991, 39, 1118–1119. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Juliano, B. A simplified assay for milled-rice amylose. Cereal Sci. Today 1971, 16, 334–360. [Google Scholar]
- Luo, H.; Duan, M.; Xing, P.; Xie, H.; Tang, X. Foliar application of procyanidins enhanced the biosynthesis of 2-acetyl-1-pyrroline in aromatic rice (Oryza sativa L.). BMC Plant Biol. 2022, 22, 376. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Cha-um, S.; Supaibulwatana, K. Proline related genes expression and physiological changes in indica rice response to water-deficit stress. Plant Omics 2012, 5, 597–603. [Google Scholar]





| Variety (V) | Silicon Fertilizer (Si) | Plant Height (cm) | Number of Tiller Plant−1 | Number of Panicle | Number of Spikelet Panicle−1 | Filled Grains (%) | 1000-Grain Weight (g) |
|---|---|---|---|---|---|---|---|
| BNM4 | Si0 | 97.7 ± 3.13 d–f | 4 ± 0.31 | 4 ± 0.22 | 117.0 ± 6.11 f | 66.5 ± 3.89 h | 35.0 ± 0.74 bc |
| Si75 | 104.2 ± 2.20 bc | 4 ± 0.17 | 4 ± 0.26 | 142.3 ± 2.84 bc | 65.5 ± 1.46 h | 35.6 ± 1.50 b | |
| Si150 | 99.3 ± 2.84 c–e | 4 ± 0.21 | 4 ± 0.17 | 150.2 ± 4.94 b | 75.5 ± 0.72 fg | 35.8 ± 0.75 b | |
| Si225 | 98.7 ± 3.02 c–e | 5 ± 0.22 | 4 ± 0.26 | 142.7 ± 3.68 bc | 78.9 ± 1.75 d–f | 35.0 ± 0.98 bc | |
| KDML105 | Si0 | 110.0 ± 1.71 b | 4 ± 0.17 | 4 ± 0.17 | 133.8 ± 10.14 c–e | 78.4 ± 2.13 ef | 31.5 ± 0.38 fg |
| Si75 | 122.7 ± 0.92 a | 4 ± 0.21 | 4 ± 0.31 | 171.0 ± 4.90 a | 84.4 ± 1.77 b–d | 32.1 ± 0.42 e–g | |
| Si150 | 125.8 ± 1.23 a | 4 ± 0.21 | 4 ± 0.31 | 169.3 ± 2.94 a | 86.8 ± 1.75 ab | 31.4 ± 0.25 fg | |
| Si225 | 126.5 ± 1.98 a | 4 ± 0.21 | 4 ± 0.22 | 168.2 ± 4.28 a | 90.7 ± 1.11 a | 33.0 ± 0.81 c–f | |
| PTT1 | Si0 | 97.7 ± 2.39 d–f | 4 ± 0.17 | 4 ± 0.17 | 101.7 ± 3.23 g | 80.0 ± 1.50 d–f | 32.3 ± 0.53 d–g |
| Si75 | 77.6 ± 1.45 g | 4 ± 0.21 | 4 ± 0.17 | 121.0 ± 4.20 ef | 85.9 ± 1.56 a–c | 33.3 ± 0.69 c–f | |
| Si150 | 75.3 ± 1.25 g | 5 ± 0.26 | 5 ± 0.33 | 125.0 ± 2.12 d–f | 80.7 ± 1.23 c–f | 33.3 ± 0.85 c–f | |
| Si225 | 74.4 ± 1.46 g | 5 ± 0.21 | 5 ± 0.22 | 128.8 ± 6.03 c–f | 79.1 ± 3.03 d–f | 34.2 ± 0.62 b–d | |
| SPR1 | Si0 | 92.7 ± 2.02 f | 5 ± 0.22 | 4 ± 0.21 | 121.2 ± 6.81 ef | 66.6 ± 1.63 h | 29.0 ± 0.59 h |
| Si75 | 101.1 ± 2.24 cd | 4 ± 0.17 | 4 ± 0.17 | 141.8 ± 5.98 bc | 70.5 ± 1.32 gh | 30.4 ± 0.66 gh | |
| Si150 | 94.2 ± 2.02 ef | 4 ± 0.21 | 4 ± 0.17 | 134.0 ± 4.49 c–e | 83.7 ± 1.33 b–e | 32.8 ± 0.65 d–f | |
| Si225 | 98.4 ± 1.48 c–f | 4 ± 0.21 | 4 ± 0.17 | 136.2 ± 6.42 b–d | 82.2 ± 2.94 b–e | 33.8 ± 0.70 b–e | |
| Analysis of variance | |||||||
| Variety (Var) | *** | ns | ns | *** | *** | *** | |
| Silicon fertilizer application (Si) | ns | ns | ns | *** | *** | *** | |
| Var × Si | *** | ns | ns | *** | *** | ns | |
| LSD0.05 (V) | 2.94 | - | - | 7.48 | 2.79 | 1.05 | |
| LSD0.05 (Si) | - | - | - | 7.49 | 2.79 | 1.05 | |
| LSD0.05 (V × Si) | 5.88 | - | - | 14.95 | 5.57 | - | |
| Proline Concentration (µmoles/g FW) | RQBadh2 Gene | RQDAO Gene | RQOAT Gene | RQP5CS Gene | Rqprodh Gene | |
|---|---|---|---|---|---|---|
| RQBadh2 gene | 0.17 ns | |||||
| RQDAO gene | 0.20 ns | 0.34 * | ||||
| RQOAT gene | 0.41 * | 0.11 ns | 0.59 *** | |||
| RQP5CS gene | 0.44 * | 0.44 ** | 0.45 *** | 0.26 ns | ||
| RQProDH gene | 0.34 * | 0.22 ns | 0.06 ns | −0.05 ns | 0.23 ns | |
| 2AP concentration (ppm) | 0.16 ns | 0.30 * | 0.34 * | −0.00 ns | 0.68 *** | 0.38 ** |
| Genes | Primer | References | |
|---|---|---|---|
| OsLsi6 | F: | AGATCGTCGTCACCTTCAACAT | [25] |
| R: | CTTGAAGGAGGAGAGCTTCTGG | ||
| DAO | F: | TGGCAAGATAGAAGCAGAAGT | [44] |
| R: | GTCCATACGGGCAACAAA | ||
| Badh2 | F: | TGTGCTAAACATAGTGACTGGA | [51] |
| R: | CTTAACCATAGGAGCAGCT | ||
| OAT | F: | ATGAAATGATGTTGCCGATGA | [84] |
| R: | CCTAATGTCCGACCATGAAAA | ||
| ProDH | F: | ATTGCTCTCGTCTTCCTCCT | [85] |
| R: | ATGACTCGATCGCTTCACTC | ||
| P5CS | F: | TGGCAATTCGAAGTGGTAAT | |
| R: | AGCAAATCTGCGATCTCATC | ||
| OsActin (housekeeping gene) | F: | GACTCTGGTGATGGTGTCAGC | [51] |
| R: | GGCTGGAAGAGGACCTCAGG | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan-in, P.; Jamjod, S.; Prom-u-thai, C.; Rerkasem, B.; Russell, J.; Pusadee, T. Application of Silicon Influencing Grain Yield and Some Grain Quality Features in Thai Fragrant Rice. Plants 2024, 13, 1336. https://doi.org/10.3390/plants13101336
Chan-in P, Jamjod S, Prom-u-thai C, Rerkasem B, Russell J, Pusadee T. Application of Silicon Influencing Grain Yield and Some Grain Quality Features in Thai Fragrant Rice. Plants. 2024; 13(10):1336. https://doi.org/10.3390/plants13101336
Chicago/Turabian StyleChan-in, Phukjira, Sansanee Jamjod, Chanakan Prom-u-thai, Benjavan Rerkasem, Joanne Russell, and Tonapha Pusadee. 2024. "Application of Silicon Influencing Grain Yield and Some Grain Quality Features in Thai Fragrant Rice" Plants 13, no. 10: 1336. https://doi.org/10.3390/plants13101336
APA StyleChan-in, P., Jamjod, S., Prom-u-thai, C., Rerkasem, B., Russell, J., & Pusadee, T. (2024). Application of Silicon Influencing Grain Yield and Some Grain Quality Features in Thai Fragrant Rice. Plants, 13(10), 1336. https://doi.org/10.3390/plants13101336








