Mineral Particles in Foliar Fertilizer Formulations Can Improve the Rate of Foliar Uptake
Abstract
:1. Introduction
2. Results
2.1. Geochemical Modelling for Assessing the Solubility of Mineral Particles
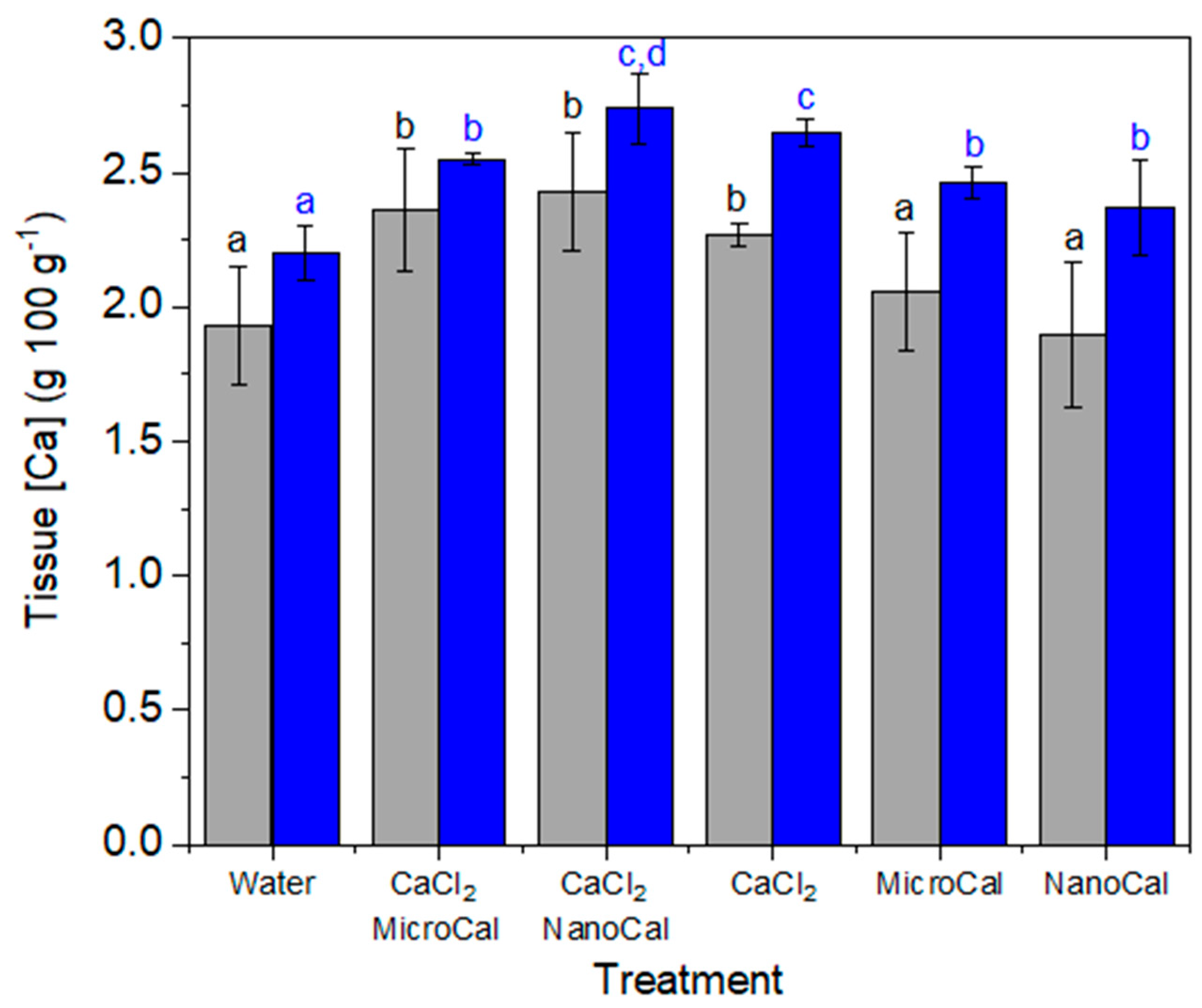
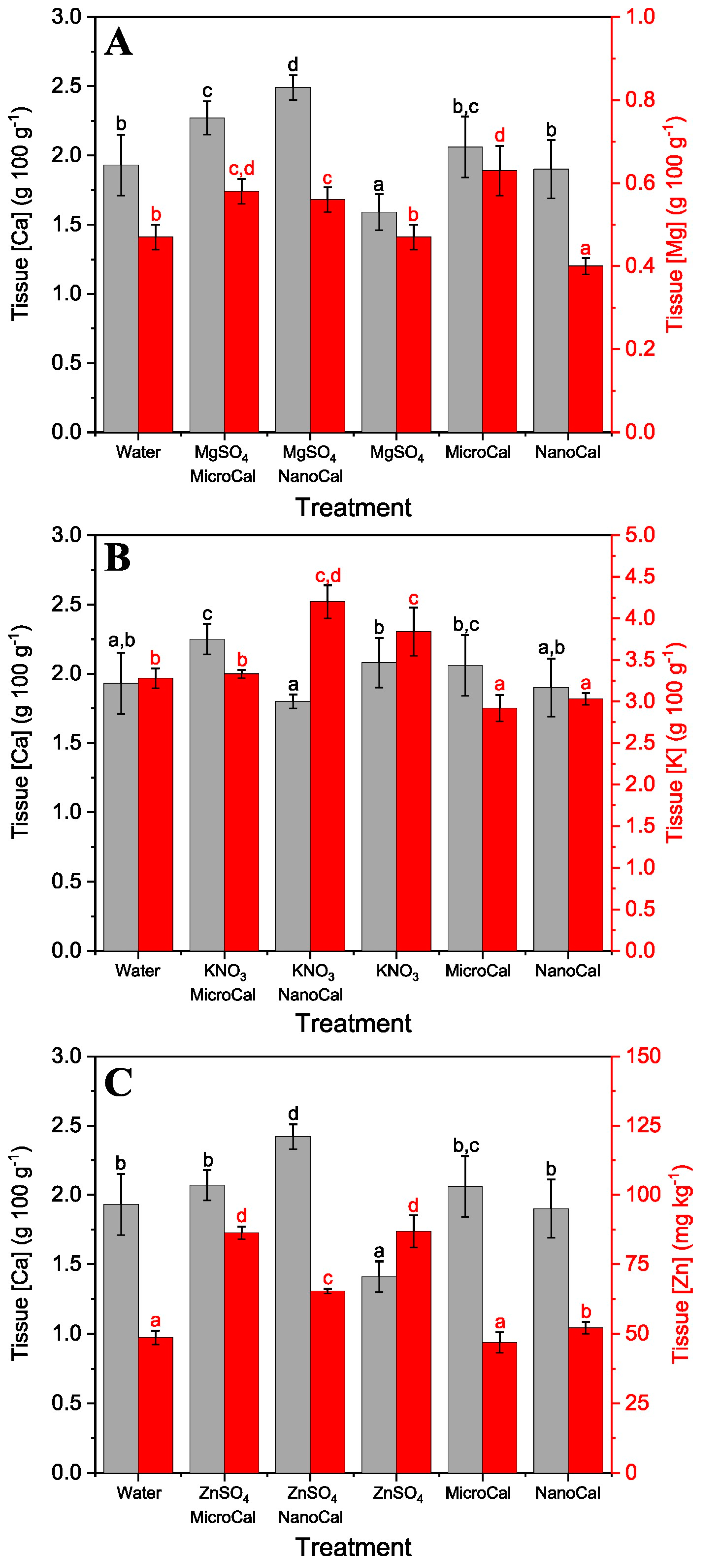
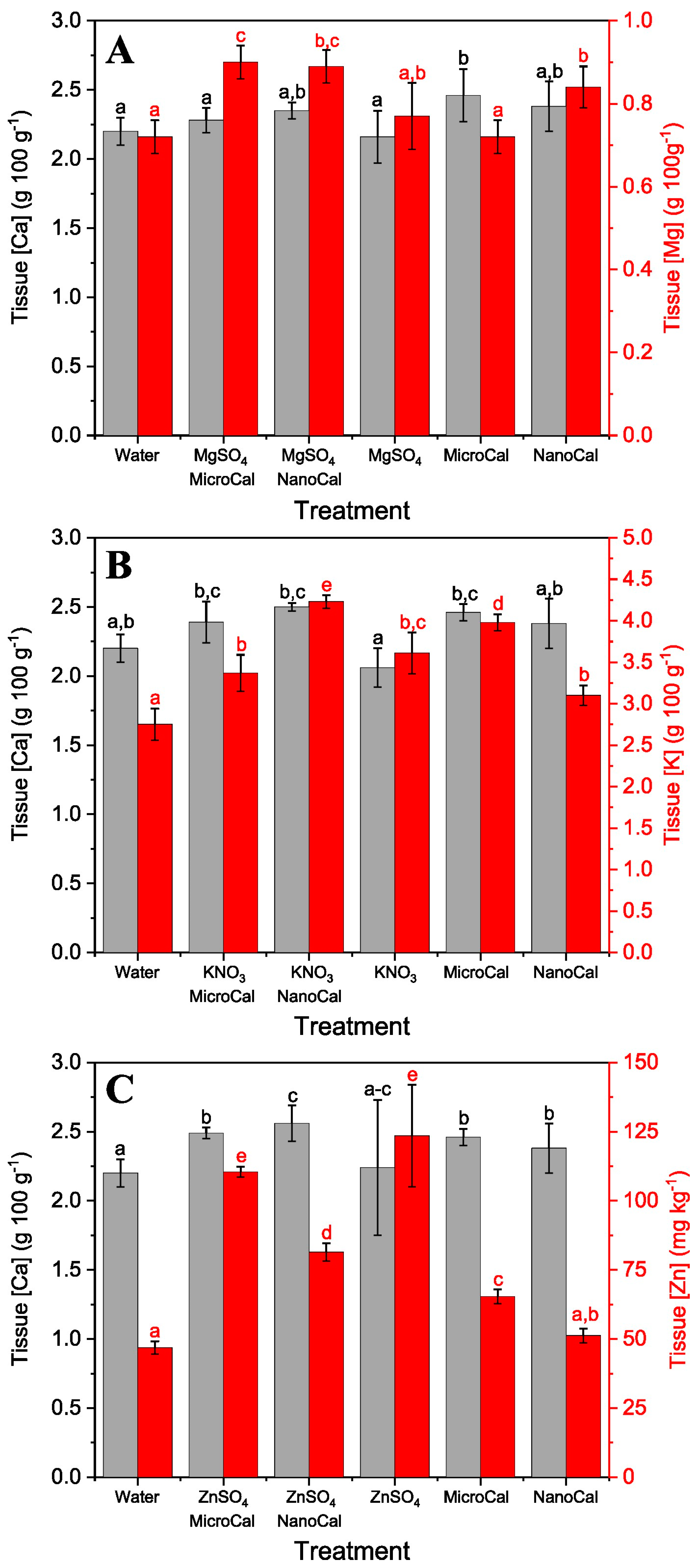
2.2. Foliar Uptake Efficiency of Spray Formulations Containing Carbonate Mineral Particles
Foliar Ca Absorption of Calcite Particles plus Model Essential Element Salts
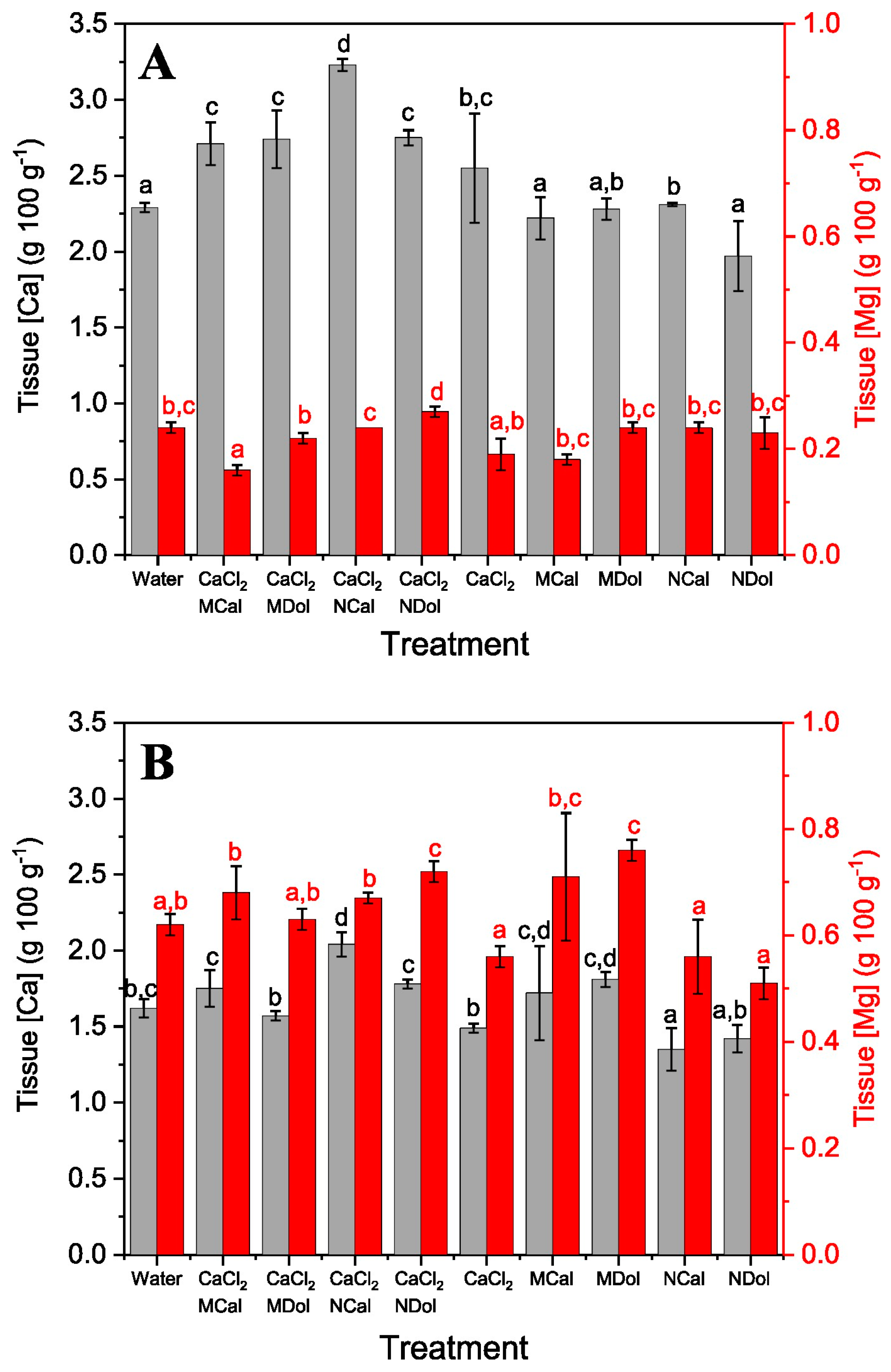
2.3. Effect of Dolomite and Calcite Particle Foliar Application in Combination with CaCl2
2.4. Foliar Ca Absorption of CaCl2 plus Calcite Particles: Effect of Leaf Re-Wetting
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Foliar Formulations
5.2. Geochemical Formulation Modelling
5.3. Foliar Application Trials
5.4. Tissue Analysis
5.5. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández, V.; Brown, P.H. From Plant Surface to Plant Metabolism: The Uncertain Fate of Foliar-Applied Nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Fernández, V.; Gil-Pelegrín, E.; Eichert, T. Foliar Water and Solute Absorption: An Update. Plant J. 2021, 105, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Holloway, P.J.; Butler Ellis, M.C.; Webb, D.A.; Western, N.M.; Tuck, C.R.; Hayes, A.L.; Miller, P.C.H. Effects of Some Agricultural Tank-Mix Adjuvants on the Deposition Efficiency of Aqueous Sprays on Foliage. Crop Prot. 2000, 19, 27–37. [Google Scholar] [CrossRef]
- Schmitz-Eiberger, M.A.; Haefs, R.; Noga, G.J. Enhancing Biological Efficacy and Rainfastness of Foliar Applied Calcium Chloride Solutions by Addition of Rapeseedoil Surfactants. J. Plant Nutr. Soil Sci. 2002, 165, 634–639. [Google Scholar] [CrossRef]
- Baur, P.; Pontzen, R. Basic Features of Plant Surface Wettability and Deposit Formation and the Impact of Adjuvants. In Proceedings of the 8th International Symposium on Adjuvants for Agrochemicals, ISAA2007, Columbus, OH, USA, 6–9 August 2007; International Society for Agrochemical Adjuvants: Bordeaux, France, 2007; pp. 1–23, ISBN 978-0-473-12388-8. [Google Scholar]
- Alexander, A.; Hunsche, M. Influence of Formulation on the Cuticular Penetration and on Spray Deposit Properties of Manganese and Zinc Foliar Fertilizers. Agronomy 2016, 6, 39. [Google Scholar] [CrossRef]
- Burkhardt, J.; Eiden, R. Thin Water Films on Coniferous Needles: A New Device for the Study of Water Vapour Condensation and Gaseous Deposition to Plant Surfaces and Particle Samples. Atmos. Environ. 1994, 28, 2001–2011. [Google Scholar] [CrossRef]
- Schönherr, J. Cuticular Penetration of Calcium Salts: Effects of Humidity, Anions, and Adjuvants. J. Plant Nutr. Soil Sci. 2001, 164, 225–231. [Google Scholar] [CrossRef]
- Fernández, V.; Pimentel, C.; Bahamonde, H.A. Salt Hydration and Drop Drying of Two Model Calcium Salts: Implications for Foliar Nutrient Absorption and Deposition. J. Plant Nutr. Soil Sci. 2020, 183, 592–601. [Google Scholar] [CrossRef]
- Bahamonde, H.A.; Pimentel, C.; Lara, L.A.; Bahamonde-Fernández, V.; Fernández, V. Foliar Application of Potassium Salts to Olive, with Focus on Accompanying Anions. Plants 2023, 12, 472. [Google Scholar] [CrossRef]
- Barlas, N.T.; Bahamonde, H.A.; Pimentel, C.; Domínguez-Huidobro, P.; Pina, C.M.; Fernández, V. Evaluating Leaf Wettability and Salt Hygroscopicity as Drivers for Foliar Absorption. Plants 2023, 12, 2357. [Google Scholar] [CrossRef] [PubMed]
- Räsch, A.; Hunsche, M.; Mail, M.; Burkhardt, J.; Noga, G.; Pariyar, S. Agricultural Adjuvants May Impair Leaf Transpiration and Photosynthetic Activity. Plant Physiol. Biochem. 2018, 132, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Bakry, B.A. Zinc-oxide and Nano ZnO Oxide Effects on Growth, some Biochemical Aspects, Yield Quantity, and Quality of flax (Linum uitatissimum L.) in Absence and Presence of Compost Under Sandy Soil. Bull. Nat. Res. Centre 2020, 44, 98. [Google Scholar] [CrossRef]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar Application of Zinc Oxide Nanoparticles: An Effective Strategy to Mitigate Drought Stress in Cucumber Seedling by Modulating Antioxidant Defense System and Osmolytes Accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef] [PubMed]
- Okey-Onyesolu, C.F.; Hassanisaadi, M.; Bilal, M.; Barani, M.; Rahdar, A.; Iqbal, J.; Kyzas, G.Z. Nanomaterials as Nanofertilizers and Nanopesticides: An Overview. ChemistrySelect 2021, 6, 8645–8663. [Google Scholar] [CrossRef]
- Kaiser, H. Stomatal Uptake of Mineral Particles from a Sprayed Suspension Containing an Organosilicone Surfactant. J. Plant Nutr. Soil Sci. 2014, 177, 869–874. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Lombi, E.; Cheng, M.; Tang, C.; Howard, D.L.; Menzies, N.W.; Kopittke, P.M. Absorption of Foliar-Applied Zn Fertilizers by Trichomes in Soybean and Tomato. J. Exp. Bot. 2018, 69, 2717–2729. [Google Scholar] [CrossRef]
- Szameitat, A.E.; Sharma, A.; Minutello, F.; Pinna, A.; Er-Rafik, M.; Hansen, T.H.; Persson, D.P.; Andersen, B.; Husted, S. Unravelling the Interactions between Nano-Hydroxyapatite and the Roots of Phosphorus Deficient Barley Plants. Environ. Sci. Nano 2021, 8, 444–459. [Google Scholar] [CrossRef]
- Huang, X.; Keller, A.A. Metabolomic Response of Early-Stage Wheat (Triticum aestivum) to Surfactant-Aided Foliar Application of Copper Hydroxide and Molybdenum Trioxide Nanoparticles. Nanomaterials 2021, 11, 3073. [Google Scholar] [CrossRef]
- Santos, E.; Montanha, G.S.; Gomes, M.H.F.; Duran, N.M.; Corrêa, C.G.; Romeu, S.L.Z.; Pereira, A.E.S.; Oliveira, J.L.; Almeida, E.; Pérez-de-Luque, A.; et al. Are Nanomaterials Leading to More Efficient Agriculture? Outputs from 2009 to 2022 Research Metadata Analysis. Environ. Sci. Nano 2022, 9, 3711–3724. [Google Scholar] [CrossRef]
- Husted, S.; Minutello, F.; Pinna, A.; Tougaard, S.L.; Møs, P.; Kopittke, P.M. What Is Missing to Advance Foliar Fertilization Using Nanotechnology? Trends Plant Sci. 2023, 28, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, T.N.M.; Savassa, S.M.; Gomes, M.H.F.; Rodrigues, E.S.; Duran, N.M.; de Almeida, E.; Martinelli, A.P.; de Carvalho, H.W.P. Shedding Light on the Mechanisms of Absorption and Transport of ZnO Nanoparticles by Plants via in Vivo X-Ray Spectroscopy. Environ. Sci. Nano 2017, 4, 2367–2376. [Google Scholar] [CrossRef]
- Sadak, M.S.; Bakr, B.A.; El, M.F. Agrophysiological variation in flax affected by folic acid and Ag nanoparticles foliar applications. Asian J. Plant Sci. 2022, 21, 339–346. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Lombi, E.; Wu, J.; Blamey, F.P.C.; Fernández, V.; Howard, D.L.; Menzies, N.W.; Kopittke, P.M. Absorption of Foliar Applied Zn Is Decreased in Zn Deficient Sunflower (Helianthus annuus) Due to Changes in Leaf Properties. Plant Soil 2018, 433, 309–322. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, Translocation, and Transformation of Metal-Based Nanoparticles in Plants: Recent Advances and Methodological Challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of Zinc Oxide Nanoparticle Entry into Wheat Seedling Leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium Translocation to Fleshy Fruit: Its Mechanism and Endogenous Control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Rengel, Z.; Cakmak, I.; White, P.J.; Marschner, H. (Eds.) Marschner’s Mineral Nutrition of Plants, 4th ed.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2023; ISBN 978-0-12-819773-8. [Google Scholar]
- Sadak, M.S.; Hanafy, R.S.; Elkady, F.M.A.M.; Mogazy, A.M.; Abdelhamid, M.T. Exogenous Calcium Reinforces Photosynthetic Pigment Content and Osmolyte, Enzymatic, and Non-Enzymatic Antioxidants Abundance and Alleviates Salt Stress in Bread Wheat. Plants 2023, 12, 1532. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Nuzzo, V.; Clearwater, M.J.; Xiloyannis, C. Internal versus External Control of Calcium Nutrition in Kiwifruit. J. Plant Nutr. Soil Sci. 2014, 177, 819–830. [Google Scholar] [CrossRef]
- Bonomelli, C.; Alcalde, C.; Aguilera, C.; Videla, X.; Rojas-Silva, X.; Nario, A.; Fernández, V. Absorption and Mobility of Radio-Labelled Calcium in Chili Pepper Plants and Sweet Cherry Trees. Sci. Agric. 2020, 78, e20200092. [Google Scholar] [CrossRef]
- Bonomelli, C.; Fernández, V.; Martiz, J.; Videla, X.; Arias, M.I.; Rojas-Silva, X.; Nario, A. Absorption and Distribution of Root, Fruit, and Foliar-Applied 45 Ca in “Clemenules” Mandarin Trees. J. Sci. Food Agric. 2020, 100, 4643–4650. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Vasilakakis, M.; Diamantidis, G.; Mignani, I. Diverse Metabolism of Cell Wall Components of Melting and Non-Melting Peach Genotypes during Ripening after Harvest or Cold Storage. J. Sci. Food Agric. 2006, 86, 243–250. [Google Scholar] [CrossRef]
- Torres, E.; Recasens, I.; Lordan, J.; Alegre, S. Combination of strategies to supply calcium and reduce bitter pit in ‘Golden Delicious’ apples. Sci. Hortic. 2017, 217, 179–188. [Google Scholar] [CrossRef]
- Val, J.; Fernández, V. In-Season Calcium-Spray Formulations Improve Calcium Balance and Fruit Quality Traits of Peach. J. Plant Nutr. Soil Sci. 2011, 174, 465–472. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size Exclusion Limits and Lateral Heterogeneity of the Stomatal Foliar Uptake Pathway for Aqueous Solutes and Water-Suspended Nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Sharma, N.; Allardyce, B.J.; Rajkhowa, R.; Agrawal, R. Controlled release fertilizer delivery system derived from rice straw cellulose nanofibres: A circular economy based solution for sustainable development. Bioengineered 2023, 14, 2242124. [Google Scholar] [CrossRef]
- Agrawal, R.; Bhagia, S.; Satlewal, A.; Ragauskas, A.J. Urban mining from biomass, brine, sewage sludge, phosphogypsum and e-waste for reducing the environmental pollution: Current status of availability, potential, and technologies with a focus on LCA and TEA. Environ. Res. 2023, 224, 115523. [Google Scholar] [CrossRef]
- Kaur, P.; Bohidar, H.B.; Nisbet, D.R.; Pfeffer, F.M.; Rifai, A.; Williams, R.; Agrawal, R. Waste to high-value products: The performance and potential of carboxymethylcellulose hydrogels via the circular economy. Cellulose 2023, 30, 2713–2730. [Google Scholar] [CrossRef]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Blanco, A.; Fernández, V.; Val, J. Improving the performance of calcium-containing spray formulations to limit the incidence of bitter pit in apple (Malus x domestica Borkh.). Sci. Hortic. 2010, 127, 23–28. [Google Scholar] [CrossRef]
- Sharma, N.; Allardyce, B.J.; Rajkhowa, R.; Agrawal, R. Delivery of Agrochemicals and Nutrients Through a Biopolymer-Based System Derived from Lignocellulosic Rice Straw. J. Polym. Environ. 2023, 31, 4606–4620. [Google Scholar] [CrossRef]
- Plummer, L.N.; Wigley, T.M.L.; Parkhurst, D.L. The Kinetics of Calcite Dissolution in CO2 -Water Systems at 5 Degrees to 60 Degrees C and 0.0 to 1.0 Atm CO2. Am. J. Sci. 1978, 278, 179–216. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. U.S. Geological Survey Techniques and Methods 6, Chapter A43; U.S. Geological Survey Techniques and Methods; USGS: Reston, VA, USA, 2013. [Google Scholar]
- Wang, Q.C.; Yang, X.D.; Shang, G.R. Hydrophilic Mechanism of sunflower leaves and simulation research. Adv. Mater. Res. 2014, 900, 275–278. [Google Scholar] [CrossRef]
- Werker, E.; Putievsky, E.; Ravid, U.; Dudai, N.; Katzir, I. Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae). Ann. Bot. 1993, 71, 43–50. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Yu, C.; Luo, S.; Peng, J.; Li, N.; Wang, T.; Jiang, L.; Dong, Z.; Wang, Y. Sustained Agricultural Spraying: From Leaf Wettability to Dynamic Droplet Impact Behavior. Glob. Chall. 2023, 7, 2300007. [Google Scholar] [CrossRef]
- Truschi, S.; Baldi, A.; Bruschi, P.; Cacciari, I.; Marvasi, M.; Lenzi, A. Foliar Roughness and Water Content Impact on Escherichia coli Attachment in Baby Leafy Greens. Biology 2023, 12, 102. [Google Scholar] [CrossRef]
| Compound | Solution | pHinitial | [Ca] (mmol L−1) | pHequilibrium | ||
|---|---|---|---|---|---|---|
| Without CO2 | With CO2 | Without CO2 | With CO2 | |||
| Calcite (CaCO3) | Pure water | 7.0 | 0.12 | 0.49 | 9.9 | 8.3 |
| 150 mM CaCl2 | 6.9 | 151.63 | 151.72 | 8.6 | 7.3 | |
| 150 mM MgSO4 | 6.7 | 0.88 | 1.42 | 9.4 | 8.4 | |
| 150 mM KNO3 | 7.0 | 0.24 | 0.76 | 10.0 | 8.4 | |
| 2 mM ZnSO4 | 6.0 | 1.26 | 1.28 | 8.3 | 8.1 | |
| Dolomite (CaMg(CO3)2) | Pure water | 7.0 | 0.08 | 0.30 | 10.0 | 8.36 |
| 150 mM CaCl2 | 6.9 | 151.73 | 151.89 | 9.3 | 7.9 | |
| Tissue [Ca] (g 100 g−1) | ||
|---|---|---|
| Treatment | No Re-Wetting | Re-Wetting |
| Water | 0.79 ± 0.07 a | - |
| MicroCal | 0.83 ± 0.13 a | 0.75 ± 0.06 a |
| MicroCal + CaCl2 | 1.14 ± 0.10 c | 1.13 ± 0.27 b |
| NanoCal | 0.81 ± 0.06 a | 0.82 ± 0.05 a |
| NanoCal + CaCl2 | 1.42 ± 0.32 d | 1.39 ± 0.13 c |
| CaCl2 | 0.98 ± 0.07 b | 1.29 ± 0.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel, C.; Pina, C.M.; Müller, N.; Lara, L.A.; Melo Rodriguez, G.; Orlando, F.; Schoelkopf, J.; Fernández, V. Mineral Particles in Foliar Fertilizer Formulations Can Improve the Rate of Foliar Uptake. Plants 2024, 13, 71. https://doi.org/10.3390/plants13010071
Pimentel C, Pina CM, Müller N, Lara LA, Melo Rodriguez G, Orlando F, Schoelkopf J, Fernández V. Mineral Particles in Foliar Fertilizer Formulations Can Improve the Rate of Foliar Uptake. Plants. 2024; 13(1):71. https://doi.org/10.3390/plants13010071
Chicago/Turabian StylePimentel, Carlos, Carlos M. Pina, Nora Müller, Luis Adrián Lara, Gabriela Melo Rodriguez, Fabrizio Orlando, Joachim Schoelkopf, and Victoria Fernández. 2024. "Mineral Particles in Foliar Fertilizer Formulations Can Improve the Rate of Foliar Uptake" Plants 13, no. 1: 71. https://doi.org/10.3390/plants13010071
APA StylePimentel, C., Pina, C. M., Müller, N., Lara, L. A., Melo Rodriguez, G., Orlando, F., Schoelkopf, J., & Fernández, V. (2024). Mineral Particles in Foliar Fertilizer Formulations Can Improve the Rate of Foliar Uptake. Plants, 13(1), 71. https://doi.org/10.3390/plants13010071








